Abstract
Interest in nanomedicines has grown rapidly over the past two decades, owing to the promising therapeutic applications they may provide, particularly for the treatment of cancer. Personalised medicine and ‘smart’ actively targeted nanoparticles represent an opportunity to deliver therapies directly to cancer cells and provide sustained drug release, in turn providing overall lower off-target toxicity and increased therapeutic efficacy. However, the successful translation of nanomedicines from encouraging pre-clinical findings to the clinic has, to date, proven arduous. In this review, we will discuss the use of nanomedicines for the treatment of cancer, with a specific focus on the use of polymeric and lipid nanoparticle delivery systems. In particular, we examine approaches exploring the surface functionalisation of nanomedicines to elicit active targeting and therapeutic effects as well as challenges and future directions for nanoparticles in cancer treatment.
Keywords: cancer, conjugation, drug delivery, nanomedicine, surface functionalisation, targeted therapeutics
Introduction
Cancer is one of the most significant global health burdens and the leading cause of mortality worldwide. Despite notable improvements in cancer treatment, one of the most prominent challenges affecting the development of novel therapeutics is controlling the biological fate of the active pharmaceutical ingredient (API) following administration. Current first-line therapies, specifically within the field of cancer, are often limited by poor stability, solubility and rapid metabolism, resulting in poor pharmacokinetics [1]. Furthermore, major off-target effects result in adverse toxicities in healthy cells, in turn contributing to reduced treatment tolerability and poorer patient outcomes. Failure of treatment is therefore frequently attributable to inadequate drug delivery to the tumour site, as opposed to the efficacy of the drug itself.
One means of overcoming this issue is through incorporation of the drug within nano-based drug delivery systems. Nanomedicine is rooted in the concept of developing improved formulation of difficult/insoluble APIs, but is now an exponentially evolving interdisciplinary field. A substantial amount of research has been carried out investigating nanoparticle design and development, including assessment of passive targeting/accumulation, active targeting, and triggered drug release.
A particular benefit of nanoparticles is the opportunity to entrap APIs within their structure, providing a transient barrier for labile therapeutics as well as enhancing the bioavailability of drugs which ordinarily exhibit poor solubility. Thus nanocarriers can be used to protect drug cargos while in circulation, improving both pharmacokinetic and pharmacodynamic profiles of APIs [2–4]. Furthermore, nanoparticles have the ability to preferentially localise within the tumour tissue due to the increased permeability of the tumour microenvironment (TME) neovasculature, resulting in sustained drug release [5–7]. Essentially this means that the therapeutic window of the payload agent can be broadened by nanoformulation through simultaneous reduction in systemic toxicity and enhancement of drug efficacy. These properties are particularly appealing within the field of oncology for sustained delivery of potent cytotoxic chemotherapeutic agents [8–15].
Although the majority of research has focused upon the entrapment of cytotoxic agents, nanoparticles for the intracellular delivery of proteins and small molecules is an avenue which has enormous potential. To date there are >200 FDA approved proteins and peptides to treat a variety of diseases, but efficacy is often compromised by poor penetration across biological membranes, their immunogenicity and short plasma half-life (due to inadequate stability and rapid clearance) [16]. Therefore, the entrapment of proteins and peptides within nanocarriers has potential to overcome these limitations. This can be achieved by either encapsulating the protein/peptide within the nanoparticle itself or via chemical conjugation to the particle surface [17]. Moreover, studies have shown that hydrophobic ion pairing is a method in which the solubility of charged hydrophilic molecules can be modulated, where pairing with oppositely charged counter molecules (e.g. nuclear localisation sequence) can be utilised to increase the loading of peptides and nucleic acids within nanoparticle cores [18,19].
The use of nanoparticles and other nanomedicines as drug delivery systems is an approach now established clinically since the first FDA approval of a nanomedicine, DOXIL® (liposomal doxorubicin) in 1995 [20]. Since then, the field of nanomedicine has developed substantially, with several other nanomedicines entering the market. However, the application for use in non-cancer related conditions is also of particular interest, especially for vaccine delivery, notably implemented within the formulation of the numerous SARS-CoV-2 vaccines approved in 2021 [21–24]. More recently, the emergence of targeted therapies has significantly altered the design process of novel drug modalities, particularly when considering the treatment of solid tumours. These next generation nanoparticles are more frequently multifunctional, where their surface can be functionalised while also entrapping therapeutics within the nanoparticle, leading to more innovative approaches in actively targeting tumour sites and eliciting biological effects that have therapeutic usefulness, independent of enhanced delivery alone.
Nanoparticles in cancer therapy
Despite the growth of the nanomedicine field and promising pre-clinical data, to date only 12 nanoformulations have been FDA/EMA approved for the treatment of cancer (Table 1).
Table 1. Advantages of clinically approved nanoparticles in the field of oncology.
| Name | Nanoparticle subtype | Clinical advantage | Cancer indication | Clinical approval |
|---|---|---|---|---|
| Doxil® | Pegylated liposomal (Doxorubicin) | Reduced systemic exposure | Ovarian cancer and multiple myeloma | FDA (1995) EMA (1996) |
| DanuoXome® | Non-pegylated Liposome (Daunorubicin) |
EPR effect | HIV-associated Kaposi's Sarcoma various leukaemia's | FDA (1996) |
| DepoCyt® | Liposomal Cytarabine | EPR effect | Lymphoma Leukaemia |
FDA (1999) |
| Myocet® | Non-pegylated Liposome (Doxorubicin) |
Sustained release | Metastatic breast cancer | EMA (2000) |
| Abraxane® | Albumin bound nanoparticle (paclitaxel) | Sustained release | Metastatic breast cancer Metastatic pancreatic cancer Non-small cell lung cancer |
FDA (2005) EMA (2008) |
| MEPACT® | Non-pegylated liposome (mifamurtide) | Sustained release | Osteosarcoma | EMA (2009) |
| NanoTherm® | Iron Oxide | Hyperthermic treatment | Brain tumours | EMA (2011) |
| Marqibo® | Non-pegylated liposome (vincristine sulfate) | Sustained release | Acute lymphoblastic leukaemia | FDA (2012) |
| Onivyde® | Pegylated Liposome (Irinotecan) |
Immune evasion | Advanced pancreatic cancer | FDA (2015) |
| Vyxeos® | Liposome (cytarabine and daunorubicin) | Combination therapy Sustained release |
Acute myeloid leukaemia | FDA (2017) EMA (2018) |
| Apealea® | Paclitaxel Micelle | EPR effect Sustained release |
Ovarian, peritoneal, and fallopian tube cancer | EMA (2018) |
| NBTXR3® | Hafnium oxide NPs | Radiotherapy | Locally advanced soft tissue sarcoma | EMA (2019) |
(This table summarised information which is available at accessdata.fda.gov (https://www.accessdata.fda.gov) and ema.europa.eu.en/medicines (https://www.ema.europa.eu/en/medicines) 22th September 2021).
Whilst initial successes in the clinic have predominantly used lipid-based carriers such as liposomes, many other opportunities exist through a broad range of polymers and materials which are FDA/EMA ‘generally regarded as safe’ (GRAS) [25]. A growing area of interest focuses on the synthesis of polymeric nanoparticles as delivery systems where GRAS biodegradable synthetic polymers are commonly utilised (e.g. poly(DL-lactic acid) (PLA), poly(glycolic acid)(PGA) and poly(lactic-co-glycolic acid)(PLGA)) [26].
Mechanisms of cellular targeting
Passive targeting nanoparticles
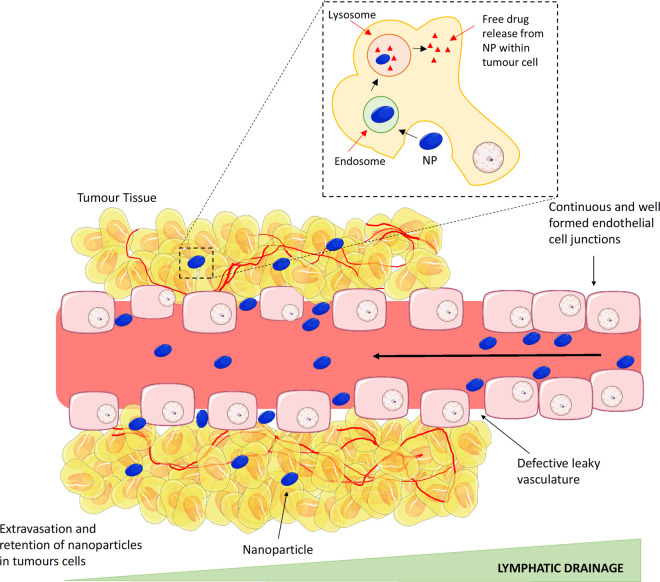
It is now well established that the presence of a leaky vasculature is a feature frequently associated within the hypoxic TME, where angiogenesis must occur simultaneously to fulfil the metabolic requirements of the rapidly proliferating tumour cells, resulting in a disorganised dense neovasculature. A particular feature of the majority of tumour vessels formed is that they are defective, with apertures (<600 nm) left between the endothelial cells [27]. Consequently, this leaky vasculature, coupled with poor lymphatic drainage in the tumour tissue, permits large macromolecules (>40 kDa) and nano-sized drugs to passively diffuse and accumulate at the disease site. This passive targeting phenomenon was first described in 1986 by Matsumura and Maeda and became known as the enhanced permeability and retention (EPR) effect [28–30] (Figure 1). Despite the approval of some nanoparticles which rely on the EPR Effect (e.g. Doxil © and Caelyx ©), robust exploitation of passive targeting for nanoparticle delivery has proven challenging. The foremost issue is the deep reliance of the EPR effect on intrinsic tumour biology, in particular: (1) intratumoural pressure, (2) degree of angiogenesis (endothelial gap size and blood supply), (3) stromal density of the tumour and perivascular growth [31]. As a result, the EPR effect is inevitably heterogenous with vast variation across tumours and individual patients [32–34]. Often only a small percentage (<1%) of nanoparticles accumulate within the tumour site [35], most often attributed to the multiple challenges aforementioned. Approaches to improve the EPR effect is an area of ongoing research with the use of vasodilating agents and vascular disruption to enhance uptake under investigation [36–40].
Figure 1. The enhanced permeability and retention (EPR) effect.
During the initial stages of tumour formation, there is a rapid pro-angiogenic neovascularisation to supply the tumour with all nutritional requirements to survive. As a result of the rapid pace of formation, blood vessels are often defective, with large apertures left between endothelial cells resulting in a leaky vasculature. Nanoparticles have the capacity to exploit this defect in vessels, allowing them to pass from the blood vessels into the tumour site. Additionally, retention of the nanoparticles occurs within the tumour site as a result of poor lymphatic drainage. Image created with the use of Smart Servier Medical Art.
Actively targeted nanoparticles
Since Paul Ehrlich suggested the concept of the ‘magic bullet’ in the early 1900's, where a therapeutic has the ability to selectively target diseased cells while avoiding/limiting intervention with healthy tissues, the concept of actively targeting tumour cells has been a focus of intense research [41]. Whilst nano-sized systems possess inherent delivery benefits, advances in synthesis/formulation approaches have resulted in the concept of active or site-specific targeting. This model has many benefits and is particularly relevant in the treatment of cancer, where the ability to selectively target the tumour site is desirable to reduce off-target toxicity towards healthy tissues.
Active targeting has already been used with success in the clinic, with a total of 11 antibody-drug conjugates (ADCs) approved (e.g. Adcetris™ and Kadcyla™) [42,43]. ADCs use monoclonal antibodies linked to a cytotoxic payload, where the antibody is employed to specifically target and deliver the payload to be internalised and released within the target cell of interest (Figure 2A). ADCs are becoming an important therapeutic development approach, proving a favourable treatment option for a variety of cancer patients. Indeed, one specific attribute of these drugs is that they can overcome resistance to a cell target, providing a second line treatment to patients who have acquired resistance. The classic example of this is the treatment of HerceptinTM resistant Her2 positive breast tumours with KadcylaTM, which uses Herceptin as the targeting agent to deliver the microtubule inhibitor maytansine payload [16]. ADC development includes the development of stable linkers adopting more innovative site-specific conjugation methods to improve conjugation stability, as well as reducing variability and optimisation of Drug:Antibody ratios (DAR). The conjugation characteristics of the linker are vital in controlling the therapeutic window and effectiveness of the ADC, where the DAR determines overall potency and toxicity. DAR represents the average number of cytotoxic drug molecules attached per single antibody (typically <10) which means that the key limiting factor for ADC effectiveness is receptor copy number on the target cell; therefore, the lower the receptor density on the cell surface, the less drug will be successfully internalised. Many studies have shown that increasing the DAR can destabilise the antibody and therefore ADCs normally explore the use of high-potency payloads to compensate, which inherently have narrower therapeutic windows [44]. The majority of ADCs under investigation utilise potent microtubule inhibitors, yet many have failed to reach the clinic due to their dose limiting toxicities. In an attempt to overcome this, current research focuses on the use of less potent cytotoxic drugs, with a lower IC50 than maytansine or auristatin, such as topoisomerase targeting agents (e.g. camptothecin derivatives), highlighted by the recent FDA approval of trastuzumab deruxtecan (approved 2019), and more recently, Sacituzumab Govitecan, (approved 2021) an ADC which targets Trop2 linked to SN-38, the active metabolite of irinotecan [45–48].
Figure 2. Structure, binding and internalisation of Antibody-drug conjugate's (ADCs) and multifunctional antibody conjugated nanoparticles.
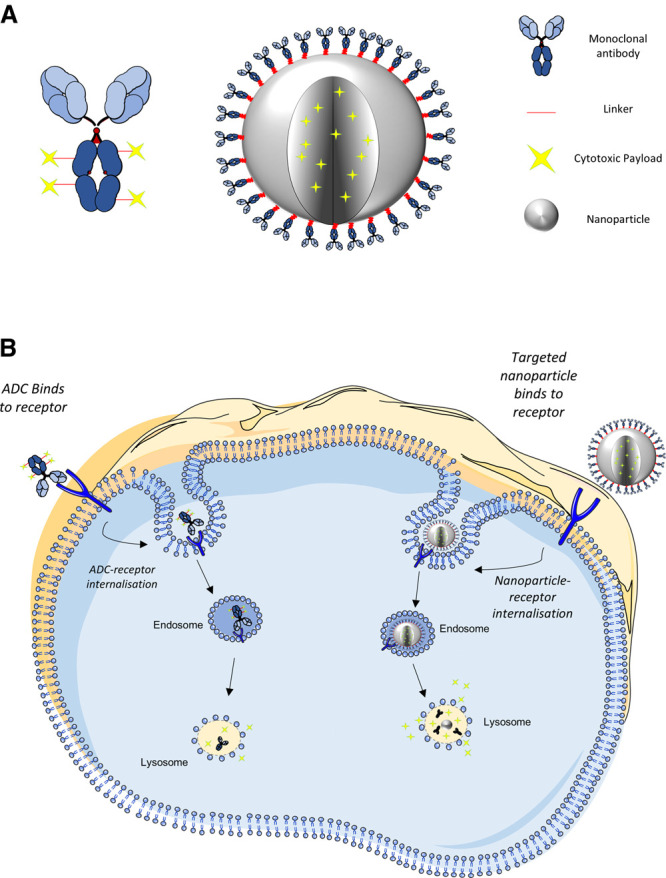
Schematic representation highlighting (A) the conceptual structure of an ADC and a multifunctional antibody conjugated nanoparticle and (B) internalisation, breakdown, and drug release within the cell. The tumour-specific ligand of the ADC or nanoparticle interacts with and binds to the cell surface expressed antigen. Upon binding, the antigen–protein complex becomes internalised via receptor mediated endocytosis. The nanoparticle or ADC linker is degraded within the endo-lysosomal system, resulting in drug release within the cell. Illustrations not to scale. Image created with the use of Smart Servier Medical Art.
Following on from the experience of developing ADCs, the ability to conjugate targeting moieties such as antibodies to the surface of nanoparticles represents an exciting approach to develop a new type of ‘magic bullet' therapy [49] (Figure 2A). As with ADCs, the binding of the tumour-specific antibody with its cognate antigen on the diseased cell can trigger internalisation of the complex to deliver the payload internally and elicit its cytotoxic effect, thus limiting payload exposure to healthy tissues [50] (Figure 2B). An interesting facet of antibody targeted nanoparticles is that they have the potential to enhance the DAR which remains one of the key efficacy-limiting factors with ADCs. This is because nanoparticles can be packed with an increased payload resulting in DARs of >100 as opposed to <10 with current ADC technologies, ensuring the internalisation of a much higher concentration of drug, which compensates for limitations such as low copy number [49].
Despite the clear opportunities provided by the exquisite selectivity and affinity of antibodies, it is important to realise that they are only one class of agent that can be used to target nanomedicines. In the following sections, the application of antibodies and other targeting agents will be further discussed.
Functionalisation of nanomedicines
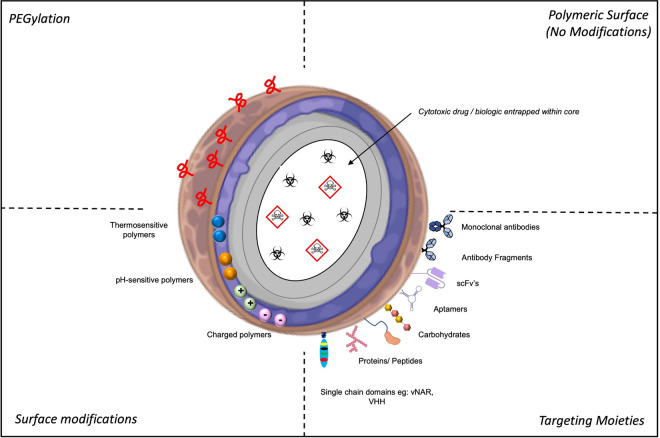
The surface of a nanoparticle represents a bioengineer's dream! The ability to coordinate the presentation of functional handles on the nanoparticle surface that can be modified in a range of different ways to achieve the exact specification required for their intended use is potentially unlimited. Not only can nanoparticles be functionalised for use in active targeting; nanoparticle surfaces can be further manipulated to change surface charge and incorporate stimuli responsive properties (Figure 3).
Figure 3. Different strategic approaches for the surface functionalisation of nanoparticles.
Nanoparticle surfaces can be modified through the incorporation of different polymers/lipids to modify surface charge as well as thermo and pH sensitive properties. PEGylation of the nanoparticle surface can be used to extend half-life of the nanoparticles in vivo. Furthermore, nanoparticles can be targeted through the conjugation of targeting moieties to the nanoparticle surface. In addition to surface modification, nanoparticle design can vary based on the nature of the therapeutic agent entrapped within the nanoparticle core. Abbreviations: PEG, Polyethylene glycol; vNAR, Variable new antigen receptor; scFv, single-chain variable fragment; sdAb, single domain antibodies. Image created with the use of Biorender & Smart Servier Medical Art.
PEGylation
For polymeric nanoparticles to reach their target tissue they should be capable of avoiding clearance through the mononuclear phagocytic system (MPS). The MPS functions to remove foreign material from the blood stream and is the first barrier which nanoparticles encounter in vivo. Through opsonisation, immunoglobulins and complement system proteins adhere to the hydrophobic nanoparticle surface effectively ‘flagging’ them for recognition by macrophages and removal via phagocytosis [51]. To avoid/limit MPS uptake, researchers have developed a strategy which involves coating the surface of the nanocarrier with a hydrophilic polymer. The presence of hydrophilic PEG chains on the hydrophobic nanoparticle surface creates a barrier which permits the nanoparticle to evade opsonisation through camouflaging the surface charge and steric repulsion, limiting the ability of opsins to bind to the nanoparticle surface [52]. This significantly reduces the rate at which nanoparticles are removed from the blood stream [53]. Whilst a range of polymers may be utilised, PEG is by far the most commonly used as it is generally regarded as safe in formulations with an extensive track record in a variety of clinically approved drugs [5,52].
Many studies have illustrated the integration of PEG within formulations to increase circulation time for both liposomes and polymeric nanoparticles [54,55]. Importantly, the use of PEGylated products is now well established in the clinic. Notably, the first formulation to be approved by the FDA, Caelyx® (DOXIL, 1995), is a doxorubicin entrapped PEGylated liposome. In vivo studies demonstrated clearly that the serum half-life of the doxorubicin payload could be significantly enhanced by the addition of PEGylation in the formulation [20,56]. Additionally, PEGylated liposome formulations also have an increased ability to penetrate the leaky tumour vasculature, thus resulting in enhanced drug accumulation [56–58]. More recently, PEGylated formulations such as Onivyde® (PEGylated Irinotecan liposome) for the treatment of advanced pancreatic cancer have been approved, whilst Genexol® (PEG-PLA micelles containing paclitaxel) for the treatment of metastatic breast cancer is currently under clinical investigation [15,53,59–61].
Targeting moieties
This approach requires the presence of a target antigen which is highly or uniquely expressed on tumour cells in comparison with normal healthy tissues, as well as the identification of a targeting moiety which binds with high affinity and specificity to the target antigen selected [50]. The ideal targeting ligand should possess a functional handle which aids stable conjugation without diminution of ligand affinity. Conjugation should result in targeted nanoparticles which display minimal aggregation and have an adequately dense coating of the targeting moieties [62]. To date, a wide range of targeting moieties have been studied to functionalise nanoparticles including traditional antibodies formats, F(Ab)2 fragments, proteins, peptides and aptamers to more innovative approaches through the use of small single chain antibodies such as ScFv's, VHH domains and vNARs [63–69]. When exploiting such ligands for the targeted delivery of nanoparticles, it is often necessary to introduce the appropriate reactive moiety on the surface of the nanoparticle, to facilitate conjugation. Countless bioconjugation methods may be applied, with the most common approach being the use of carbodiimide and maleimide chemistries. However, alternative approaches, including ‘click' chemistries, are now being evaluated with superior conjugation efficiencies and biological functionalities reported [64,65,70–72].
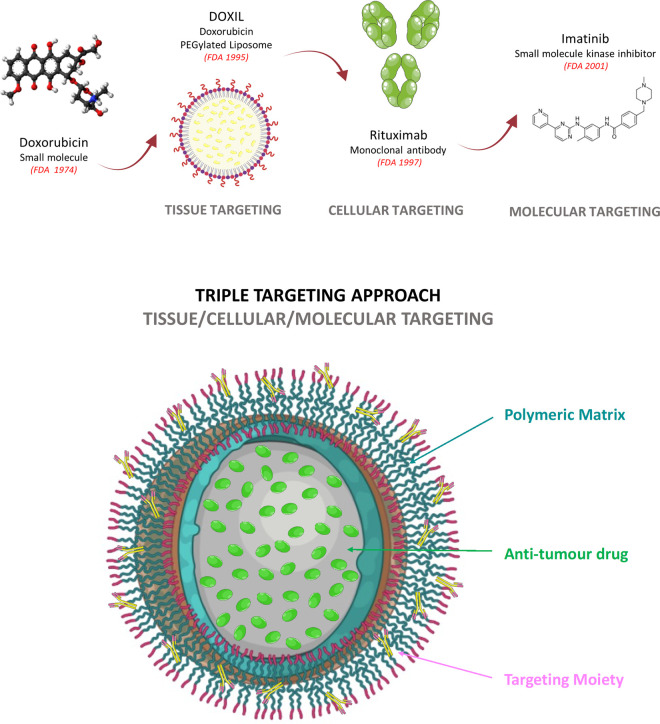
A significant volume of research has been carried out investigating surface functionalisation of nanoparticles. BIND-014 (Accurins), a polymeric nanoparticle with a therapeutic payload (Docetaxel) designed to target tumours with an anti-PMSA (Prostate-specific membrane antigen) targeting ligand grafted on the nanoparticle surface was the first to enter clinical trials [73,74]. Despite not achieving clinical approval, experience gained from the development of BIND-014 has highlighted the need for a programmable nanoformulation, a combination of controlled release polymer and a targeting moiety which has the capacity to target the tumour at three levels: (1) tissue, (2) cellular and (3) molecular (Figure 4).
Figure 4. Evolution of targeted therapies from the approval of small molecules to the first approval of nanomedicines and molecular targeting small molecule kinase inhibitors.
A schematic representation of the progression of targeted therapies from the use of small molecules alone, through to targeting at a tissue, cellular and molecular level. Such advancement has paved the way for potential triple targeting approaches, wherein future targeted nanoparticle formulations, possessing a range of targeting moieties and advanced polymer chemistries, may target all three levels simultaneously and in turn greatly enhance treatment specificity and efficacy. Image created with the use of Biorender & Smart Servier Medical Art.
As a result, many researchers have exploited this model of triple targeting, where studies have indicated that conjugation of Trastuzumab (anti-HER2) or Rituximab (anti-CD20) to PLA-nanoparticles results in a six-fold increase in the rate of nanoparticle uptake compared with similar controls lacking antibody functionalisation [75]. Further studies have focused on optimising the conjugation of both Trastuzumab and Cetuximab F(ab)2 fragments to PLGA-PEG nanoparticles, through a selectively re-bridged strained alkyne handle on the F(ab)2 fragments to permit azide ‘click’ conjugation chemistry [65,76]. This advanced conjugation method resulted in significant improvement in antigen binding owing to the production of a highly uniform nanoparticle where the targeting moiety is conjugated in a site-selective and optimally orientated manner, when compared with conventional amine-selective carbodiimide chemistry [71]. In addition to the use of monoclonal antibodies and fragments, research has also more recently focused on the use of antibody like molecules (e.g. vNARs), peptides and aptamers as next generation targeting agents [77,78]. As a result of their unique properties these new targeting agents have the capability to enhance tissue penetration and extend tumour retention times, improving upon previously utilised nanoparticle targeting ligands [79,80]. Whilst their potential use for nanoparticle functionalisation has already been demonstrated within the literature, they are yet to enter clinical investigation [63,64,81–83].
The role of active targeting can be further enhanced in some settings through receptor activation, which can be utilised as a dual approach concurrently with active targeting. Some receptor targeted monoclonal antibodies have showed limited efficacy in clinic (e.g. AMG655 and Death Receptor 5 (DR5)), which is thought to be as a result of weak receptor activation [84]. For instance, DR5 requires cross-linking or receptor clustering for activation and therefore recent DR5-targeted therapeutic design has progressed towards multivalent agents, whereas first-generation DR5-targeting antibodies were bivalent [85–87]. Alternatively however, through the use of a multi-functional antibody-conjugated nanoparticle, a high number of antibody molecules can be conjugated per particle, resulting in a sufficient density of antibody paratopes to induce apoptosis via effective DR5 clustering, unlike free antibody alone [66,88,89]. Furthermore, studies have indicated that the entrapment of a cytotoxic payload can enhance drug targeting to antigen-expressing cells resulting in superior toxicity [65].
Surface charge
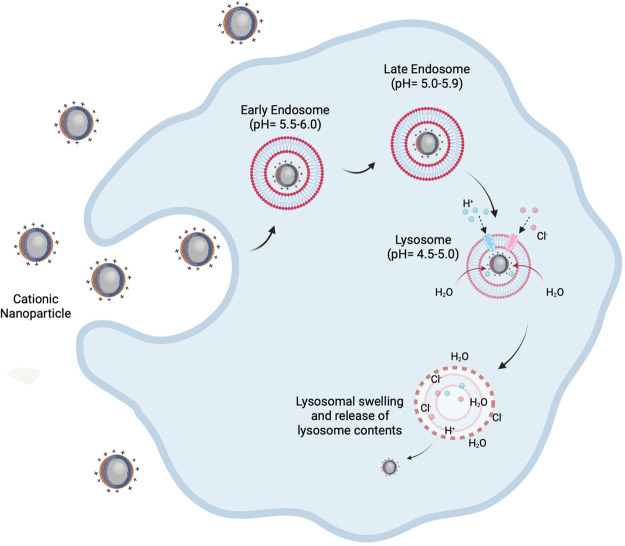
Altering the surface charge of nanoparticles is another potential avenue to tailor nanoparticle delivery systems, where nanoparticles can possess an anionic, cationic, or neutral charge. To date, the use of anionic/neutral nanoparticles have shown success within the clinic (Table 1), however utilisation of cationic nanoparticles is of much interest due to their ability to enhance cellular uptake (in comparison with neutral/anionic nanoparticles), and manipulate intracellular trafficking once internalised (e.g. endo/lysosomal escape) [90]. Cationic nanoparticle interactions with the cell are promoted via electrostatic attraction owing to the net negative charge which is characteristic of the plasma membrane. This in turn enhances clathrin-mediated endocytosis of the nanoparticle, when compared with anionic or neutral nanoparticles [91]. Additionally, studies have indicated uptake may also be ATP-independent as a result of direct translocation into the cell [90,92–94]. One drawback however in the use of cationic nanoparticles is the associated increased cell cytotoxicity, which is significantly increased compared with anionic/neutral nanoparticles. The exact mechanism of action associated with the toxicity is not fully elucidated, however it has been suggested that cationic nanoparticles activate caspase 3/7 and cleave PARP, signifying stimulation of apoptosis and cell necrosis. In addition to cytotoxicity, cationic nanoparticles are more rapidly cleared from the blood stream as a result of their charge, however this can be lessened by incorporation of PEG within the nanoparticle formulation to increase plasma circulation time, as discussed above. Aside from cationic nanoparticle toxicity and clearance, with the correct formulation, cationic nanoparticles may reduce cargo degradation and elicit desired intracellular effects at a rapid pace via escaping the endo-lysosomal pathway. This can be achieved through exploiting phenomenon such as the proton sponge effect, where pH buffering agents can be manipulated due to the acidic nature of endosomal/lysosomal vesicles to stimulate cargo release as a result of membrane destabilisation [95,96]. Under acidic conditions, numerous macromolecules which exhibit low pKa amine groups (e.g. PEI) promote release, whereby unprotonated amines within the cationic polymer become protonated, which in turn leads to an influx of chlorine ions and water into the lysosome. This influx of water results in osmotic swelling and lysis, whilst paired forces of repulsion in the cationic polymer results in further disruption of the lysosomal membrane (Figure 5) [96–99].
Figure 5. The proton sponge theory.
Cationic nanoparticles have the ability to escape the endo-lysosomal pathway, allowing cargo to enter the cytosol using methods such as the proton sponge effect. Endosomes fuse into lysosomes, where unprotonated amine groups on cationic nanoparticles become protonated. The protonation results in the influx of chlorine ions and water causing the osmotic rupturing of the lysosomal membrane, stimulating stimulate cargo release. Image created with the use of Biorender.
pH/thermo sensitive nanoparticles
Stimuli responsive nanoparticles have generated interest due to their potential to improve drug delivery by releasing therapeutics in response to pH and/or temperature alterations. pH sensitive nanoparticles are often tailored to respond to a fluctuation in pH found within the TME, in particular the acidified vesicles of the endo/lysosomal systems where nanoparticles accumulate following endocytosis. Thus pH-sensitive polymers such as PDPA (poly(2-diisopropylamino)ethyl methacrylate) and PPAA (Poly(propylacrylic acid) can be utilised to exploit the acidic pH of endosomes and lysosomes [99–101]. Cytosolic delivery of therapeutic agents from the endosome is crucial in order to protect drugs susceptible to degradation by lysosomal enzymes. In such cases, nanoparticle formulation can be tailored through incorporation of polymers which disassemble at endosomal pH, promoting endosomal escape, or via the incorporation of cell penetrating molecules, such as peptides which exclusively promote endosomal escape [102]. Activated upon a fall in pH (4.5–6.0), these nanoparticles enable the release of their cargo within these more acidic environments and subsequent release into the cytoplasm [103,104]. Furthermore, thermosensitive liposomes (e.g. ThermoDox), modified via the addition of thermosensitive lipids (e.g. distearoyl phosphocholine and 1,2-dipalmitoyl-sn-glyce-ro-3-phosphocholine) and/or polymers [e.g. poly(N-isopropylacrylamide)] are considered favourable for site-specific delivery, where particles remain in a stable state at their lower critical solution temperature. Upon undergoing slight change in temperature, most often induced by radiofrequency ablation, which induces mild hyperthermia of the tumour site (∼40–43°C), thermosensitive nanoparticles have the ability to trigger cargo release predominantly within the TME [105,106]. However, issues have been raised on delivery effectiveness, as localised therapy depends on the ability to accurately maintain a controlled elevated temperature, specifically within the tumour site while minimising exposure to surrounding healthy tissue. As a result, the ability to target deep within tissues has proven problematic, thus currently limiting treatment to superficial tumours. However research is progressing through the use of focused high density ultrasound to allow more controlled and specific targeting [107].
The future of nanomedicine
The amalgamation of a small size, high surface area: volume ratio and ability for extensive functionalisation endow nanoparticles with the capability to address a range of drug delivery challenges [1,8]. However, successful clinical application of nanomedicines has been limited. Whilst the total number of nanoformulations which are FDA approved has increased in the last decade, many of these are for non-biomedical implications. Whilst early-stage research demonstrates the advantages of nanomedicine delivery approaches, several issues must be addressed to translate this more readily from bench to clinic, such as better understanding the biological fate of nanomaterials in vivo, better appreciation of their interactions within the blood stream and further investigation of their trafficking through intracellular compartments. In addition to investigations into their biological fate, issues relating to manufacturing on large scale must also be considered. Whilst complexity of design may theoretically aid delivery, this must be implemented in a manner which is both reproducible and scalable. Such manufacturing issues have recently been addressed through the advancement of microfluidic technology, which holds great promise for nanomedicine production at an industrial scale [108,109]. Whilst nanomedicines have been granted approval in the oncology setting, advancements of the field such as improved targeting strategies, has led to the development of innovative formulations, some of which are now undergoing clinical trials (Table 2).
Table 2. A promising future: a selection of nanoparticles currently undergoing clinical trials within the field of oncology (with clinical stage).
| Name | Nanoparticle subtype | Clinical advantage | Cancer indication | Status | Clinicaltrial. gov identifier |
|---|---|---|---|---|---|
| MM-310 | Liposome (Ephrin type-A receptor targeted docetaxel) | Targeted therapy | Solid tumours | Phase I ongoing | NCT03076372 |
| PROMITIL | Pegylated liposome (mitomycin-C) | Immune evasion | Solid tumours | Phase I complete Phase Ib recruiting |
NCT03823989 |
| ANTI-EGFR-IL dox | Liposome (Doxorubicin) Anti-EGFR |
Targeted therapy | Advanced triple negative EGFR positive breast cancer | Phase I/II recruiting |
NCT02833766 NCT03603379 |
| ThermoDox | Liposome Thermosensitive (Doxorubicin) |
Temperature triggered sustained release | Breast Cancer Liver tumours Hepatocellular carcinoma |
Phase I/II/III |
NCT02536183 NCT02181075 NCT02112656 NCT00826085 |
| Genexol-PM | Polymeric micelle nanoparticle (Paclitaxel) |
Sustained release | Ovarian cancer | Phase II | NCT01276548 |
| NK105 | Polymeric nanoparticle (Paclitaxel) |
Sustained release | Breast cancer | Phase III | NCT01644890 |
| CTX-Somatostatin | Polymeric nanoparticle (Cetuximab), somatostatin decorated | Targeted therapy Sustained release |
Colon cancer | Phase I | NCT03774680 |
| PRECIOUS-01 | PLGA nanoparticle (immunomodulating agent) | Sustained release Cargo delivery |
NY-ESO-1 positive cancers | Phase I | NCT04751786 |
| AR160 | Paclitaxel albumin-stabilized/Rituximab-coated nanoparticle | Targeted therapy Sustained release |
Non-Hodgkin's lymphoma | Phase I | NCT03003546 |
| NK012 | Polymeric nanoparticle (SN38) |
Sustained release | Solid tumours | Phase II | NCT00951613 NCT00951054 |
| Anti-EGFR-IL-dox | EGFR-targeting liposome (Doxorubicin) | Targeted therapy Sustained release |
Solid tumours Breast cancer |
Phase I Phase II |
NCT01702129 NCT02833766 |
(This table summarised information which is available at Clinicaltrials.gov (https://clinicaltrials.gov) as of 22nd September 2021).
Advanced nanotechnology provides the opportunity to combine numerous treatment avenues within the same nanoparticle to develop multifunctional nanoparticles combining chemotherapies, targeting agents, immunotherapies and photodynamic therapies, highlighting the potential scope for ‘precision' nanoparticle development, which could be particularly important for addressing the need of targeting metastatic cancer in complex evolving tissue milieu [110]. Furthermore, nanoparticles can be designed to respond to specific stimuli, such as fluctuations in temperature or pH, as well as hypoxia and the presence of specific enzymes within the TME [110–115].
In addition to chemotherapeutic indications; immune therapy, gene delivery and gene silencing are rapidly emerging treatments within the field of oncology, offering a variety of advantages including high specificity, potency, reduced off-target effects, as well as the possibility of targeting multiple genes, thereby concurrently targeting many of the hallmarks of cancer [116,117]. Immunotherapy is a catch-all term that involves harnessing or manipulating the patient's own immune system to not only to recognise but kill cancer cells. Nanomedicine may offer opportunities to enhance this revolutionary approach, for example through delivering chemotherapies such as oxaliplatin which can induce immunogenic cell death, thus not only killing the tumour cell, but changing the TME to unleash an anti-tumour immune response [118]. Additionally, surface modification of nanoparticles to incorporate immune stimulation such as the use of an anti-CD40 monoclonal antibody, has been shown to induce potent CD8+ T cell responses resulting in tumour growth attenuation and prolonged survival in preclinical models [118,119].
Other than the more conventional use for entrapment of APIs, nanocarriers have the potential to target specific genes. Gene therapy has shown great promise in the literature, both as single agents and in combination with cytotoxic agents, where RNA molecules such as small interfering RNAs (siRNAs) and micro-RNAs (miRNAs) are capable of regulating expression of certain genes which contribute to cancer development. Despite preliminary success, clinical application remains largely limited; a result of poor pharmacokinetics and potency as well as off target effects and intolerable levels of toxicity. Delivery of large negatively charged nucleic acids which are fragile inside the cell can also be problematic, therefore the use of a nanocarrier could be a solution to protect the cargo while aiding delivery to the TME as well as minimising toxicity [120]. Since the introduction of the first liposome based siRNA delivery system in clinical trials in 2008, the development of DNA/RNA based nanocarriers has been an evolving field [121]. Studies have looked at systemic delivery of RNAs using scFv modified liposome-polycation-hyaluronan nanoparticles in lung cancer models, which through inducing apoptosis led to significant tumour inhibition [122]. Additional studies have investigated the use of a nanosized immunoliposome-based anti-HER-2 siRNA delivery complex to preferentially target tumour cells, where efficiency of the complex was improved by the inclusion of a pH-sensitive histidine-lysine peptide [123]. The complex demonstrates the ability to sensitise tumours to chemotherapeutic agents and silence the target gene of interest, effecting downstream pathways in vivo, as well as significantly inhibiting tumour growth in a pancreatic cancer model [123].
Within the past year, the subject of mRNA has become more of a ubiquitous topic, owing to the approval of RNA-based vaccine nanocarriers for COVID-19 worldwide [23]. The remarkable speed at which these vaccines were advanced is majorly owed to the fact that delivery of nucleic acids using lipid nanoparticles has been widely examined, where structure, uptake, surface-functionalisation endosomal escape, cargo release, clearance and, importantly, safety had to a degree already been optimised. This recent interest in nanomedicines highlights the abundance of research which is on-going, where combination with other therapies as well as further optimisation of the delivery system may hold the key to improve mRNA-based therapies and gene editing.
Conclusion
Despite many advances in cancer treatment, there is still a clinical need for superior, kinder, and more effective therapies. The extensive application of nanomedicines within oncology has yielded some significant benefits but there is much greater potential to be still realised. With formulation advancements in terms of entrapping therapeutics within the nanoparticle core, as well as the concept of surface functionalisation aiding active targeting, an ever-growing number of formulations are likely to enter clinical trials for the treatment of cancer in the coming years. Further work to better understanding the biological fate of nanomaterials in vivo as well as improvements on large scale manufacturing and stability will further increase the success of the nanomedicine field within oncology.
Perspectives
In summary, nanoparticle drug delivery systems offer significant advantages when compared with their free drug counterparts, overcoming limitations in delivery via controlling the biological fate of drugs in vivo, including higher drug deposition at the tumour site, in turn increasing drug efficacy and reducing undesirable off-target side effects.
Advancement within the field, particularly in regard to improved targeting strategies, has led to the development of innovative formulations, with a particular focus on surface functionalisation using targeting ligands, many which are currently in clinical trials.
Whilst 12 nanomedicine therapies have been clinically approved for an oncology indication, these have primarily focused on entrapment and PEGylation to improve API performance. Therefore, combination of entrapment with advancements in active targeting and surface modifications of nanomedicines have the potential to realise tangible patient benefit via exploiting a triple targeting approach. This, in combination with improved manufacture scale-up to ensure a commercially viable system, should facilitate the advent of next generation nanomedicines into the clinic.
Abbreviations
- ADCs
antibody-drug conjugates
- API
active pharmaceutical ingredient
- DAR
Drug:Antibody ratios
- DR5
Death Receptor 5
- EPR
enhanced permeability and retention
- MPS
mononuclear phagocytic system
- PGA
poly(glycolic acid)
- PLA
poly(DL-lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- TME
tumour microenvironment
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors acknowledge the Northern Ireland Department for Economy for funding the Ph.D. studentships of S.T. This work was also partially funded through the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/R009112/1).
Open Access Statement
Open access for this article was enabled by the participation of Queen's University Belfast in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contributions
S.T. wrote the manuscript and prepared figures. P.S., C.J.B. and C.J.S. edited and revised the manuscript.
References
- 1.Hare, J.I., Lammers, T., Ashford, M.B., Puri, S., Storm, G. and Barry, S.T. (2017) Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv. Drug Deliv. Rev. 108, 25–38 10.1016/j.addr.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 2.De Jong, W.H. and Borm, P.J.A. (2008) Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomedicine 3, 133–149 10.2147/IJN.S596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapley, N. and Mello, C. (2011) Nanoparticles and their applications in cell. Anal. Chem. 6, 9–26 10.1039/c3ib40165k [DOI] [Google Scholar]
- 4.Wu, L., Zhang, J. and Watanabe, W. (2011) Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 63, 456–469 10.1016/j.addr.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Ngoune, R., Peters, A., von Elverfeldt, D., Winkler, K. and Pütz, G. (2016) Accumulating nanoparticles by EPR: a route of no return. J. Control Release 238, 58–70 10.1016/j.jconrel.2016.07.028 [DOI] [PubMed] [Google Scholar]
- 6.Fang, J., Nakamura, H. and Maeda, H. (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 63, 136–151 10.1016/j.addr.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 7.Desai, N. (2012) Challenges in development of nanoparticle-based therapeutics. AAPS J. 14, 282–295 10.1208/s12248-012-9339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen, T.M. and Cullis, P.R. (2004) Drug delivery systems: entering the mainstream. Science 303, 1818–1822 10.1126/science.1095833 [DOI] [PubMed] [Google Scholar]
- 9.Awasthi, R., Roseblade, A., Hansbro, P.M., Rathbone, M.J., Dua, K. and Bebawy, M. (2018) Nanoparticles in cancer treatment: opportunities and obstacles. Curr. Drug Targets 19, 1696–1709 10.2174/1389450119666180326122831 [DOI] [PubMed] [Google Scholar]
- 10.Anselmo, A.C. and Mitragotri, S. (2019) Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4, 1–16 10.1002/btm2.10100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari, A., Yadav, S.K. and Yadav, S.C. (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 75, 1–18 10.1016/j.colsurfb.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 12.Masood, F. (2016) Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 60, 569–578 10.1016/j.msec.2015.11.067 [DOI] [PubMed] [Google Scholar]
- 13.Anselmo, A.C. and Mitragotri, S. (2016) Nanoparticles in the clinic. Bioeng. Transl. Med. 1, 10–29 10.1002/btm2.10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarron, P.A., Marouf, W.M., Quinn, D.J., Fay, F., Burden, R.E., Olwill, S.A.et al. (2008) Antibody targeting of camptothecin-loaded PLGA nanoparticles to tumor cells. Bioconjug. Chem. 19, 1561–1569 10.1021/bc800057g [DOI] [PubMed] [Google Scholar]
- 15.Tran, S., DeGiovanni, P.-J., Piel, B. and Rai, P. (2017) Cancer nanomedicine: a review of recent success in drug delivery. Clin. Transl. Med. 6, 44 10.1186/s40169-017-0175-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu, Y., Yang, J. and Sega, E. (2006) Issues related to targeted delivery of proteins and peptides. AAPS J. 8, E466–E478 10.1208/aapsj080355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins, S., Sarmento, B., Ferreira, D.C. and Souto, E.B. (2007) Lipid-based colloidal carriers for peptide and protein delivery: liposomes versus lipid nanoparticles. Int. J. Nanomedicine 2, 595–607 [PMC free article] [PubMed] [Google Scholar]
- 18.Ristroph, K.D. and Prud'homme, R.K. (2019) Hydrophobic ion pairing: encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 1, 4207–4237 10.1039/C9NA00308H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik, S. and Bahal, R. (2019) Investigation of PLGA nanoparticles in conjunction with nuclear localization sequence for enhanced delivery of antimiR phosphorothioates in cancer cells in vitro. J. Nanobiotechnology 17, 1–13 10.1186/s12951-019-0490-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barenholz, Y. (2012) Doxil®: the first FDA-approved nano-drug: lessons learned. J. Control Release 160, 117–134 10.1016/j.jconrel.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 21.Aldosari, B.N., Alfagih, I.M. and Almurshedi, A.S. (2021) Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics 13, 1–29 10.3390/pharmaceutics13020206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zewail, M. (2021) Folic acid decorated chitosan-coated solid lipid nanoparticles for the oral treatment of rheumatoid arthritis. Ther. Deliv. 12, 297–310 10.4155/tde-2020-0123 [DOI] [PubMed] [Google Scholar]
- 23.Khurana, A., Allawadhi, P., Khurana, I., Allwadhi, S., Weiskirchen, R., Banothu, A.K.et al. (2021) Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 38, 101142 10.1016/j.nantod.2021.101142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrichs, S. and Bowman, D.M. (2021) COVID-19 may become nanomedicines finest hour yet. Nat. Nanotechnol. 16, 358–361 10.1038/s41565-021-00901-8 [DOI] [PubMed] [Google Scholar]
- 25.Wang, Y., Qu, W. and Choi, S.H. (2016) FDA's regulatory science program for generic PLA/PLGA-based drug products. Am. Pharm. Rev. 20, 92 10.1208/s12248-021-00611-y [DOI] [Google Scholar]
- 26.Bobo, D., Robinson, K.J., Islam, J., Thurecht, K.J. and Corrie, S.R. (2016) Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm. Res. 33, 2373–2387 10.1007/s11095-016-1958-5 [DOI] [PubMed] [Google Scholar]
- 27.Yuan, F., Dellian, M., Fukumura, D., Leunig, M., Berk, D.A., Torchilin, V.P.et al. (1995) Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size1. Cancer Res. 55, 3752–3757 PMID: [PubMed] [Google Scholar]
- 28.Moghimi, S.M., Hunter, A.C. and Murray, J.C. (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 53, 283–318 PMID: [PubMed] [Google Scholar]
- 29.Maeda, H. (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 41, 189–207 10.1016/S0065-2571(00)00013-3 [DOI] [PubMed] [Google Scholar]
- 30.Matsumura, Y. and Maeda, H. (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 PMID: [PubMed] [Google Scholar]
- 31.Attia, M.F., Anton, N., Wallyn, J., Omran, Z. and Vandamme, T.F. (2019) An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 71, 1185–1198 10.1111/jphp.13098 [DOI] [PubMed] [Google Scholar]
- 32.Maeda, H. (2015) Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 91, 3–6 10.1016/j.addr.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 33.Prabhakar, U., Maeda, H., Jain, R.K., Sevick-Muraca, E.M., Zamboni, W., Farokhzad, O.C.et al. (2013) Challenges and key considerations of the enhanced permeability and retention (EPR) effect for nanomedicine drug delivery in oncology. Cancer Res. 73, 2412–2417 10.1158/0008-5472.CAN-12-4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golombek, S.K., May, J., Theek, B. and Appold, L. (2018) Tumor targeting via EPR: strategies to enhance patient responses. Adv. Drug Deliv. Rev., 17–38 10.1016/j.addr.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilhelm, S., Tavares, A.J., Dai, Q., Ohta, S., Audet, J., Dvorak, H.F.et al. (2016) Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 10.1038/natrevmats.2016.14 [DOI] [Google Scholar]
- 36.Maeda, H. (2021) The 35th anniversary of the discovery of EPR effect: a new wave of nanomedicines for tumor-targeted drug delivery-personal remarks and future prospects. J. Pers. Med. 11, 229 10.3390/jpm11030229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang, J., Islam, R., Islam, W., Yin, H., Subr, V., Etrych, T.et al. (2019) Augmentation of epr effect and efficacy of anticancer nanomedicine by carbon monoxide generating agents. Pharmaceutics 11, 343 10.3390/pharmaceutics11070343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda, H. (2012) Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control Release 164, 138–144 10.1016/j.jconrel.2012.04.038 [DOI] [PubMed] [Google Scholar]
- 39.Chen, D., Qu, X., Shao, J., Wang, W. and Dong, X. (2020) Anti-vascular nano agents: a promising approach for cancer treatment. J. Mater. Chem. B 8, 2990–3004 10.1039/C9TB02957E [DOI] [PubMed] [Google Scholar]
- 40.Fang, J., Islam, W. and Maeda, H. (2020) Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 157, 142–160 10.1016/j.addr.2020.06.005 [DOI] [PubMed] [Google Scholar]
- 41.Strebhardt, K. and Ullrich, A. (2008) Paul Ehrlich's magic bullet concept: 100 years of progress. Nat. Rev. Cancer 8, 473–480 10.1038/nrc2394 [DOI] [PubMed] [Google Scholar]
- 42.Amiri-Kordestani, L., Blumenthal, G.M., Xu, Q.C., Zhang, L., Tang, S.W., Ha, L.et al. (2014) FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin. Cancer Res. 20, 4436–4441 10.1158/1078-0432.CCR-14-0012 [DOI] [PubMed] [Google Scholar]
- 43.Zhao, P., Zhang, Y., Li, W., Jeanty, C., Xiang, G. and Dong, Y. (2020) Recent advances of antibody drug conjugates for clinical applications. Acta Pharm. Sin. B 10, 1589–1600 10.1016/j.apsb.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponziani, S., Di Vittorio, G., Pitari, G., Cimini, A.M., Ardini, M., Gentile, R.et al. (2020) Antibody-drug conjugates: the new frontier of chemotherapy. Int. J. Mol. Sci. 21, 5510 10.3390/ijms21155510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, W., Veale, K.H., Qiu, Q., Sinkevicius, K.W., Maloney, E.K., Costoplus, J.A.et al. (2019) Synthesis and evaluation of camptothecin antibody-drug conjugates. ACS Med. Chem. Lett. 10, 1386–1392 10.1021/acsmedchemlett.9b00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narayan, P., Osgood, C.L., Singh, H., Chiu, H.-J., Ricks, T.K., Chow, E.C.Y.et al. (2021) Geruxtecan-Nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin. Cancer Res. 27, 4478–4485 10.1158/1078-0432.CCR-20-4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Criscitiello, C., Morganti, S. and Curigliano, G. (2021) Antibody–drug conjugates in solid tumors: a look into novel targets. J. Hematol. Oncol. 14, 1–18 10.1186/s13045-021-01035-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fenn, K.M. and Kalinsky, K. (2019) Sacituzumab govitecan: antibody-drug conjugate in triple-negative breast cancer and other solid tumors. Drugs Today 55, 575 10.1358/dot.2019.55.9.3039669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston, M.C. and Scott, C.J. (2018) Antibody conjugated nanoparticles as a novel form of antibody drug conjugate chemotherapy. Drug Discov. Today Technol. 30, 63–69 10.1016/j.ddtec.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 50.Sechi, M., Sanna, V. and Pala, N. (2014) Targeted therapy using nanotechnology: focus on cancer. Int. J. Nanomedicine 9, 467 10.2147/IJN.S36654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suk, J.S., Xu, Q., Kim, N., Hanes, J. and Ensing, L.M. (2017) PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 99, 28–51 10.1016/j.addr.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bazak, R., Houri, M., El Achy, S., Hussein, W. and Refaat, T. (2014) Passive targeting of nanoparticles to cancer: a comprehensive review of the literature. Mol. Clin. Oncol. 2, 904–908 10.3892/mco.2014.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papi, M., Caputo, D., Palmieri, V., Coppola, R., Palchetti, S., Bugli, F.et al. (2017) Clinically approved PEGylated nanoparticles are covered by a protein corona that boosts the uptake by cancer cells. Nanoscale 9, 10327–10334 10.1039/C7NR03042H [DOI] [PubMed] [Google Scholar]
- 54.Kreuter, J. (2007) Nanoparticles-a historical perspective. Int. J. Pharm. 331, 1–10 10.1016/j.ijpharm.2006.10.021 [DOI] [PubMed] [Google Scholar]
- 55.Schmid, D., Jarvis, G.E., Fay, F., Small, D.M., Greene, M.K., Majkut, J.et al. (2014) Nanoencapsulation of ABT-737 and camptothecin enhances their clinical potential through synergistic antitumor effects and reduction of systemic toxicity. Cell Death Dis. 5, e1454–e1411 10.1038/cddis.2014.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabizon, A., Shmeeda, H. and Barenholz, Y. (2003) Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 42, 419–436 10.2165/00003088-200342050-00002 [DOI] [PubMed] [Google Scholar]
- 57.Jiang, W., Lionberger, R. and Yu, L.X. (2011) In vitro and in vivo characterizations of PEGylated liposomal doxorubicin. Bioanalysis 3, 333–344 10.4155/bio.10.204 [DOI] [PubMed] [Google Scholar]
- 58.Hadjidemetriou, M., Al-Ahmady, Z. and Kostarelos, K. (2016) Time-evolution of in vivo protein corona onto blood-circulating PEGylated liposomal doxorubicin (DOXIL) nanoparticles. Nanoscale 8, 6948–6957 10.1039/C5NR09158F [DOI] [PubMed] [Google Scholar]
- 59.Frampton, J.E. (2020) Liposomal irinotecan: a review in metastatic pancreatic adenocarcinoma. Drugs 80, 1007–1018 10.1007/s40265-020-01336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee, S.W., Kim, Y.M., Cho, C.H., Kim, Y.T., Kim, S.M., Hur, S.Y.et al. (2018) An open-label, randomized, parallel, phase ii trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of paclitaxel as first-line treatment for ovarian cancer: a Korean gynecologic oncology group study (KGOG-3021). Cancer Res. Treat. 50, 195–203 10.4143/crt.2016.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee, K.S., Chung, H.C., Im, S.A., Park, Y.H., Kim, C.S., Kim, S.B.et al. (2008) Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 108, 241–250 10.1007/s10549-007-9591-y [DOI] [PubMed] [Google Scholar]
- 62.Friedman, A., Claypool, S. and Liu, R. (2013) The smart targeting of nanoparticles. Curr. Pharm. Des. 19, 6315–6329 10.2174/13816128113199990375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nogueira, J.C.F., Greene, M.K., Richards, D.A., Furby, A.O., Steven, J., Porter, A.et al. (2019) Oriented attachment of VNAR proteins: via site-selective modification, on PLGA-PEG nanoparticles enhances nanoconjugate performance. Chem. Commun. 55, 7671–7674 10.1039/C9CC02655J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leach, A., Smyth, P., Ferguson, L., Steven, J., Greene, M.K., Branco, C.M.et al. (2020) Anti-DLL4 VNAR targeted nanoparticles for targeting of both tumour and tumour associated vasculature. Nanoscale 12, 14751–14763 10.1039/D0NR02962A [DOI] [PubMed] [Google Scholar]
- 65.Greene, M.K., Nogueira, J.C.F., Tracey, S.R., Richards, D.A., McDaid, W.J., Burrows, J.F.et al. (2020) Refined construction of antibody-targeted nanoparticles leads to superior antigen binding and enhanced delivery of an entrapped payload to pancreatic cancer cells. Nanoscale 12, 11647–11658 10.1039/D0NR02387F [DOI] [PubMed] [Google Scholar]
- 66.Johnston, M.C., Nicoll, J.A., Redmond, K.M., Smyth, P., Greene, M.K., McDaid, W.J.et al. (2020) DR5-targeted, chemotherapeutic drug-loaded nanoparticles induce apoptosis and tumor regression in pancreatic cancer in vivo models. J. Control Release 324, 610–619 10.1016/j.jconrel.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ubah, O.C., Buschhaus, M.J., Ferguson, L., Kovaleva, M., Steven, J., Porter, A.J.et al. (2018) Next-generation flexible formats of VNAR domains expand the drug platform's utility and developability. Biochem. Soc. Trans. 46, 1559–1565 10.1042/BST20180177 [DOI] [PubMed] [Google Scholar]
- 68.Kovaleva, M., Ferguson, L., Steven, J., Porter, A. and Barelle, C. (2014) Shark variable new antigen receptor biologics: a novel technology platform for therapeutic drug development. Expert Opin. Biol. Ther. 14, 1527–1539 10.1517/14712598.2014.937701 [DOI] [PubMed] [Google Scholar]
- 69.Togtema, M., Hussack, G., Dayer, G., Teghtmeyer, M.R., Raphael, S., Tanha, J.et al. (2019) Single-domain antibodies represent novel alternatives to monoclonal antibodies as targeting agents against the human papillomavirus 16 E6 protein. Int. J. Mol. Sci. 20, 2088 10.3390/ijms20092088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martínez-Jothar, L., Doulkeridou, S., Schiffelers, R.M., Sastre Torano, J., Oliveira, S., van Nostrum, C.F.et al. (2018) Insights into maleimide-thiol conjugation chemistry: conditions for efficient surface functionalization of nanoparticles for receptor targeting. J. Control Release 282, 101–109 10.1016/j.jconrel.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 71.Thorek, D.L.J., Elias, D.R. and Tsourkas, A. (2009) Comparative analysis of nanoparticle-antibody conjugations: carbodiimide versus click chemistry. Mol. Imaging 8, 221–229 PMID: [PubMed] [Google Scholar]
- 72.Koo, H., Lee, S., Na, J.H., Kim, S.H., Hahn, S.K., Choi, K.et al. (2012) Bioorthogonal copper-free click chemistry in vivo for tumor-targeted delivery of nanoparticles. Angew. Chem. Int. Ed. Engl. 124, 12006–12010 10.1002/ange.201206703 [DOI] [PubMed] [Google Scholar]
- 73.Autio, K.A., Dreicer, R., Anderson, J., Garcia, J.A., Alva, A., Hart, L.L.et al. (2018) Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 4, 1344–1351 10.1001/jamaoncol.2018.2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Autio, K.A., Garcia, J.A., Alva, A.S., Hart, L.L., Milowsky, M.I., Posadas, E.M.et al. (2016) A phase 2 study of BIND-014 (PSMA-targeted docetaxel nanoparticle) administered to patients with chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 34, 233 10.1200/jco.2016.34.2_suppl.233 [DOI] [Google Scholar]
- 75.Nobs, L., Buchegger, F., Gurny, R. and Allémann, E. (2006) Biodegradable nanoparticles for direct or two-step tumor immunotargeting. Bioconjug Chem. 17, 139–145 10.1021/bc050137k [DOI] [PubMed] [Google Scholar]
- 76.Greene, M., Richards, D.A., Nogueira, J., Campbell, K., Smyth, P., Fernandez, M.et al. (2017) Generating next-generation antibody-nanoparticle conjugates through the oriented installation of non-engineered antibody fragments. Chem. Sci 9, 79–87 10.1039/C7SC02747H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alibolandi, M., Ramezani, M., Abnous, K., Sadeghi, F., Atyabi, F., Asouri, M.et al. (2015) In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J. Control Release 209, 88–100 10.1016/j.jconrel.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 78.Ubah, O.C., Steven, J., Porter, A.J. and Barelle, C.J. (2019) An anti-hTNF-α variable new antigen receptor format demonstrates superior in vivo preclinical efficacy to humira® in a transgenic mouse autoimmune polyarthritis disease model. Front. Immunol. 10, 526 10.3389/fimmu.2019.00526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiang, D., Zheng, C., Zhou, S.F., Qiao, S., Tran, P.H.L., Pu, C.et al. (2015) Superior performance of aptamer in tumor penetration over antibody: implication of aptamer-based theranostics in solid tumors. Theranostics 5, 1083–1097 10.7150/thno.11711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhar, P., Samarasinghe, R.M. and Shigdar, S. (2020) Antibodies, nanobodies, or aptamers—which is best for deciphering the proteomes of non-model species? Int. J. Mol. Sci. 21, 2485 10.3390/ijms21072485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu, Z. and Xiang, J. (2020) Aptamer-functionalized nanoparticles in targeted delivery and cancer therapy. Int. J. Mol. Sci. 21, 9123 10.3390/ijms21239123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li, Y., Duo, Y., Bao, S., He, L., Ling, K., Luo, J.et al. (2017) EpCAM aptamer-functionalized polydopamine-coated mesoporous silica nanoparticles loaded with DM1 for targeted therapy in colorectal cancer. Int. J. Nanomedicine 12, 6239–6257 10.2147/IJN.S143293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cotton, G., Thom, J., Trumper, P., Bell, S., Kamenski, A., Wappett, M.et al. (2019) Abstract 222: novel protein drug conjugates targeting ROR1 through the development and exploitation of a drug discovery platform based on small, engineered VNAR domains. Cancer Res. 79, 222 10.1158/1538-7445.AM2019-222 [DOI] [Google Scholar]
- 84.Dubuisson, A. and Micheau, O. (2017) Antibodies and derivatives targeting DR4 and DR5 for cancer therapy. Antibodies 6, 16 10.3390/antib6040016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valldorf, B., Fittler, H., Deweid, L., Ebenig, A., Dickgiesser, S., Sellmann, C.et al. (2016) An apoptosis-inducing peptidic heptad that efficiently clusters death receptor 5. Angew. Chem. Int. Ed. Engl. 55, 5085–5089 10.1002/anie.201511894 [DOI] [PubMed] [Google Scholar]
- 86.Walczak, H., Miller, R.E., Ariail, K., Gliniak, B., Griffith, T.S., Kubin, M.et al. (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 5, 157–163 10.1038/5517 [DOI] [PubMed] [Google Scholar]
- 87.Von Karstedt, S., Montinaro, A. and Walczak, H. (2017) Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 17, 352–366 10.1038/nrc.2017.28 [DOI] [PubMed] [Google Scholar]
- 88.Fay, F., McLaughlin, K.M., Small, D.M., Fennell, D.A., Johnston, P.G., Longley, D.B.et al. (2011) Conatumumab (AMG 655) coated nanoparticles for targeted pro-apoptotic drug delivery. Biomaterials 32, 8645–8653 10.1016/j.biomaterials.2011.07.065 [DOI] [PubMed] [Google Scholar]
- 89.Schmid, D., Fay, F., Small, D.M., Jaworski, J., Riley, J.S., Tegazzini, D.et al. (2014) Efficient drug delivery and induction of apoptosis in colorectal tumors using a death receptor 5-targeted nanomedicine. Mol. Ther. 22, 2083–2092 10.1038/mt.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abbasi, S., Paul, A., Shao, W. and Prakash, S. (2012) Cationic albumin nanoparticles for enhanced drug delivery to treat breast cancer: preparation and in vitro assessment. J. Drug Deliv. 2012, 686108 10.1155/2012/686108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Donahue, N.D., Acar, H. and Wilhelm, S. (2019) Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 143, 68–96 10.1016/j.addr.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 92.Bilensoy, E. (2010) Cationic nanoparticles for cancer therapy. Expert Opin. Drug Deliv. 7, 795–809 10.1517/17425247.2010.485983 [DOI] [PubMed] [Google Scholar]
- 93.Shen, S., Zhang, Y., Chen, K.G., Luo, Y.L. and Wang, J. (2018) Cationic polymeric nanoparticle delivering CCR2 siRNA to inflammatory monocytes for tumor microenvironment modification and cancer therapy. Mol. Pharm. 15, 3642–3653 10.1021/acs.molpharmaceut.7b00997 [DOI] [PubMed] [Google Scholar]
- 94.Arvizo, R.R., Miranda, O.R., Thompson, M.A., Pabelick, C.M., Bhattacharya, R., David Robertson, J.et al. (2010) Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 10, 2543–2548 10.1021/nl101140t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hughes, C.S., Colhoun, L.M., Bains, B.K., Kilgour, J.D., Burden, R.E., Burrows, J.F.et al. (2016) Extracellular cathepsin S and intracellular caspase 1 activation are surrogate biomarkers of particulate-induced lysosomal disruption in macrophages. Part Fibre Toxicol. 13, 1–13 10.1186/s12989-016-0129-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang, L. and Guo, S. (2011) Nanoparticles escaping RES and endosome: challenges for siRNA delivery for cancer therapy. J. Nanomater. 2011, 742895 10.1155/2011/742895 [DOI] [Google Scholar]
- 97.Hu, Q.D., Fan, H., Ping, Y., Liang, W.Q., Tang, G.P. and Li, J. (2011) Cationic supramolecular nanoparticles for co-delivery of gene and anticancer drug. Chem. Commun. 47, 5572–5574 10.1039/C1CC10721F [DOI] [PubMed] [Google Scholar]
- 98.Farshbaf, M., Davaran, S., Zarebkohan, A., Annabi, N., Akbarzadeh, A. and Salehi, R. (2018) Significant role of cationic polymers in drug delivery systems. Artif. Cells Nanomed. Biotechnol. 46, 1872–1891 10.1080/21691401.2017.1395344 [DOI] [PubMed] [Google Scholar]
- 99.Wang, W.L., Ma, X.J. and Yu, X.F. (2017) pH-responsive polymersome based on PMCP-b-PDPA as a drug delivery system to enhance cellular internalization and intracellular drug release. Chinese J. Polym. Sci. 35, 1352–1362 10.1007/s10118-017-1982-x [DOI] [Google Scholar]
- 100.Pearson, R.T., Warren, N.J., Lewis, A.L., Armes, S.P. and Battaglia, G. (2013) Effect of pH and temperature on PMPC–PDPA copolymer self-assembly. Macromolecules 46, 1400–1407 10.1021/ma302228m [DOI] [Google Scholar]
- 101.Gibson, T.J., Smyth, P., Mcdaid, W.J., Lavery, D., Thom, J., Cotton, G.et al. (2018) Single-domain antibody-functionalized pH-responsive amphiphilic block copolymer nanoparticles for epidermal growth factor receptor targeted cancer therapy. ACS Macro Lett. 7, 1010–1015 10.1021/acsmacrolett.8b00461 [DOI] [PubMed] [Google Scholar]
- 102.Pei, D. and Buyanova, M. (2019) Overcoming endosomal entrapment in drug delivery. Bioconjug. Chem. 30, 273–283 10.1021/acs.bioconjchem.8b00778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deirram, N., Zhang, C., Kermaniyan, S.S., Johnston, A.P.R. and Such, G.K. (2019) pH-responsive polymer nanoparticles for drug delivery. Macromol. Rapid Commun. 40, 1–23 10.1002/marc.201800917 [DOI] [PubMed] [Google Scholar]
- 104.Johnson, L., Gray, D.M., Niezabitowska, E. and McDonald, T.O. (2021) Multi-stimuli-responsive aggregation of nanoparticles driven by the manipulation of colloidal stability. Nanoscale, 7879–7896 10.1039/D1NR01190A [DOI] [PubMed] [Google Scholar]
- 105.Kono, K. (2001) Thermosensitive polymer-modified liposomes. Adv. Drug Deliv. Rev. 53, 307–319 10.1016/S0169-409X(01)00204-6 [DOI] [PubMed] [Google Scholar]
- 106.Dou, Y., Hynynen, K. and Allen, C. (2017) To heat or not to heat: challenges with clinical translation of thermosensitive liposomes. J. Control Release 249, 63–73 10.1016/j.jconrel.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 107.Ta, T. and Porter, T. (2013) Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control Release 169, 112–125 10.1016/j.jconrel.2013.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Webb, C., Forbes, N., Roces, C.B., Anderluzzi, G., Lou, G., Abraham, S.et al. (2020) Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: a case study using protein-loaded liposomes. Int. J. Pharm. 582, 119266 10.1016/j.ijpharm.2020.119266 [DOI] [PubMed] [Google Scholar]
- 109.Roces, C.B., Port, E.C., Daskalakis, N.N., Watts, J.A., Aylott, J.W., Halbert, G.W.et al. (2020) Rapid scale-up and production of active-loaded PEGylated liposomes. Int. J. Pharm. 586, 119566 10.1016/j.ijpharm.2020.119566 [DOI] [PubMed] [Google Scholar]
- 110.Ma, L., Young, J., Prabhala, H., Pan, E., Mestdagh, P., Muth, D.et al. (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 12, 247–256 10.1038/ncb2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trewyn, B.G., Giri, S., Slowing, I.I. and Lin, V.S.Y. (2007) Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun. 31, 3236–3245 10.1039/b701744h [DOI] [PubMed] [Google Scholar]
- 112.Bae, Y., Fukushima, S., Harada, A. and Kataoka, K. (2003) Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. Engl. 42, 4640–4643 10.1002/anie.200250653 [DOI] [PubMed] [Google Scholar]
- 113.Portney, N.G. and Ozkan, M. (2006) Nano-oncology: drug delivery, imaging, and sensing. Anal. Bioanal. Chem. 384, 620–630 10.1007/s00216-005-0247-7 [DOI] [PubMed] [Google Scholar]
- 114.De Angelis, B., Depalo, N., Petronella, F., Quintarelli, C., Curri, M.L., Pani, R.et al. (2020) Stimuli-responsive nanoparticle-assisted immunotherapy: a new weapon against solid tumours. J. Mater. Chem. B 8, 1823–1840 10.1039/C9TB02246E [DOI] [PubMed] [Google Scholar]
- 115.Yang, M., Li, J., Gu, P. and Fan, X. (2021) The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact Mater 6, 1973–1987 10.1016/j.bioactmat.2020.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanahan, D. and Weinberg, R.A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 117.Hanahan, D. and Weinberg, R.A. (2000) The hallmarks of cancer. Cell 100, 57–70 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 118.Zhao, X., Yang, K., Zhao, R., Ji, T., Wang, X., Yang, X.et al. (2016) Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials 102, 187–197 10.1016/j.biomaterials.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 119.Rosalia, R.A., Cruz, L.J., van Duikeren, S., Tromp, A.T., Silva, A.L., Jiskoot, W.et al. (2015) CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 40, 88–97 10.1016/j.biomaterials.2014.10.053 [DOI] [PubMed] [Google Scholar]
- 120.Wang, K., Kievit, F.M. and Zhang, M. (2016) Nanoparticles for cancer gene therapy: recent advances, challenges, and strategies. Pharmacol. Res. 114, 56–66 10.1016/j.phrs.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 121.Ozpolata, B., Sood, A. and Lopez-Beresteina, G. (2014) Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 66, 110–116 10.1016/j.addr.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen, Y., Zhu, X., Zhang, X., Liu, B. and Huang, L. (2010) Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 18, 1650–1656 10.1038/mt.2010.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pirollo, K.F., Rait, A., Zhou, Q., Sung, H.H., Dagata, J.A., Zon, G.et al. (2007) Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Res. 67, 2938–2943 10.1158/0008-5472.CAN-06-4535 [DOI] [PubMed] [Google Scholar]