Abstract
In somatic cells, RNA polymerase II (Pol II) transcription initiation starts by the binding of the general transcription factor TFIID, containing the TATA-binding protein (TBP) and 13 TBP-associated factors (TAFs), to core promoters. However, in growing oocytes active Pol II transcription is TFIID/TBP-independent, as during oocyte growth TBP is replaced by its vertebrate-specific paralog TBPL2. TBPL2 does not interact with TAFs, but stably associates with TFIIA. The maternal transcriptome is the population of mRNAs produced and stored in the cytoplasm of growing oocytes. After fertilization, maternal mRNAs are inherited by the zygote from the oocyte. As transcription becomes silent after oocyte growth, these mRNAs are the sole source for active protein translation. They will participate to complete the protein pool required for oocyte terminal differentiation, fertilization and initiation of early development, until reactivation of transcription in the embryo, called zygotic genome activation (ZGA). All these events are controlled by an important reshaping of the maternal transcriptome. This procedure combines cytoplasmic readenylation of stored transcripts, allowing their translation, and different waves of mRNA degradation by deadenylation coupled to decapping, to eliminate transcripts coding for proteins that are no longer required. The reshaping ends after ZGA with an almost total clearance of the maternal transcripts. In the past, the murine maternal transcriptome has received little attention but recent progresses have brought new insights into the regulation of maternal mRNA dynamics in the mouse. This review will address past and recent data on the mechanisms associated with maternal transcriptome dynamic in the mouse.
Keywords: early embryo, mouse oocytes, readenylation, RNA decay, RNA polymerase II transcription, TBPL2
In Eukaryotes, three DNA-dependent RNA polymerases transcribe the nuclear genome: RNA polymerases I, II and III (Pol I, Pol II and Pol III, respectively). In plants, there are also Pol IV and Pol V, involved in gene silencing [1]. RNA produced by Pol I (long ribosomal RNA precursors; 45S rRNA) and by Pol III (short non translated RNAs such as transfer RNA (tRNA) and 5S rRNA) represent the vast majority of the RNA present in the cell. Pol II is responsible for the generation of the messenger RNAs (mRNAs), long non-coding RNAs, and short non-coding RNAs. These Pol II transcribed RNAs, the mRNA in particular, represent only a minor fraction of the total RNA molecules in a given cell, but functionally the most interesting part. First, the proteins encoded by these mRNAs are crucial for the function, structure and specificity of each cell. Among these proteins, transcription factors control gene expression and transcription. Second, the mRNA content is variable, depending on the cell type, but also on the cell state: it represents the cell identity and fate at a given time. The population of mRNA can evolve upon changes of the cell state, such as during differentiation or during development. For these reasons, the notion of transcriptome is usually restricted to Pol II transcripts.
The half-life of mRNA in different somatic cells is variable, but is generally short (few hours) [2,3]. The population of steady-state RNAs that form the transcriptome at a given time during the life of a given cell is defined by the sum of active transcription (also called nascent transcription) and active degradation of these transcripts [4]. Therefore, regulation of RNA synthesis and RNA degradation define the transcriptome in a given cell at a given time point.
The generation of the cellular diversity of a multicellular organism results from the regulation of specific gene expression. Remarkably, the zygote is initially transcriptionally silent and all the initial developmental events are dependent on RNA and proteins inherited from the gametes. Due to the difference of size between the male and female gametes, the cytoplasm of the fertilized egg is almost exclusively contributed by the oocyte. As a consequence, the initial pool of transcripts of the zygote, prior to the zygotic genome activation (ZGA), is inherited from the oocyte and is called the maternal transcriptome. The oocyte is particularly rich in very stable mRNA: the general half-life of these mRNA is ∼2.5 weeks (compared with a few hours, see above) [5–8] and 20% of the total RNA of a fully grown oocyte is mRNA, which is ∼10 times higher than in any somatic cell [9]. The maternal transcriptome is established by active transcription in growing oocytes (Figure 1). At the end of the oocyte growth, the transcription becomes silent and all the events up to and beyond fertilization are controlled by the stability and translatability of the maternal transcriptome.
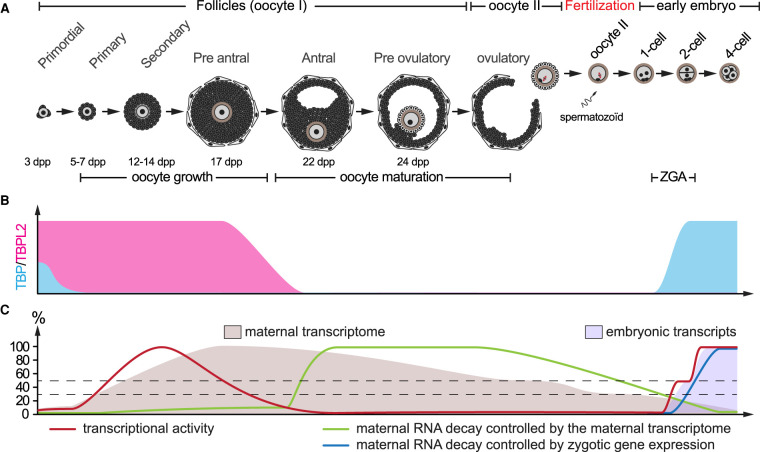
Figure 1. Establishment and reshaping of the maternal transcriptome in the mouse.
(A) Summary of the folliculogenesis and early development in the mouse. Each follicle (from primordial to pre-ovulatory) contains a single primary oocyte arrested at the end of prophase I. Oocyte growth occurs from the primary to the pre-antral follicle stages in a 3-weeks period. Oocyte maturation leads to the resumption of meiosis and ovulation of a secondary oocyte arrested in metaphase II. Meiosis II is completed upon fertilization. Zygotic genome activation (ZGA) is initiated in late 1-cell embryo and complete in 2-cell embryo stages. The indicative timing of folliculogenesis is indicated below [15]. dpp, days post partum. (B) Dynamic expression of TBP (blue) and TBPL2 (pink) proteins. While both TBP and TBPL2 proteins are present in primordial follicle oocytes, TBP is not present during oocyte growth, being replaced by TBPL2. After oocyte growth, TBPL2 protein is not detected anymore. Only TBP protein reappears after fertilization. (C) Evolution of the maternal transcriptome (beige). Oocyte growth is characterized by an important increase in RNA polymerase II transcription activity (red curve) driven by the TBPL2 machinery. Transcription ceases after oocyte growth and remains silent until ZGA. A major reshaping of the maternal transcriptome begins with oocyte maturation by maternally controlled mRNA decay (green curve) degrading 50% of the transcripts, while only 30% remain at fertilization. The degradation is reinforced after ZGA by embryonically controlled mRNA decay products (blue curve).
Here we will review our knowledge about maternal transcriptome establishment during oogenesis and how it is reshaped until the reactivation of zygotic transcription. We will focus mostly on the mouse model, as recent studies allowed a better understanding of the mechanisms associated with the evolution of the maternal transcriptome.
What is the role of the maternal transcriptome?
The male and female gametes are transcriptionally silent. An important step after fertilization is the activation of the transcription of the embryonic genome, also called ZGA, which happens several hours after fertilization. ZGA is occurring in two waves, a minor and a major [10].
Depending on the species, little or lot happens between fertilization and ZGA. In species like insects, nematodes, fish or amphibians, which develop rather quickly, several very fast cell divisions occur in the total absence of transcription. In mammals, which are developing more slowly, initiation of ZGA occurs prior to the first division and is complete at the two-cell stage in the mouse [10]. Altogether, these observations indicate that the early development of embryos is not controlled by active transcription, but by distinct RNA and protein products inherited from the oocyte. At this stage, in the absence of active transcription, post-transcriptional modifications, translation or degradation of mRNAs, as well as post-translational modifications or degradation of proteins are the mechanisms by which early development occurs. In fast developing clades, asymmetric distribution of specific mRNAs inherited from the oocyte is crucial for the patterning of the embryo or establishment of the germline [11]. Thus maternal transcription is functionally important to direct early development almost up to the initiation of gastrulation [10]. In mammals, the situation is different as ZGA is initiated already before the first division [10]. One explanation is that in mammals, the embryonic development stricto sensu is delayed to favor the implantation of the embryo in the uterus for the establishment of the maternal support of embryonic development via the formation of the extra-embryonic annexes [12].
Independent of the extent of maternal control of embryonic development, in all clades the maternal transcriptome is important before fertilization, as it is required for the resumption of meiosis, a crucial step before ovulation and fertilization [13] (Figure 1). This process is controlled by active degradation of specific transcripts, but also by the translatability of the available transcripts (see below). While the maternal transcriptome is important for the competence of the oocyte to resume meiosis and to initiate early zygotic development, its degradation is also required for proper development [14]. Active degradation initiated during meiotic maturation (Figure 1) and persisting up to the ZGA is required for the almost complete elimination of the maternal transcriptome [10]. Defects in this process lead to impaired oocyte maturation characterized by failure to (i) properly resume meiosis I and/or (ii) early embryonic development arrests [15–23]. Thus, while the maternal transcriptome is important to prepare fertilization and to drive early embryonic development, it has to be eliminated and replaced by the embryonic transcriptome, which will control the development of the zygotic genome.
Establishment of the maternal transcriptome
In mice, the maternal transcriptome is built during oogenesis. Oogenesis starts during embryonic development. After a phase of proliferation, all the oogonia initiate the first meiotic division by embryonic day (E) 13.5. Remarkably, all these primary oocytes become arrested at the end of the prophase I and will be maintained during adulthood in this arrested state until the periovulation phase. Therefore, oocytes are unique because they do not divide and they contain 4 copies of the genome. Around birth, the primordial follicles containing a single oocyte associated with few follicular cells are formed. They constitute a limited pool of germ cells that will support the reproductive life of the females. A subset of these primordial follicles is continuously selected for folliculogenesis (i.e. the development of the follicle up to the release of the oocyte). During this process, the follicles increase in size and complexity via the proliferation of the granulosa cells, the oocyte is growing and increases its volume by ∼150-fold in a 3-weeks period (Figure 1). The growth of the oocyte is the consequence of a dramatic increase in specific transcription activity, allowing the accumulation of transcripts and proteins that are required for the acquisition of the competence for meiosis, fertilization and embryonic development.
Pol II-mediated transcription requires the assembly of the pre-initiation complex (PIC) on the promoters [24]. The PIC is formed by the sequential addition of general transcription factors (GTF), including TFIID, which is important to correctly position Pol II on core promoters for specific transcription initiation [25]. Most of the Pol II GTFs are multisubunit complexes. An important particularity in growing oocytes is that Pol II transcription initiation is mediated by an oocyte-specific protein complex [26]. In somatic cells, TFIID composed of the TATA-binding protein (TBP) associated with 13 TBP-associated factors (TAFs), is the first GTF to bind to promoters. TFIID is also present at the early stage of folliculogenesis, as TBP protein is detected in the oocytes of primordial follicles. Interestingly, during oocyte growth, Tbp is still actively transcribed, but Tbp transcripts are only stored and not translated [27]. As a consequence, TBP protein is not detected in the oocytes of primary follicles and onward, and only reappears in 1-cell stage embryos at the time of the ZGA [28] (Figure 1). In growing oocytes, TBP is replaced by a vertebrate specific paralog: TBP-like 2 or TBPL2 (also called TRF3 or TBP2) [28]. The DNA binding domains of mouse TBP and TBPL2 share 95% conservation, and similarly to TBP, TBPL2 is able to interact with TATA-boxes and with the Pol II GTFs TFIIA and TFIIB [29,30]. TBP and TBPL2 however differ in their N terminal domains, showing basically no conservation [29,31]. In mice, TBPL2 is only expressed in oocytes and is initially detected at the primordial follicle stage, up to antral stage [28,32] (Figure 1). TBPL2 is not detected in the oocytes of pre-ovulatory follicles. TBPL2 is absolutely required for oocyte growth as Pol II transcription stops in Tbpl2−/− mutant oocytes resulting in an arrest of growth between the primary and secondary follicle stages [33]. As a result, Tbpl2−/− females are viable, but sterile [33]. Similarly, TBPL2 is highly enriched in the oocytes of Xenopus and zebrafish and a similar TBP/TBPL2 switch has been documented [30], suggesting that this transition is conserved in vertebrate oocytes. Recently, a recurrent null mutation in TBPL2 has been identified in human female patients with fertility problems [34,35], further suggesting that the role of TBPL2 in transcription initiation during oocyte growth is conserved in all vertebrates. However, a main difference is that in Xenopus and zebrafish embryos, TBPL2 is still present after fertilization and is involved in early development [29,30,32].
The murine TBPL2-containing complex has been recently characterized in vivo by immunoprecipitation coupled to mass spectrometry. Contrary to TBP, TBPL2 does not assemble in a TFIID-like structure as it does not interact with TAFs, but strongly associates with TFIIA [26]. The non-functionality of any TFIID-like complex was further demonstrated by the apparent absence of TAF7 in the oocyte nuclei and by the lack of oogenesis defects in Taf7 oocyte-specific conditional mutant females [26]. Remarkably, cap analysis of gene expression (CAGE) during oocyte growth has suggested that TFIID/TBP and TBPL2 recognize different types of promoters [26]. While TFIID/TBP-promoters are devoid of recognizable TATA-elements and have a broad transcription start site (TSS) architecture, TBPL2-dependent promoters are enriched in TATA-like elements and have a sharp TSS pattern. As a consequence, TBPL2 imposes a specific signature of TSS usage that characterizes the maternal transcriptome [26]. Remarkably, this signature is different from the TFIID/TBP-type embryonic signature that takes over at ZGA [36,37].
Another characteristic of Pol II activity during oocyte growth it the strong expression of endogenous retroviral elements (ERV) [38]. These elements are retrotransposons derived from retroviruses usually silenced in somatic cells. During oocyte growth, a particular class of ERV, ERVL-MaLR (endogenous retrovirus like mammalian apparent LTR retrotransposons), is highly expressed via transcription mediated by TBPL2 [26]. The activity of these ERVL-MaLR elements helps to shape the maternal transcriptome as they provide alternative oocyte-specific promoters and first exons to transcripts [38,39]. It is also conceivable that ERVL-MaLR transcripts are important to organize the oocyte chromatin at these developmental stages.
The reason of this overhaul in the transcription initiation machinery is still unclear, but could be associated with a distinct mode of transcription regulation. Seminal experiments in the 90's have suggested that enhancers and co-activators may start to function only in two-cell stage embryos [40–43]. In growing oocytes, the absence of long range interactions is supported by the loss of the canonical histone H3K4 mono-methylation (H3K4me1) mark at distal enhancers [44] and by the atypical chromatin architecture characterized by the progressive loss of topological associating domains (TAD) [45–48].
How is transcription stopped?
The factors controlling the transcriptional arrest at the end of the oocyte growth remain to be clearly identified. In Xenopus, TBPL2 is actively degraded upon meiotic maturation, but transcriptional silencing occurs even if high levels of TBPL2 are artificially maintained [49]. Similarly in mice, the arrest of transcription is not directly associated with TBPL2's disappearance as TBPL2 is still detected in transcriptionally quiescent oocytes of antral follicles [28]. While Pol II remains in the nucleus of transcriptionally quiescent oocytes, it is dissociated from the chromatin [50]. During oocyte growth, the chromatin structure is becoming more organized and more compacted around a very dense area called the nucleolar body (NLB) [51]. Fully grown oocytes can be classified in two categories [52]: (i) NSN (non-surrounded nucleoli) oocytes are still transcriptionally active and are characterized by a diffuse chromatin network with small dense clumps, associated with an incomplete ring of dense chromatin juxtaposed to the NLB, and (ii) SN (surrounded nucleoli) oocytes are transcriptionally silent and characterized by a complete ring of condensed chromatin around the NLB. Therefore, it seems that chromatin compaction correlates with the arrest of active transcription.
Growing oocytes are also characterized by the establishment of non-canonical epigenetic marks. PRC2-dependent H3K27me3 and PRC1-dependent H2A119Ub marks are organized in atypical broad domains [53–55]. These marks are associated with the repression of developmental genes, but not with the termination of transcription [55,56]. These marks are actually important for the organization of the chromatin and maternal epigenetic inheritance after fertilization [54,55]. The gene bodies of actively transcribed genes become heavily methylated [57]. Moreover, global histone methylation, including H3K4me3 and H3K9me3, increases during oocyte growth and culminates in transcriptionally silent fully grown oocytes [58]. Surprisingly, in oocytes, high levels of H3K4me3 correlate with transcription silencing and transcription is still active in oocytes unable to generate H3K4me3 marks [59]. This non canonical association between H3K4me3 marks and transcription silencing can be explained by the fact that H3K4me3 is redistributed in atypical intergenic broad domains during oocyte growth [60,61]. Remarkably, persistence of H3K4me3 and H3K9me3 demethylase activity during oocyte growth prevents the accumulation of these marks, resulting in the maintenance of active transcription in fully grown oocyte despite their SN configuration [62]. Furthermore, histone deacetylase activity is also associated with the arrest of transcription in fully grown oocytes [63]. Altogether, the transformation of the oocyte-specific chromatin landscape is the major mechanism involved in the transcriptional arrest, independently of the degree of chromatin compaction, leading to the eviction of Pol II from the chromatin.
Different machineries and mechanisms to overhaul the maternal transcriptome
At the end of oocyte growth, the oocytes have acquired the competency to resume meiosis and to support the early development before the initiation of the zygotic transcription. As transcription becomes inactive after oocyte growth (Figure 1), all the steps from the initiation of oocyte maturation until the activation of the zygotic transcription will depend on the post transcriptional regulation of previously accumulated maternal transcripts via two main mechanisms: (i) (re-)activation of translation by cytoplasmic readenylation and (ii) degradation of maternal mRNA initiated by deadenylation (Figure 2). The degradation of maternal mRNA that is completed by the four-cell stage [64] and the ZGA constitute the maternal to zygote transition (MZT). Several waves of degradation of specific mRNAs, associated with translation activation of other mRNAs, are important to allow the resumption of meiosis, and after fertilization, erasure of the oocyte identity, establishment of the totipotency state and transition from meiosis to active mitosis. These sequential waves are crucial to eliminate transcripts that are no longer of use and/or could be detrimental to the next developmental steps, in order to clear the way to the newly translated regulators [14].
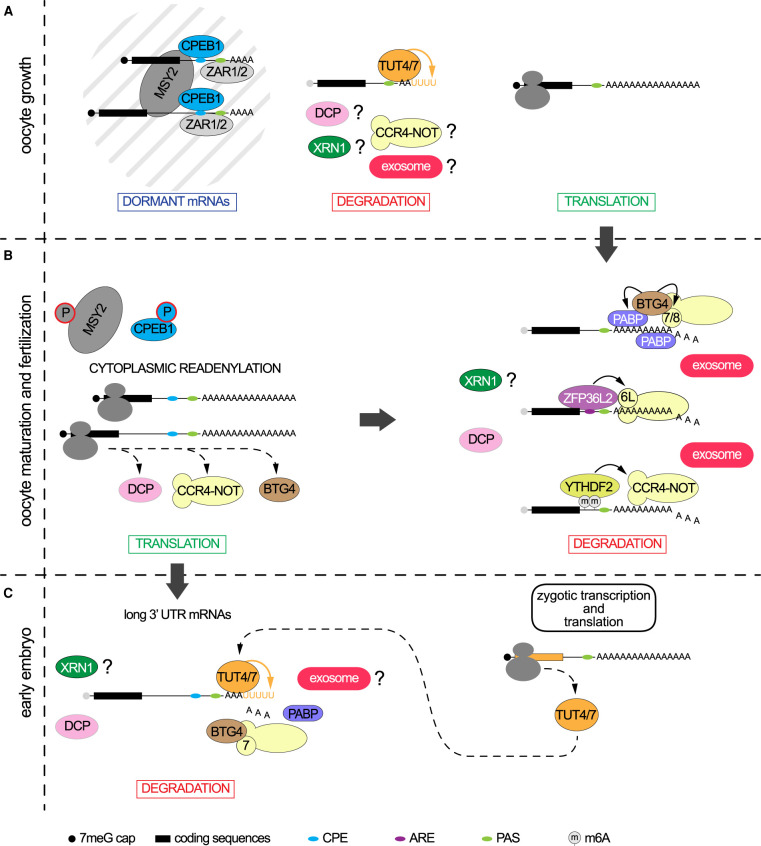
Figure 2. Dynamics of maternal transcriptome translation and degradation during oocyte growth and MZT.
(A) During oocyte growth, dormant mRNAs have cytoplasmic polyadenylation elements (CPE) close to their poly(A) signal (PAS) in their 3′ UTR and have a short poly(A). They are associated with RNA binding proteins CPEB1, ZAR1/2 and MSY2 in the cytoplasmic lattices (CPL) and are not translated. The degradation of mRNA during this period is not well characterized, but terminal 3′ uridylation by TUT4 or TUT7 has been shown to be involved. (B) Upon initiation of oocyte maturation, phosphorylation of MSY2 and CPEB1 leads to the readenylation of the dormant mRNAs and activation of their translation. The combination of the CPE and PAS elements contribute to different regulation of translatability. Translation of proteins coding for the MZT licensing factor BTG4, or subunits of the decapping (DCP) and CCR4-NOT complexes is induced during this period. In parallel, the transcripts that were translated during oocyte growth are degraded. At least three pathways of CCR4-NOT recruitment have been described: (i) the MZT licensing factor BTG4 which interacts with the poly(A) binding proteins (PABP), PABPC1L and PABPN1L and with the CNOT7 or CNOT8 deadenylase subunits of the CCR4-NOT complex, (ii) the AU-rich element (ARE) binding protein ZFP36L2, which recruits CCR4-NOT via its CNOT6L deadenylase subunit, and (iii) the m6A reader YTFHDF2. Activity of the DCP complex is associated with the degradation of maternal mRNAs during this period. The implication XRN1 exoribonuclease has not been studied, however, activity of EXOSC10 exoribonuclease of the exosome complex is important during this phase. (C) Zygotic gene expression of TUT4 and TUT7 is reinforcing the action of the maternal decay proteins, such as BTG4 and CCR4-NOT, and accelerating the degradation of maternal mRNA with longer 3′ UTR that resisted to the maternally controlled decay. Question marks indicate that the implication of these proteins have not yet been studied.
Stability and translatability of mRNA is controlled by the action of several complexes including the CCR4-NOT deadenylase complex, which shortens poly(A) tails, the decapping complex DCP1/DCP2, which removes the 7-methyl guanosine cap, leading to translation inhibition and mRNA body decay. RNA degradation is assured by the 5′–3′ exoribonuclease XRN1, or by the 3′–5′ exoribonuclease exosome complex [65]. The maternal transcriptome contains actively translated mRNAs, but also very stable untranslated transcripts also called dormant maternal mRNAs that will be recruited for translation during oocyte maturation or early development.
Dormant transcripts are deadenylated and stored in the cytoplasm of growing oocytes (Figure 2A). One of the most abundant protein of the growing oocyte is the conserved MSY2 protein (also called YBX2) associated with mRNA stabilization and inhibition of translation [66]. MSY2 and mRNA are found in different related cytoplasmic structures such as the oocyte cytoplasmic lattices (CPL) [51]. MSY2 is associated with the cytoskeleton [67] and binds to 70–80% of mRNA with little sequence specificity [68]. MSY2 is important for the storage and stabilization of mRNA as Msy2−/− oocytes are 90% smaller and contain less mRNAs than their wild-types counterparts [69,70]. The RNA binding proteins ZAR1 and ZAR2 interact with the 3′ UTR of the maternal mRNAs associated with MSY2, as well as with components of the CPL, playing a major role in maternal mRNA stabilization, but also in translational activation upon meiotic maturation [23]. The phosphorylation of MSY2 by CDK1 when meiosis resumes, controls the transition between mRNA stability to instability or translation [69].
Degradation during oocyte growth
Little is known about mRNA degradation prior to meiotic maturation. Very few transcripts with half-live less than 12 days have been identified in fully grown oocytes [71]. Stabilization of a large number of transcripts was observed in the Tbpl2−/− mutant growing oocytes [26], where no active transcription persists [33], strongly suggesting that the RNA decay pathways are active in growing wild-type oocytes. A recent study has indicated that 3′ uridylation of 700 mRNAs with very short poly(A) tails by the terminal 3′ uridylyl transferases, TUT4 and TUT7, is important for the elimination of transcripts during oocyte growth, but also for the ability of the oocytes to undergo meiotic maturation and to initiate development after fertilization [72] (Figure 2A). In mouse oocytes, the miRNA pathway is not active [73,74], however, the endogenous siRNAs and piRNAs are abundant and required for meiotic progression [75], therefore, RNAi could also be involved in the regulation of transcripts, including retrotransposons derived mRNAs, prior to the resumption of the meiosis.
Degradation induced by the meiotic maturation, controlled by the maternal transcriptome
A fast transition occurs at the induction of meiotic maturation. Translation is derepressed for dormant transcripts important for the resumption of meiosis, whereas actively translated mRNAs are deadenylated and degraded [76] (Figure 2B).
The dormant transcripts are characterized by their short poly(A) tails (20–40 A) [77,78]. They also possess specific U-rich sequences in their 3′ UTR such as CPEs (cytoplasmic polyadenylation elements) bound by CPEB1. CPEB1 is a conserved protein important for the control of polyadenylation and translation during oocyte growth and maturation [15]. Combination of multiple CPEs and polyadenylation sequences (PAS) in the 3′ UTR contributes to the regulation of translatability of these transcripts [79]. Activation of ERK1/2 upon meiotic maturation leads to the phosphorylation and the degradation of CPEB1, inducing the readenylation of mRNA such as Dazl and Btg4, and their translation [80,81]. DAZL is a translation activator [82] that enhances the translation of the MZT licensing factor BTG4 of the TOB/BTG family, via the binding to the DAZL binding sites of Btg4 3′ UTR [81].
During oocyte maturation, the degradation of mRNA is intense as 50% of the poly(A)+ RNA present in fully grown oocyte will be degraded in MII oocytes [17,83] and only 30% will be left prior to fertilization [84] (Figure 1). Degradation of the translated transcripts is initiated by deadenylation that a rate-limiting step. One CCR4 deadenylase (CNOT6 or CNOT6L), and one CAF1 deadenylase (CNOT7 or CNOT8) are responsible for CCR4-NOT deadenylase activity [85]. Remarkably, transcripts coding for CNOT6L and CNOT7, as well as for the decapping enzyme DCP2 and its regulator DCP1A, are dormant transcripts, translated upon meiotic maturation (note that Dcp1b is not expressed in oocytes) [16,17]. CNOT6 is expressed at very low levels but is involved in the deadenylation of a specific mRNA population during maturation, however, it is not associated with regulation of mRNA stability or translatability [21]. CNOT6L and CNOT7 activities are important for meiotic progression and correct ZGA [17,20,22]. While CNOT7 and CNOT8 are recruited to mRNA via their interaction with the MZT licensing factor BTG4 [18] that also interacts with poly(A) binding proteins PABPC1L and PABPN1L [86,87], CNOT6L is recruited via the RNA binding protein ZFP36L2 on mRNA containing ARE (A/U-rich element) sequences in their 3′ UTR [20]. The CCR4-NOT complex can also be recruited to mRNAs by the m6A reader YTHDF2 [19,88]. Altogether, these different modes of CCR4-NOT recruitment allow the targeting of different mRNA populations. The accumulation of DCP1A and DCP2 proteins induced by the meiotic maturation is important for the degradation of maternal mRNA indicating that the decapping complex is also involved [16]. It is not known whether XRN1 is involved downstream of CCR4-NOT, however, the implication of the exosome has been demonstrated during oocyte maturation [89] (Figure 2B).
Degradation of the maternal transcriptome controlled by the zygotic transcriptome
At fertilization, 30% of the maternal poly(A)+ transcripts are still present [84]. These mRNA tend to have longer 3′ UTR with more CPEs and PASs and tend to be more actively translated, explaining their resistance to the maternally controlled decay. However, their elimination by a zygotically controlled decay is important for development beyond the four-cell stage [64] (Figure 2C). Contrary to zebrafish or Xenopus, where miRNAs play a major role in the final clearance of maternal transcripts after fertilization, miRNAs are not involved in mouse maternal transcriptome degradation [74]. The zygotic RNA decay machinery still relies on maternal BTG4 and CCR4-NOT complex, but it also requires the activation of zygotic re-expression of Tut4 and Tut7 by the zygotically expressed transcription factor TEAD4 and its activator YAP1 [64]. Terminal 3′ uridylation activity allows the rapid degradation of deadenylated mRNA in two-cell stage embryos [90].
Remarkably, recent published data indicate that some of these mechanisms are conserved between mouse and human. Homozygous mutations in the human BTG4 gene have been associated with early developmental defects and female infertility [91]. Interestingly, one of these mutations did not affect BTG4 protein expression, but resulted in the loss of BTG4 and CNOT7 interaction, strongly suggesting that BTG4 is also involved in the recruitment of the CCR4-NOT complex for the clearance of maternal mRNA in human [91]. It has also been shown that although the transcriptomes of fully grown human and mouse oocytes only partially overlap, in both species, the degradation of maternal transcripts is controlled by both maternally and zygotically controlled decay pathways [92].
Conclusions
The maternal transcriptome is a transient group of mRNAs that have the particularity to be transcribed by a unique transcription initiation machinery but also, to be translated and degraded in a very controlled manner. This dynamic is important to support different sequential steps before the embryo genome takes over the control of gene expression. The presence of a maternal transcriptome is conserved among all animals and while the general phases of transcription, differential translation and degradation are shared, its functional importance differs from most of the clades in mammals, where it is not involved in the establishment of early embryonic patterning, probably due to divergent modes of development. Nevertheless, in all animals, the maternal transcriptome is remodeled and fully degraded to allow proper development, even if some of the maternal and the zygotic transcripts are transcribed from the same genes. It is tempting to speculate that degrading the whole maternal transcriptome to clear the path to the embryonic transcription is a better basic solution than differentially degrading some maternal mRNA while preserving others. Remarkably, at the onset of ZGA, there is another complete change in transcription initiation machinery. This transition is associated with the complete disappearance of maternal transcripts. In the future, it would be important to know whether mRNAs produced by TFIID/TBP-dependent transcription initiation, are similarly degraded at the beginning of oocyte growth (Figure 3).
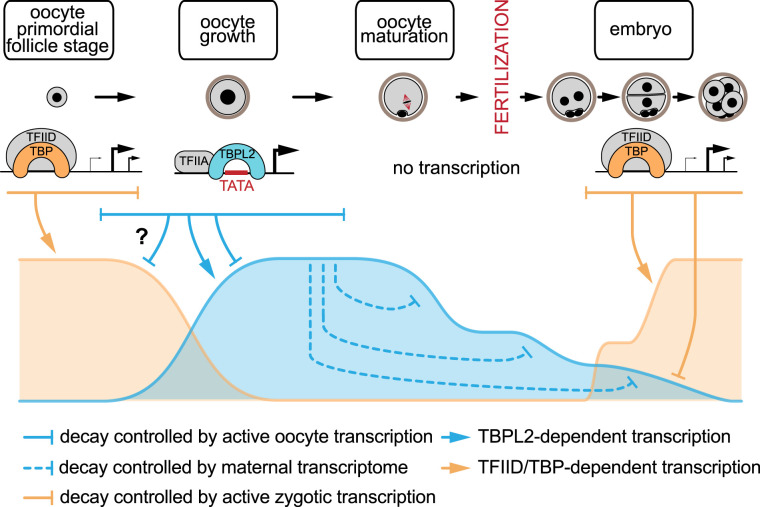
Figure 3. The maternal transcriptome degradation is regulated by decay pathways controlled by two transcriptional initiation machineries.
Two initiation machinery switches occur in the life time of the maternal transcriptome: at the beginning of oocyte growth and at the zygotic genome activation (ZGA) after fertilization. The bulk of the maternal transcriptome is produced by transcription initiated by the TBPL2/TFIIA complex (blue) that interacts preferentially with TATA boxes (TATA) of core promoters with sharp promoter architecture. Active RNA decay during this phase has been recently suggested [26], probably controlled by TBPL2/TFIIA initiated transcripts (blue line). After growth, transcription is silent and the different phases of maternal transcriptome degradation are under the control of proteins translated from dormant transcripts transcribed during the oocyte growth (dashed blue line). After ZGA, the degradation of the maternal transcriptome is reinforced by zygotically expressed proteins (orange line), leading to the disappearance of the maternal transcriptome after the four-cell stage. An interesting hypothesis is that a similar complete degradation of the initial transcriptome inherited from the TFIID/TBP transcription initiation occurs during oocyte growth (question mark). Note that there is an available resource on transcripts dynamics during maternal to zygotic transition [93].
Perspectives
Initiation of the development occurs in a transcription free environment and is controlled by the reshaping of the maternal transcriptome composed of maternally deposited mRNA during oocyte growth.
A specific transcription initiation machinery is responsible for the establishment of the maternal transcriptome. Sequential transition of maternal mRNA stability and translatability reshape the maternal transcriptome.
A second transcription initiation machinery switch occurs at the beginning of the ZGA and is associated with active degradation of the maternal transcripts which have been generated by the TBPL2/TFIIA initiation machinery. It will be interesting to test whether mRNAs transcribed before the first transcription initiation machinery transition, by the TFIID/TBP-dependent initiation machinery, are also similarly specifically eliminated at the beginning of oocyte growth.
Acknowledgements
We are grateful to Ferenc Müller for helpful discussions, Didier Devys, Ferenc Müller and Bertand Séraphin for critically reading the manuscript.
Abbreviations
- CAGE
cap analysis of gene expression
- CPL
cytoplasmic lattices
- ERV
endogenous retroviral elements
- GTF
general transcription factors
- MZT
maternal to zygote transition
- NLB
nucleolar body
- PAS
polyadenylation sequences
- PIC
pre-initiation complex
- SN
surrounded nucleoli
- TAFs
TBP-associated factors
- TBP
TATA-binding protein
- TSS
transcription start site
- ZGA
zygotic genome activation
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by funds from the Agence Nationale de la Recherche (PICen-19-CE11-0003-02, and EpiCAST-19-CE12-0029-01) and NIH grant 1RO1GM131626-01 to L.T. and Fondation pour la Recherche Médicale (EQU202103012631) to S.D.V. and L.T. This work was supported by funds from CNRS, INSERM, Strasbourg University, and Investissements d'Avenir grants (ANR-10-IDEX-0002-02 and ANR-10-LABX-0030-INRT).
Author Contributions
L.T. and S.D.V. wrote the review. S.D.V. created the figures.
References
- 1.Roeder, R.G. (2019) 50+ years of eukaryotic transcription: an expanding universe of factors and mechanisms. Nat. Struct. Mol. Biol. 26, 783–791 10.1038/s41594-019-0287-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lubimova, E.V., Chernovskaja, T.V. and Lerman, M.I. (1975) Three mRNA populations differing in turnover and processing in mouse liver. Mol. Biol. Rep. 2, 269–275 10.1007/BF00357013 [DOI] [PubMed] [Google Scholar]
- 3.Kidder, G.M. and Pedersen, R.A. (1982) Turnover of embryonic messenger RNA in preimplantation mouse embryos. J. Embryol. Exp. Morph. 67, 37–49 PMID: [PubMed] [Google Scholar]
- 4.Timmers, H.T.M. and Tora, L. (2018) Transcript buffering: a balancing act between mRNA synthesis and mRNA degradation. Mol. Cell 72, 10–17 10.1016/j.molcel.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 5.Bachvarova, R. and Leon, V.D. (1980) Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev. Biol. 74, 1–8 10.1016/0012-1606(80)90048-2 [DOI] [PubMed] [Google Scholar]
- 6.Brower, P.T., Gizang, E., Boreen, S.M. and Schultz, R.M. (1981) Biochemical studies of mammalian oogenesis: Synthesis and stability of various classes of RNA during growth of the mouse oocyte in vitro. Dev. Biol. 86, 373–383 10.1016/0012-1606(81)90195-0 [DOI] [PubMed] [Google Scholar]
- 7.Leon, V.D., Johnson, A. and Bachvarova, R. (1983) Half-lives and relative amounts of stored and polysomal ribosomes and poly(A) + RNA in mouse oocytes. Dev. Biol. 98, 400–408 10.1016/0012-1606(83)90369-X [DOI] [PubMed] [Google Scholar]
- 8.Bachvarova, R. (1985) Gene expression during oogenesis and oocyte development in mammals. Dev. Biol. 1, 453–524 10.1007/978-1-4615-6814-8_11 [DOI] [PubMed] [Google Scholar]
- 9.Piko, L. and Clegg, K.B. (1982) Quantitative changes in total RNA, total poly(A), and ribosomes in early mouse embryos. Dev. Biol. 89, 362–378 10.1016/0012-1606(82)90325-6 [DOI] [PubMed] [Google Scholar]
- 10.Vastenhouw, N.L., Cao, W.X. and Lipshitz, H.D. (2019) The maternal-to-zygotic transition revisited. Development (Cambridge, England) 146, dev161471 10.1242/dev.161471 [DOI] [PubMed] [Google Scholar]
- 11.Blower, M.D. (2013) Chapter one molecular insights into intracellular RNA localization. Int. Rev. Cell Mol. Biol. 302, 1–39 10.1016/B978-0-12-407699-0.00001-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molè, M.A., Weberling, A. and Zernicka-Goetz, M. (2019) Comparative analysis of human and mouse development: from zygote to pre-gastrulation. Curr. Top. Dev. Biol. 136, 113–138 10.1016/bs.ctdb.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 13.He, M., Zhang, T., Yang, Y. and Wang, C. (2021) Mechanisms of oocyte maturation and related epigenetic regulation. Front. Cell Dev. Biol. 9, 654028 10.3389/fcell.2021.654028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svoboda, P., Fulka, H. and Malik, R. (2017) Clearance of parental products. Adv. Exp. Med. Biol. 953, 489–535 10.1007/978-3-319-46095-6_10 [DOI] [PubMed] [Google Scholar]
- 15.Racki, W.J. and Richter, J.D. (2006) CPEB controls oocyte growth and follicle development in the mouse. Development 133, 4527–4537 10.1242/dev.02651 [DOI] [PubMed] [Google Scholar]
- 16.Ma, J., Flemr, M., Strnad, H., Svoboda, P. and Schultz, R.M. (2013) Maternally recruited DCP1A and DCP2 contribute to messenger RNA degradation during oocyte maturation and genome activation in mouse. Biol. Reprod. 88, 11 10.1095/biolreprod.112.105312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma, J., Fukuda, Y. and Schultz, R.M. (2015) Mobilization of dormant Cnot7 mRNA promotes deadenylation of maternal transcripts during mouse oocyte maturation. Biol. Reprod. 93, 48 10.1095/biolreprod.115.130344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu, C., Ji, S.-Y., Sha, Q.-Q., Dang, Y., Zhou, J.-J., Zhang, Y.-L.et al. (2016) BTG4 is a meiotic cell cycle-coupled maternal-zygotic-transition licensing factor in oocytes. Nat. Struct. Mol. Biol. 23, 387–394 10.1038/nsmb.3204 [DOI] [PubMed] [Google Scholar]
- 19.Ivanova, I., Much, C., Giacomo, M.D., Azzi, C., Morgan, M., Moreira, P.N.et al. (2017) The RNA m6A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol. Cell 67, 1059–1067.e4 10.1016/j.molcel.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sha, Q.-Q., Yu, J.-L., Guo, J.-X., Dai, X.-X., Jiang, J.-C., Zhang, Y.-L.et al. (2018) CNOT6L couples the selective degradation of maternal transcripts to meiotic cell cycle progression in mouse oocyte. EMBO J. 37, e99333 10.15252/embj.201899333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vieux, K.-F. and Clarke, H.J. (2018) CNOT6 regulates a novel pattern of mRNA deadenylation during oocyte meiotic maturation. Sci. Rep. 8, 6812 10.1038/s41598-018-25187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvat, F., Fulka, H., Jankele, R., Malik, R., Jun, M., Solcova, K.et al. (2018) Role of Cnot6l in maternal mRNA turnover. Life Sci. Alliance 1, e201800084 10.26508/lsa.201800084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong, Y., Ji, S.-Y., Zhu, Y.-Z., Wu, Y.-W., Shen, L. and Fan, H.-Y. (2019) ZAR1 and ZAR2 are required for oocyte meiotic maturation by regulating the maternal transcriptome and mRNA translational activation. Nucleic acids Res. 47, 11387–11402 10.1093/nar/gkz863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haberle, V. and Stark, A. (2018) Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 19, 621–637 10.1038/s41580-018-0028-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen, X., Qi, Y., Wu, Z., Wang, X., Li, J., Zhao, D.et al. (2021) Structural insights into preinitiation complex assembly on core promoters. Science 372, eaba8490 10.1126/science.aba8490 [DOI] [PubMed] [Google Scholar]
- 26.Yu, C., Cvetesic, N., Hisler, V., Gupta, K., Ye, T., Gazdag, E.et al. (2020) TBPL2/TFIIA complex establishes the maternal transcriptome through oocyte-specific promoter usage. Nat. Commun. 11, 6439 10.1038/s41467-020-20239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veenstra, G.J., Destrée, O.H. and Wolffe, A.P. (1999) Translation of maternal TATA-binding protein mRNA potentiates basal but not activated transcription in Xenopus embryos at the midblastula transition. Mol. Cell. Biol. 19, 7972–7982 10.1128/MCB.19.12.7972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazdag, E., Rajkovic, A., Torres-Padilla, M.-E. and Tora, L. (2007) Analysis of TATA-binding protein 2 (TBP2) and TBP expression suggests different roles for the two proteins in regulation of gene expression during oogenesis and early mouse development. Reproduction (Cambridge, England) 134, 51–62 10.1530/REP-06-0337 [DOI] [PubMed] [Google Scholar]
- 29.Bártfai, R., Balduf, C., Hilton, T., Rathmann, Y., Hadzhiev, Y., Tora, L.et al. (2004) TBP2, a vertebrate-specific member of the TBP family, is required in embryonic development of zebrafish. Curr. Biol. 14, 593–598 10.1016/j.cub.2004.03.034 [DOI] [PubMed] [Google Scholar]
- 30.Jallow, Z., Jacobi, U.G., Weeks, D.L., Dawid, I.B. and Veenstra, G.J.C. (2004) Specialized and redundant roles of TBP and a vertebrate-specific TBP paralog in embryonic gene regulation in Xenopus. Proc. Natl Acad. Sci. U.S.A. 101, 13525–13530 10.1073/pnas.0405536101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persengiev, S.P., Zhu, X., Dixit, B.L., Maston, G.A., Kittler, E.L.W. and Green, M.R. (2003) TRF3, a TATA-box-binding protein-related factor, is vertebrate-specific and widely expressed. Proc. Natl Acad. Sci. U.S.A. 100, 14887–14891 10.1073/pnas.2036440100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao, L., Kim, M. and DeJong, J. (2006) Developmental and cell type-specific regulation of core promoter transcription factors in germ cells of frogs and mice. Gene Expr. Patterns 6, 409–419 10.1016/j.modgep.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 33.Gazdag, E., Santenard, A., Ziegler-Birling, C., Altobelli, G., Poch, O., Tora, L.et al. (2009) TBP2 is essential for germ cell development by regulating transcription and chromatin condensation in the oocyte. Genes Dev. 23, 2210–2223 10.1101/gad.535209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He, W.-B., Zhang, Y.-X., Tan, C., Meng, L.-L., Liu, G., Li, Y.et al. (2020) A recurrent mutation in TBPL2 causes diminished ovarian reserve and female infertility. J. Genet. Genomics 47, 785–788 10.1016/j.jgg.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 35.Yang, P., Chen, T., Hou, Z., Zou, Y., Li, M., Zhang, X.et al. (2021) A homozygous variant in TBPL2 was identified in women with oocyte maturation defects and infertility. Hum. Reprod. 36, 2011–2019 10.1093/humrep/deab094 [DOI] [PubMed] [Google Scholar]
- 36.Haberle, V., Li, N., Hadzhiev, Y., Plessy, C., Previti, C., Nepal, C.et al. (2014) Two independent transcription initiation codes overlap on vertebrate core promoters. Nature 507, 381–385 10.1038/nature12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cvetesic, N., Borkowska, M., Hatanaka, Y., Leitch, H.G., Müller, F., Yu, C.et al. (2020) Global regulatory transitions at core promoters demarcate the mammalian germline cycle. bioRxiv 10.1101/2020.10.30.361865 [DOI] [Google Scholar]
- 38.Peaston, A.E., Evsikov, A.V., Graber, J.H., de Vries, W.N., Holbrook, A.E., Solter, D.et al. (2004) Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev. Cell 7, 597–606 10.1016/j.devcel.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 39.Brind'Amour, J., Kobayashi, H., Albert, J.R., Shirane, K., Sakashita, A., Kamio, A.et al. (2018) LTR retrotransposons transcribed in oocytes drive species-specific and heritable changes in DNA methylation. Nat. Commun. 9, 3331–3314 10.1038/s41467-018-05841-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majumder, S., Miranda, M. and DePamphilis, M.L. (1993) Analysis of gene expression in mouse preimplantation embryos demonstrates that the primary role of enhancers is to relieve repression of promoters. EMBO J. 12, 1131–1140 10.1002/j.1460-2075.1993.tb05754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiekowski, M., Miranda, M. and DePamphilis, M.L. (1993) Requirements for promoter activity in mouse oocytes and embryos distinguish paternal pronuclei from maternal and zygotic nuclei. Dev. Biol. 159, 366–378 10.1006/dbio.1993.1248 [DOI] [PubMed] [Google Scholar]
- 42.Majumder, S., Zhao, Z., Kaneko, K. and DePamphilis, M.L. (1997) Developmental acquisition of enhancer function requires a unique coactivator activity. EMBO J. 16, 1721–1731 10.1093/emboj/16.7.1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawinger, P., Rastelli, L., Zhao, Z. and Majumder, S. (1999) Lack of enhancer function in mammals is unique to oocytes and fertilized eggs. J. Biol. Chem. 274, 8002–8011 10.1074/jbc.274.12.8002 [DOI] [PubMed] [Google Scholar]
- 44.Hanna, C.W., Taudt, A., Huang, J., Gahurova, L., Kranz, A., Andrews, S.et al. (2018) MLL2 conveys transcription-independent H3K4 trimethylation in oocytes. Nat. Struct. Mol. Biol. 25, 73–82 10.1038/s41594-017-0013-5 [DOI] [PubMed] [Google Scholar]
- 45.Du, Z., Zheng, H., Huang, B., Ma, R., Wu, J., Zhang, X.et al. (2017) Allelic reprogramming of 3D chromatin architecture during early mammalian development. Nature 547, 232–235 10.1038/nature23263 [DOI] [PubMed] [Google Scholar]
- 46.Flyamer, I.M., Gassler, J., Imakaev, M., Brandão, H.B., Ulianov, S.V., Abdennur, N.et al. (2017) Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature 544, 110–114 10.1038/nature21711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ke, Y., Xu, Y., Chen, X., Feng, S., Liu, Z., Sun, Y.et al. (2017) 3D chromatin structures of mature gametes and structural reprogramming during mammalian embryogenesis. Cell 170, 367–381.e20 10.1016/j.cell.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 48.Du, Z., Zheng, H., Kawamura, Y.K., Zhang, K., Gassler, J., Powell, S.et al. (2020) Polycomb group proteins regulate chromatin architecture in mouse oocytes and early embryos. Mol. Cell 77, 825–839.e7 10.1016/j.molcel.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 49.Akhtar, W. and Veenstra, G.J.C. (2009) TBP2 is a substitute for TBP in Xenopus oocyte transcription. BMC Biol. 7, 45 10.1186/1741-7007-7-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abe, K.-I., Inoue, A., Suzuki, M.G. and Aoki, F. (2010) Global gene silencing is caused by the dissociation of RNA polymerase II from DNA in mouse oocytes. J. Reprod. Dev. 56, 502–507 10.1262/jrd.10-068A [DOI] [PubMed] [Google Scholar]
- 51.Zuccotti, M., Piccinelli, A., Rossi, P.G., Garagna, S. and Redi, C.A. (1995) Chromatin organization during mouse oocyte growth. Mol. Reprod. Dev. 41, 479–485 10.1002/mrd.1080410410 [DOI] [PubMed] [Google Scholar]
- 52.Bouniol-Baly, C., Hamraoui, L., Guibert, J., Beaujean, N., Szöllösi, M.S. and Debey, P. (1999) Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol. Reprod. 60, 580–587 10.1095/biolreprod60.3.580 [DOI] [PubMed] [Google Scholar]
- 53.Liu, X., Wang, C., Liu, W., Li, J., Li, C., Kou, X.et al. (2016) Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature 537, 558–562 10.1038/nature19362 [DOI] [PubMed] [Google Scholar]
- 54.Chen, Z., Djekidel, M.N. and Zhang, Y. (2021) Distinct dynamics and functions of H2AK119ub1 and H3K27me3 in mouse preimplantation embryos. Nat. Genet. 53, 551–563 10.1038/s41588-021-00821-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mei, H., Kozuka, C., Hayashi, R., Kumon, M., Koseki, H. and Inoue, A. (2021) H2AK119ub1 guides maternal inheritance and zygotic deposition of H3K27me3 in mouse embryos. Nat. Genet. 53, 539–550 10.1038/s41588-021-00820-3 [DOI] [PubMed] [Google Scholar]
- 56.Posfai, E., Kunzmann, R., Brochard, V., Salvaing, J., Cabuy, E., Roloff, T.C.et al. (2012) Polycomb function during oogenesis is required for mouse embryonic development. Gene Dev. 26, 920–932 10.1101/gad.188094.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veselovska, L., Smallwood, S.A., Saadeh, H., Stewart, K.R., Krueger, F., Maupetit-Méhouas, S.et al. (2015) Deep sequencing and de novo assembly of the mouse oocyte transcriptome define the contribution of transcription to the DNA methylation landscape. Genome Biol. 16, 209 10.1186/s13059-015-0769-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kageyama, S., Liu, H., Kaneko, N., Ooga, M., Nagata, M. and Aoki, F. (2007) Alterations in epigenetic modifications during oocyte growth in mice. Reproduction (Cambridge, England) 133, 85–94 10.1530/REP-06-0025 [DOI] [PubMed] [Google Scholar]
- 59.Andreu-Vieyra, C.V., Chen, R., Agno, J.E., Glaser, S., Anastassiadis, K., Stewart, A.F.et al. (2010) MLL2 is required in oocytes for bulk histone 3 lysine 4 trimethylation and transcriptional silencing. PLoS Biol. 8, e1000453 10.1371/journal.pbio.1000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dahl, J.A., Jung, I., Aanes, H., Greggains, G.D., Manaf, A., Lerdrup, M.et al. (2016) Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature 537, 548–552 10.1038/nature19360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, B., Zheng, H., Huang, B., Li, W., Xiang, Y., Peng, X.et al. (2016) Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature 537, 553–557 10.1038/nature19361 [DOI] [PubMed] [Google Scholar]
- 62.Dumdie, J.N., Cho, K., Ramaiah, M., Skarbrevik, D., Mora-Castilla, S., Stumpo, D.J.et al. (2018) Chromatin modification and global transcriptional silencing in the oocyte mediated by the mRNA decay activator ZFP36L2. Dev. Cell 44, 392–402.e7 10.1016/j.devcel.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borsuk, E. and Milik, E. (2005) Fully grown mouse oocyte contains transcription inhibiting activity which acts through histone deacetylation. Mol. Reprod. Dev. 71, 509–515 10.1002/mrd.20300 [DOI] [PubMed] [Google Scholar]
- 64.Sha, Q.-Q., Zhu, Y.-Z., Li, S., Jiang, Y., Chen, L., Sun, X.-H.et al. (2020) Characterization of zygotic genome activation-dependent maternal mRNA clearance in mouse. Nucleic Acids Res. 48, 879–894 10.1093/nar/gkz1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mugridge, J.S., Coller, J. and Gross, J.D. (2018) Structural and molecular mechanisms for the control of eukaryotic 5′–3′ mRNA decay. Nat. Struct. Mol. Biol. 25, 1077–1085 10.1038/s41594-018-0164-z [DOI] [PubMed] [Google Scholar]
- 66.Yu, J., Hecht, N.B. and Schultz, R.M. (2001) Expression of MSY2 in mouse oocytes and preimplantation embryos1. Biol. Reprod. 65, 1260–1270 10.1095/biolreprod65.4.1260 [DOI] [PubMed] [Google Scholar]
- 67.Liu, X., Morency, E., Li, T., Qin, H., Zhang, X., Zhang, X.et al. (2017) Role for PADI6 in securing the mRNA-MSY2 complex to the oocyte cytoplasmic lattices. Cell Cycle 16, 360–366 10.1080/15384101.2016.1261225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu, J., Deng, M., Medvedev, S., Yang, J., Hecht, N.B. and Schultz, R.M. (2004) Transgenic RNAi-mediated reduction of MSY2 in mouse oocytes results in reduced fertility. Dev. Biol. 268, 195–206 10.1016/j.ydbio.2003.12.020 [DOI] [PubMed] [Google Scholar]
- 69.Medvedev, S., Yang, J., Hecht, N.B. and Schultz, R.M. (2008) CDC2A (CDK1)-mediated phosphorylation of MSY2 triggers maternal mRNA degradation during mouse oocyte maturation. Dev. Biol. 321, 205–215 10.1016/j.ydbio.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medvedev, S., Pan, H. and Schultz, R.M. (2011) Absence of MSY2 in mouse oocytes perturbs oocyte growth and maturation, RNA stability, and the transcriptome. Biol. Reprod. 85, 575–583 10.1095/biolreprod.111.091710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puschendorf, M., Stein, P., Oakeley, E.J., Schultz, R.M., Peters, A.H.F.M. and Svoboda, P. (2006) Abundant transcripts from retrotransposons are unstable in fully grown mouse oocytes. Biochem. Biophys. Res. Commun. 347, 36–43 10.1016/j.bbrc.2006.06.106 [DOI] [PubMed] [Google Scholar]
- 72.Morgan, M., Much, C., DiGiacomo, M., Azzi, C., Ivanova, I., Vitsios, D.M.et al. (2017) mRNA 3′ uridylation and poly(A) tail length sculpt the mammalian maternal transcriptome. Nature 548, 347–351 10.1038/nature23318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma, J., Flemr, M., Stein, P., Berninger, P., Malik, R., Zavolan, M.et al. (2010) MicroRNA activity is suppressed in mouse oocytes. Curr. Biol. 20, 265–270 10.1016/j.cub.2009.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suh, N., Baehner, L., Moltzahn, F., Melton, C., Shenoy, A., Chen, J.et al. (2010) MicroRNA function is globally suppressed in mouse oocytes and early embryos. Curr. Biol. 20, 271–277 10.1016/j.cub.2009.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe, T., Totoki, Y., Toyoda, A., Kaneda, M., Kuramochi-Miyagawa, S., Obata, Y.et al. (2008) Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453, 539–543 10.1038/nature06908 [DOI] [PubMed] [Google Scholar]
- 76.Luong, X.G., Daldello, E.M., Rajkovic, G., Yang, C.-R. and Conti, M. (2020) Genome-wide analysis reveals a switch in the translational program upon oocyte meiotic resumption. Nucleic Acids Res. 48, 3257–3276 10.1093/nar/gkaa010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bachvarova, R.F. (1992) A maternal tail of poly(A): the long and the short of it. Cell. 69, 895–897 10.1016/0092-8674(92)90606-D [DOI] [PubMed] [Google Scholar]
- 78.Paynton, B.V. and Bachvarova, R. (1994) Polyadenylation and deadenylation of maternal mRNAs during oocyte growth and maturation in the mouse. Mol. Reprod. Dev. 37, 172–180 10.1002/mrd.1080370208 [DOI] [PubMed] [Google Scholar]
- 79.Dai, X.-X., Jiang, J.-C., Sha, Q.-Q., Jiang, Y., Ou, X.-H. and Fan, H.-Y. (2019) A combinatorial code for mRNA 3’-UTR-mediated translational control in the mouse oocyte. Nucleic Acids Res. 47, 328–340 10.1093/nar/gky971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang, Y., Yang, C.-R., Han, S.J., Daldello, E.M., Cho, A., Martins, J.P.S.et al. (2017) Maternal mRNAs with distinct 3’ UTRs define the temporal pattern of Ccnb1 synthesis during mouse oocyte meiotic maturation. Genes Dev. 31, 1302–1307 10.1101/gad.296871.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sha, Q.-Q., Dai, X.-X., Dang, Y., Tang, F., Liu, J., Zhang, Y.-L.et al. (2016) MAPK cascade couples maternal mRNA translation and degradation to meiotic cell cycle progression in mouse oocyte. Development 144, 452–463 10.1242/dev.144410 [DOI] [PubMed] [Google Scholar]
- 82.Chen, J., Melton, C., Suh, N., Oh, J.S., Horner, K., Xie, F.et al. (2011) Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Gene Dev. 25, 755–766 10.1101/gad.2028911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paynton, B.V., Rempel, R. and Bachvarova, R. (1988) Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev. Biol. 129, 304–314 10.1016/0012-1606(88)90377-6 [DOI] [PubMed] [Google Scholar]
- 84.Bachvarova, R., Leon, V.D., Johnson, A., Kaplan, G. and Paynton, B.V. (1985) Changes in total RNA, polyadenylated RNA, and actin mRNA during meiotic maturation of mouse oocytes. Dev. Biol. 108, 325–331 10.1016/0012-1606(85)90036-3 [DOI] [PubMed] [Google Scholar]
- 85.Shirai, Y.-T., Suzuki, T., Morita, M., Takahashi, A. and Yamamoto, T. (2014) Multifunctional roles of the mammalian CCR4-NOT complex in physiological phenomena. Front. Genet. 5, 286 10.3389/fgene.2014.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu, Y., Lu, X., Shi, J., Yu, X., Zhang, X., Zhu, K.et al. (2016) BTG4 is a key regulator for maternal mRNA clearance during mouse early embryogenesis. J. Mol. Cell Biol. 8, 366–368 10.1093/jmcb/mjw023 [DOI] [PubMed] [Google Scholar]
- 87.Zhao, L., Zhu, Y., Chen, H., Wu, Y., Pi, S., Chen, L.et al. (2020) PABPN1L mediates cytoplasmic mRNA decay as a placeholder during the maternal-to-zygotic transition. EMBO Rep. 21, e49956 10.15252/embr.201949956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du, H., Zhao, Y., He, J., Zhang, Y., Xi, H., Liu, M.et al. (2016) YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 7, 12626–12611 10.1038/ncomms12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu, D. and Dean, J. (2020) EXOSC10 sculpts the transcriptome during the growth-to-maturation transition in mouse oocytes. Nucleic Acids Res. 48, 5349–5365 10.1093/nar/gkaa249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang, H., Yeo, J., Kim, J.-G., Kim, H., Lim, J., Lee, M.et al. (2018) Terminal uridylyltransferases execute programmed clearance of maternal transcriptome in vertebrate embryos. Mol. Cell 70, 72–82.e7 10.1016/j.molcel.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 91.Zheng, W., Zhou, Z., Sha, Q., Niu, X., Sun, X., Shi, J.et al. (2020) Homozygous mutations in BTG4 cause zygotic cleavage failure and female infertility. Am. J. Hum. Genet. 107, 24–33 10.1016/j.ajhg.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sha, Q.-Q., Zheng, W., Wu, Y.-W., Li, S., Guo, L., Zhang, S.et al. (2020) Dynamics and clinical relevance of maternal mRNA clearance during the oocyte-to-embryo transition in humans. Nat. Commun. 11, 4917–4916 10.1038/s41467-020-18680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park, S.-J., Shirahige, K., Ohsugi, M. and Nakai, K. (2015) DBTMEE: a database of transcriptome in mouse early embryos. Nucleic Acids Res. 43, D771–D776 10.1093/nar/gku1001 [DOI] [PMC free article] [PubMed] [Google Scholar]