Abstract
Parkinson's disease (PD) is an age-related neurodegenerative disorder, clinically characterized by bradykinesia, rigidity, and resting tremor. Leucine-Rich Repeat Kinase 2 (LRRK2) is a large, multidomain protein containing two enzymatic domains. Missense mutations in its coding sequence are amongst the most common causes of familial PD. The physiological and pathological impact of LRRK2 is still obscure, but accumulating evidence supports a role for LRRK2 in membrane and vesicle trafficking, mainly functioning in the endosome-recycling system, (synaptic) vesicle trafficking, autophagy, and lysosome biology. LRRK2 binds and phosphorylates key regulators of the endomembrane systems and is dynamically localized at the Golgi. The impact of LRRK2 on the Golgi may reverberate throughout the entire endomembrane system and occur in multiple intersecting pathways, including endocytosis, autophagy, and lysosomal function. This would lead to overall dysregulation of cellular homeostasis and protein catabolism, leading to neuronal dysfunction and accumulation of toxic protein species, thus underlying the possible neurotoxic effect of LRRK2 mutations causing PD.
Keywords: Golgi apparatus, LRRK2, lysosomes
Introduction
Leucine-Rich Repeat Kinase 2 (LRRK2) is a large, multidomain protein containing two enzymatic domains (GTPase and kinase) where many mutations linked to familial Parkinson's disease (PD) reside [1]. The most common mutation is the G2019S substitution, located in the kinase domain as the I2020T. A mutational hotspot is found in the GTPase Roc domain, with the rarer R1441G/C/H mutations. Altogether, LRRK2 mutations are amongst the most common causes of familial PD. LRRK2 mutations have an incomplete penetrance that depends on ageing and present a clinical progression similar to idiopathic PD but with pleomorphic pathology [2]. The G2019S mutation is linked to alpha-synuclein (aSyn) neuropathology in ∼50% of carriers, albeit several patients present tauopathy, which could be even more relevant than Lewy bodies (LBs) [3]. This spectrum of similar pathological presentations is intriguing and muddies the waters when attempting to elucidate the underlying molecular mechanisms. Biochemically, the PD mutations in LRRK2 generally lead to an increase in kinase activity, with the G2019S presenting the clearest consensus in enhancing this enzymatic function across independent studies [4,5].
In this respect, intense research work addressed the pathways whereby LRRK2 mutations affect neuronal viability and ultimately cause neurodegeneration. This has been hampered so far by the lack of information on the physiological role of LRRK2 itself. Since its genetic discovery, LRRK2 has been implicated in a plethora of cellular and neuronal functions. LRRK2 appears to consistently regulate synaptic transmission and plasticity [6–11], with pathogenic mutations progressively impairing synaptic efficacy [12,13]. Consistent with this, a role for LRRK2 in intracellular organelle and vesicle trafficking has been emerging, with reports of function in the endosome-recycling system, (synaptic) vesicle trafficking, autophagy, and lysosome biology [14–16]. The identification of the exact neuronal function(s) of LRRK2 found an additional hurdle in the uncertainty about the substrates of its kinase activity. Many cellular targets have been proposed with different methodologies until a robust proteomic approach identified members of the Rab subfamily of GTPases as bona fide phosphorylation targets [17]. Of note, Rabs regulate protein trafficking between the plasma membrane and cellular organelles. This finding further supported a role for LRRK2 in vesicle trafficking.
In this review, we focus on the processes modulated by LRRK2, which could underlie its pathogenic role in PD. Several lines of evidence agree on the negative impact of LRRK2 PD-linked mutations on endosome and autophagy pathways, both impinging on lysosomal function. Interestingly, most intracellular trafficking events involve the Golgi apparatus. We discuss here a cell biology perspective that centers on this organelle to recapitulate the observations reported so far (Figure 1).
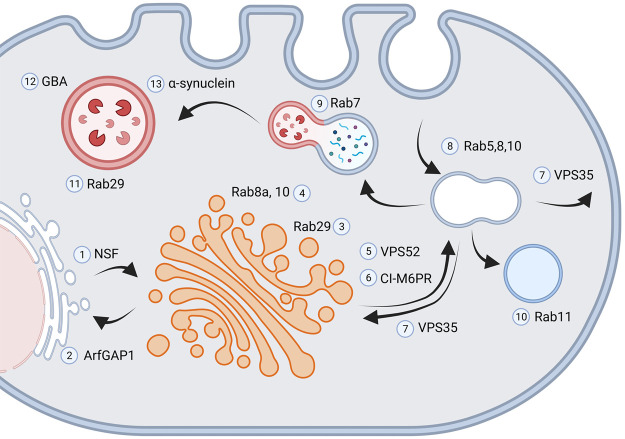
Figure 1. LRRK2 at the endolysosomal system.
LRRK2 interacts with NSF (1) and ArfGAP1 (2) and promotes ER-Golgi antero- and retrograde trafficking, respectively. Both NSF and ArfGAP1 are substrates of LRRK2 kinase activity. Genetic manipulation of NSF and ArfGAP1 affects the neurite phenotype observed in G2019S-LRRK2 models. Rab29 (3) localizes and activates LRRK2 at the trans-Golgi membrane. Rab29-LRRK2 complex triggers the recruitment of Rab10 and Rab8a (4) mediating the clearance of Golgi-derived vesicles. The expression of LRRK2 mutants affects Golgi integrity in a kinase-dependent manner. LRRK2 interacts VPS52 (5), component of the GARP complex that ensures proper trafficking between TGN and the endosome. The cation-independent mannose 6-phosophate receptor (CI-M6PR, 6) delivers the lysosomal hydrolases to endosomes. LRRK2 mutations impair CI-M6PR levels and lysosomal sorting. LRRK2 functionally interacts with VPS35 (7), the core component of the retromer complex, to regulate endosome-to-Golgi and endosome-to-plasma membrane vesicular trafficking. LRRK2 mutations could affect retromer activity via Rab proteins, such as Rab29 or Rab7A. LRRK2 phosphorylation of Rab5, Rab8 and Rab10 (8) impairs the endocytosis of membrane proteins. LRRK2 influences the activity of Rab7 (9) and Rab11 (10), thus altering the trafficking of late and recycling endosomes. The Rab29-LRRK2 complex acts also at the membrane of stressed lysosomes (11). LRRK2 mutants impair the activation of Rab29-LRRK2 healing mechanism. LRRK2 kinase inhibition enhances the activity of the lysosomal enzyme Glucocerebrosidase (GBA, 12). LRRK2 mutation decreases aSyn (13) clearance and enhances aSyn toxicity (created on BioRender.com).
LRRK2 and the endolysosomal system
Accumulating evidence localizes LRRK2 within the endolysosomal system. LRRK2 is localized to endosomes of the degradative pathway, where it interacts with clathrin and modulates actin remodeling [18]. Studies in the brains of iPD subjects revealed endolysosomal pathology in nigral DA neurons, such as the accumulation of Rab5-positive early endosomes, reduction of late endosomes, and depletion of lysosomes [19,20]. Noteworthy, by using proximity ligation assays (PLA), Di Maio and colleagues proposed an increase in LRRK2 kinase activity to underlie endolysosomal alterations. Rotenone exposure in rats triggers LRRK2 activation, as measured by this technique, and mimics the endolysosomal defects observed in iPD brains, including aSyn accumulation. The treatment with a brain penetrant LRRK2 inhibitor prevented endolysosomal pathology and DA toxicity observed upon rotenone insult [20]. Our group has obtained similar results in mice injected in the midbrain with AAV-A53T-aSyn, which causes an increase in PLA signal reflecting LRRK2 kinase activity in DA neurons (BioRxiv 10.1101/2020.10.21.348144). Nevertheless, additional validation from independent laboratories is warranted. Indeed, LRRK2 interacts with several proteins active along the endolysosomal pathway, such as AP3B1, ArfGAP1, N-ethylmaleimide-sensitive fusion (NSF), and the markers Rab proteins (reviewed in [21]). The genetic or pharmacological manipulation of LRRK2 kinase activity directly impacts vesicle trafficking and protein clearance, but the underlying mechanisms are not yet fully clarified. One plausible explanation may arise from the physical and functional interaction between LRRK2 and Rab proteins, such as Rab5, Rab7, Rab8a, Rab10, Rab12, and Rab29 [17,22] (and reviewed in [23,24]). Rabs orchestrate a molecular fingerprint ensuring membrane identity and act as key players for the transport between organelles through vesicles. Specific Rab proteins are active on endosome and lysosome membranes. Rab5 decorates endosomes, but when early endosomes mature into late endosomes, Rab7 replaces Rab5. Further Rab proteins, namely Rab4, Rab11, and Rab14, support the recycling pathways at early endosomes, while Rab9 supports the exchange between the Golgi and the lysosomes (reviewed in [25]).
While a clear LRRK2-dependent Rab phosphorylation has been measured in complementary LRRK2-PD rodent models [17,26–28], the link between the increased Rab phosphorylation and endolysosomal defects and, eventually, neuronal death is still missing. Three studies focusing on the trafficking of the epidermal growth factor receptor (EGFR) showed that overexpression of PD-linked mutant LRRK2 hampered endosomal trafficking of EGFR by decreasing Rab7, Rab8, and Rab10 activity [29–31]. In particular, by comparing mutants mimicking active Rab variants to specific knockdown studies, the authors concluded that pathogenic LRRK2 inhibits Rabs function via their phosphorylation and thus alters membrane trafficking events. Likely, LRRK2 impacts the endolysosomal system through interaction with, or direct phosphorylation of, several Rab proteins. Eventually, functional deficits in Rabs that regulate the late endocytic step may alter the lysosomal degradative processes.
LRRK2 and the Golgi network
Early studies demonstrated that genetic ablation of LRRK2 causes Golgi fragmentation and disrupts vesicular trafficking homeostasis in human renal proximal tubule epithelial cells [32].
Indeed, LRRK2 plays a crucial role in the Golgi compartment, where it influences integrity and vesicle trafficking. The expression of LRRK2 mutants affected Golgi integrity via a LRRK2 kinase-dependent mechanism in a number of PD-relevant models, such as immortalized cell lines, primary neuron cultures, and striatal tissue [33–36]. Recent evidence demonstrated that Rab29 (a.k.a. Rab7L1) recruits LRRK2 to specific cellular compartments, including the trans-Golgi network (TGN). The binding of Rab29 at the LRRK2 N-terminal domain boosts its kinase activity in a GTPase-dependent fashion and triggers the recruitment of Rab10 and Rab8a to the membrane of specific organelles, such as enlarged lysosomes, TGN or phagosomes [35,37–40]. However, these results have been obtained upon Rab29 overexpression. A recent study indicates that endogenous levels of Rab29 do not significantly modulate LRRK2 kinase activity [41]. Further investigations on this aspect are thus required. Of note, Rab10, Rab8a, and Rab29 themselves are substrates of LRRK2 kinase activity [34,36,38]. Phosphorylation operated by LRRK2 may stabilize the Rabs at the TGN membrane and influence their interactions with downstream targets [38]. This Rab29-LRRK2 pathway promotes the clearance of Golgi-derived vesicles through an autophagy-dependent mechanism [33]. The recently described interaction with VPS52, a member of the Golgi-associated retrograde protein (GARP) complex, brings further support to a role for LRRK2 within Golgi physiology [42]. The LRRK2-GARP complex stabilizes the interaction of VPS52 with the SNARE protein STX-6 and eventually modulates both anterograde and retrograde trafficking. ArfGAP1 acts as a GTPase-activating protein for Arf1, a cis-Golgi resident protein involved in the Golgi-ER retrograde pathway [43,44]. LRRK2 interacts with ArfGAP1 within the cytoplasm and at the membrane surface of the Golgi complex and Golgi-derived vesicles [36]. In vitro, ArfGAP1 sustains LRRK2 GTPase and kinase activities and acts as a LRRK2 substrate [36,45]. Genetic manipulation of ArfGAP1 affects the phenotype observed in G2019S-LRRK2 models, such as neurite shortening [36] and DA neuron loss [45]. In particular, ArfGAP1 silencing can revert the effect of G2019S-LRRK2, suggesting the occurrence of an ArfGAP1-LRRK2 mechanism underlying neuronal toxicity.
Lastly, the function of LRRK2 at the Golgi may involve the NSF protein. NSF is an ATPase that facilitates SNARE complex disassembly, thus promoting intracellular membrane dynamics [46]. NSF catalyzes vesicle trafficking within the Golgi [47] and from the endoplasmic reticulum to the Golgi [48]. Eventually, it promotes the reorganization of the Golgi upon mitosis [49]. NSF acts as an interactor and substrate for LRRK2, which increases NSF ATPase activity in vitro upon phosphorylation [50] and leads to the formation of toxic aggregates that induce neurite shortening [11].
Clearly, since the Golgi apparatus sits as a major hub within the endomembrane system, its dysfunctions can affect downstream organelles and processes, such as endosome and lysosome function, synaptic vesicle trafficking and, eventually, neuronal plasticity and maintenance.
The addition of lipids and proteins to target membranes is required for neuronal dendritic growth and takes place through the Golgi (reviewed in [51]). Not surprisingly, genetic alteration of the LRRK2 interactors VPS52, ArfGAP1, and NSF affects neurite outgrowth [36,52,53]. Thus, the Golgi dysfunction observed upon expression of PD mutant LRRK2 may explain dendritic shortening. Instead, the membrane sources allowing axonal growth are less characterized. However, complementary observations suggest a role for late and recycling endosomes via mechanisms involving several Rab proteins, including the LRRK2 substrates Rab8 and Rab5 (albeit evidence is weaker for the latter; reviewed in [54]).
Lysosomes are the last step of the endocytic pathway. Internalization of extracellular and membrane proteins through endosomes culminates in the fusion of late endosomes with lysosomes, forming endolysosomes that degrade the endocytic cargo. Alterations in this pathway could critically imbalance the recycling and degradation of specific protein substrates, further progressing cellular dysfunction [14,16]. The cation-independent mannose 6-phosphate receptor (CI-M6PR, also known as IGF-IIR) delivers the lysosomal hydrolases to endosomes [55]. To execute this function, the receptor must efficiently move from the TGN to the endosomes. Beilina and colleagues brought evidence that LRRK2 mutations impair CI-M6PR levels and trafficking, along with its cargo cathepsin D, implying a lysosomal alteration that eventually resulted in DA neurotoxicity [42]. Noteworthy, postmortem analysis of frontal cortex specimens from PD patients harboring G2019S and I2020T LRRK2 mutations found a robust reduction in CI-M6PR protein expression [56].
VPS35 is a core component of the retromer complex that also includes VPS29 and VPS26. The retromer facilitates both endosome-to-Golgi and endosome-to-plasma membrane transport, thus allowing the recycling of transmembrane protein cargo [57,58]. The retromer is also crucial to target acidic hydrolases to the endosomes that eventually mature in lysosomes. Genetic evidence links VPS35 to familial PD: after the first reports describing the functional impact of the D620N mutation [59,60], other mutations located within the VPS35 gene have been identified in PD patients (reviewed in [61]). VPS35-associated PD is rare, with an estimated frequency of 0.4% of all PD cases. Structural insights suggest that the D620N-VPS35 mutation may impact VPS35 protein interactions [62]. Although the pathological cascade is still obscure, the data gathered so far are supportive for both a gain and loss of function mechanism (reviewed in [63]). LRRK2 functionally interacts with VPS35 in regulating intracellular vesicular trafficking. VPS35 overexpression rescues the sorting defects and neurite shortening observed in G2019S-LRRK2 models. Conversely, the expression of mutant LRRK2 reduces the levels of retromer proteins, including VPS35, in rodent models [39]. However, the investigation of specimens from the frontal cortex of G2019S-LRRK2 PD brains did not enlighten a substantial VPS35 reduction [64]. LRRK2 mutations could affect retromer activity via the phosphorylation of Rab proteins, such as Rab29. To further complicate the picture, D620N-VPS35 indirectly enhances LRRK2 kinase activity towards Rab8A, Rab10, and Rab12, while genetic ablation of VPS35 reduces LRRK2-dependent Rab10 phosphorylation [62]. Insights from D620N-VPS35 models suggest that retromer dysfunctions may induce neurodegeneration potentially via three main pathways: defective autophagy, disruption of neuronal receptor trafficking, and altered mitochondrial plasticity (reviewed in [65]). Accordingly, LRRK2 mutations can affect autophagy (reviewed in [66]), synaptic receptor trafficking [67–69], and mitochondrial physiology (reviewed in [70]). Thus, VPS35 and LRRK2 may participate in the same pathway and control retromer-dependent sorting to different cellular compartments, including the lysosomes. It is important to note here that the vast majority of the studies reported above employ overexpression methods, leaving the exact mechanics of the physiological conditions still to be accurately determined. Still, the data reported above suggests that alteration of Golgi-mediated protein trafficking toward the lysosome has a role in LRRK2-related PD.
The endosome and autophagy systems: convergence of LRRK2 on the lysosome
The common neuropathological feature of neurodegenerative diseases is the accumulation of intracellular protein aggregates, such as the LBs in PD [71]. The autophagy-lysosome pathway (ALP) has been causally implicated in the onset of proteinopathies [72] and in the clearance of aSyn (the main component of LBs) [73]. Indeed, blockade of lysosome function impairs the degradation of WT aSyn [74]. Lysosome abnormalities are recognized as a feature of PD [75], and the efficacy of autophagy declines with aging [66,76], possibly contributing to the risk of neurodegeneration. The ALP is one of the processes mostly studied in the context of LRRK2 pathophysiology. Early studies localized LRRK2 to autophagic vesicles and multivesicular bodies [77]. A wealth of reports indicates a link between ALP dysfunction and LRRK2-PD onset and progression [15,66,76,78,79]. Correct autophagy function is required for neuronal survival and its impairment causes neurodegeneration, including loss of midbrain DA neurons and proteinopathy [80,81]. The alterations of autophagy due to LRRK2 mutants might underlie the pathogenic role of LRRK2 in PD. In addition, it has been reported that the impairment of chaperone-mediated autophagy (CMA) operated by mutant LRRK2 impedes the degradation of aSyn [82], providing a direct link to neuropathology. Autophagy is important for the clearance of other proteins prone to amyloid formation, including Tau [83]. Tauopathy is relatively common in LRRK2 PD, and this could indicate the protein catabolism as a common denominator for different neuropathologies. Nevertheless, it remains mysterious what factors determine the impairment in the degradation of one specific protein substrate.
Despite the flourishing of studies, controversies on the specific effect(s) of LRRK2 on autophagic flux still exist. Inhibition of LRRK2 kinase activity can stimulate the autophagic process [84]. The effects of PD-linked mutations appear more complex and might depend on several parameters, including cell type [85]. Of note, LRRK2 expression increases in activated microglia and localizes to autophagosomes. Stimulation of microglial cells induces autophagy, and LRRK2 could be involved in this process and modulate a neuroinflammatory response. In concordance, LRRK2 silencing in microglia impairs autophagy, indicating it is required for a correct autophagic response [86–88]. On the other hand, the physiological role of LRRK2 in neuronal autophagy is more complex to unequivocally define. Recent evidence indicates an increase in lysosomal activity in LRRK2 KO primary neurons [89], contrasting with previous evidence suggesting the loss of LRRK2 in animals did not affect autophagy in the brain [90].
It is generally accepted that the G2019S mutation causes abnormalities in both macroautophagy and CMA, albeit it is not clear at which step of the ALP these take place. LRRK2 might phosphorylate p62, with G2019S increasing this process and thus impacting autophagy initiation and neurotoxicity [87]. However, the regulation of phagophore biogenesis and autophagosome formation by LRRK2 is straightforward, as reviewed previously [85].
LRRK2 appears to consistently modulate lysosome function, maybe regulating the ALP downstream of its initial stages. The Rab29-LRRK2 complex described above also occurs at the lysosomal membrane. In particular, Rab29 promotes the recruitment of LRRK2 onto overloaded and thus enlarged lysosomes. The subsequent LRRK2-dependent phosphorylation and lysosomal targeting of several Rab proteins, including Rab8a and Rab10, restores the functionality of this organelle. The expression of LRRK2 mutants prevented lysosomal enlargement, thus possibly impairing the activation of the Rab29-LRRK2 healing mechanism [37]. This pathway constitutes a stress-response mechanism to attenuate the consequences of lysosomal overload [37]. However, the participation of Rab29 to this pathway has been recently queried [41]. In addition, LRRK2 inhibition promotes the activity of Glucocerebrosidase (GBA), a critical lysosomal enzyme genetically linked to PD [91]. This suggests a possible link between LRRK2 and lysosomal activity. We and others reported that G2019S-LRRK2 negatively affects lysosome activity via different mechanisms [82,91–94]. These findings indicate a functional relationship between LRRK2 and lysosomes, and suggest that LRRK2 kinase inhibition may improve lysosomal function in PD patients.
LRRK2-dependent modulation of aSyn processing
The observations that aSyn is degraded by lysosomes [95] and that LRRK2 affects autophagy and lysosome activity [82,84] sparked the exploration of a possible role for LRRK2 in aSyn handling and accumulation. Indeed, LB pathology is a prominent feature of LRRK2-PD, albeit not exclusive [96]. However, recent evidence suggested that endogenous LRRK2 may be hyperactive in idiopathic PD presenting with aSyn pathology [19], further supporting a functional interaction between these proteins. Preclinical studies first demonstrated that genetic LRRK2 deletion is protective against aSyn neurotoxicity elicited by viral overexpression [97]. The same authors later reinforced their findings showing that G2019S-LRRK2 sensitizes animals to aSyn-induced neurodegeneration and inflammation [98], which is consistent with the expression and function of LRRK2 in immune cells [87]. Early studies indicated increased aSyn neuropathology in aSyn and LRRK2 double transgenic mice, suggesting a role for LRRK2 in regulating PD pathology and progression [99]. Unfortunately, these results were not replicated by other groups, who failed to identify regulation of aSyn pathology by varied levels of LRRK2 expression [100,101]. It is important to note that these early studies relied on the use of transgenic animal models where expression is regulated differently than the physiological situation. Indeed, the advent of more subtle modeling paradigms, such as knock-in (KI) models and aSyn pre-formed fibrils (PFFs), led to increased literature on the topic. Progressive aSyn neuropathology elicited in neuronal cultures and experimental rodents by exogenous aSyn PFFs [102,103] is worsened by G2019S-LRRK2 expression and ameliorated with pharmacological LRRK2 kinase inhibition [104]. Importantly, brain silencing of LRRK2 using an antisense oligonucleotide strongly prevented PFF-induced aSyn neuropathology in non-transgenic animals, further supporting a permissive role of endogenous LRRK2 in the onset and progression of aSyn proteinopathy [105]. In parallel, KI mouse models expressing PD-causing LRRK2 mutations at physiologic levels became available [8,106–108]. The G2019S-LRRK2 KI mice appear more sensitive to neurodegeneration and neuropathology caused by virally expressed aSyn [109]. In addition, while not showing overt PD-like pathologies, different LRRK2 KI mouse models display age-dependent accumulation of oligomeric and phosphorylated aSyn [110,111]. Indeed, the influence of LRRK2 on the activity of the lysosomal enzyme GBA and the modulation of pathologic aSyn levels supports this view [91]. Our groups also recently reported that LRRK2 modulates lysosome function in a kinase-dependent fashion with a direct impact on pS129-aSyn levels [93]. The field is constantly expanding and providing more detailed information on this complex mechanistic relationship, albeit a ‘full circle' that comes back to modulation of lysosomal function seems to be preponderant [82]. Future studies will address with increased specificity the direct mechanistic link between LRRK2 kinase activity, lysosomal degradation, and accumulation of aSyn (and possibly other aggregation-prone proteins, such as Tau [3]). Indeed, LRRK2 dysfunction caused by PD-linked mutations is hypothesized to promote proteinopathy via impaired aSyn degradation. Identifying the lysosome as an important cellular substrate of LRRK2 supports the view of intracellular vesicle trafficking as a critical event in PD pathogenesis. For this reason, understanding the impact of LRRK2 mutation on the ALP appeared crucial for the development of efficacious therapies.
Perspectives
LRRK2 is dynamically localized at the Golgi where it organizes protein complexes; LRRK2 mutations affect Golgi functionality and integrity.
The impact of LRRK2 on the Golgi may reverberate throughout the entire endolysosomal system and occur in multiple intersecting pathways, including endocytosis, autophagy and lysosomal function.
Lysosomes appear to be particularly sensitive to changes in LRRK2 expression and kinase activity. Impaired lysosomal degradation capacity and impaired autophagy are emerging as recurring themes in distinct PD models. Of note, lysosomes are druggable targets.
Abbreviations
- ALP
autophagy-lysosome pathway
- AP3B1
adaptor related protein complex 3 subunit beta 1
- ArfGAP1
ADP ribosylation factor GTPase activating protein 1
- aSyn
alpha-synuclein
- CI-M6PR
cation-independent mannose 6-phosphate receptor
- CMA
chaperone-mediated autophagy
- DA
dopamine
- EGFR
epidermal growth factor receptor
- GARP
Golgi-associated retrograde protein
- GTPase
guanosine triphosphatase
- iPD
idiopathic Parkinson's disease
- KI
knock-in
- KO
knock-out
- LB
Lewy bodies
- LRRK2
leucine-rich repeat kinase
- NSF
N-ethylmaleimide-sensitive fusion
- PD
Parkinson's disease
- PFFs
aSyn pre-formed fibrils
- SNARE
soluble NSF attachment protein receptor
- STX-6
syntaxin 6
- TGN
trans-Golgi network
- VPS35
vacuolar protein sorting ortholog 35
- VPS52
vacuolar protein sorting-associated protein 52 homolog
- WT
wild-type
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was funded by the Autonomous Province of Bolzano and Weston Brain Institute (grant number RR191071) to MV. G.P. received support by Fondazione Telethon (grant TDPG00514TA), MIUR (PRIN-2017ENN4FY), and Fondazione Cariplo (project 2019-3415). The authors thank the Department of Innovation, Research, University and Museums of the Autonomous Province of Bozen/Bolzano for covering the Open Access publication costs.
Author Contributions
The authors have equally contributed to conception, writing, editing and finalizing this manuscript.
References
- 1.Cookson, M.R. (2015) LRRK2 pathways leading to neurodegeneration. Curr. Neurol. Neurosci Rep. 15, 42 10.1007/s11910-015-0564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalia, L.V., Lang, A.E., Hazrati, L.N., Fujioka, S., Wszolek, Z.K., Dickson, D.W.et al. (2015) Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 72, 100–105 10.1001/jamaneurol.2014.2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson, M.X., Sengupta, M., Trojanowski, J.Q. and Lee, V.M.Y. (2019) Alzheimer's disease tau is a prominent pathology in LRRK2 Parkinson's disease. Acta Neuropathol. Commun. 7, 183 10.1186/s40478-019-0836-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry, A.G., Aghamohammadzadeh, S., Samaroo, H., Chen, Y., Mou, K., Needle, E.et al. (2015) Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum. Mol. Genet. 24, 6013–6028 10.1093/hmg/ddv314 [DOI] [PubMed] [Google Scholar]
- 5.West, A.B. (2015) Ten years and counting: moving leucine-rich repeat kinase 2 inhibitors to the clinic. Mov. Disord. 30, 180–189 10.1002/mds.26075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beccano-Kelly, D.A., Volta, M., Munsie, L.N., Paschall, S.A., Tatarnikov, I., Co, K.et al. (2015) LRRK2 overexpression alters glutamatergic presynaptic plasticity, striatal dopamine tone, postsynaptic signal transduction, motor activity and memory. Hum. Mol. Genet. 24, 1336–1349 10.1093/hmg/ddu543 [DOI] [PubMed] [Google Scholar]
- 7.Lamonaca, G. and Volta, M. (2020) Alpha-synuclein and LRRK2 in synaptic autophagy: linking early dysfunction to late-stage pathology in Parkinson's disease. Cells 9, 1115 10.3390/cells9051115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matikainen-Ankney, B.A., Kezunovic, N., Mesias, R.E., Tian, Y., Williams, F.M., Huntley, G.W.et al. (2016) Altered development of synapse structure and function in striatum caused by Parkinson's disease-linked LRRK2-G2019S mutation. J. Neurosci. 36, 7128–7141 10.1523/JNEUROSCI.3314-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parisiadou, L., Yu, J., Sgobio, C., Xie, C., Liu, G., Sun, L.et al. (2014) LRRK2 regulates synaptogenesis and dopamine receptor activation through modulation of PKA activity. Nat. Neurosci. 17, 367–376 10.1038/nn.3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccoli, G., Condliffe, S.B., Bauer, M., Giesert, F., Boldt, K., De Astis, S.et al. (2011) LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J. Neurosci. 31, 2225–2237 10.1523/JNEUROSCI.3730-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pischedda, F., Cirnaru, M.D., Ponzoni, L., Sandre, M., Biosa, A., Carrion, M.P.et al. (2021) LRRK2 g2019s kinase activity triggers neurotoxic NSF aggregation. Brain 144, 1509–1525 10.1093/brain/awab073 [DOI] [PubMed] [Google Scholar]
- 12.Tozzi, A., Durante, V., Bastioli, G., Mazzocchetti, P., Novello, S., Mechelli, A.et al. (2018) Dopamine D2 receptor activation potently inhibits striatal glutamatergic transmission in a G2019S LRRK2 genetic model of Parkinson's disease. Neurobiol. Dis. 118, 1–8 10.1016/j.nbd.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 13.Tozzi, A., Tantucci, M., Marchi, S., Mazzocchetti, P., Morari, M., Pinton, P.et al. (2018) Dopamine D2 receptor-mediated neuroprotection in a G2019S Lrrk2 genetic model of Parkinson's disease. Cell Death Dis. 9, 204 10.1038/s41419-017-0221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abeliovich, A. and Gitler, A.D. (2016) Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature 539, 207–216 10.1038/nature20414 [DOI] [PubMed] [Google Scholar]
- 15.Roosen, D.A. and Cookson, M.R. (2016) LRRK2 at the interface of autophagosomes, endosomes and lysosomes. Mol. Neurodegener. 11, 73 10.1186/s13024-016-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volta, M., Milnerwood, A.J. and Farrer, M.J. (2015) Insights from late-onset familial parkinsonism on the pathogenesis of idiopathic Parkinson's disease. Lancet Neurol. 14, 1054–1064 10.1016/S1474-4422(15)00186-6 [DOI] [PubMed] [Google Scholar]
- 17.Steger, M., Tonelli, F., Ito, G., Davies, P., Trost, M., Vetter, M.et al. (2016) Phosphoproteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 5, e12813 10.7554/eLife.12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreij, A.M., Chaineau, M., Ruan, W., Lin, S., Barker, P.A., Fon, E.A.et al. (2015) LRRK2 localizes to endosomes and interacts with clathrin-light chains to limit Rac1 activation. EMBO Rep. 16, 79–86 10.15252/embr.201438714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Maio, R., Hoffman, E.K., Rocha, E.M., Keeney, M.T., Sanders, L.H., De Miranda, B.R.et al. (2018) LRRK2 activation in idiopathic Parkinson's disease. Sci. Transl. Med. 10, eaar5429 10.1126/scitranslmed.aar5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocha, E.M., De Miranda, B.R., Castro, S., Drolet, R., Hatcher, N.G., Yao, L.et al. (2020) LRRK2 inhibition prevents endolysosomal deficits seen in human Parkinson's disease. Neurobiol. Dis. 134, 104626 10.1016/j.nbd.2019.104626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erb, M.L. and Moore, D.J. (2020) LRRK2 and the endolysosomal system in Parkinson's disease. J. Parkinsons Dis. 10, 1271–1291 10.3233/JPD-202138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong, G.R., Jang, E.H., Bae, J.R., Jun, S., Kang, H.C., Park, C.H.et al. (2018) Dysregulated phosphorylation of Rab GTPases by LRRK2 induces neurodegeneration. Mol. Neurodegener. 13, 8 10.1186/s13024-018-0240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham, L.A. and Moore, D.J. (2020) Endosomal sorting pathways in the pathogenesis of Parkinson's disease. Prog. Brain Res. 252, 271–306 10.1016/bs.pbr.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwahara, T. and Iwatsubo, T. (2020) The emerging functions of LRRK2 and Rab GTPases in the endolysosomal system. Front. Neurosci. 14, 227 10.3389/fnins.2020.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langemeyer, L., Frohlich, F. and Ungermann, C. (2018) Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol. 28, 957–970 10.1016/j.tcb.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 26.Ito, G., Katsemonova, K., Tonelli, F., Lis, P., Baptista, M.A., Shpiro, N.et al. (2016) Phos-tag analysis of Rab10 phosphorylation by LRRK2: a powerful assay for assessing kinase function and inhibitors. Biochem. J. 473, 2671–2685 10.1042/BCJ20160557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lis, P., Burel, S., Steger, M., Mann, M., Brown, F., Diez, F.et al. (2018) Development of phospho-specific Rab protein antibodies to monitor in vivo activity of the LRRK2 Parkinson's disease kinase. Biochem. J. 475, 1–22 10.1042/BCJ20170802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steger, M., Diez, F., Dhekne, H.S., Lis, P., Nirujogi, R.S., Karayel, O.et al. (2017) Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife 6, e31012 10.7554/eLife.31012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Suaga, P., Rivero-Rios, P., Fdez, E., Blanca Ramirez, M., Ferrer, I., Aiastui, A.et al. (2014) LRRK2 delays degradative receptor trafficking by impeding late endosomal budding through decreasing Rab7 activity. Hum. Mol. Genet. 23, 6779–6796 10.1093/hmg/ddu395 [DOI] [PubMed] [Google Scholar]
- 30.Rivero-Rios, P., Romo-Lozano, M., Fernandez, B., Fdez, E. and Hilfiker, S. (2020) Distinct roles for RAB10 and RAB29 in pathogenic LRRK2-mediated endolysosomal trafficking alterations. Cells 9, 1719 10.3390/cells9071719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivero-Rios, P., Romo-Lozano, M., Madero-Perez, J., Thomas, A.P., Biosa, A., Greggio, E.et al. (2019) The G2019S variant of leucine-rich repeat kinase 2 (LRRK2) alters endolysosomal trafficking by impairing the function of the GTPase RAB8A. J. Biol. Chem. 294, 4738–4758 10.1074/jbc.RA118.005008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanning, N.J., VanOpstall, C., Goodall, M.L., MacKeigan, J.P. and Looyenga, B.D. (2018) LRRK2 deficiency impairs trans-Golgi to lysosome trafficking and endocytic cargo degradation in human renal proximal tubule epithelial cells. Am. J. Physiol. Renal Physiol. 315, F1465–F1477 10.1152/ajprenal.00009.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beilina, A., Rudenko, I.N., Kaganovich, A., Civiero, L., Chau, H., Kalia, S.K.et al. (2014) Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc. Natl Acad. Sci. U.S.A. 111, 2626–2631 10.1073/pnas.1318306111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujimoto, T., Kuwahara, T., Eguchi, T., Sakurai, M., Komori, T. and Iwatsubo, T. (2018) Parkinson's disease-associated mutant LRRK2 phosphorylates Rab7L1 and modifies trans-Golgi morphology. Biochem. Biophys. Res. Commun. 495, 1708–1715 10.1016/j.bbrc.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 35.Purlyte, E., Dhekne, H.S., Sarhan, A.R., Gomez, R., Lis, P., Wightman, M.et al. (2019) Rab29 activation of the Parkinson's disease-associated LRRK2 kinase. EMBO J. 38, e101237 10.15252/embj.2018101237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stafa, K., Trancikova, A., Webber, P.J., Glauser, L., West, A.B. and Moore, D.J. (2012) GTPase activity and neuronal toxicity of Parkinson's disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet. 8, e1002526 10.1371/journal.pgen.1002526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eguchi, T., Kuwahara, T., Sakurai, M., Komori, T., Fujimoto, T., Ito, G.et al. (2018) LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc. Natl Acad. Sci. U.S.A. 115, E9115–E9124 10.1073/pnas.1812196115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, Z., Bryant, N., Kumaran, R., Beilina, A., Abeliovich, A., Cookson, M.R.et al. (2018) LRRK2 phosphorylates membrane-bound rabs and is activated by GTP-bound Rab7L1 to promote recruitment to the trans-Golgi network. Hum Mol Genet. 27, 385–395 10.1093/hmg/ddx410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacLeod, D.A., Rhinn, H., Kuwahara, T., Zolin, A., Di Paolo, G., McCabe, B.D.et al. (2013) RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron 77, 425–439 10.1016/j.neuron.2012.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madero-Perez, J., Fernandez, B., Lara Ordonez, A.J., Fdez, E., Lobbestael, E., Baekelandt, V.et al. (2018) RAB7L1-mediated relocalization of LRRK2 to the Golgi complex causes centrosomal deficits via RAB8A. Front. Mol. Neurosci. 11, 417 10.3389/fnmol.2018.00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalogeropulou, A.F., Freemantle, J.B., Lis, P., Vides, E.G.. Polinski, N.K. and Alessi, D.R. (2020) Endogenous Rab29 does not impact basal or stimulated LRRK2 pathway activity. Biochem. J. 477, 4397–4423 10.1042/BCJ20200458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beilina, A., Bonet-Ponce, L., Kumaran, R., Kordich, J.J., Ishida, M., Mamais, A.et al. (2020) The Parkinson's disease protein LRRK2 interacts with the GARP complex to promote retrograde transport to the trans-Golgi network. Cell Rep. 31, 107614 10.1016/j.celrep.2020.107614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamber, E.P., Siedenburg, A.C. and Barr, F.A. (2019) Rab regulation by GEFs and GAPs during membrane traffic. Curr. Opin. Cell Biol. 59, 34–39 10.1016/j.ceb.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 44.Spang, A. (2013) Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 5, a013391 10.1101/cshperspect.a013391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong, Y., Yuan, C., Chen, R., Dawson, T.M. and Dawson, V.L. (2012) ArfGAP1 is a GTPase activating protein for LRRK2: reciprocal regulation of ArfGAP1 by LRRK2. J. Neurosci. 32, 3877–3886 10.1523/JNEUROSCI.4566-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao, C., Slevin, J.T. and Whiteheart, S.W. (2007) Cellular functions of NSF: not just SNAPs and SNAREs. FEBS Lett. 581, 2140–2149 10.1016/j.febslet.2007.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Block, M.R., Glick, B.S., Wilcox, C.A., Wieland, F.T. and Rothman, J.E. (1988) Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc. Natl Acad. Sci. U.S.A. 85, 7852–7856 10.1073/pnas.85.21.7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckers, C.J., Block, M.R., Glick, B.S., Rothman, J.E. and Balch, W.E. (1989) Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature 339, 397–398 10.1038/339397a0 [DOI] [PubMed] [Google Scholar]
- 49.Rabouille, C., Kondo, H., Newman, R., Hui, N., Freemont, P. and Warren, G. (1998) Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell 92, 603–610 10.1016/S0092-8674(00)81128-9 [DOI] [PubMed] [Google Scholar]
- 50.Belluzzi, E., Gonnelli, A., Cirnaru, M.D., Marte, A., Plotegher, N., Russo, I.et al. (2016) LRRK2 phosphorylates pre-synaptic N-ethylmaleimide sensitive fusion (NSF) protein enhancing its ATPase activity and SNARE complex disassembling rate. Mol. Neurodegener. 11, 1 10.1186/s13024-015-0066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sann, S., Wang, Z., Brown, H. and Jin, Y. (2009) Roles of endosomal trafficking in neurite outgrowth and guidance. Trends Cell Biol. 19, 317–324 10.1016/j.tcb.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 52.Ibuchi, K., Fukaya, M., Shinohara, T., Hara, Y., Shiroshima, T., Sugawara, T.et al. (2020) The Vps52 subunit of the GARP and EARP complexes is a novel Arf6-interacting protein that negatively regulates neurite outgrowth of hippocampal neurons. Brain Res. 1745, 146905 10.1016/j.brainres.2020.146905 [DOI] [PubMed] [Google Scholar]
- 53.Yu, F., Guan, Z., Zhuo, M., Sun, L., Zou, W., Zheng, Z.et al. (2002) Further identification of NSF* as an epilepsy related gene. Brain Res. Mol. Brain Res. 99, 141–144 10.1016/S0169-328X(01)00345-X [DOI] [PubMed] [Google Scholar]
- 54.Petrova, V., Nieuwenhuis, B., Fawcett, J.W. and Eva, R. (2021) Axonal organelles as molecular platforms for axon growth and regeneration after injury. Int. J. Mol. Sci. 22, 1798 10.3390/ijms22041798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castino, R., Demoz, M. and Isidoro, C. (2003) Destination ‘lysosome’: a target organelle for tumour cell killing? J. Mol. Recognit. 16, 337–348 10.1002/jmr.643 [DOI] [PubMed] [Google Scholar]
- 56.Zhao, Y., Perera, G., Takahashi-Fujigasaki, J., Mash, D.C., Vonsattel, J.P.G., Uchino, A.et al. (2018) Reduced LRRK2 in association with retromer dysfunction in post-mortem brain tissue from LRRK2 mutation carriers. Brain 141, 486–495 10.1093/brain/awx344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cullen, P.J. and Korswagen, H.C. (2011) Sorting nexins provide diversity for retromer-dependent trafficking events. Nat. Cell Biol. 14, 29–37 10.1038/ncb2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seaman, M.N. (2012) The retromer complex: endosomal protein recycling and beyond. J. Cell Sci. 125, 4693–4702 10.1242/jcs.103440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vilarino-Guell, C., Wider, C., Ross, O.A., Dachsel, J.C., Kachergus, J.M., Lincoln, S.J.et al. (2011) VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 89, 162–167 10.1016/j.ajhg.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimprich, A., Benet-Pages, A., Struhal, W., Graf, E., Eck, S.H., Offman, M.N.et al. (2011) A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 89, 168–175 10.1016/j.ajhg.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sassone, J., Reale, C., Dati, G., Regoni, M., Pellecchia, M.T. and Garavaglia, B. (2021) The role of VPS35 in the pathobiology of Parkinson's disease. Cell. Mol. Neurobiol. 41, 199–227 10.1007/s10571-020-00849-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mir, R., Tonelli, F., Lis, P., Macartney, T., Polinski, N.K., Martinez, T.N.et al. (2018) The Parkinson's disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem. J. 475, 1861–1883 10.1042/BCJ20180248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohan, M. and Mellick, G.D. (2017) Role of the VPS35 D620N mutation in Parkinson's disease. Parkinsonism Relat. Disord. 36, 10–18 10.1016/j.parkreldis.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 64.Tsika, E., Glauser, L., Moser, R., Fiser, A., Daniel, G., Sheerin, U.M.et al. (2014) Parkinson's disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum. Mol. Genet. 23, 4621–4638 10.1093/hmg/ddu178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams, E.T., Chen, X. and Moore, D.J. (2017) VPS35, the retromer complex and Parkinson's disease. J. Parkinsons Dis. 7, 219–233 10.3233/JPD-161020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Albanese, F., Novello, S. and Morari, M. (2019) Autophagy and LRRK2 in the aging brain. Front. Neurosci. 13, 1352 10.3389/fnins.2019.01352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bono, F., Mutti, V., Devoto, P., Bolognin, S., Schwamborn, J.C., Missale, C.et al. (2021) Impaired dopamine D3 and nicotinic acetylcholine receptor membrane localization in iPSCs-derived dopaminergic neurons from two Parkinson's disease patients carrying the LRRK2 G2019S mutation. Neurobiol. Aging 99, 65–78 10.1016/j.neurobiolaging.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 68.Migheli, R., Del Giudice, M.G., Spissu, Y., Sanna, G., Xiong, Y., Dawson, T.M.et al. (2013) LRRK2 affects vesicle trafficking, neurotransmitter extracellular level and membrane receptor localization. PLoS ONE 8, e77198 10.1371/journal.pone.0077198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rassu, M., Del Giudice, M.G., Sanna, S., Taymans, J.M., Morari, M., Brugnoli, A.et al. (2017) Role of LRRK2 in the regulation of dopamine receptor trafficking. PLoS ONE 12, e0179082 10.1371/journal.pone.0179082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh, F. and Ganley, I.G. (2021) Parkinson's disease and mitophagy: an emerging role for LRRK2. Biochem. Soc. Trans. 49, 551–562 10.1042/BST20190236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brettschneider, J., Del Tredici, K., Lee, V.M. and Trojanowski, J.Q. (2015) Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat. Rev. Neurosci. 16, 109–120 10.1038/nrn3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scrivo, A., Bourdenx, M., Pampliega, O. and Cuervo, A.M. (2018) Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 17, 802–815 10.1016/S1474-4422(18)30238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bandyopadhyay, U. and Cuervo, A.M. (2007) Chaperone-mediated autophagy in aging and neurodegeneration: lessons from alpha-synuclein. Exp. Gerontol. 42, 120–128 10.1016/j.exger.2006.05.019 [DOI] [PubMed] [Google Scholar]
- 74.Vogiatzi, T., Xilouri, M., Vekrellis, K. and Stefanis, L. (2008) Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J. Biol. Chem. 283, 23542–23556 10.1074/jbc.M801992200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein, A.D. and Mazzulli, J.R. (2018) Is Parkinson's disease a lysosomal disorder? Brain 141, 2255–2262 10.1093/brain/awy147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Obergasteiger, J., Frapporti, G., Pramstaller, P.P., Hicks, A.A. and Volta, M. (2018) A new hypothesis for Parkinson's disease pathogenesis: GTPase-p38 MAPK signaling and autophagy as convergence points of etiology and genomics. Mol. Neurodegener. 13, 40 10.1186/s13024-018-0273-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alegre-Abarrategui, J., Christian, H., Lufino, M.M., Mutihac, R., Venda, L.L., Ansorge, O.et al. (2009) LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 18, 4022–4034 10.1093/hmg/ddp346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alegre-Abarrategui, J. and Wade-Martins, R. (2009) Parkinson disease, LRRK2 and the endocytic-autophagic pathway. Autophagy 5, 1208–1210 10.4161/auto.5.8.9894 [DOI] [PubMed] [Google Scholar]
- 79.Manzoni, C. (2017) The LRRK2-macroautophagy axis and its relevance to Parkinson's disease. Biochem. Soc. Trans. 45, 155–162 10.1042/BST20160265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hara, T., Nakamura, K., Matsui, M., Yamamoto, A., Nakahara, Y., Suzuki-Migishima, R.et al. (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 10.1038/nature04724 [DOI] [PubMed] [Google Scholar]
- 81.Sato, S., Uchihara, T., Fukuda, T., Noda, S., Kondo, H., Saiki, S.et al. (2018) Loss of autophagy in dopaminergic neurons causes Lewy pathology and motor dysfunction in aged mice. Sci. Rep. 8, 2813 10.1038/s41598-018-21325-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orenstein, S.J., Kuo, S.H., Tasset, I., Arias, E., Koga, H., Fernandez-Carasa, I.et al. (2013) Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 16, 394–406 10.1038/nn.3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez-Vicente, M. and Cuervo, A.M. (2007) Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol. 6, 352–361 10.1016/S1474-4422(07)70076-5 [DOI] [PubMed] [Google Scholar]
- 84.Manzoni, C., Mamais, A., Dihanich, S., Abeti, R., Soutar, M.P.M., Plun-Favreau, H.et al. (2013) Inhibition of LRRK2 kinase activity stimulates macroautophagy. Biochim. Biophys. Acta 1833, 2900–2910 10.1016/j.bbamcr.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madureira, M., Connor-Robson, N. and Wade-Martins, R. (2020) LRRK2: autophagy and lysosomal activity. Front. Neurosci. 14, 498 10.3389/fnins.2020.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deretic, V. (2012) Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr. Opin. Immunol. 24, 21–31 10.1016/j.coi.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moehle, M.S., Webber, P.J., Tse, T., Sukar, N., Standaert, D.G., DeSilva, T.M.et al. (2012) LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 32, 1602–1611 10.1523/JNEUROSCI.5601-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schapansky, J., Nardozzi, J.D., Felizia, F. and LaVoie, M.J. (2014) Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum. Mol. Genet. 23, 4201–4214 10.1093/hmg/ddu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallings, R., Connor-Robson, N. and Wade-Martins, R. (2019) LRRK2 interacts with the vacuolar-type H+-ATPase pump a1 subunit to regulate lysosomal function. Hum. Mol. Genet. 28, 2696–2710 10.1093/hmg/ddz088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tong, Y., Yamaguchi, H., Giaime, E., Boyle, S., Kopan, R., Kelleher, III, R.J.et al. (2010) Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc. Natl Acad. Sci. U.S.A. 107, 9879–9884 10.1073/pnas.1004676107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ysselstein, D., Nguyen, M., Young, T.J., Severino, A., Schwake, M., Merchant, K.et al. (2019) LRRK2 kinase activity regulates lysosomal glucocerebrosidase in neurons derived from Parkinson's disease patients. Nat. Commun. 10, 5570 10.1038/s41467-019-13413-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hockey, L.N., Kilpatrick, B.S., Eden, E.R., Lin-Moshier, Y., Brailoiu, G.C., Brailoiu, E.et al. (2015) Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 128, 232–238 10.1242/jcs.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Obergasteiger, J., Frapporti, G., Lamonaca, G., Pizzi, S., Picard, A., Lavdas, A.A.et al. (2020) Kinase inhibition of G2019S-LRRK2 enhances autolysosome formation and function to reduce endogenous alpha-synuclein intracellular inclusions. Cell Death Discov. 6, 45 10.1038/s41420-020-0279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schapansky, J., Khasnavis, S., DeAndrade, M.P., Nardozzi, J.D., Falkson, S.R., Boyd, J.D.et al. (2018) Familial knockin mutation of LRRK2 causes lysosomal dysfunction and accumulation of endogenous insoluble alpha-synuclein in neurons. Neurobiol. Dis. 111, 26–35 10.1016/j.nbd.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cuervo, A.M., Stefanis, L., Fredenburg, R., Lansbury, P.T. and Sulzer, D. (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 10.1126/science.1101738 [DOI] [PubMed] [Google Scholar]
- 96.Zimprich, A., Biskup, S., Leitner, P., Lichtner, P., Farrer, M., Lincoln, S.et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 10.1016/j.neuron.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 97.Daher, J.P., Volpicelli-Daley, L.A., Blackburn, J.P., Moehle, M.S. and West, A.B. (2014) Abrogation of alpha-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc. Natl Acad. Sci. U.S.A. 111, 9289–9294 10.1073/pnas.1403215111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daher, J.P., Abdelmotilib, H.A., Hu, X., Volpicelli-Daley, L.A., Moehle, M.S., Fraser, K.B.et al. (2015) Leucine-rich repeat kinase 2 (LRRK2) pharmacological inhibition abates alpha-synuclein gene-induced neurodegeneration. J. Biol. Chem. 290, 19433–19444 10.1074/jbc.M115.660001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin, X., Parisiadou, L., Gu, X.L., Wang, L., Shim, H., Sun, L.et al. (2009) Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron 64, 807–827 10.1016/j.neuron.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Daher, J.P., Pletnikova, O., Biskup, S., Musso, A., Gellhaar, S., Galter, D.et al. (2012) Neurodegenerative phenotypes in an A53T alpha-synuclein transgenic mouse model are independent of LRRK2. Hum. Mol. Genet. 21, 2420–2431 10.1093/hmg/dds057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herzig, M.C., Bidinosti, M., Schweizer, T., Hafner, T., Stemmelen, C., Weiss, A.et al. (2012) High LRRK2 levels fail to induce or exacerbate neuronal alpha-synucleinopathy in mouse brain. PLoS ONE 7, e36581 10.1371/journal.pone.0036581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luk, K.C., Kehm, V., Carroll, J., Zhang, B., O'Brien, P., Trojanowski, J.Q.et al. (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Volpicelli-Daley, L.A., Luk, K.C., Patel, T.P., Tanik, S.A., Riddle, D.M., Stieber, A.et al. (2011) Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 10.1016/j.neuron.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Volpicelli-Daley, L.A., Abdelmotilib, H., Liu, Z., Stoyka, L., Daher, J.P., Milnerwood, A.J.et al. (2016) G2019S-LRRK2 expression augments alpha-synuclein sequestration into inclusions in neurons. J. Neurosci. 36, 7415–7427 10.1523/JNEUROSCI.3642-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao, H.T., John, N., Delic, V., Ikeda-Lee, K., Kim, A., Weihofen, A.et al. (2017) LRRK2 antisense oligonucleotides ameliorate alpha-synuclein inclusion formation in a Parkinson's disease mouse model. Mol. Ther. Nucleic Acids 8, 508–519 10.1016/j.omtn.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Volta, M., Beccano-Kelly, D.A., Paschall, S.A., Cataldi, S., MacIsaac, S.E., Kuhlmann, N.et al. (2017) Initial elevations in glutamate and dopamine neurotransmission decline with age, as does exploratory behavior, in LRRK2 G2019S knock-in mice. eLife 6, e28377 10.7554/eLife.28377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volta, M. and Melrose, H. (2017) LRRK2 mouse models: dissecting the behavior, striatal neurochemistry and neurophysiology of PD pathogenesis. Biochem. Soc. Trans. 45, 113–122 10.1042/BST20160238 [DOI] [PubMed] [Google Scholar]
- 108.Yue, M., Hinkle, K.M., Davies, P., Trushina, E., Fiesel, F.C., Christenson, T.A.et al. (2015) Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol. Dis. 78, 172–195 10.1016/j.nbd.2015.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Novello, S., Arcuri, L., Dovero, S., Dutheil, N., Shimshek, D.R., Bezard, E.et al. (2018) G2019s LRRK2 mutation facilitates alpha-synuclein neuropathology in aged mice. Neurobiol. Dis. 120, 21–33 10.1016/j.nbd.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 110.Ho, P.W., Leung, C.T., Liu, H., Pang, S.Y., Lam, C.S., Xian, J.et al. (2020) Age-dependent accumulation of oligomeric SNCA/alpha-synuclein from impaired degradation in mutant LRRK2 knockin mouse model of Parkinson disease: role for therapeutic activation of chaperone-mediated autophagy (CMA). Autophagy 16, 347–370 10.1080/15548627.2019.1603545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Longo, F., Mercatelli, D., Novello, S., Arcuri, L., Brugnoli, A., Vincenzi, F.et al. (2017) Age-dependent dopamine transporter dysfunction and Serine129 phospho-alpha-synuclein overload in G2019S LRRK2 mice. Acta Neuropathol. Commun. 5, 22 10.1186/s40478-017-0426-8 [DOI] [PMC free article] [PubMed] [Google Scholar]