To the Editor:
Survival after intensive induction chemotherapy for patients with newly diagnosed, previously untreated acute myeloid leukemia (AML) is limited by both treatment-related toxicity and disease resistance to chemotherapy. Early post-treatment mortality, defined as death within 2 months of treatment initiation, ranges from less than 10% to greater than 50%, depending on the patient population, and has been associated with older age and poor performance status, secondary AML, higher hematopoietic cell transplantation comorbidity index score, unfitness by Ferrara criteria,1,2 and abnormal pretreatment laboratory values.3 Limitations of prior analyses include a selective focus on either early post-treatment mortality or on overall survival (OS), an absence of genetic annotation, and reliance on selected clinical trial cohorts which may not be broadly representative of treated AML patients. Moreover, early mortality remains a consequential problem even among patients predicted to have lower risk based on Ferrara criteria.2 In order to identify distinct factors associated with early post-treatment mortality and with OS, we analyzed an unselected, consecutive series of generally fit, previously untreated adult AML patients treated with intensive induction chemotherapy.
We identified 290 adult patients who received intensive induction chemotherapy as the first treatment for newly diagnosed AML at our institution from 2014 to 2019. Patients received “high-dose” anthracycline (60–90 mg/m2 for 3 days), “standard-dose” cytarabine (100–200 mg/m2 for 7 days), and may have received an additional agent, in some cases on a clinical trial (eg FLT3 inhibitor). We collected pretreatment clinical, pathology, and laboratory data, and annotated clinical outcomes. Gene mutations were determined at the time of diagnosis using targeted next-generation sequencing of genes recurrently mutated in AML. We used univariate and multivariate regression models to identify associations between pretreatment clinical or genetic factors and clinical outcomes, including the proportion of patients alive at 60 days, the proportion of patients in remission at 60 days, OS landmarked at 60 days, and relapse-free survival (RFS) landmarked at 60 days (Supplementary Methods).
The median age at diagnosis was 61 years (range 19–76); 15% of patients had clinically defined secondary AML and 14% had therapy-related AML (Table S1). Only 2 of 290 patients (0.7%) were identified as unfit by Ferrara criteria.1 CR was reported in 72% (209/290) of patients, of whom 194 had CR with hematologic recovery and 15 had CR with incomplete hematologic recovery. For patients surviving past the first 60 days, median OS and RFS, both landmarked at day 60, were 33.9 months and 28.0 months, respectively.
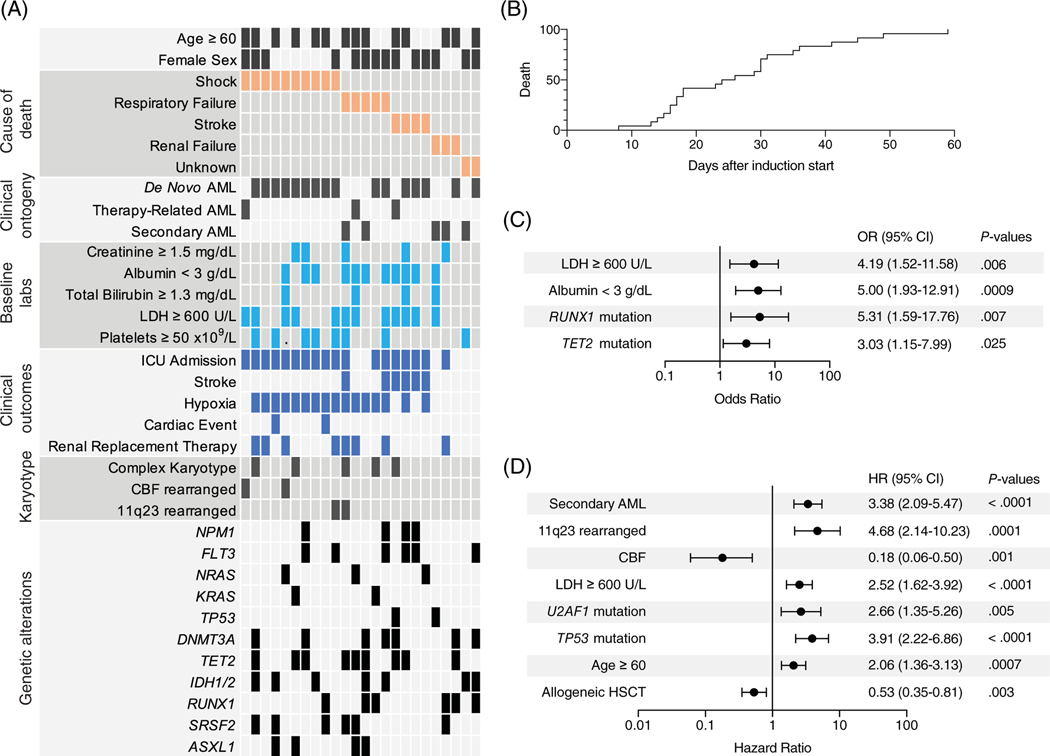
In total, 24 of 290 (8%) patients died within 60 days of starting induction chemotherapy (Figure 1A). Among these patients, median time to death from the start of induction was 25 days; 17% of patients died within the first 15 days, and 71% died within the first 30 days (Figure 1B). Shock (predominantly septic shock) was the most common primary cause of death (42%), followed by respiratory failure (21%) and stroke (hemorrhagic or ischemic; 17%) (Figure 1A).
FIGURE 1.
Pretreatment variables associated with early post-treatment mortality and with AML outcomes in patients undergoing induction. (A) Clinical and genetic characteristics of patients (n = 24) who died within the first 60 days of intensive induction. Each column represents an individual patient. (B) Cumulative incidence of death over the first 60 days from the start of induction chemotherapy for patients dying during this time period. (C) Forest plot of multivariable analysis of pretreatment risk factors associated with mortality within the first 60 days of initiating induction chemotherapy. The odds ratio reflects the presence of the variable compared with the absence. (D) Forest plot of multivariable model of pretreatment risk factors associated with OS landmarked at 60 days. Model was constrained to include allogeneic hematopoietic stem cell transplant (HSCT) as a time-varying covariate. The hazard ratio reflects the presence of the variable compared with the absence
We first examined factors associated with early post-treatment mortality, defined as death within 60 days of AML induction chemotherapy initiation (Figure S1A). In a multivariable logistic regression model, pretreatment albumin <3 g/dL (OR 5.00, p = .009), pretreatment LDH ≥600 U/L (OR 4.19, p = .006), and mutations in RUNX1 (OR 5.31, p = .007) or TET2 (OR 3.03, p = .025) were independently associated with death within 60 days (Figure 1C). Factors not independently associated with early mortality included older age (<60 vs 60 years or older), clinical ontogeny (secondary or treatment-related AML), complex karyotype, and pretreatment blood counts.
We next examined factors associated with survival in those alive at 60 days. We generated a multivariable model with allogeneic stem cell transplantation as a time-varying covariate based on variables significant in the univariate setting and found that age ≥ 60 years (HR 2.06, p = .0007), AML arising after antecedent myeloid disorder (HR 3.38, p < .0001), pretreatment LDH ≥600 U/L (HR 2.52, p < .0001), and the presence of TP53 mutation (HR 3.91, p < .0001), U2AF1 mutation (HR 2.66, p = .005), or 11q23 rearrangement (HR 4.68, p = .0001) were independently associated with inferior OS after day 60, whereas the presence of CBF rearrangement (HR 0.18, p = .001) was independently associated with superior OS after day 60 (Figure 1D and Figure S1B).
Disease refractoriness and relapse have previously been shown as important mediators of survival in AML patients after induction chemotherapy. To determine the contribution of resistant disease to OS landmarked at day 60, we identified independent variables for failure to achieve remission by day 60 and for RFS landmarked at day 60 and applied those variables to OS (Figure S2A,B). All variables in the failure to achieve remission and the landmarked RFS models remained significant when applied to landmarked OS, indicating that treatment resistant disease, rather than treatment-related complications, is the main driver of survival after 60 days.
Intensive anthracycline-based chemotherapy remains a highly effective remission-induction approach for patients with newly diagnosed AML. Prior studies have defined specific clinical variables that can predict which patients are “fit” to undergo intensive induction most safely or “unfit” and should be prioritized for less intensive treatment or supportive care.1,2 However, early mortality after intensive induction chemotherapy remains a risk even for patients determined to be “fit.” Moreover, predictive models for quantifying the risk of treatment-related mortality in newly diagnosed AML patients receiving intensive induction were developed in cohorts treated in the pre-molecular era. We therefore sought to define pretreatment variables that predict early adverse outcomes among fit patients receiving initial intensive anthracycline-based induction chemotherapy by analyzing a consecutive series of AML patients with complete annotation of pretreatment genetic (cytogenetic and molecular), pathologic, and laboratory features.
We found that the risk of early post-treatment mortality is associated with specific pretreatment variables, including baseline laboratory values (low albumin and high LDH) and somatic mutations (TET2 and RUNX1). The adverse effect of hypoalbuminemia may reflect the acute decrease in albumin that can occur due to physiologic stress or more chronic decreases due to malnutrition or end-organ (hepatic or renal) dysfunction. The effect of increased LDH may relate to the overall burden of disease or augmented turnover in a number of cellular compartments, including spontaneous lysis of leukemia blasts, hemolysis, or tissue injury.
The effect of TET2 and RUNX1 mutations on early mortality was not attributable to treatment resistance, and thus contrasted sharply with the effects of genetic alterations associated with later outcomes, such as TP53 or U2AF1 mutations and CBF or 11q23 rearrangements. TET2 mutations occur early in the evolution of disease, and in the context of clonal hematopoiesis, contribute to adverse non-hematologic outcomes such as cardiovascular disease and cause potentiated inflammatory signaling in mature immune effectors.4 TET2 mutations have further been shown to persist in clonal remissions, and their persistence has been linked to delayed count recovery after induction chemotherapy. TET2 mutations could thus magnify early risks of infection, hemorrhage, or stroke via multiple mechanisms related to the pleiotropic effects in mutated hematopoietic progenitors or more mature immune cells. A similar link between somatic GATA2 mutations and development of treatment-associated invasive fungal disease has been reported in MDS and AML patients.5
RUNX1 mutations occur late in disease evolution, often at the time of transformation from MDS to AML, and have been associated with primary treatment resistance in AML. Somatic RUNX1 mutations have been further associated with severe thrombocytopenia in MDS, and germline RUNX1 mutations have been linked to functional and quantitative platelet defects.6 As such, RUNX1 mutations could contribute to early mortality through a number of mechanisms, including treatment-refractoriness, poor hematopoietic reserve from underlying MDS, or platelet defects.
Among patients surviving induction, survival was driven primarily by disease resistance and was associated with established predictors of long-term survival in AML, such as age, clinical ontogeny, and disease genetics.
Specific strengths of our study are its analysis of a consecutive series of uniformly treated patients receiving intensive induction as initial AML therapy, the majority of whom were not enrolled on clinical trials, with complete baseline clinical, laboratory, and genetic characterization and detailed annotation of induction clinical course. Recently, the Ferrara criteria1 have been shown to predict early post-treatment mortality and OS in a heterogenous group of myeloid neoplasm patients receiving several different intensive induction or re-induction regimens.2 In contrast, our study focuses on a fit, previously-untreated cohort (where only two patients would be categorized as Ferrara-unfit) and thus addresses factors predictive of early mortality in the Ferrara-fit patients routinely offered intensive induction therapy given perceived favorable risk ratio.
Overall, our results indicate that distinct pretreatment characteristics predict early versus late adverse outcomes in fit patients with newly diagnosed AML. Improved ability to identify patients with high risk of early death after intensive AML induction may allow development of mitigation strategies. Unanswered questions include whether these fit patients who are at high risk for early mortality after intensive induction would have improved outcomes with alternative therapeutic approaches, or whether the risk of early mortality is inherent to disease biology at presentation. Further investigations into the molecular mechanisms of the gene mutations associated with early post-treatment mortality may yield novel mechanistic links between disease biology and adverse clinical outcomes in specific treatment contexts.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funding from the NIH (1K08CA204734) and the James A. and Lois J. Champy Family Fund (R.C.L.).
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CONFLICTS OF INTEREST
All authors declare no relevant conflicts of interest.
REFERENCES
- 1.Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997–999. [DOI] [PubMed] [Google Scholar]
- 2.Palmieri R, Othus M, Halpern AB, et al. Accuracy of SIE/SIES/GITMO consensus criteria for unfitness to predict early mortality after intensive chemotherapy in adults with AML or other high-grade myeloid neoplasm. J Clin Oncol. 2020;38:4163–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley SA, Othus M, Vainstein V, Abkowitz JL, Estey EH, Walter RB. Prediction of adverse events during intensive induction chemotherapy for acute myeloid leukemia or high-grade myelodysplastic syndromes. Am J Hematol. 2014;89(4):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vedula RS, Cheng MP, Ronayne CE, et al. Somatic GATA2 mutations define a subgroup of myeloid malignancy patients at high risk for invasive fungal disease. Blood Adv. 2021;5(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glembotsky AC, Sliwa D, Bluteau D, et al. Downregulation of TREM-like transcript-1 and collagen receptor α2 subunit, two novel RUNX1-targets, contributes to platelet dysfunction in familial platelet disorder with predisposition to acute myelogenous leukemia. Haematologica. 2019;104 (6):1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.