Abstract
We undertook a study to evaluate Streck tissue fixative (STF) as a substitute for formalin and polyvinyl alcohol (PVA) in fecal preservation. A comparison of formalin, PVA, (mercuric chloride based), and STF was done by aliquoting fecal samples into each fixative. Stool specimens were collected in Haiti, and parasites included Cyclospora cayetanensis, Giardia intestinalis, Entamoeba coli, Iodamoeba butschlii, Endolimax nana, Ascaris lumbricoides, Trichuris trichiura, Strongyloides stercoralis, and Necator americanus. Preserved stools were examined at various predetermined times (1 week, 1 month, and 3 months) to establish the quality of the initial preservation as well as the suitability of the fixative for long-term storage. At each time point, stool samples in fixatives were examined microscopically as follows: (i) in wet mounts (with bright-field and epifluorescence microscopy), (ii) in modified acid-fast-, trichrome-, and safranin-stained smears, and (iii) with two commercial test kits. At the time points examined, morphologic features remained comparable for samples fixed with 10% formalin and STF. For comparisons of STF- and 10% formalin-fixed samples, specific findings showed that Cyclospora oocysts retained full fluorescence, modified acid-fast- and safranin-stained smears of Cryptosporidium and Cyclospora oocysts were equal in staining quality, and results were comparable in the immunofluorescence assay and enzyme immunoassay commercial kits. Stool fixed in STF and stained with trichrome showed less-than-acceptable staining quality compared with stool fixed in PVA. STF provides an excellent substitute for formalin as a fixative in routine examination of stool samples for parasites. However, modifications to the trichrome staining procedures will be necessary to improve the staining quality for protozoal cysts fixed in STF to a level comparable to that with PVA.
For decades, formalin and polyvinyl alcohol (PVA) have been the two standard fixatives for stool specimens collected for subsequent microscopic examination for intestinal parasites (1). Recent developments have raised serious concerns regarding the safety of formalin in the workplace due to potential carcinogenic properties and high toxicity from inhalation and dermal absorption (4, 6). Neutralization of formalin for drain disposal with laundry bleach is cumbersome and time-consuming (7). Therefore, many laboratories, especially pathology laboratories that use large volumes of formalin, are moving towards a formalin-free workplace (2).
PVA (mercuric chloride base) is an excellent fixative that is used side by side with formalin but specifically for staining of protozoan parasites. PVA is a potential carcinogen and contains highly toxic and corrosive mercuric chloride; therefore, waste disposal of staining products calls for careful measures (5). Proper disposal is costly for all laboratories and is not always readily available in developing countries. A fixative for both protozoa and helminths that can be decanted down the drain would be ideal.
Hence, an effective but more user-friendly, environmentally safe fixative is needed. A single fixative that maintains the morphology as well as the staining capabilities of parasites such as those achievable with formalin and PVA would be highly advantageous. An added benefit of a single fixative would be in the collection of specimens in outbreak settings or large surveys, where aliquoting of specimens into several vials is costly and time-consuming. Our laboratory set out to determine whether a commercially available, non-formalin-based fixative, Streck tissue fixative (STF), would provide acceptable fixation and preservation of stool specimens for parasitologic examination.
MATERIALS AND METHODS
A study site in Haiti, an area where intestinal parasites are highly endemic, was chosen for collecting stool specimens. One hundred forty individual stool samples were collected, divided into 5-ml aliquots, and preserved in 15 ml of the fixative being investigated, i.e., 10% formalin, PVA (mercuric chloride base), or Streck tissue fixative (STF) (Streck Laboratories, Omaha, Nebr.). STF has two key components, diazoiidinyl urea and 2-bromo-2-nitropropane-1,3-diol, but does include zinc sulfate and sodium citrate (8). An initial 140 samples were examined as formalin-ethyl acetate concentrate wet mounts at the 1-week time point, and of these, 36 samples that possessed the greatest number and assortment of organisms were examined at the later times. In Haiti, we rarely encounter Cryptosporidium in this study population, so we aliquoted fresh feces from a calf experimentally infected with Cryptosporidium parvum into each of the three fixatives. The selected human samples and the sample containing Cryptosporidium were examined at the predetermined times of 1 week (time zero) and 1 and 3 months. At each time point, stool samples preserved in 10% formalin and STF were concentrated by using a standard ethyl acetate procedure (1).
Aliquots from each preserved concentrate were examined as wet mounts by using bright-field and epifluorescence microscopy; in modified acid-fast-, trichrome-, and safranin-stained smears; and with commercial immunofluorescence assay (IFA) (Meridian Diagnostics, Inc., Cincinnati, Ohio) and enzyme immunoassay (EIA) (Alexon-Trend, Inc., Ramsey, Minn.) kits for Cryptosporidium and Giardia. Particular attention was paid to the morphologic condition of protozoa, whether the fixative halted embryonation of helminth eggs, and the quality of staining if the commercial assay kits performed satisfactorily. The commercial kits were examined for fluorescing parasites, and samples were graded accordingly. The scale was as follows: rare, fewer than 20 parasites in the sample; few, fewer than one parasite per field at ×20; moderate, one to five parasites per field at ×20; and many, more than five parasites per field at ×20. No modifications to the standard formalin-ethyl acetate concentration procedure were used for the samples fixed in STF, other than the substitution of STF for 10% formalin.
RESULTS
Of the 140 samples examined initially, we focused on 36 preserved stool samples that possessed the greatest number and assortment of organisms to better establish the quality of preservation. At the 1-month time point, we examined 27 samples by wet mounting, 19 samples by trichrome staining, and 28 samples by using the commercial test kits. Because samples varied in starting amounts and were processed at each time point, some of the samples ran low at the 3-month time point. Therefore, at the 3-month interval we examined 16 samples by wet mounting, 6 samples by trichrome staining, and 25 samples with the commercial test kits. The samples available for trichrome staining were limited in number since we had to choose those known to be positive for protozoa for comparing stain intensities. Stools assayed by using the IFA and EIA kits were processed by one of us blinded to the microscopic results for Giardia or Cryptosporidium. An individual familiar with the microscopic results selected an equal number of positive and negative Giardia samples; the positive samples included a range of samples with few to many cysts. In addition, an aliquot of the samples containing Cryptosporidium was also included.
Wet mounts.
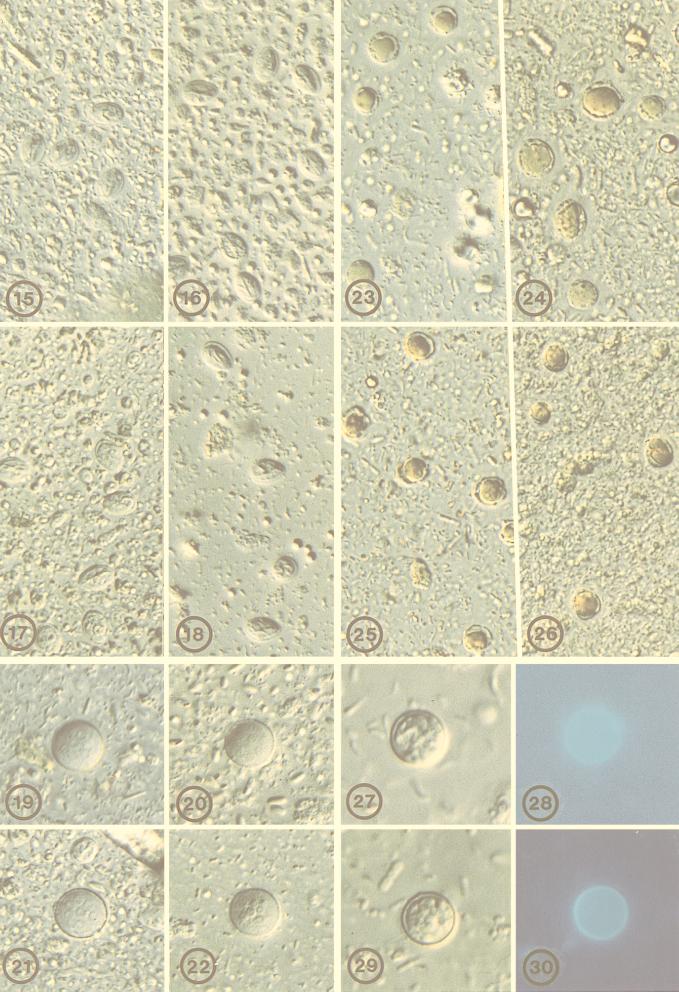
Microscopic examination of wet mounts demonstrated that parasite eggs, larvae, and cysts fixed in STF were as well preserved as those fixed in 10% formalin. This was true at 1 week and 3 months of storage (Fig. 1 to 12). At 1 and 3 months, some Ascaris eggs were observed to have embryonated and contained larvae. This was noted to occur equally in 10% formalin- and STF-fixed material (Fig. 1 to 14). Strongyloides rhabditiform larvae were equally well preserved in STF and in 10% formalin (Fig. 13 and 14). Giardia and Entamoeba coli cysts and Blastocystis maintained excellent morphology with STF, which was easily visible with differential interference contrast (DIC) (Fig. 15 to 26). STF also did not alter the morphology of Cyclospora oocysts (Fig. 27 and 29), nor did it inhibit their strong autofluorescence (Fig. 28 and 30).
Staining.
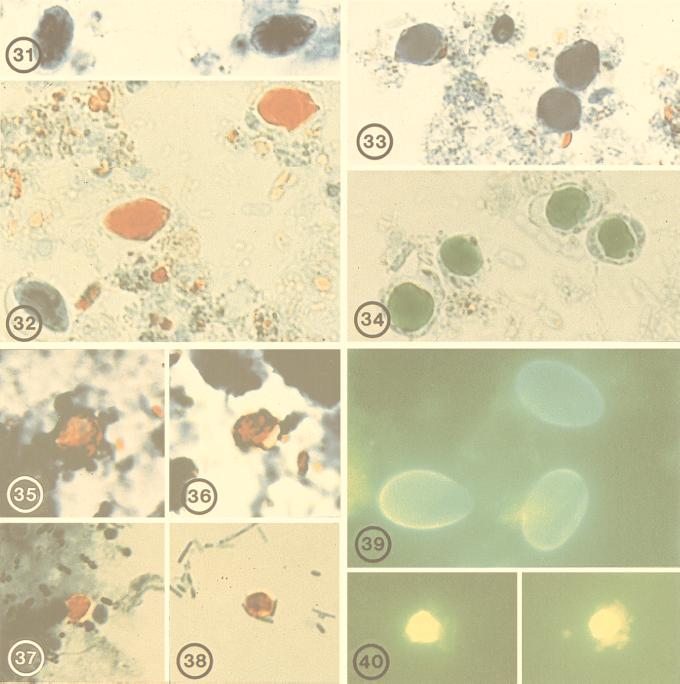
Examination of trichrome-stained smears revealed that PVA provided the best staining of protozoal cysts and trophozoites. STF provided less-than-acceptable staining quality, and, as expected, formalin performed the poorest (not shown). The most significant differences in staining of STF-fixed material were the absence of sharp, discrete nuclei and cytoplasmic definition and a marked shift in color toward a red or green hue (Fig. 32 and 34). This represents a marked difference from the normal blue-gray coloration seen in protozoan parasites preserved in PVA and stained with trichrome (Fig. 31 and 33). This staining feature did not change with the age of the preserved sample. The color shift itself was less troubling than the absence of discernible nuclei, upon which differentiation of various amebae is based.
Modified acid-fast- and safranin-stained smears were evaluated for staining of Cryptosporidium and Cyclospora oocysts, and the STF worked excellently with both stains (Fig. 35 to 38).
Kits.
With the commercial IFA kit that was used for detection of Giardia and Cryptosporidium with immunofluorescence-labeled antibodies, STF did not alter the detection of parasites (Fig. 39 and 40). It was noted, however, that the number of parasites seen in the IFA dropped off more rapidly with time in stools fixed in STF than in those fixed in formalin (Table 1). While the parasite number in STF fixative decreased with time, the difference in counts between STF- and formalin-fixed samples was insignificant. Samples positive in formalin were positive in STF at all times. The EIA commercial kits, using a monoclonal antibody for the qualitative detection of Giardia or Cryptosporidium antigen in fecal suspensions, showed no statistically significant difference between formalin-fixed and STF-fixed samples at either time point (Table 2).
TABLE 1.
Results of stool testing with IFA
| Genus | Time (mo) and fixative | No. of samples negative with kit/no. negative by microscopy | No. of samples positive with kit/no. positive by microscopy (%) | No. of samples with the following no. of parasitesa/total no. of samples (%):
|
|||
|---|---|---|---|---|---|---|---|
| Rare | Few | Moderate | Many | ||||
| Giardia | 0 | ||||||
| Formalin | 5/5 | 25/27 (92) | 1/27 (3) | 4/27 (15) | 7/27 (26) | 13/27 (48) | |
| STF | 4/4 | 24/26 (92) | 6/26 (23) | 7/26 (27) | 6/26 (23) | 5/26 (19) | |
| 3 | |||||||
| Formalin | 6/6 | 17/19 (89) | 2/19 (11) | 2/19 (11) | 5/19 (25) | 8/19 (42) | |
| STF | 6/6 | 18/19 (94) | 7/19 (37) | 3/19 (16) | 3/19 (16) | 5/19 (26) | |
| Cryptosporidium | 0 | ||||||
| Formalin | 31/31 | 1/1 (100) | 0 | 1/1 (100) | 0 | 0 | |
| STF | 29/29 | 1/1 (100) | 1/1 (100) | 0 | 0 | 0 | |
| 3 | |||||||
| Formalin | 24/24 | 1/1 (100) | 0 | 1/1 (100) | 0 | 0 | |
| STF | 24/24 | 0/1 (0) | 0 | 0 | 0 | 0 | |
See Materials and Methods.
TABLE 2.
Results of stool testing with a commercial EIA
| Genus | Time (mo) and fixative | No. of samples negative with kit/no. negative by microscopy | No. of samples positive with kit/no. positive by microscopy (%) | Mean optical density |
|---|---|---|---|---|
| Giardia | 0 | |||
| Formalin | 5/5 | 25/27 (93) | 2.72 | |
| STF | 4/4 | 24/26 (92) | 1.30 | |
| 3 | ||||
| Formalin | 6/6 | 17/19 (89) | 2.14 | |
| STF | 6/6 | 16/19 (84) | 1.58 | |
| Cryptosporidium | 0 | |||
| Formalin | 31/31 | 1/1 (100) | 2.91 | |
| STF | 29/29 | 1/1 (100) | 3.44 | |
| 3 | ||||
| Formalin | 24/24 | 1/1 (100) | 3.09 | |
| STF | 24/24 | 1/1 (100) | 3.42 |
DISCUSSION
For examination of wet preparations, STF provided excellent preservation of key morphologic features, and this did not diminish during the 3-month examination time. Diagnostic anatomical structure was maintained throughout the various parasites, including some of the more notable structures, such as the buccal cavity, esophagus, and genital primordium in Strongyloides larvae and the nuclei and axonemes in Giardia cysts. In the other amebae, nuclear integrity, including the nucleolus, and inclusions such as food granules or glycogen vacuoles were well preserved. It is well known that Ascaris eggs are resistant to preservatives, including 10% formalin. That we observed embryonated Ascaris eggs in both formalin- and STF-fixed samples is not surprising. In addition to the results of bright-field observation of the wet mounts, Cyclospora oocysts retained brilliant autofluorescence in STF, as in 10% formalin, making this an excellent alternative.
Cryptosporidium and Cyclospora oocysts stained equally well with modified acid-fast or safranin staining following fixation in STF or 10% formalin. However, stool specimens fixed in STF and stained with trichrome gave less-than-adequate staining quality compared with stools fixed in PVA. Although the major protozoa, such as Giardia and E. coli cysts and Blastocystis, could be readily recognized in STF-fixed, trichrome-stained preparations, the crispness and detail of nuclear staining necessary for differential diagnosis that have come to be expected in PVA-fixed trichrome-stained smears are lacking. Simple modifications to the trichrome staining protocol may render STF suitable for staining of protozoa to allow accurate identification.
Stool samples preserved in 10% formalin and STF performed equally in commercial assays for Giardia and Cryptosporidium. While the numbers of oocysts visibly fluorescing per positive sample decreased over time in the STF-fixed samples when the IFA kit was used, all positive samples remained so during the 3 months of evaluation. There was no visible difference in fluorescing of parasites with IFA kits. The only difference seen was in the parasite numbers that were detected. STF-fixed and formalin-fixed samples performed equally well at both time points when the EIA kit was used.
Because STF is free of formalin, other carcinogens, or noxious chemicals, it is safer to work with in the laboratory. STF is not alcohol based; therefore, there are no fire or shipping hazards. Furthermore, laboratory usage of STF over formalin can help cut costs because of convenient “down-the sink” disposal (2, 8).
As laboratory regulations governing the use of formalin and mercuric chloride become more rigorous, many clinical, research, and commercial laboratories will want to switch from formalin and PVA to other types of fixatives. Since disposal of mercury compounds is a growing concern, EcoFix, another formalin-free fixative, was tested recently not only as a fixative for stools but also for its staining qualities (3). A comparison of Wheatley's trichrome stain to EcoStain found that the two were comparable in retaining protozoan morphology but that trichrome staining was preferable when the organism numbers were low. It should be noted that STF has been substituted for formalin as a routine tissue fixative in many histopathology laboratories for these same reasons (2, 8). STF provides an excellent alternative to formalin in regard to safety issues and disposal costs, more so because STF performs equally as well as formalin as a fixative. Also worth noting is that STF compared favorably with formalin fixative in two of the more widely used commercial test kits for detection of Cryptosporidium and Giardia. With some modification, STF may prove suitable for trichrome staining and hence may replace PVA also. Having a fixative that would both simplify transport from field to laboratory and allow laboratories to accomplish all microscopic assays normally done to diagnose intestinal parasites would be especially helpful. The ability to dispose of such a fixative down the sink would be a real benefit. A fixative such as STF may provide such a solution. In conclusion, STF provides an excellent substitute for formalin and may ultimately become a preferred fixative for preservation of samples for subsequent microscopic examination of intestinal parasites.
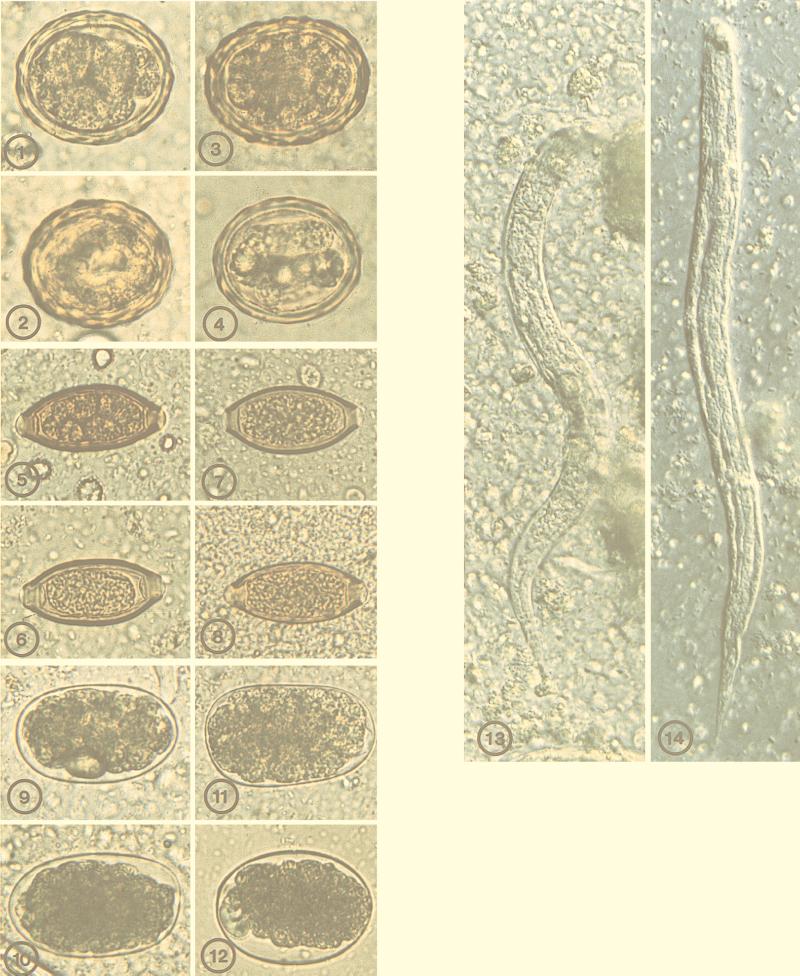
FIG. 1-14.
Vaious helminth eggs, preserved in either 10% formalin or STF, at time zero or after 3 months in wet preparation following sedimentation concentration. FIG. 1. Ascaris lumbricoides, 10% formalin, time zero. FIG. 2. A. lumbricoides, 10% formalin, 3 months. FIG. 3. A. lumbricoides, STF, time zero. FIG. 4. A. lumbricoides, STF, 3 months. FIG. 5. Trichuris trichiura, 10% formalin, time zero. FIG. 6. T. trichiura, 10% formalin, 3 months. FIG. 7. T. trichiura, STF, time zero. FIG. 8. T. trichiura, STF, 3 months. FIG. 9. Necator americanus, 10% formalin, time zero. FIG. 10. N. americanus, 10% formalin, 3 months. FIG. 11. N. americanus, STF, time zero. FIG. 12. N. americanus, STF, 3 months. FIG. 13. and 14. Strongyloides stercoralis rhabditiform larvae preserved in 10% formalin (Fig. 13) or STF (Fig. 14) after 3 months, shown in DIC wet mount.
FIG. 15-30.
Varoius protozoal cysts and oocysts, preserved in 10% formalin or STF, at time zero or after 3 months in wet preparation following sedimentation concentration. FIG. 15. Giardia intestinalis, 10% formalin, time zero, DIC. FIG. 16. Giardia intestinalis, 10% formalin, 3 months, DIC. FIG. 17. G. intestinalis, STF, time zero, DIC. FIG. 18. G. intestinalis, STF, 3 months, DIC. FIG. 19. Entamoeba coli, 10% formalin, time zero, DIC. FIG. 20. E. coli, 10% formalin, 3 months, DIC. FIG. 21. E. coli, STF, time zero, DIC. FIG. 22. E. coli, STF, 3 months, DIC. FIG. 23. Blastocystis hominis, 10% formalin, time zero. FIG. 24. B. hominis, 10% formalin, 3 months. FIG. 25. B. hominis, STF, time zero. FIG. 26. B. hominis, STF, 3 months. FIG. 27. Cyclospora cayetanensis, 10% formalin, time zero, DIC. FIG. 28. C. cayetanensis, 10% formalin, time zero, epifluorescence. FIG. 29. C. cayetanensis, STF, time zero, DIC. FIG. 30. C. cayetanensis, STF, time zero, epifluorescence.
FIG. 31-40.
Various protozoal cysts or oocysts, fixed in either 10% formalin, PVA, or STF, in stained smears. FIG. 31. Giardia intestinalis, PVA, trichrome stain, time zero. FIG. 32.G. intestinalis, STF, trichrome stain, time zero. FIG. 33.Blastocystis hominis, PVA, trichrome stain, time zero. FIG. 34.B. hominis, STF, trichrome stain, time zero. FIG. 35.Cyclospora cayetanensis, 10% formalin, safranin stain, 3 months. FIG. 36.C. cayetanensis, STF, safranin stain, 3 months. FIG. 37.Cryptosporidium parvum, 10% formalin, safranin stain, 3 months. FIG. 38.C. parvum, STF, safranin stain, 3 months. FIG. 39.G. intestinalis, STF, IFA staining, time zero. FIG. 40.C. parvum, STF, IFA staining, time zero.
ACKNOWLEDGMENT
This work was partially supported by Streck Laboratories, Omaha, Nebr.
REFERENCES
- 1.Ash L R, Orihel T C. Formalin-ethyl acetate or formalin-ether sedimentation technique for fresh material. In: Ash L R, editor. Parasites: a guide to laboratory procedures and identification. Chicago, Ill: American Society of Clinical Pathologists Press; 1991. pp. 23–25. [Google Scholar]
- 2.Chapman B. Fixing to switch. College Am Pathol Today. 1996;11:54–62. [Google Scholar]
- 3.Garcia L S, Shimizu R Y. Evaluation of intestinal protozoan morphology in human fecal specimens preserved in EcoFix: comparison of Wheatley's trichrome stain and EcoStain. J Clin Microbiol. 1998;36:1974–1976. doi: 10.1128/jcm.36.7.1974-1976.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald G. Chemical hazards: regulations, identification and resources. J California Dent Assoc. 1989;17:32–34. [PubMed] [Google Scholar]
- 5.Meridian Diagnostics, Inc. Meridian catalog. Cincinnati, Ohio: Meridian Diagnostics Inc.; 1998. [Google Scholar]
- 6.Morgan K T. A brief review of formaldehyde carcinogenesis in relation to rat nasal pathology and human health risk assessment. Toxicol Pathol. 1997;25:291–307. doi: 10.1177/019262339702500307. [DOI] [PubMed] [Google Scholar]
- 7.National Research Council. Prudent practices in the laboratory: handling and disposal of chemicals. Washington, D.C: National Academy Press; 1995. pp. 139–170. [Google Scholar]
- 8.Streck Laboratories, Inc. Streck catalog. Omaha, Nebr: Streck Laboratories, Inc.; 1995. [Google Scholar]