Abstract
Objectives:
Previous work has shown effects of transcranial direct current stimulation (tDCS) on clinical pain measures, qualitative sensory testing measures, and peripheral inflammation. The present report extends this research to investigate the effect of tDCS on brain-derived neurotrophic factor (BDNF) levels.
Methods:
This secondary analysis examined a sample of 40 older adults (50–70 years old) with symptomatic knee osteoarthritis (OA) randomly assigned in a 1:1 fashion to active (n = 20) or sham (n = 20) tDCS for 20 minutes on 5 consecutive days. BDNF was measured before the first session and after the final treatment session. Generalized linear modeling (GLM) evaluated BDNF plasma levels as a function of tDCS group, adjusted for baseline. Bayesian statistical inference was used to quantify the probability that effects of the treatment exist.
Results:
GLM indicated a 90.4% posterior probability (PP) that the sham condition had 49.9% higher BDNF at the end of treatment, controlling for baseline. Follow-up analyses within the active TDCS group supported an association between change in BDNF and change in clinical pain, and exploratory analyses found an effect of tDCS on irisin.
Discussion:
Results indicated that tDCS could be a potential nonpharmacological treatment to decrease BDNF levels, which may in turn decrease pain. This study adds to a growing literature suggesting that tDCS affects cortical excitability, and consequentially, the neural circuits implicated in pain modulation. In addition to a direct connection to analgesia, BDNF changes may reflect tDCS-induced changes in different cortical areas and/or neural circuits.
Keywords: transcranial direct current stimulation, knee osteoarthritis, brain-derived neurotrophic factor, BDNF
Introduction
Osteoarthritis (OA) is a pervasive source of pain among older adults,1 caused by cartilage degeneration. This chronic condition affects approximately 10% of men and 18% of women over 60 years of age, and represents a growing public health problem.2,3 Pain is the main symptom of OA and the major reason for disability and reduction in quality of life of affected people.4 Although OA pain is primarily peripheral, central sensitization mechanisms are also implicated. The neurobiological mechanisms underlying OA pain are quite complex and multifactorial, which makes it a difficult-to-manage symptom.4 Pharmacological and nonpharmacological strategies have been used in the management of OA pain.3 The available pharmacological treatments have side effects and may decrease effectiveness over time.5,6 In this context, nonpharmacological strategies, such as supervised physical activity and physiotherapy, have gained prominence, displaying modulatory effects on pathways related to pain control.3
Transcranial direct current stimulation (tDCS) is one such nonpharmacological strategy for managing OA pain. tDCS is a noninvasive brain stimulation technique that is involved in multiple neurotransmitter functions and has been shown to have a promising effect in the treatment of chronic pain conditions. This technique involves applying two electrodes of direct current over the scalp: (a) the anode, increasing local cortical excitability, and (b) the cathode, which decreases excitability.7,8 The present authors -investigated the relative effects of sham versus active tDCS on measures of clinical pain, qualitative sensory testing measures, and peripheral inflammatory markers.9–11
The neurobiological mechanisms of action for tDCS are an active subject of investigation in the literature, and heterogeneity in anode/cathode placement complicates the issue. A recent review12 summarized the current understanding of these mechanisms: in brief, multi-session tDCS is thought to regulate cortical information processing efficiency and induce continuous enhancement of signal transduction between neurons (i.e., long-term potentiation (LTP). Anodal stimulation modulates the dopamine system, enhances transmissions of glutamate and serotonin, and suppresses acetylcholine and GABA (gamma-aminobutyric acid) neurotransmission.
With respect to analgesia, multiple physiological mechanisms have been implicated as potential mediators of tDCS effects that involve changes in the emotional and perceptual processing of pain.13 For example, tDCS may achieve analgesic effects via reduced pathological excitability in the S1 area. Further, the aforementioned modulation of the dopamine system in the frontal/prefrontal cortex mediates mood regulation and emotional processing; activating these brain structures modifies the emotional assessment of pain, leading to analgesic effects. Some evidence has also suggested that anodal tDCS may induce analgesic effects via decreased mu opioid receptor binding of an exogenous receptor ligand (i.e., directly increasing endogenous opioid release).14
Convergent evidence has also suggested that the neurotrophin brain-derived neurotrophic factor (BDNF) is a critical determinant of tDCS effects.15 BDNF is a synaptically-related neurotrophin that has been implicated in pain modulation16,17 involved in both central and peripheral nociceptive pathways.18 Secreted by pre- and post-synaptic terminals in an activity-dependent manner, it selectively activates two main classes of receptors: the tropomyosin-related kinase (TrkB) receptor and the p75 neurotrophin (p75NTR) receptor.19 BDNF plays an important role in neurogenesis, neuronal growth, development, and survival, which suggests that it is involved in compensatory mechanisms for the harmful effects caused by injuries or diseases.18 Increased circulating levels of BDNF have been reported in patients with different pain-related conditions, including fibromyalgia,20 migraine,21 and OA.22 However, imbalance among the TrkB receptors may be involved in maladaptive response to disease that results in aberrant signaling;23 when the truncated receptor is up-regulated due to inflammation, axonal repair is inhibited, aiding the development of neuropathic pain.
The current research extends recent efforts by the present research team in reporting the results of a sham-controlled trial of tDCS for OA pain. Blood/plasma data collected during the trial were sequenced for the present secondary data analysis to extend previous examinations of tDCS mechanisms and evaluate the putative involvement of BDNF in the tDCS effect in OA patients. While processing samples to measure BDNF, three additional molecules were quantified: adiponectin, osteocalcin, and irisin. To optimize the utility of the collected data, changes over time in these molecules across groups were also evaluated in exploratory fashion.
Materials and Methods
Selection and enrollment procedures for the present secondary analysis have been described in previous work.5 A total of 40 older adults (ages 50 to 70) with symptomatic knee OA were recruited in north central Florida. They were randomly assigned in a 1:1 fashion to active (n = 20) or sham (n = 20) tDCS for 20 minutes on 5 consecutive days.
Exclusion criteria required that participants have no concurrent medical conditions that could confound the effect of tDCS on outcomes, risk patient safety, or impede protocol completion, including prosthetic knee replacement/non-arthroscopic surgery to the affected knee; history of acute myocardial infarction, uncontrolled hypertension, or heart failure; systemic lupus erythematosus; peripheral neuropathy; rheumatoid arthritis; fibromyalgia; history of stroke, seizure, brain tumor, brain surgery, or intracranial metal implantation; pregnancy or lactation; cognitive impairment; alcohol/substance use; or hospitalization due to psychiatric illness within the preceding year. The study was approved by the Institutional Review Board of the University of Florida prior to commencement. All participants were provided detailed information regarding the protocol, and written informed consent was obtained from all participants.
tDCS Intervention
tDCS (2 mA intensity) was applied for 20 min once per day for 5 consecutive days using saline-saturated rectangular sponge electrodes (35 cm2). The anode was placed at C3/C4 contralateral to the affected knee. The cathode was placed over the supraorbital (SO) contralateral to the anode (M1-SO montage). The resting cortex was then stimulated without any movement or other intervention. The sham condition utilized an identical configuration; however, to emulate the sensation of active tDCS, participants were exclusively stimulated for 30-second periods at the beginning and end of treatment (i.e., during ramp-up/ramp-down).
Outcome Measures
BDNF was collected via blood draws at baseline (pre-treatment on Day 1) and after the final treatment (Day 5) into ethylenediaminetetraacetic acid (EDTA) plasma tubes. Each sample was inverted five times and put on ice pending further processing. Within 30 minutes, samples were centrifuged at 1600 × g for 15 min at 4°C, aliquoted, and immediately stored in a −80°C freezer. Plasma levels of BDNF were assessed using Enzyme-Linked Immunosorbent Assay (ELISA) via manufacturer-supplied procedures (DuoSet, R&D Systems, Minneapolis, MN, USA). Samples were then reconstituted using the original sample volume in assay buffer. Although the current analysis is primarily concerned with BDNF, three additional compounds were measured through ELISA and evaluated in exploratory fashion: osteocalcin, adiponectin, and irisin.
Data Analytic Strategy
Descriptive statistics (frequency, central tendency, and dispersion) were used to characterize the sample. Established procedures for evaluating spurious associations24 were used to inspect the potential for other variables to confound the relationship between BDNF and treatment condition. None of the tested variables (age, race, sex, income, education, employment status, marital status, OA severity) met criteria for confounding.
Generalized linear modeling (GLM) was used to model end-of-study levels of each outcome as a function of tDCS treatment group, adjusted for baseline via covariate inclusion. This formulation provides unbiased estimates even in the case of baseline nonequality.25 Baseline and end-of-treatment levels of BDNF were log-transformed to address variable skew. Finally, within the active treatment group, GLM was used to (separately) model change in log BDNF as a function of change in each of two measures of pain: (a) the numeric rating scale (NRS) for current knee pain, scaled from 0 (no pain) to 100 (worst pain imaginable), and (b) the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) for OA-related pain symptoms.26
To quantify the probability that an effect of active tDCS treatment exists, Bayesian inference was utilized for the present analysis because it provides the ability to directly evaluate the alternative hypothesis that an effect exists. This evaluation of the alternative hypothesis provides a more readily accessible conceptualization of probability; in contrast, traditional frequentist analyses (whether parametric or nonparametric) focus on a circuitous path to inference by providing the probability of the observed data, or data more extreme, given that the null hypothesis is true. Previous work provides elaborate detail on the use of Bayesian inference in the present context.9,10 Weakly informative priors (b ~ Normal [μ = 0, σ2 = 100]) were employed to maximize the influence of the observed data on the posterior distribution. The median and 95% credible intervals (CrI) of the posterior distribution were used to provide a point estimate and corresponding range of uncertainty for the magnitude of the treatment effect. Posterior predictive checking graphical plots were visually evaluated27 to confirm that the observed distribution of each outcome fell within the range of distributions produced by 1,000 replications from the posterior predictive distribution of the outcome. This visual inspection involves ensuring that the observed distribution of the data lies completely within a shaded region that is generated by drawing the density of all simulated replications in a single plot; a more detailed description of this visualization procedure may be found in the literature.28 The R statistical computing environment29 was used for all analyses via packages rstan30 and brms.31
Bayesian inference relies on interpreting the posterior probabilty (PP) that an effect exists, given the data and a stipulated prior probability. Notably, the PP is not the inverse of a frequentist p-value (i.e., PP ≥ 0.95 is not equal to p ≤ .05), and there is no single monolithic PP value that is suggestive of a “significant” effect akin to p ≤ .05.32 Instead, decision making regarding the meaningfulness of a given PP is dependent on the context. For example, consider a hypothetical novel medication for depression: many alternative medications already exist, with varying degrees of efficacy and side effects. Given that alternatives exist, researchers may want a stronger chance that an effect of the novel medication exists, particularly if there are many side effects to that new drug. In this case, perhaps a 9-in-10 chance (PP ≥ 90%) would be meaningful. Conversely, consider a hypothetical novel medication for a rare cancer for which no other treatments currently exist; in such a scenario, any improvement over chance may be sufficient to support the new treatment. In this case, maybe a 2-in-3 chance (PP ≥ 66.67%) or even a lower threshold would be meaningful.
Some work has been done to describe generalized thresholds for interpreting posterior probabilities:33,34 (a) no evidence: PP = 50%; (b) anecdotal evidence: PP 51% – 74%; (c) moderate evidence: PP 75% – 90%; (d) strong evidence: PP 91% – 96%; (e) very strong evidence: PP 97% – 99%; (f) extreme evidence: PP > 99%. These heuristics provide a broad evidence base; however, as noted, researchers can and should consider their own subjective probability threshold that an effect exists. In the view of the present research team, PP = 75% provided the minimum threshold of interest to consider effects to be potentially worthy of future investigation. This threshold was chosen for consistency with previous work using data collected during this trial,9,10 as well as similarity to thresholds that researchers chose in other recent trials investigating treatment effects.35,36
Bayesian analyses provide findings that may be directly compared to previous findings in this research area that used frequentist analyses. As in the frequentist approach, Bayesian methods provide a point estimate and a credible range of values for model effects; the difference here is that the values are more probabiliistically intuitive than those derived by frequentist methods. With respect to inference, the Bayesian findings may still be directly compared to frequentist analyses via decision making based on thresholds of evidence. The frequentist method relies on dichotomous decision-making via the p-value, whereas Bayesian decision making requires researchers to specify a PP threshold of interest. In this sense, the Bayeisan approach is sometimes considered to be more subjective; however, the usual p-value threshold is itself a firmly entrenched (but inherently subjective) threshold. In any case, Bayesian inference may dichotomize at a given PP threshold to provide directly comparable decision making to previous research in this area; for example, using the minimum treshold suggested above for the current research, one might fail to reject the null if given a p-value > .05 or if given a PP < 75%. Finally, with specific respect to secondary data analyses, the intuitive evaluation of probability provided by Bayesian methods is equivalently benefiical as it is in primary analyses. Examples may be found in the literature in which secondary Bayesian reanalyses were used to capitalize on these intuitive benefits.37–40
Results
Sample Characteristics
Participants (N = 40) were stratified by sex (50% female/male) and race (50% Asian/Caucasian) and randomly assigned in a 1:1 ratio to tDCS condition (active vs. sham). The mean age of the participants was 60 years (SD = 9.1). The sample was predominantly married (75%) with education beyond high school (85%) and either retired (27.5%) or currently employed (52.5%). Table 1 summarizes the characteristics of the sample by tDCS group.
Table 1.
Sample Characteristics
| Sham (n = 19) | Treatment (n = 19) | Group Differences χ2 (p-value) | |
|---|---|---|---|
| Sex, N | 0.00 (> 0.99) | ||
| Male | 9 | 9 | |
| Female | 10 | 10 | |
| Race, N | 0.00 (> 0.99) | ||
| Asian | 10 | 10 | |
| White | 9 | 9 | |
| Education, N | 0.87 (0.93) | ||
| < High School | 0 | 0 | |
| High School | 3 | 3 | |
| 2 Years College | 4 | 2 | |
| College | 5 | 6 | |
| Master’s | 3 | 3 | |
| Doctoral | 4 | 5 | |
| Employment, N | 3.14 (0.68) | ||
| Working | 10 | 9 | |
| Laid Off | 0 | 1 | |
| Unemployed | 0 | 0 | |
| Retired | 5 | 6 | |
| Disabled | 0 | 0 | |
| Student | 0 | 1 | |
| Keeping House | 1 | 1 | |
| Marital Status, N | 1.33 (0.86) | ||
| Married | 14 | 14 | |
| Widowed | 1 | 1 | |
| Divorced | 1 | 0 | |
| Separated | 0 | 2 | |
| Never Married | 2 | 0 | |
| Living w/ Partner | 1 | 2 | |
| M (SD) | M (SD) | t (p-value) | |
| Age, M (SD) | 59.9 (8.7) | 61.1 (9.8) | 0.48 (0.64) |
| BMI, M (SD) | 26.0 (4.2) | 26.9 (3.3) | 0.75 (0.46) |
Note. N = number, M = mean, SD = standard deviation.
Generalized Linear Modeling
Raw plasma BDNF levels at baseline were higher in the active tDCS group (M = 330.9, SD = 303.2) than in the sham group (M = 261.3, SD = 306.9). This pattern reversed by the end of treatment, such that raw BDNF was lower in the active condition (M = 220.9, SD = 250.7) than in the sham condition (M = 384.7, SD = 409.4). Bayesian GLM showed that, adjusted for baseline, sham tDCS demonstrated moderate evidence (PP = 90.4%) for higher end-of-treatment levels of log BDNF relative to active tDCS (b = 0.41, 95% CrI [−0.20, 1.02]). Regression coefficients for the log-transformed outcome were then exponentiated to provide an index of the percentage difference between groups in BDNF levels: sham tDCS demonstrated higher BDNF at the end of treatment (+49.9%, 95% CrI [−18.1%, +178.4%]). Figure 1 displays the predicted BDNF values (transformed) at end of treatment for each group, conditional on baseline values.
Figure 1.
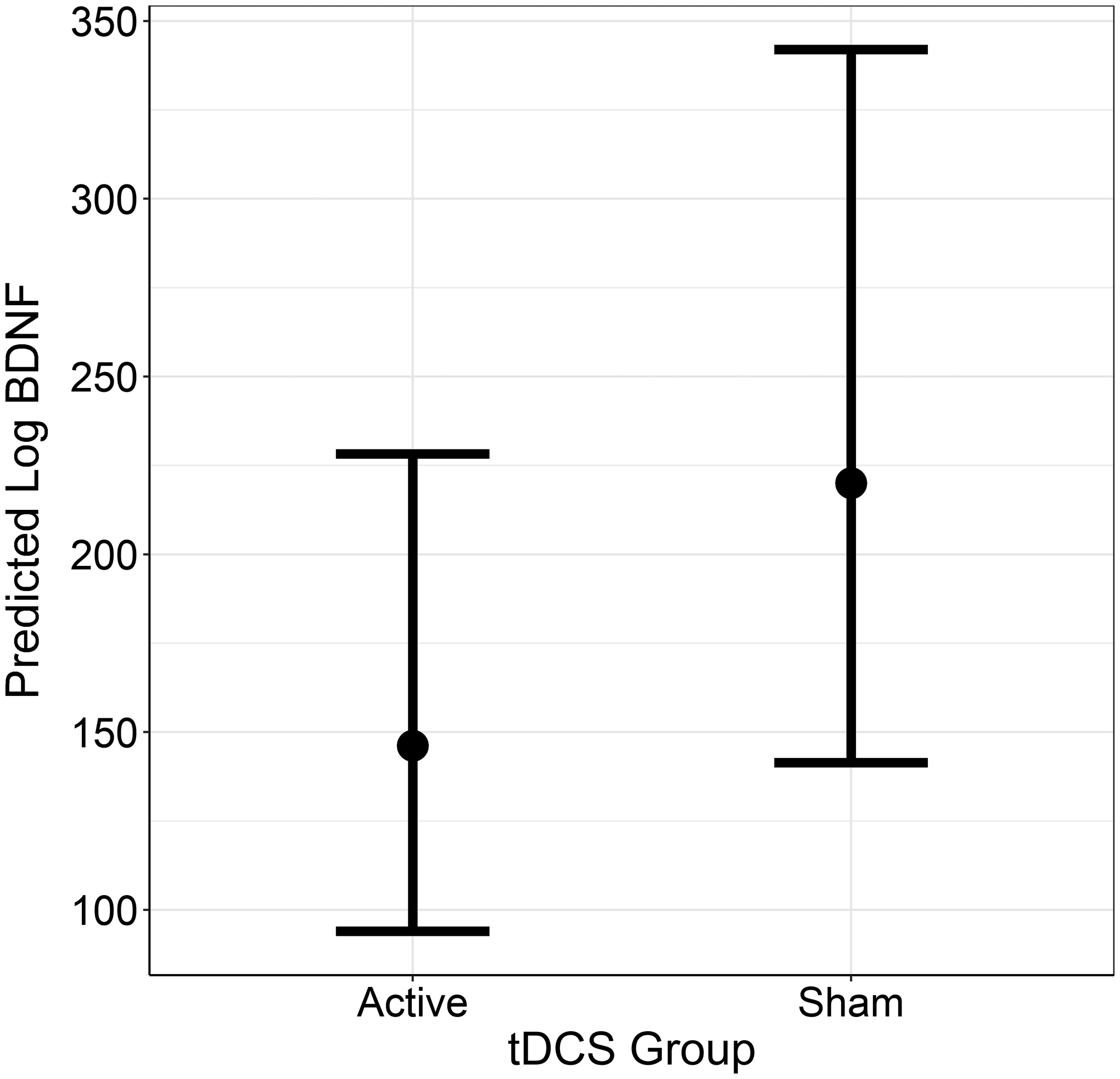
Plot of predicted log-BDNF values at the end of treatment for each group, with 95% credible intervals (controlling for baseline).
Follow-up analyses evaluated change in log BDNF as a function of change in pain scores (NRS; WOMAC). Models supported positive relationships between changes in pain scores and change in BDNF. For the NRS, strong evidence (PP = 91.2%) supported this relationship (b = 0.021, 95% CrI [−0.01, 0.05]); for the WOMAC, moderate evidence (PP = 88.1) supported a relationship (b = 0.095, 95% CrI [−0.08, 0.26]).
Finally, exploratory models evaluated three additional compounds: adiponectin, irisin, and osteocalcin. Bayesian GLM did not find noteworthy PP for either adiponectin (PP = 65.2%) or osteocalcin (PP = 67.3%). However, analyses did find very strong evidence (PP = 91.1%) for an effect of tDCS on irisin (b = 0.046, 95% CrI [−0.02, 0.11]); exponentiating the effect described this difference as a percentage (+4.70%, 95% CrI [0.98, 1.12]).
Discussion
The present study investigated the effects of tDCS on BDNF levels in patients with knee OA. Patients subjected to active tDCS presented lower levels of BDNF at the end of treatment after adjusting for baseline, and follow-up analyses demonstrated an association between change in clinical pain and change in BDNF for the active tDCS group. These findings extend previous findings investigating the effects of tDCS on pain and inflammation in older adults with knee OA.9,10 Specifically concerning BDNF, previous study findings41 demonstrated that active tDCS was able to decrease BDNF levels in the spinal cord and brainstem only in unstressed animals. In another study,42 tDCS reverted behavioral changes (e.g., decreased locomotion) associated with neuropathic pain in humans or animals, indicating a possible analgesic effect of tDCS and decreased BDNF levels. Therefore, decreased BDNF levels might directly influence pain. As suggested elsewhere,23 future research may be able to explicate the relationship between BDNF and neuropathic pain by focusing on the mechanistic pathways involved. The present study supports existing research that tDCS affects cortical excitability, and consequentially, the neural circuits implicated in pain modulation.13 Besides directly implicated in analgesia, BDNF changes might also reflect tDCS-induced changes in different cortical areas and/or neural circuits, as demonstrated in other clinical contexts,43,44 but this hypothesis must be investigated in OA. The overall role of BDNF in OA pain is still unclear and more research in this area should be conducted.45
Although exploratory analyses did not find support for an effect of tDCS on adiponectin or osteocalcin, the active treatment was shown to be related to lower irisin (relative to sham), controlling for baseline. To date, the literature has not evaluated connections between tDCS and irisin, a hormone-like peptide produced mainly by myocytes (but also present in the brain) that has traditionally been implicated in beneficial effects of exercise in humans (e.g., weight loss; thermoregulation; counter-regulation of chronic inflammation)46 among others; however, the parallel findings between BDNF and irisin in the present study (i.e., lower values among those receiving active treatment) supports recent literature endorsing a connection between these compounds.47,48 Inferences regarding this exploratory analysis follow from this connection; a preliminary consideration of the issue suggests that there may be some additive or otherwise synergistic effect between these compounds with respect to the positive effects of tDCS.
The present study was primarily limited by the small sample size available for the experiment. This sample size may influence the generalizability of the findings and the precision of the estimates in the statistical model. Also, the outcomes evaluated for the present analysis were secondary; future research could improve on this by directly evaluating BDNF and other compounds, such as irisin, as a primary focus. Further, these results may be exclusive to older adults with knee OA only; in the larger population, it is possible that such individuals are relatively rare, compared to older adults with other chronic conditions.
The timing of blood sample collection across the varying daily routines of participants may have resulted in some heterogeneity in the sample, as accommodations were given to permit flexibility in scheduling baseline and final blood draws. BDNF levels are influenced by light and dark and thus may fluctuate with circadian rhythm.49 Although there were no systematic differences in the application of tDCS across groups (and thus no readily apparent reason that it could have confounded24,50 the relationship between tDCS and BDNF), such naturally occurring fluctuations in BDNF could have been in part responsible for the observed differences between groups.
Finally, the present findings should also be considered in context of flaws in tDCS research, such as questionable reliability within participants,51 difficult-to-control sources of heterogeneity such as anatomical differences,52 and variability in saline application across practitioners.53 Given the strength of the present evidence, future research should seek to replicate and extend the present results to larger and more diverse samples. Future studies would also benefit from collecting data at several time points in order to capture the functional form of change in BDNF levels over time within and between treatment conditions. The present research provides an incremental update to an evolving literature on peripheral neurotrophic factors in tDCS and, more generally, in neuromodulatory therapies.
Conflicts of Interest and Source of Funding
The authors declare no conflicts of interest. This research was supported in part by the University of Florida Claude D. Pepper Older Americans Independence Center (P30 AG028740), Isla Carroll Turner Endowment from The University of Texas Health Science Center at Houston, and NIH/NINR R01NR019051.The funding agencies had no role in the study design, methods, or data collection and analysis or in the preparation of the manuscript.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthr Cartil. 2018;26(3):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol. 2019;37(5):3–6. [PubMed] [Google Scholar]
- 4.Fu K, Robbins SR, McDougall JJ. Osteoarthritis: The genesis of pain. Rheumatol (United Kingdom). 2018;57:iv43–iv50. [DOI] [PubMed] [Google Scholar]
- 5.Ahn H, Woods A, Kunik M, et al. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: An experimenter- and participant-bl. Brain Stimul. 2017;10(5):902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinecke H, Weber C, Lange K, Simon M, Stein C, Sorgatz H. Analgesic efficacy of opioids in chronic pain: Recent meta-analyses. Br J Pharmacol. 2015;172(2):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasilva AF, Mendonca ME, Zaghi S, et al. TDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012;52(8):1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- 9.Ahn H, Suchting R, Woods AJ, et al. Bayesian analysis of the effect of transcranial direct current stimulation on experimental pain sensitivity in older adults with knee osteoarthritis: Randomized sham-controlled pilot clinical study. J Pain Res. 2018;11:2071–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suchting R, Colpo GD, Rocha NP, Ahn H. The effect of transcranial direct current stimulation on inflammation in older adults with knee osteoarthritis: A Bayesian residual change analysis. Biol Res Nurs. 2020;22(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suchting R, Kapoor S, Mathis KB, Ahn H. Changes in experimental pain sensitivity from using home-based remotely supervised transcranial direct current stimulation in older adults with knee osteoarthritis. Pain Med. 2020;21(11):2676–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada Y, Sumiyoshi T. Neurobiological Mechanisms of Transcranial Direct Current Stimulation for Psychiatric Disorders; Neurophysiological, Chemical, and Anatomical Considerations. Front Hum Neurosci. 2021;15. doi: 10.3389/fnhum.2021.631838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knotkova H, Nitsche MA, Cruciani RA. Putative physiological mechanisms underlying tDCS analgesic effects. Front Hum Neurosci. 2013;(SEP). doi: 10.3389/fnhum.2013.00628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D MF, M IK, N TD, et al. Immediate effects of tDCS on the mu-opioid system of a chronic pain patient. Front Psychiatry. 2012;3. http://europepmc.org/search?query=(DOI:10.3389/fpsyt.2012.00093)%0Ahttps://www.frontiersin.org/articles/10.3389/fpsyt.2012.00093/pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocco S, Podda MV, Grassi C. Role of BDNF signaling in memory enhancement induced by transcranial direct current stimulation. Front Neurosci. 2018;12(JUN). doi: 10.3389/fnins.2018.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55(1):1–10. doi: 10.1016/j.neures.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 17.Merighi A, Salio C, Ghirri A, et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85(3):297–317. doi: 10.1016/j.pneurobio.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 18.Smith PA. BDNF: No gain without pain? Neuroscience. 2014;283:107–123. doi: 10.1016/j.neuroscience.2014.05.044 [DOI] [PubMed] [Google Scholar]
- 19.Miranda-Lourenço C, Ribeiro-Rodrigues L, Fonseca-Gomes J, et al. Challenges of BDNF-based therapies: From common to rare diseases. Pharmacol Res. 2020;162. doi: 10.1016/j.phrs.2020.105281 [DOI] [PubMed] [Google Scholar]
- 20.Laske C, Stransky E, Eschweiler GW, et al. Increased BDNF serum concentration in fibromyalgia with or without depression or antidepressants. J Psychiatr Res. 2007;41(7):600–605. [DOI] [PubMed] [Google Scholar]
- 21.Cai X, Shi X, Zhang X, Zhang A, Zheng M, Fang Y. The association between brain-derived neurotrophic factor gene polymorphism and migraine: a meta-analysis. J Headache Pain. 2017;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simão AP, Mendonça VA, De Oliveira Almeida TM, et al. Involvement of BDNF in knee osteoarthritis: The relationship with inflammation and clinical parameters. Rheumatol Int. 2014;34(8):1153–1157. [DOI] [PubMed] [Google Scholar]
- 23.Cao T, Matyas JJ, Renn CL, Faden AI, Dorsey SG, Wu J. Function and Mechanisms of Truncated BDNF Receptor TrkB.T1 in Neuropathic Pain. Cells. 2020;9(5). doi: 10.3390/cells9051194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisions in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–2930. [DOI] [PubMed] [Google Scholar]
- 25.Senn S Change from baseline and analysis of covariance revisited. Stat Med. 2006;25(4):4334–4344. [DOI] [PubMed] [Google Scholar]
- 26.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 27.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis. Third Edit. New York: Chapman and Hall/CRC; 2013. [Google Scholar]
- 28.Gabry J, Simpson D, Vehtari A, Betancourt M, Gelman A. Visualization in Bayesian workflow. J R Stat Soc Ser A Stat Soc. 2019;182(2):389–402. doi: 10.1111/rssa.12378 [DOI] [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing. 2020.
- 30.Stan Development Team. RStan: the R interface to Stan. R package version 2.26.0. 2020.
- 31.Bürkner P brms: An R package for Bayesian multilevel models using Stan. J Stat Softw. 2017;80(1):1–28. [Google Scholar]
- 32.Makowski D, Ben-Shachar MS, Chen SHA, Lüdecke D. Indices of Effect Existence and Significance in the Bayesian Framework. Front Psychol. 2019;10. doi: 10.3389/fpsyg.2019.02767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MD, Wagenmakers E. Bayesian Cognitive Modeling: A Practical Course. Cambridge, UK: Cambridge University Press; 2013. [Google Scholar]
- 34.Jeffreys H Theory of Probability. 3rd ed. Oxford, UK: Oxford University Press; 1961. [Google Scholar]
- 35.Bauer IE, Suchting R, Cazala F, et al. Changes in amygdala, cerebellum, and nucleus accumbens volumes in bipolar patients treated with lamotrigine. Psychiatry Res - Neuroimaging. 2018;278:13–20. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz JM, Green CE, Hasan KM, et al. PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: A double-blind randomized controlled pilot trial. Addiction. 2017;112(10):1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green CE, Moeller FG, Schmitz JM, et al. Evaluation of heterogeneity in pharmacotherapy trials for drug dependence: A bayesian approach. Am J Drug Alcohol Abuse. 2009;35(2):95–102. doi: 10.1080/00952990802647503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klitgaard TL, Schjørring OL, Lange T, et al. Bayesian and heterogeneity of treatment effect analyses of the HOT-ICU trial—A secondary analysis protocol. Acta Anaesthesiol Scand. 2020;64(9):1376–1381. doi: 10.1111/aas.13669 [DOI] [PubMed] [Google Scholar]
- 39.Shehabi Y, Neto AS, Howe BD, et al. Early sedation with dexmedetomidine in ventilated critically ill patients and heterogeneity of treatment effect in the SPICE III randomised controlled trial. Intensive Care Med. 2021;47:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blini E, Tilikete C, Farnè A, Hadj-Bouziane F. Probing the role of the vestibular system in motivation and reward-based attention. Cortex. 2018;103:82–99. doi: 10.1016/j.cortex.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spezia Adachi LN, Quevedo AS, de Souza A, et al. Exogenously induced brain activation regulates neuronal activity by top-down modulation: conceptualized model for electrical brain stimulation. Exp Brain Res. 2015;233(5):1377–1389. [DOI] [PubMed] [Google Scholar]
- 42.Filho PRM, Vercelino R, Cioato SG, et al. Transcranial direct current stimulation (tDCS) reverts behavioral alterations and brainstem BDNF level increase induced by neuropathic pain model: Long-lasting effect. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;64:44–51. [DOI] [PubMed] [Google Scholar]
- 43.Chan MMY, Yau SSY, Han YMY. The neurobiology of prefrontal transcranial direct current stimulation (tDCS) in promoting brain plasticity: A systematic review and meta-analyses of human and rodent studies. Neurosci Biobehav Rev. 2021;125:392–416. doi: 10.1016/j.neubiorev.2021.02.035 [DOI] [PubMed] [Google Scholar]
- 44.Adam O, Psomiades M, Rey R, et al. Frontotemporal transcranial direct current stimulation decreases serum mature brain-derived neurotrophic factor in schizophrenia. Brain Sci. 2021;11(5). doi: 10.3390/brainsci11050662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Wang X, Wang W, Lu YG, Pan ZZ. Brain-derived neurotrophic factor-mediated downregulation of brainstem K+-Cl- cotransporter and cell-type-specific GABA impairment for activation of descending pain facilitation. Mol Pharmacol. 2013;84(4):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckel J Chapter 3 - Skeletal Muscle: A Novel Secretory Organ. In: Eckel J, ed. The Cellular Secretome and Organ Crosstalk. Academic Press; 2018:65–90. [Google Scholar]
- 47.Huang L, Yan S, Luo L, Yang L. Irisin regulates the expression of BDNF and glycometabolism in diabetic rats. Mol Med Rep. 2019;19(2):1074–1082. doi: 10.3892/mmr.2018.9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conti E, Grana D, Stefanoni G, et al. Irisin and BDNF serum levels and behavioral disturbances in Alzheimer’s disease. Neurol Sci. 2019;40(6):1145–1150. doi: 10.1007/s10072-019-03781-y [DOI] [PubMed] [Google Scholar]
- 49.Begliuomini S, Lenzi E, Ninni F, et al. Plasma brain-derived neurotrophic factor daily variations in men: Correlation with cortisol circadian rhythm. J Endocrinol. 2008;197(2):429–435. doi: 10.1677/JOE-07-0376 [DOI] [PubMed] [Google Scholar]
- 50.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355(9209):1064–1069. [DOI] [PubMed] [Google Scholar]
- 51.Dyke K, Kim S, Jackson GM, Jackson SR. Intra-Subject Consistency and Reliability of Response Following 2 mA Transcranial Direct Current Stimulation. Brain Stimul. 2016;9(6):819–825. doi: 10.1016/j.brs.2016.06.052 [DOI] [PubMed] [Google Scholar]
- 52.Filmer HL, Ehrhardt SE, Shaw TB, Mattingley JB, Dux PE. The efficacy of transcranial direct current stimulation to prefrontal areas is related to underlying cortical morphology. Neuroimage. 2019;196:41–48. doi: 10.1016/j.neuroimage.2019.04.026 [DOI] [PubMed] [Google Scholar]
- 53.Horvath JC, Carter O, Forte JD. Transcranial direct current stimulation: Five important issues we aren’t discussing (but probably should be). Front Syst Neurosci. 2014;8(JAN). doi: 10.3389/fnsys.2014.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]


