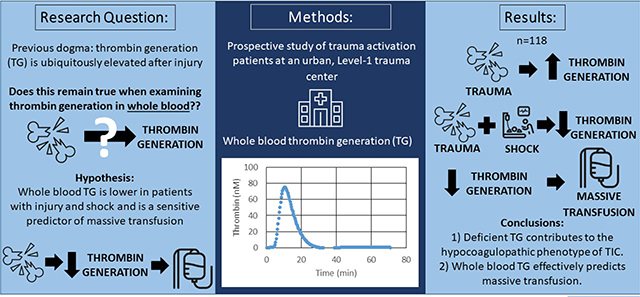
Abstract
Background
Despite the prevalence of hypocoagulability after injury, the majority of trauma patients paradoxically present with elevated thrombin generation (TG). While several studies have examined plasma TG post-injury, this has not been assessed in whole blood. We hypothesize that whole blood TG is lower in hypocoagulopathy, and TG effectively predicts massive transfusion.
Study Design
Blood was collected from trauma activation patients at an urban Level-1 trauma center. Whole blood TG was performed with a prototype point-of-care device. Whole blood TG values in healthy volunteers were compared to trauma patients, and TG values were examined in trauma patients with shock and massive transfusion requirement (MT).
Results
Overall, 118 patients were included (Table 1). Compared to healthy volunteers, trauma patients overall presented with more robust TG; however, those arriving in shock (n=23) had a depressed TG, with significantly lower peak thrombin (88.3 versus 133.0 nM, p=0.01) and slower maximum rate of thrombin generation (27.4 versus 48.3 nM/min, p=0.04). Patients who required massive transfusion (n=26) had significantly decreased TG, with a longer lag time (median 4.8 min versus 3.9 min, p=0.04), decreased peak thrombin (median 71.4 nM versus 124.2 nM, p=0.0003), and lower maximum rate of TG (median 15.8 nM/min versus 39.4 nM/min, p=0.01). AUROC analysis revealed lag time (AUROC 0.6), peak thrombin (AUROC 0.7), and maximum rate of TG (AUROC 0.7) predict early MT.
Conclusion
These data challenge the prevailing bias that all trauma patients present with elevated TG and highlight that deficient thrombin contributes to the hypocoagulopathic phenotype of trauma-induced coagulopathy. Further, whole blood TG predicts massive transfusion, suggesting point of care whole blood TG may be a useful tool for diagnostic and therapeutic strategies in trauma.
Keywords: Thrombin generation, thrombelastography, trauma-induced coagulopathy, massive transfusion, hemostasis
Precis
Whole blood was collected from trauma activation patients at a single trauma center. Whole blood thrombin generation (TG) was measured. Whole blood TG was depressed in patients with shock and it effectively predicted massive transfusion, challenging the prevailing bias that all trauma patients present with elevated TG.
Graphical Abstract
Introduction
Trauma-induced coagulopathy (TIC) is one of the leading causes of preventable death in injured patients(1). While it has been established that TIC constitutes a spectrum of dynamic phenotypes from a hypocoagulable to hypercoagulable state, the mechanisms remain elusive. Approximately 15% of severely injured patients present with a hypocoagulable phenotype, driven by enzymatic and cellular coagulation derangements, endotheliopathy, fibrinolysis and physiologic demise(2–4). Despite this pathologic cascade leading to inadequate clot formation, previous reports examining thrombin generation (TG) in trauma patients have led to a belief that all patients present with an elevated TG after injury(5–7). This prevailing dogma is based on work performed in plasma from severely injured patients, but the description of whole blood thrombin generation (TG) has been limited to experimental work(6, 7). This is relevant because, in line with previous work describing cellular contributions to thrombin generation(8–13), whole blood is known to be distinct from plasma TG and has been proposed as a superior assessment of hemostatic capacity(14, 15).
Studies examining TG in blood from severely injured patients have employed platelet-poor plasma (PPP)(5, 16), which ultimately does not account for the platelet contribution to thrombin generation. Further, recent work indicates that plasma and whole blood TG do not correlate, with plasma TG overestimating the coagulation capacity of injured patients(14). While several studies have reported that plasma TG is elevated following trauma, this has yet to be assessed in whole blood. Moreover, whole blood TG has yet to be evaluated in a heterogenous group of patients spanning the spectrum of TIC phenotypes. This study aims to examine whole blood TG in severely injured trauma patients. We hypothesize that TG in whole blood is depressed in patients with overt hypocoagulopathy and is a sensitive predictor of massive transfusion.
Methods
Study Design
This study is an analysis of prospectively collected data from the Trauma Activation Protocol (TAP), a registry which includes trauma activation patients from 2018 to 2020 at the Ernest E Moore Shock Trauma Center at DH, an American College of Surgeons verified and Colorado state certified academic Level-1 trauma center. The TAP study was approved by the Colorado Multiple Institution Review Board (COMIRB#13–3087) and performed under waiver of consent. Clinical data were collected by trained research professional assistants and included: age, sex, BMI, mechanism of injury, new injury severity score (NISS), field and hospital arrival systolic blood pressure (SBP), Glasgow Coma Scale (GCS), base deficit, complete blood count, PT/INR, partial thromboplastin time (PTT), number of blood products transfused, clinical outcomes, and mortality.
Participants
Criteria for inclusion in TAP were adult patients (≥18 years old) who presented to the hospital as a trauma activation, an emergency department walk-in, or non-transfer patients upgraded to a trauma activation upon arrival. The criteria for activation are traumatic injury with any of the following: 1) GCS < 8 with presumed thoracic, abdominal or pelvic injury, 2) respiratory compromise with presumed thoracic, abdominal, or pelvic injury, 3) blunt trauma with SBP < 90 mm Hg, 4) mechanically unstable pelvic fracture, 5) penetrating injuries to neck or torso with SBP < 90 mm Hg or requiring endotracheal intubation, 6) amputation proximal to the ankle or wrist, or 7) the emergency medicine attending or chief surgical resident believes urgent operative intervention may be required. Exclusion criteria include any patient < 18 years, patients whose initial blood sample is not collected within one hour of injury, consultations from external hospitals, documented chronic liver disease (total bilirubin > 2.0 mg/dL or advanced cirrhosis discovered on laparotomy), known inherited defects of coagulation function, and patients who are known prisoners.
Procedures
Whole blood samples were collected (in field by trained paramedics or upon emergency department presentation within one hour of injury) in citrated vacuum tubes (3.5 mL, 3.2% sodium citrate) for TEG and sodium citrate-corn trypsin inhibitor (CTI) vacuum tubes (3.2% sodium citrate with 100 μg/mL CTI) for whole blood TG. Whole blood TG and TEG were performed within one and two hours of venipuncture respectively. Citrated rapid (CR-) TEG was performed with the TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics, Niles, IL) according to manufacturer instructions(17) and whole blood TG was performed with the prototype point-of-care WB TG device (Near Patient Testing Thrombin Generation [NPT-TG], Stago, Asnières-sur-Seine, France). This latter point-of-care prototype was created for the evaluation of TG in WB. The device functions by activating thrombin generation by relipidated tissue factor and then continuously monitoring by means of a thrombin-specific fluorogenic substrate(18). Change in fluorescence intensity produced by the cleavage of the fluorogenic substrate by thrombin is monitored over time and compared to an internal calibrator with known stable thrombin activity. Whole blood TG was initiated using 6.5 pM relipidated tissue factor reagent and 15 mM CaCl2 (Stago, Asnieres-sur-Seine, France), and TG curves were recorded continuously for 90 minutes at a rate of three readings per minute alongside an internal calibrator.
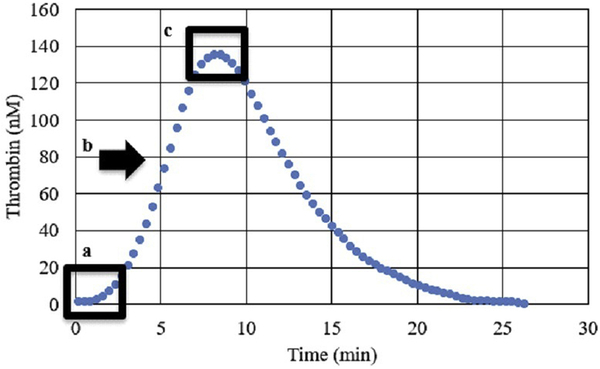
TG yields the following measurements (Figure 1): lag time (minutes from calcium addition to 10 nM of thrombin detected), peak thrombin (the maximum thrombin concentration [in nM] during the assay), and maximum rate of thrombin generation (nM/min; also known as velocity index).
Figure 1.
Standard thrombin generation curve, including measurements of lag time (a), maximum rate of thrombin generation (b), and peak thrombin (c).
Statistical Analysis
The outcomes of interest were whole blood TG measurements: lag time, peak thrombin, and maximum rate of thrombin generation. Descriptive analyses were performed, followed by a comparison of trauma patients’ whole blood TG to that of healthy volunteers based on an established database of normal values with Mann-Whitney test(14), and then comparisons were made between patients who presented with and without shock (lactic acidosis of >4 mmol/L) with Mann-Whitney test. Statistical analyses were performed using R software(19). Significance was established at p < 0.05.
Results
Overall, 118 trauma activation patients presented during the study time period and were included in this investigation. The median age was 33.3 years (interquartile range [IQR] 27.2–47.0), and the majority (75%) were male. Approximately half (52%) of the patients sustained blunt mechanism, and the majority were severely injured, with a median NISS of 22 (IQR 10–34). Median arrival systolic blood pressure (SBP) was 120 mm Hg (IQR 90–140), with 28 (24%) presenting with a SBP < 90 mm Hg. During the first six hours, 48 (41%) received a packed red blood cell (RBC) transfusion (range 1–36 units), 44 (37%) received fresh frozen plasma (FFP) (range 1–41 units), 20 (17%) received platelets (range 1–8 units), and 7 (6%) received cryoprecipitate (range 2–7 units). Early massive transfusion (defined as ≥4U RBC in 1st hour) was required in 26 (22%) of the patients. The overall mortality rate was 18% (21).
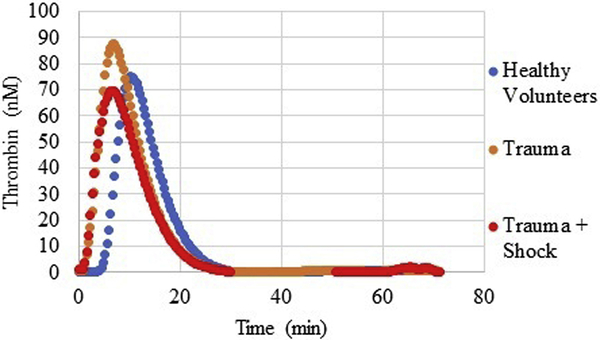
Compared to healthy volunteers, trauma patients overall presented with robust TG, in the form of shortened lag time (4.0 min versus 6.6 min, p<0.0001), higher peak thrombin (122.2 nM versus 86.3 nM, p=0.009), and faster rate of thrombin generation (39.3 nM/min versus 22.8 nM/min, p=0.007). However, when stratifying trauma patients by physiologic status, those arriving in shock (SBP < 90 mm Hg, n=23) had a depressed TG. Patients with shock presented with significantly lower peak thrombin (88.3 versus 133.0 nM, p=0.01) and slower maximum rate of thrombin generation (27.4 versus 48.3 nM/min, p=0.04), whereas there was no difference by lag time (4.3 versus 3.8 min, p=0.72). Interestingly, even when comparing TG values to healthy volunteers, while trauma patients, when examined altogether, had elevated TG as compared to healthy volunteers, when the effect of shock was examined, patients with severe shock had lower TG values than both their non-shock trauma counterparts, and compared to healthy volunteers (Figure 2).
Figure 2.
Thrombin generation curves of healthy volunteers, as compared with trauma patients and trauma patients with shock. Healthy volunteers: mean of 20 patients, Trauma: mean of 118 patients, Trauma + Shock: mean of 23 patients. nM, nanomolar
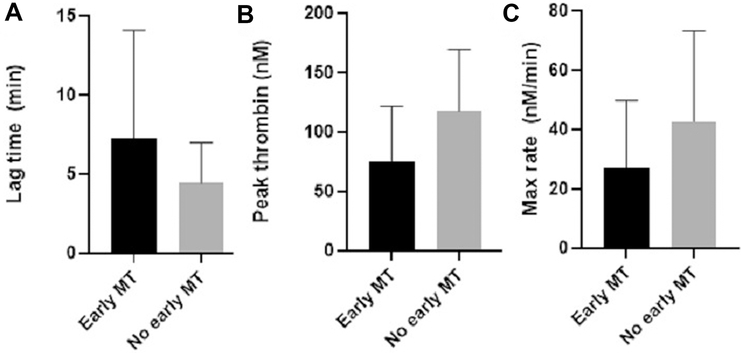
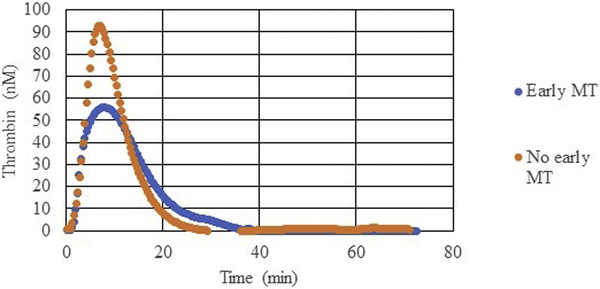
Not only was there a relationship between tissue injury, shock, and whole blood TG, but whole blood TG was also associated with other hematologic markers of coagulopathy and predictive of clinical outcomes associated with coagulopathy. Specifically, patients who presented with decreased maximum amplitude (MA, MA < 55mm), a marker of clot strength reflecting the combined effects of platelets and fibrinogen on TEG, had decreased peak thrombin (median of 87.0 nM versus 125.4 nM in patients with normal MA, p=0.04). Additionally, patients who required an early massive transfusion (n=26) had significantly decreased thrombin generation, with a longer lag time (median 4.8 min [3.4–7.8 IQR] versus 3.9 min [3.0–5.0 IQR] in patients without early MT [n=92], p=0.04), decreased peak thrombin (median 71.4 nM [38.6–113.1 IQR] versus 124.2 nM [80.4–150.4 IQR], p=0.0003), and lower maximum rate of TG (median 15.8 nM/min [8.5–43.9 IQR] versus 39.4 nM/min [20.7–58.4 IQR], p=0.01) (Figure 3 & 4). AUROC analysis revealed lag time (AUOC 0.6), peak thrombin (AUOC 0.7), and maximum rate of TG (AUROC 0.7) predicted early MT. Additionally, patients who required FFP transfusion in the 1st six hours (n=44), had a significantly lower peak thrombin (median 102.5 nM [53.9–127.9 IQR] versus 125.6 nM [78.4–156.4 IQR] in patients who did not require FFP transfusion [n=74], p=0.01). Peak thrombin predicted FFP transfusion in the 1st six hours with an AUROC of 0.6.
Figure 3.
Thrombin generation values of patients who require a massive transfusion (MT)) compared with those who did not require early MT. nM, nanomolar
Figure 4.
Thrombin generation of trauma patients who required early massive transfusion (MT) compared with those who did not require early MT. Early MT: mean of 26 patients, no early MT: mean of 92 patients. nM, nanomolar.
Discussion
The changes in thrombin biology after trauma remains controversial. While several studies have identified that plasma TG is elevated after injury, TG has not been assessed in whole blood not has it been examined in a heterogenous group of patients spanning the spectrum of TIC phenotypes. This study aimed to examine whole blood TG in severely injured trauma patients through analysis of blood collected in a prospective cohort study of all trauma activation patients at an urban, level-1 trauma center. In our 118 trauma activation patients, we confirmed that, compared to healthy volunteers, trauma patients overall presented with robust TG. However, when stratifying trauma patients by physiologic status, those arriving in shock had a depressed TG, with significantly lower peak thrombin and slower maximum rate of thrombin generation. When comparing TG values to healthy volunteers, trauma patients when examined altogether, had elevated TG as compared to healthy volunteers. However, when the effect of shock was examined, patients with severe shock had lower TG values than both their non-shock trauma counterparts, and compared to healthy volunteers. Lastly, the depressed TG seen in shocked patients effectively predicted massive transfusion and FFP transfusion in the first six hours. To our knowledge, this study is the first comprehensive description of whole blood TG in trauma patients and challenges the historical dogma that TG is wholly elevated after injury and not a significant player in early TIC.
Previous work examining plasma TG in animal models of trauma and in trauma patients describes robust thrombin biology with shortened lag time, elevated peak thrombin, and greater rate of thrombin generation(16, 20–23). The underlying mechanism suspected in these plasma-based works postulated that elevated TG in trauma patients was due to high levels of inflammatory cytokines and tissue factor after injury with a concomitant, significant deficit in antithrombin, leading to early robust TG. This early TG is postulated to be followed early consumption of coagulation factors and consequent TIC(24, 25); suggested to be similar mechanistically to disseminated intravascular coagulation (DIC)(26–28). However, this unifying concept has been challenged in a recent consensus forum, which is supported by the presented data(29). Additionally, elevated TG post-injury is not unanimously supported by the plasma-based literature, with some reporting impaired thrombin generation after in vitro and in vivo studies of traumatic injury, coagulopathy, and hemorrhage(30–33). In fact, in a hierarchical clustering analysis of trauma patients (including data with plasma TG), White et al describe three phenotypes of trauma patients, including 1) those with platelet activation, preserved plasma TG, decreased fibrinogen, and normal clot formation, 2) platelet activation, preserved plasma TG, and preserved fibrinogen, or 3) patients with concomitant platelet dysfunction, reduced plasma TG, decreased fibrinogen, and overt coagulopathy(34). Our work, based on whole blood thrombin generation, which is known to be distinct from plasma TG(14), challenges previous literature by identifying that, when shock is taken into consideration, thrombin generation is significantly depressed. This is likely mediated by a myriad of factors, but notably, increasing shock with hypoperfusion has been associated with a rise in soluble thrombomodulin(35), which complexes with thrombin and ultimately has an antithrombotic effect, and acidosis is known to impair thrombin generation in in vitro and animal work(36, 37). The presented work also details an interplay between thrombin biology and cellular contributions to hemostasis with a strong association between decreased peak thrombin and decreased maximum amplitude on TEG (an effect predominantly of platelets), suggesting that patients with aberrant TG are predisposed to decreased interval clot strength.
Not only was whole blood TG significantly depressed in the presence of shock, but this aberrant profile predicted massive transfusion and FFP transfusion in the early window. Our data highlight the potential use of TG as a tool which may outperform current hemostatic assays in identifying patients at risk of coagulopathic demise, in particular in the case of patients who had deranged TG yet normal time to clot formation on TEG. TG as a superior tool for diagnosing TIC has been suggested in plasma-based work in surgical and trauma patients. Matijevic et al examined plasma TG in 40 trauma patients, as compared to 25 healthy controls, and found that, similar to our work, while trauma patients overall had a higher TG, those who had substantial bleeding, mortality, and other established markers of coagulopathy had decreased plasma TG(31). Bosch et al examined plasma TG in patients undergoing cardiac surgery with cardiopulmonary bypass and found that TG reflects hemostatic capacity better than conventional clotting assays and predicted postoperative blood loss effectively(38). Plasma TG has also been explored as a tool for diagnosing pathologic hypercoagulability and venous thromboembolic complications, with a robust TG predicting deep venous thrombosis and thrombotic events despite thromboprophylaxis post-injury(39, 40). This area has yet to be explored in whole blood TG and highlights another future application of this assay.
Describing depressed TG in patients with injury and shock and an association of this TG profile with clinically relevant hemorrhage highlights the potential future for targeting TG in resuscitative efforts. For one, these data emphasize the role of fresh frozen plasma in resuscitation of TIC(41), and the potential for novel therapeutics which target TG specifically. For example, red blood cells are known to release microparticles capable of supporting prothrombinase function(42), and these microparticles, which have been isolated in previous work, may serve as a future therapeutic adjunct. Further, upon activation, platelets release of platelet extracellular vesicles (PEVs) after traumatic injury which result in robust thrombin generation, and these PEVs, when administered in a murine model of uncontrolled hemorrhage mitigate blood loss, representing another potential therapeutic strategy in the future(43, 44).
There are several limitations to this work. First, while this is the first study to examine whole blood thrombin generation in trauma patients and includes over 100 patients, we are limited by a relatively small sample of trauma patients with severe shock or severe tissue injury. Therefore, we are limited to small sample sizes for our sub-cohort analyses. Additionally, these values are limited to a single value in time and a dynamic description of thrombin generation over time and during resuscitation is absent, highlighting potential for future study. The complexity of thrombin biology will need to be investigated further in a larger-scale study of whole blood TG.
Conclusions
Severely injured patients present with robust whole blood thrombin generation as compared to healthy volunteers. However, within the heterogeneous group of trauma patients, those with overt coagulopathy, as identified on conventional coagulation assays, TEG, and clinical markers including transfusion, present with a significantly decreased peak thrombin. These data challenge the prevailing bias that all injured patients present with elevated TG and highlight that inadequate thrombin infusion contributes to the hypocoagulopathic phenotype of TIC. Further, whole blood TG effectively predicts both early massive transfusion and FFP transfusion requirement, suggesting point of care whole blood TG may be a useful tool in the future for guided diagnostic and therapeutic strategies in trauma patients.
Table 1.
Demographics, Injury Characteristics, and Clinical Outcomes of Study Population (n=118).
| Characteristic | Study population (n = 118) |
|---|---|
| Demographic | |
| Age, y, median (IQR) | 33.3 (27.2–47.0) |
| Sex, m, n (%) | 89 (75) |
| Injury characteristic | |
| Time from injury to arrival, min, median (IQR) | 26 (20–34) |
| Blunt mechanism, n (%) | 61 (52) |
| New injury severity score, median (IQR) | 10 (22–34) |
| Glasgow Coma Score, median (IQR) | 6 (14–15) |
| Traumatic brain injury, n (%) | 41 (35) |
| Physiologic marker, median (IQR) | |
| Systolic blood pressure, mmHg | 5.7 (3.4–11.0) |
| Base deficit, meq/L | 11.0 (3.4–5.7) |
| Lactate, mmol/L | 3.6 (2.4–7.0) |
| Hematology/coagulation assay, median (IQR) | |
| Hemoglobin, g/dL | 14.2 (12.9–15.2) |
| Platelets, 109/L | 251 (200–303) |
| International normalized ratio | 1.1. (1.0–1.3) |
| Partial thromboplastin time, sec | 26.7 (23.6–30.9) |
| TEG measurement, median (IQR) | |
| Activating clotting time, sec | 105 (97–121) |
| Angle, degrees | 74.8 (69.2–77.8) |
| Maximum amplitude, mm | 62.0 (55.8–66.8) |
| LY30, % | 1.4 (0.5–2.9) |
| Outcomes, n (%) | |
| Mortality | 21 (18) |
| Massive transfusion | 18 (15) |
IQR, interquartile range; LY30, lysis at 30 minutes; TEG, thromboelastography.
Acknowledgments
Support: Research reported in this publication was supported by the National Institute of General Medical Sciences of the NIH [T32 GM008315 and P50 GM049222]. Drs Silliman, Cohen, and Moore are supported by grants from National Institutes of General Medical Sciences (RM1 grant under review), the National Heart, Lung and Blood Institute [TACTIC - 5 UM 1HL120877], and the Department of Defense [USAMRAA W81XWH-12-2-0028]. Dr Silliman is supported by funding from the Foundation for Women and Girls with Bleeding Disorders.
Disclosure Information: This research was supported with materials from Instrumentation Laboratory and Diagnostica Stago, Inc
Footnotes
Disclosures outside the scope of this work: Dr Silliman is a paid scientific advisory board member of Hemanext, Inc. Dr Moore is cofounder of Thrombo Therapeutics, Inc, and holds a patent on tPA-challenge TEG. Other authors have nothing to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other sponsors of the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. The Journal of Trauma. 1995;38(2):185–93. [DOI] [PubMed] [Google Scholar]
- 2.Gando S, Hayakawa M. Pathophysiology of Trauma-Induced Coagulopathy and Management of Critical Bleeding Requiring Massive Transfusion. Seminars in Thrombosis and Hemostasis. 2016;42(2):155–65. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MJ, Christie SA. Coagulopathy of Trauma. Critical Care Clinics. 2017;33(1):101–18. [DOI] [PubMed] [Google Scholar]
- 4.Moore HB, Moore EE, Liras IN, et al. Targeting resuscitation to normalization of coagulating status: Hyper and hypocoagulability after severe injury are both associated with increased mortality. American Journal of Surgery. 2017;214(6):1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas JC, Rahbar E, Pommerening MJ, et al. Measuring thrombin generation as a tool for predicting hemostatic potential and transfusion requirements following trauma. The Journal of Trauma and Acute Care Surgery. 2014;77(6):839–45. [DOI] [PubMed] [Google Scholar]
- 6.Selby R, Geerts W, Ofosu FA, et al. Hypercoagulability after trauma: hemostatic changes and relationship to venous thromboembolism. Thrombosis Research. 2009;124(3):281–7. [DOI] [PubMed] [Google Scholar]
- 7.Voils SA, Lemon SJ, Jordan J, et al. Early thrombin formation capacity in trauma patients and association with venous thromboembolism. Thrombosis Research. 2016;147:13–5. [DOI] [PubMed] [Google Scholar]
- 8.Gertz JM, Bouchard BA. Mechanisms Regulating Acquisition of Platelet-Derived Factor V/Va by Megakaryocytes. Journal of Cellular Biochemistry. 2015;116(10):2121–6. [DOI] [PubMed] [Google Scholar]
- 9.Hong J, Larsson A, Ekdahl KN, et al. Contact between a polymer and whole blood: sequence of events leading to thrombin generation. The Journal of Laboratory and Clinical Medicine. 2001;138(2):139–45. [DOI] [PubMed] [Google Scholar]
- 10.Ninivaggi M, Apitz-Castro R, Dargaud Y, et al. Whole-blood thrombin generation monitored with a calibrated automated thrombogram-based assay. Clinical Chemistry. 2012;58(8):1252–9. [DOI] [PubMed] [Google Scholar]
- 11.Peyrou V, Lormeau JC, Herault JP, et al. Contribution of erythrocytes to thrombin generation in whole blood. Thrombosis and Haemostasis. 1999;81(3):400–6. [PubMed] [Google Scholar]
- 12.Whelihan MF, Kiankhooy A, Brummel-Ziedins KE. Thrombin generation and fibrin clot formation under hypothermic conditions: an in vitro evaluation of tissue factor initiated whole blood coagulation. Journal of Critical Care. 2014;29(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu S, Lu Y, Sinno T, Diamond SL. Dynamics of Thrombin Generation and Flux from Clots during Whole Human Blood Flow over Collagen/Tissue Factor Surfaces. The Journal of Biological Chemistry. 2016;291(44):23027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman JR, Moore EE, Samuels JM, et al. Whole blood thrombin generation is distinct from plasma thrombin generation in healthy volunteers and after severe injury. Surgery. 2019;166(6):1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripodi A The long-awaited whole-blood thrombin generation test. Clinical Chemistry. 2012;58(8):1173–5. [DOI] [PubMed] [Google Scholar]
- 16.Cardenas JC, Cap AP, Swartz MD, et al. Plasma Resuscitation Promotes Coagulation Homeostasis Following Shock-Induced Hypercoagulability. Shock. 2016;45(2):166–73. [DOI] [PubMed] [Google Scholar]
- 17.Haemonetics. TEG 5000 System User Manual. P/N 06–510-US, Manual revision: AC. Niles, IL: Haemonetics Corporation, Haemoscope Division; 2010. [Google Scholar]
- 18.Prior SM, Mann KG, Freeman K, Butenas S. Continuous thrombin generation in whole blood: New applications for assessing activators and inhibitors of coagulation. Analytical biochemistry. 2018;551:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 20.Albert V, Arulselvi S, Agrawal D, et al. Early posttraumatic changes in coagulation and fibrinolysis systems in isolated severe traumatic brain injury patients and its influence on immediate outcome. Hematology/Oncology and Stem Cell Therapy. 2019;12(1):32–43. [DOI] [PubMed] [Google Scholar]
- 21.Dunbar NM, Chandler WL. Thrombin generation in trauma patients. Transfusion. 2009;49(12):2652–60. [DOI] [PubMed] [Google Scholar]
- 22.Gangloff C, Mingant F, Theron M, et al. New considerations on pathways involved in acute traumatic coagulopathy: the thrombin generation paradox. World Journal of Emergency Surgery. 2019;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaz BH, Winkler AM, James AB, et al. Pathophysiology of early trauma-induced coagulopathy: emerging evidence for hemodilution and coagulation factor depletion. The Journal of Trauma. 2011;70(6):1401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gando S, Sawamura A, Hayakawa M. Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Annals of Surgery. 2011;254(1):10–9. [DOI] [PubMed] [Google Scholar]
- 25.Prior SM, Cohen MJ, Conroy AS, et al. Correlation between factor (F)XIa, FIXa and tissue factor and trauma severity. The Journal of Trauma and Acute Care Surgery. 2017;82(6):1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gando S, Nanzaki S, Sasaki S, Kemmotsu O. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thrombosis and Haemostasis. 1998;79(6):1111–5. [PubMed] [Google Scholar]
- 27.Gando S, Otomo Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Critical Care. 2015;19(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagida Y, Gando S, Sawamura A, et al. Normal prothrombinase activity, increased systemic thrombin activity, and lower antithrombin levels in patients with disseminated intravascular coagulation at an early phase of trauma: comparison with acute coagulopathy of trauma-shock. Surgery. 2013;154(1):48–57. [DOI] [PubMed] [Google Scholar]
- 29.Moore HB, Gando S, Iba T, et al. Defining trauma-induced coagulopathy with respect to future implications for patient management: Communication from the SSC of the ISTH. Journal of Thrombosis and Haemostasis. 2020;18(3):740–7. [DOI] [PubMed] [Google Scholar]
- 30.Martini WZ, Cortez DS, Dubick MA, Blackbourne LH. Different recovery profiles of coagulation factors, thrombin generation, and coagulation function after hemorrhagic shock in pigs. The Journal of Trauma and Acute Care Surgery. 2012;73(3):640–7. [DOI] [PubMed] [Google Scholar]
- 31.Matijevic N, Wang YW, Wade CE, et al. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thrombosis Research. 2014;134(3):652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahbar E, Cardenas JC, Baimukanova G, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. Journal of Translational Medicine. 2015;13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schols SE, Feijge MA, Lance MD, et al. Effects of plasma dilution on tissue-factor-induced thrombin generation and thromboelastography: partly compensating role of platelets. Transfusion. 2008;48(11):2384–94. [DOI] [PubMed] [Google Scholar]
- 34.White NJ, Contaifer D Jr., Martin EJ, et al. Early hemostatic responses to trauma identified with hierarchical clustering analysis. Journal of Thrombosis and Haemostasis. 2015;13(6):978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Annals of Surgery. 2007;245(5):812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martini WZ. Coagulopathy by hypothermia and acidosis: mechanisms of thrombin generation and fibrinogen availability. The Journal of Trauma. 2009;67(1):202–8. [DOI] [PubMed] [Google Scholar]
- 37.Mitrophanov AY, Rosendaal FR, Reifman J. Mechanistic Modeling of the Effects of Acidosis on Thrombin Generation. Anesthesia and Analgesia. 2015;121(2):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosch YP, Al Dieri R, ten Cate H, et al. Measurement of thrombin generation intra-operatively and its association with bleeding tendency after cardiac surgery. Thrombosis Research. 2014;133(3):488–94. [DOI] [PubMed] [Google Scholar]
- 39.Goldman S, Frączek P, Szklanny K, et al. Altered Plasma Clot Properties and Trauma-Related Venous Thromboembolism despite Thromboprophylaxis. Thrombosis and Haemostasis. 2018;118(4):654–63. [DOI] [PubMed] [Google Scholar]
- 40.Park MS, Spears GM, Bailey KR, et al. Thrombin generation profiles as predictors of symptomatic venous thromboembolism after trauma: A prospective cohort study. The Journal of Trauma and Acute Care Surgery. 2017;83(3):381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schols SE, van der Meijden PE, van Oerle R, et al. Increased thrombin generation and fibrinogen level after therapeutic plasma transfusion: relation to bleeding. Thrombosis and Haemostasis. 2008;99(1):64–70. [DOI] [PubMed] [Google Scholar]
- 42.Bouchard BA, Orfeo T, Keith HN, et al. Microparticles formed during storage of red blood cell units support thrombin generation. The Journal of Trauma and Acute Care Surgery. 2018;84(4):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyer MR, Alexander W, Hassoune A, et al. Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice. Journal of Thrombosis and Haemostasis. 2019;17(10):1733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez E, Srivastava AK, Burchfield J, et al. Platelet-derived- Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock. Scientific eports. 2019;9(1):17676. [DOI] [PMC free article] [PubMed] [Google Scholar]