Abstract
Chronic wounds represent an economic burden to healthcare systems worldwide and a societal burden to patients, deeply impacting their quality of life. The incidence of recalcitrant wounds has been steadily increasing since the population more susceptible, the elderly and diabetic, are rapidly growing. Chronic wounds are characterised by a delayed wound healing process that takes longer to heal under standard of care than acute (i.e. healthy) wounds. Two of the most common problems associated with chronic wounds are inflammation and infection, with the latter usually exacerbating the former. With this in mind, researchers and wound care companies have developed and marketed a wide variety of wound dressings presenting different compositions but all aimed at promoting healing. This makes it harder for physicians to choose the correct therapy, especially given a lack of public quantitative data to support the manufacturers’ claims. This review aims at giving a brief introduction to the clinical need for chronic wound dressings, focusing on inflammation and evaluating how bio-derived and synthetic dressings may control excess inflammation and promote healing.
Keywords: chronic ulcers, collagen, inflammation, wound dressing, wound healing
Introduction
Wound healing is a complex process that involves numerous cell types, cytokines, chemokines, growth factors and extracellular matrix (ECM) components, which work synergistically to achieve healing [1,2]. It consists of four overlapping stages: haemostasis, inflammation, proliferation and remodelling, each with its own function and role valuable for the next phase to occur smoothly and with no delay [1,2]. In a healthy wound (i.e. acute wound), these stages usually occur with no obstacle, resulting in a completely healed wound with minimal scar tissue, albeit with slightly reduced mechanical properties (∼80%), when compared with the skin before injury [3].
A chronic wound is defined as a wound that has failed to proceed through the wound healing process readily, and it does not heal within 3 months under standard of care [2,4]. It usually results when patients present comorbidities such as diabetes, obesity, immune system deficiencies, peripheral vascular disease and cardiopulmonary disease [1,2,5]. These wounds often stall in the inflammatory stage, whereby excess and persistent inflammation creates a hostile wound environment. A wound that is slow or fails to heal is at greater risk of infection. If infection does occur, the heightened inflammation is further exacerbated [1,2]. Chronic wounds include diabetic foot ulcers (DFUs), pressure ulcers (PUs) and leg ulcers (LUs), which include venous leg ulcers, arterial leg ulcers and ulcers of mixed aetiology [4].
Wound care and chronic wounds, in particular, represent a health economic burden worldwide, accounting for an NHS annual expenditure in the management of chronic wounds of £5.3billion with a mean cost of £3700 per unhealed wound [6,7]. With such a demand, the global advanced wound dressings market targeting chronic and surgical wounds is expected to exceed ∼£16.5 billion by 2024 [5]. Furthermore, as population and life expectancy increases, the effect of wound management and chronic wounds on global health services will increase further, calling for the development of therapies that can relieve both patients and healthcare systems of this economic and societal burden [8,9].
Two main factors that are usually addressed as common underlying issues in non-healing wounds are inflammation and infection [2]. Infection occurs when immune cells fail to readily eliminate harmful microorganisms infiltrating the wound [10]. Different grades of infection require different therapies and present different degrees of severity: local infection (when infection is contained only at the wound site, usually easy to address); spreading infection (signs and symptoms of infection outside wound border in neighbouring tissues); systemic infection (affects the whole body and may present a severe issue for the health of the patient) [2]. Infection is a major contributing factor to the failure of a wound to heal [2].
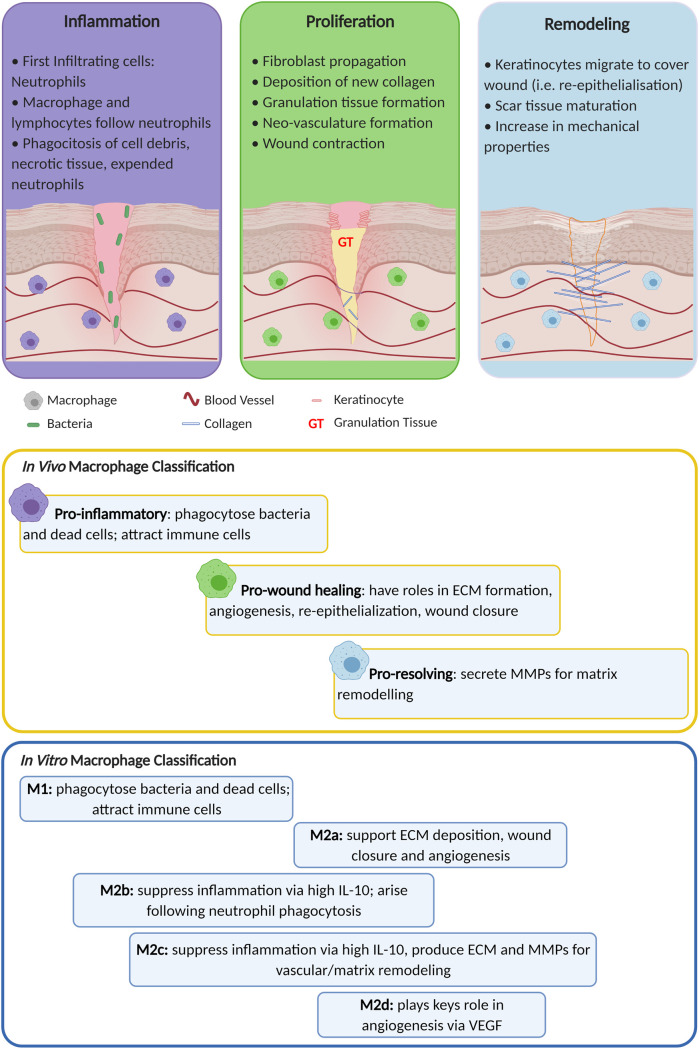
Inflammation represents the body's immune system response to foreign agents such as microorganisms or damaged host tissue; therefore, it is necessary and essential to achieving healing [11]. Inflammation occurs in two stages: early and late inflammation [1,2]. During early inflammation, the innate immune system is activated, and neutrophils are recruited in the wound to remove microorganisms, cellular debris and non-functional tissue [1,9,11]. Neutrophils are followed by infiltrating monocytes that differentiate into ‘M1’ pro-inflammatory macrophages as a response to pathogen-associated molecular patterns (PAMPs), danger-associated molecular patterns (DAMPs), IL-2, IFN-γ and TNF-α [9,12,13]. ‘M1’ macrophages present a high phagocytic capability, and their primary role is to remove any harmful agents. They also secrete pro-inflammatory cytokines such as IL-1, TNF-α, IL-6, IL-12, reactive oxygen species (ROS) and matrix metalloproteinase (MMPs) [9,12,13]. ROS are produced as a mechanism of killing microorganisms, but in excess, when inflammation is out of control, they cause direct tissue damage to the ECM and result in premature cell senescence [14]. MMPs degrade the damaged ECM to allow infiltration of pro-healing cells and factors [9,13]. It is thought that elevated levels of MMPs participate in stalling and delaying the wound healing process in chronic wounds [9,13]. This is because there is a delicate balance between MMPs and their inhibitors, tissue inhibitors of metalloproteinases (TIMPS) [9,13]. When this balance is tipped in favour of MMPs, degradation of healthy tissue and subsequent persistent inflammation creates a negative feedback loop that contributes to delayed healing [9,13]. In an acute wound, once inflammation is resolved and the wound is cleared of contamination, healing progresses into the proliferative stage, where granulation tissue is formed. Keratinocytes start to proliferate and migrate across the wound bed to re-epithelialise the wound [9,13]. More macrophages switch from the pro-inflammatory ‘M1’ phenotype to the pro-healing ‘M2’ phenotype, becoming the most common leukocyte in the wound [9,12,13]. At this point, a chronic wound tends to stall since ‘M1’ macrophages persist without switching to the ‘M2’ phenotype, resulting in elevated levels of pro-inflammatory cytokines produced by ‘M1’ macrophages and by the delayed removal of expended neutrophils [9,11–13]. It is worth noting that macrophages do not present a binary classification of phenotype, but rather a spectrum, as shown in Figure 1; however, in this review, ‘M1’ and ‘M2’ terminology will be used for simplicity [11,13]. A prolonged and heightened state of inflammation, no matter the cause, results in delayed healing. Various means have been proved to curb inflammation in wound healing [15,16]; in this paper, we review how wound dressings reduce excess inflammation allowing chronic wounds to heal more readily.
Figure 1. Schematic of the wound healing stages focusing on macrophage phenotypes classification in vivo and in vitro with their respective functions.
Adapted with permission from [13]. Created with BioRender.com.
Wound dressings: an introduction
The increasing prevalence of chronic wounds highlights the importance of developing innovative products to promote healing [2,17]. In a clinical setting, when a patient presents a wound, clinicians apply a set of principles that aids them to understand the wound aetiology and subsequent wound bed preparation [17,18]. These principles are represented by the acronym TIME, which is defined as Tissue assessment and management, Infection/Inflammation management, Moisture imbalance/management and Edge of wound observation and management [17,18]. Even when TIME management is followed, some wounds fail to heal and require more advanced interventions to restart the healing process [17,18].
Over time, wound management has evolved drastically, from assuming that a dry wound environment would aid healing to prioritise moisture retention when developing new wound dressings [19]. This shift in knowledge was mirrored in the shift in the type of wound dressings used. The more traditional wound dressings (e.g. gauze, lint and cotton wool), which aimed at only covering the wound, have now been replaced by sophisticated materials (e.g. hydrogels, hydrocolloids, foams, films, etc.) that aim to [19–21]:
Provide or maintain a moist environment;
Enhance cellular migration;
Promote angiogenesis and new tissue synthesis;
Allow gas exchange from/to wound;
Maintain appropriate tissue temperature;
Protect against bacterial infection;
Be easily removed after healing or between dressing changes;
Promote autolytic debridement;
Be sterile, non-toxic and hypoallergenic.
One of the more recent advantages introduced into wound dressing formulations, and an indirect way to reduce inflammation by resolving bioburden, is the addition of antimicrobial agents such as antiseptics, antibiotics and natural products [22]. Several reviews provide additional information for a more detailed analysis of the different types of antimicrobial agents and dressings [23–25].
There are thousands of wound dressings on today's market, some claiming the same benefits but presenting different compositions [2,21]. This large number of options makes choosing an appropriate dressing an ever so complicated task for physicians [2,21,26]. Choosing an appropriate dressing is essential to achieve faster healing and depends on many factors, including the type and location of the wound, the level of exudate, the integrity of the surrounding skin, and whether the wound is infected or stalled in the inflammatory phase of healing [21,23,24,27]. A summary of different types of wound dressings is given in Table 1.
Table 1. Summary of different types of dressing with the corresponding description.
| Type of dressings | Description | Ref |
|---|---|---|
| Gauze | Drying Cheap May produce painful removal |
[19–21] |
| Foams | Soft and conformable High porosity, moderately absorbent Thermal insulating Wounds may dry out if little or no exudate |
[23,24] |
| Films | Occlusive, retains moisture No absorbent properties Protects against infection Can cause fluid collection |
[19,20,102,103] |
| Hydrogels | Maintain moist environment Aid in autolytic debridement Rehydrates dry wound Easy removal Not suitable for heavily exuding wounds May require secondary dressing |
[20,103–105] |
| Hydrocolloids | Occlusive Highly absorbent May cause peri-wound maceration May adhere to the wound and damage fragile tissue |
[23,24,103,106] |
| Alginates | Moderate-highly absorbent Haemostatic Maintain moist environment Not suitable for dry wounds May require secondary dressing |
[20,103,107,108] |
| Gelling Fibres | Moderate-highly absorbent Maintain moist environment Forms gel when in contact with exudate Easy to remove Not suitable for dry wounds |
[109,110] |
| Superabsorbent dressings | Highly absorbent Conformable Prevents maceration Not suitable for dry wounds |
[111–114] |
As described before, pathologically extensive inflammation plays an essential role in the delayed wound healing process of chronic ulcers [28]. This is usually caused by multifactorial stimuli that create a hostile microenvironment (e.g. excess levels of inflammatory cells) in which the balance between pro-inflammatory mediators (e.g. chemokines, cytokines, proteases) and their inhibitors is disrupted [28]. The large number of biological entities involved in the inflammatory process makes anti-inflammatory wound dressings varied in their mechanisms of action. For example, some only address locking in exudate away from the wound bed along with the inflammatory components present in wound exudate [29,30]. Others instead focus on regulating pro- and anti-inflammatory cytokines either directly or via macrophage phenotype regulation [31–35]. Nonetheless, the ultimate aim of anti-inflammatory wound dressings is to remove the perpetuating cause and provide a healthy wound microenvironment to promote granulation tissue formation and to promote the healing processes [28].
Bio-derived wound dressings to regulate inflammation
In the last decade, more researchers investigated bio-derived materials as building blocks for new wound dressings [36,37]. These aim to mimic the skin's ECM to provide the chronic wound with a substrate that can function as a healthy ECM or as a sacrificial scaffold for proteases degradation, thereby protecting the native ECM and rebalancing the wound microenvironment allowing for faster healing. The ECM is more than a passive physical substrate for cells; it actively participates in cell–cell communications via cell–matrix interactions, as a result of and resulting in the activation of biochemical mediators, cytokines and growth factors, which play a significant role in wound physiology [38–40]. Although bio-derived materials can provide a high degree of biomimicry, they can be limited by their reproducibility during manufacturing, given by variance between batches [37,41]. The utilisation of animal sources raises concern also from the transmission of pathogens standpoint [42].
The skin ECM comprises the epidermal ECM (i.e. a basement membrane, which separates the epidermis from the dermis) and the dermal ECM. The latter is composed of fibroblasts embedded in connective tissue fibres, interstitial fluid, cell adhesion proteins (e.g. laminin, vitronectin, fibronectin), glycosaminoglycans (GAGs) and proteoglycans [43]. Collagen is the major component of dermal ECM, accounting for ∼70% of skin (dry weight) [44]. It presents 28 genetically different variants, with type I and III variants being the most abundant in the skin [44–46].
Refer to Table 2 for a summary of currently commercially available dressings or in the research stage of development.
Table 2. Non-exhaustive summary of wound dressings with anti-inflammatory effects currently on the market (M) or in the research (R) stage, showing their composition and reported anti-inflammatory ability.
| Dressing | Composition | Anti-inflammatory effects | Market (jurisdiction) or research | Ref. |
|---|---|---|---|---|
| Bio-derived Dressings | ||||
| Endoform® Antimicrobial Dermal Template | Decellularised ovine forestomach matrix with 0.3%w/w silver chloride | Broad-spectrum of MMPs inactivation capability Retention of structural molecules and growth factors |
M(US) | [32,57,61] |
| Puracol® Ultra Matrix | Decellularised porcine mesothelium matric | Retention of growth factors (FGF-basic, VEGF, and TGF-β1) MMPs inactivation capability |
M(US) | [58] |
| Integra® Dermal Regeneration Template | Cross-linked bovine tendon collagen type I, shark chondroitin-6-sulfate GAG, silicon membrane | Chondroitin-6-sulfate GAG has anti-inflammatory properties Allows quick permeation of cells |
M(US & EU) | [49–53] |
| OASIS® Ultra Tri-Layer Matrix | Decellularised porcine small intestinal submucosa | Retention of structural molecules and growth factors | M(US) | [54,115–117] |
| Apligraf® | Bovine type I collagen seeded with human neonatal fibroblasts and keratinocytes | ↑ VEGF, IL-6, IL-8 ↓ fibrotic TGF-β1 Restore fibroblasts function |
M(US) | [118–123] |
| Q-peptide | Chitosan-collagen hydrogel functionalised with QHREDGS peptide | Provide resistance to oxidative stress Induce shift in macrophage polarisation |
R | [33,86–88] |
| Co-modified CBD-VEGF-SDF-1α collagen scaffold | Collagen scaffold modified with CBD-VEGF-SDF-1α | ↓ Infiltration of ‘M1’ macrophages ↓ IL-1β and TNF-α |
R | [34] |
| NAg-CSS | Chitosan-collagen loaded with silver nanoparticles | Modulate macrophage polarisation ↓ IL-6, TNF-α and TGF-β ↑ IL-10 and IFN-γ |
R | [35] |
| Promogran™ Matrix | Freeze-dried matrix composed of 55% bovine type I collagen with 45% ORC |
Bind and inactivate proteases by means of ORC Bind and protect naturally occurring growth factors Demonstrate free radical scavenging properties and anti-inflammatory activity in vitro |
M(US & EU) | [8,82–85,124] |
| Promogran Prisma™ Matrix | Freeze-dried matrix composed of 55% bovine type I collagen with 44% ORC and 1% silver-ORC |
Same benefits as Promogran™ Matrix Silver provides both antimicrobial and anti-inflammatory properties. |
M(US & EU) | [8,82–85,124] |
| ColActive® PLUS | Porcine collagen, sodium alginate, CMC, EDTA and silver chloride |
EDTA and collagen target and deactivate elevated MMP Activity | M(US & EU) | [125–127] |
| Suprasorb® X + PHMB | Biocellulose dressing made up of small-pored HydroBalance fibres that are produced using Acetobacter xylinium | Exudate control | M(US & EU) | [128–132] |
| BIOSTEP™ Collagen Matrix | Porcine gelatin and type I collagen matrix, EDTA, CMC and alginate | EDTA and collagen target and deactivate elevated MMPs activity | M(US) | [74,75] |
| Cutimed® Epiona | Fenestrated substrate made of 90% native bovine-derived collagen and 10% alginate | MMPs sequestered by the substrate due to collagen-binding properties. | M(US & EU) | [76,77] |
| Grafix® | Cryopreserved placental membrane, comprised of native viable cells, GFs and ECM | Retention of epithelial cells, fibroblasts and mesenchymal stem cells ↓ TNF-α and IL-1α ↑ IL-10 |
M(US) | [133–136] |
| Synthetic Dressings | ||||
| UrgoStart® | Soft-adherent foam dressing with TLC and NOSF | Neutralisation of excess proteases | M(EU) | [95–101] |
| PVA Sponge + MCG | Polyvinyl alcohol sponge impregnated with modified collagen gel | Promote shift of macrophage phenotype from ‘M1’ to ‘M2’ ↑ IL-10, IL-4 and VEGF |
R | [31] |
| Drawtex® | Hydroconductive dressing obtained using LevaFibre™ technology, made of two absorbent, cross-action structures of viscose (63.2%) and polyester (26.8%) | Locks exudate and components away from the wound through capillary action ↓ MMPs levels |
M(US & EU) | [29,30,137] |
| Biatain® Ag | Polyurethane foam with semi-permeable, bacteria- and top waterproof film | Exudate control Minimise risk of maceration and leakage |
M(EU) | [138–141] |
| Dermagraft® | Polyglactin mesh with neonatal foreskin fibroblasts | Stimulate granulation tissue formation Stimulate secretion of cytokines and growth factors |
M(US) | [142–146] |
Legend: ↑: up-regulate; ↓: down-regulate, (US): approved to market in the United States; (EU): approved to market in the European Union; (US & EU): approved to market in both US and EU.
Skin substitutes
Skin substitutes are bioengineered dressings made of natural or synthetic polymers set to mimic the physiological geometry and function of native skin [47]. Skin substitutes for recalcitrant wounds are usually either acellular or cellular dermal components or dermo-epidermal components obtained through chemical synthesis of biological components or decellularisation of native ECM [48].
Integra® Dermal Regeneration Template (Integra Life Sciences Corporation, Plainsboro, New Jersey, US) was the first dermal skin substitute product approved by the US Food and Drug Administration (FDA). It consists of a porous matrix of cross-linked bovine tendon collagen type I, shark chondroitin-6-sulfate GAG and covered by a semi-permeable silicone membrane [47,49–53]. Chondroitin-6-sulfate GAG has been shown to have anti-inflammatory effect on macrophages; however, when the effects of Integra® on macrophage phenotype was analysed, a temporal down-regulation of ‘M2a’ macrophages (ECM deposition macrophages) was observed [54,55]. This is assumed to be due to the presence of glutaraldehyde as cross-linking agent in Integra® [54]. Nonetheless, clinical data show that Integra® induces deposition of collagen, histologically indistinguishable from native dermal collagen, achieving good quality tissue with no hypertrophic or keloid scar formation [54,56].
Decellularised xenografts have shown promising results in wound healing management, as they retain the native structure of the ECM, which comprises not only collagen but also structural, adhesion and signalling molecules (e.g. laminin, GAGs, elastin, fibronectin) [57,58]. Endoform® Antimicrobial Dermal Template (Aroa Biosurgery Ltd., Auckland, NZ) presents a matrix of ovine forestomach (OFM) with 0.3% w/w silver chloride [59,60]. Its main component, OFM, has been shown to promote wound healing in recalcitrant wounds (wound closure achieved within 4–24 weeks) with no reports of adverse reactions when used as Endoform® Dermal Template [61–63]. OFM/silver has been demonstrated to be effective at inhibiting a broad spectrum of MMPs and exhibits low cytotoxicity in vitro [32,57,64]. This large MMP-spectrum is assumed to be due to the retained native ECM structure [32,57,64]. A preliminary in vivo study showed positive results on wounds characterised by different aetiologies [65].Puracol® Ultra ECM (Puracol® Ultra ECM, Medline Industries Inc., Northfield, IL) is a decellularised porcine mesothelium matrix that has also been shown to have a high retention of growth factors (FGF-basic, VEGF, and TGF-β1) after the decellularisation process, high angiogenetic potential in vitro and similar MMPs-inhibition abilities to OFM/silver [58]. Although more research is needed on skin substitutes’ full effects in curbing inflammation, they represent an attractive, albeit expensive, way of modulating inflammation and promoting healing [66].
Collagen dressings
Collagen plays many roles in wound management, including chemotaxis of fibroblasts, wound contraction, induction of growth factors and cytokines, activation and inhibition of MMPs [67,68]. Together with its biocompatibility, biodegradable, and non-toxic attributes, these advantages make collagen an attractive candidate material for treating recalcitrant wounds. Numerous clinical studies highlight the benefit of collagen-based dressings for treating chronic wounds, showing faster healing rates or shorter healing times, inactivation of proteases and maintenance of moist wound environment [69–72].
There are many commercially available collagen-based wound dressings, all with different composition but similar claims. They are made of collagen or a combination of collagen and other ECM components, such as elastin, hyaluronic acid and chitosan or in conjunction with other biologically derived materials like alginate and cellulose [73]. BIOSTEP™ Collagen Matrix dressing (Smith & Nephew, London, U.K.) is a matrix presenting both type I and denatured (gelatin) porcine collagen with the addition of EDTA, CMC and alginate [74]. The collagen acts as a sacrificial layer for excess MMPs, while EDTA binds and permanently inactivate them [74,75]. The presence of both collagen and gelatin is claimed to attract both collagenase (MMP-1) and gelatinase (MMP-2, MMP-9) [74]. Cutimed® Epiona (Essity Medical Solutions, Stockholm, Sweden) is a fenestrated substrate made of 90% native bovine-derived collagen and 10% alginate [76,77]. The dressing structure is claimed to be nearly identical to human dermis, which allows for MMP binding and reduction in inflammation [76,77]. Despite the common use of collagen-based wound dressings in wound care, more research is needed to understand their mechanisms of action. Many dressings lack satisfactory evidence-based data due to poorly-designed studies that, in many cases, are industry-funded or present traditional dressing (i.e. saline moistened gauze) as control [69,70].
Cellulose
Cellulose is a naturally occurring polymer used in biomedical applications for its biocompatibility, biodegradability, low-toxicity and good absorption properties [78,79]. There are different cellulose sources and derivatives. An example is given by oxidised regenerated cellulose (ORC), a chemically modified form of cellulose with haemostatic abilities [80,81]. The Promogran™ Matrix family (3M, Saint Paul, MN, US) of wound dressings are collagen-based dressings containing ORC (3M™ Promogran™ Protease Modulating Matrix) or ORC and silver-ORC (3M™ Promogran Prisma™ Wound Balancing Matrix). Promogran™ Matrix is composed of a freeze-dried matrix made of 55% type I bovine collagen and 45% oxidised regenerated cellulose (ORC), whereas Promogran Prisma™ Matrix replaces 1% of ORC with silver-ORC to provide the dressing with antimicrobial properties. The combination of collagen and ORC presents a series of anti-inflammatory benefits. Firstly, ORC has been shown to passively affect protease levels through its negative charge that attracts positively charged metal ions which are essential for the activation of MMPs and thereby reduces MMPs activity [82]. ORC has the additional benefit of reducing elastase activity, an enzyme that breaks down elastin fibres within the ECM and contributes to non-healing wounds’ chronicity. Studies have shown that collagen/ORC dressings reduce elastase activity and inhibit MMP-2 and MMP-9 activity in chronic wound exudate, resulting in increased healing rates [83,84]. Additionally, collagen/ORC binds platelet-derived growth factor (PDGF) and shields it from degradation within the wound and γ-irradiation when loaded into the dressing during the manufacturing process, opening a series of opportunities to locally deliver exogenous growth factors and protection of endogenous growth factors within the wound [82,83]. These benefits have also been analysed and recognised by many clinical reviews [8,85].
Functionalised collagen dressings
Numerous studies showed functionalisation of collagen-based dressings incorporating growth factor or peptides to provide a more instructive wound microenvironment to aid cellular behaviour and tissue regeneration [86]. An example is given by a chitosan-collagen hydrogel which incorporates an angiopoietin-1 (Ang1) mimetic peptide, QHREDGS (glutamine-histidine-arginine-glutamic acid-aspartic acid-glycine-serine) [33,86–88]. Ang1 has been widely recognised to positively participate in several cellular processes such as vascular protection, wound healing and inflammation, providing skin cells protection from oxidative stress [87]. This product is in development as an injectable gel and a pre-gelled patch and presents the QHREDGS peptide conjugated to the dressing's chitosan component to avoid systemic circulation of the peptide and to promote a more localised activity [88]. The QHREDGS-functionalised hydrogel has been shown to improve in vitro keratinocytes resistance to oxidative stress caused by elevated ROS levels, which is common in diabetic chronic wounds [87]. In addition, when co-cultured with macrophages, the Q-peptide hydrogel induces a shift in macrophages polarisation, resulting in the expression of both pro-inflammatory and anti-inflammatory cytokines; it, therefore, offers the potential to modulate the stalled inflammatory process in chronic wounds [33].
Growth factors have also shown promising results when included in a scaffold as topical therapies [34,89,90]. Long et al. [34] produced a collagen scaffold co-modified with VEGF and SDF-1α. These were loaded onto the scaffold after separately being fused with a collagen-binding domain (CBD); this allows for a more controlled release of the growth factors once implanted [34]. This co-modified CBD-VEGF-SDF-1α collagen scaffold showed reduced infiltration of ‘M1’ macrophages together with reduced expression of IL-1β and TNF-α, two pro-inflammatory cytokines found at high levels in chronic wounds [34]. Furthermore, the synergic action of VEGF and SDF-1α promotes blood vessel formation, which is thought to reduce hypoxia at the wound site [34].
Silver
Ionic silver (Ag+) has been shown to provide anti-inflammatory properties in addition to its antimicrobial activity, although the mechanism of action is not well understood [15,91]. A silver nanoparticle loaded collagen/chitosan scaffold (NAg-CSS) claimed to modulate fibroblast migration and macrophage activation to promote healing has been devised by You and colleagues [35]. Compared with non-loaded collagen/chitosan scaffold, NAg-CSS significantly decreased the expression of CD68 (a macrophage marker), further proven by the inhibition of pro-inflammatory cytokines IL-6, TNF-α and TGF-β while up-regulating the anti-inflammatory cytokines, IL-10 and IFN-γ [35]. Even though silver nanoparticles’ exact anti-inflammatory mechanism is not yet fully understood, the different NAg-CSS components’ combinations could offer a synergic effect. Chitosan has been shown to stabilise collagen scaffolds’ mechanical properties while also providing practical benefits such as antioxidant and antimicrobial properties [92].
Synthetic wound dressings to regulate inflammation
Synthetic wound dressings are considered not as competitive as bio-derived as they do not mimic the native EMC in a similar manner to bio-derived dressings [19]. The most common types of polymers used in wound dressings include polyurethane, polyester, poly(glycolic acid), poly-l-lactide and poly(lactic-co-glycolic acid) [93,94]. Compared with bio-derived dressings, synthetic dressings present advantages in terms of reproducibility and tailoring as their synthesis is more easily controlled. However, they tend to be more inert, not provide a physiological microenvironment to promote healing, and consideration needs to be given to degradation components left in the host tissue [37,41].
Refer to Table 2 for a summary of dressings currently available or in the research stage.
Technology lipido colloid (TLC)
The synthetic dressing UrgoStart® (Urgo Medical, Chenôve, France) presents a polyester mesh saturated with a sucrose octasulfate potassium salt (Nano Oligo Saccharide Factor, NOSF) embedded lipido-colloid matrix (Technology Lipido-Colloid, TLC). NOSF and TLC composition are protected under the manufacturer's patent. UrgoStart (TLC-NOSF) turns into a colloidal solution, allowing for conformability to the wound bed [95]. Oligosaccharides (NOSF) have been shown to reduce MMPs level and restore growth factors biological functions, while the TLC matrix creates a moist wound environment [96]. Furthermore, in vitro data report decreased levels of gelatinases and an initial decrease of collagenases (MMP-1 and MMP-8) [97]. Multiple clinical studies report its effectiveness in DUs, VUs and PUs; however, there is a lack of data regarding its mechanism of action [96,98–101].
Polyvinyl alcohol (PVA) sponge
An exciting example of a collagen-functionalised synthetic dressing is presented by Das and co-workers [31]. The authors saturated a polyvinyl alcohol (PVA) sponge with a modified collagen gel (MCG), implanted it subcutaneously in a mouse model and assessed the effects on inflammation after 3 and 7 days [31]. They found MCG increased macrophage recruitment in situ, decreased pro-inflammatory ‘M1’ phenotype and promoted an ‘M2’ phenotype [31]. This was further proved by the increased levels of anti-inflammatory IL-10 and IL-4 and pro-angiogenic VEGF production [31]. Furthermore, they showed MCG induces IL-10 production via the miR-21-JNK pathway; however, the lack of testing in a wound model calls for further research [31].
Summary
Wound treatment represents one of the most expensive burdens on healthcare systems worldwide.
Many chronic wounds become stuck in the inflammatory phase, which prevents healing from progressing.
A vast market of wound dressings presents different composition but similar claims, making it difficult for healthcare practitioners to decide the appropriate treatment.
Collagen is one of the most used materials in wound dressings since it helps mimic the native wound microenvironment.
Additional research is needed to fully understand the mechanisms of action of collagen-based wound dressings to curb inflammation, and a better clinical study design needs to be implemented so that the results obtained are truly valuable.
Funding
The authors would like to thank the EPSRC grant reference EP/S022201/1 for funding this work.
Abbreviations
- Ang1
angiopoietin-1
- CBD
collagen binding domain
- CMC
carboxymethylcellulose
- DAMPs
danger-associated molecular patterns
- DFUs
diabetic foot ulcers
- ECM
extracellular matrix
- EDTA
ethylenediaminetetraacetic acid
- FDA
US Food and Drug Administration
- GAGs
glycosaminoglycans
- IFN-γ
interferon-γ
- IL-x
interleukin-x (e.g. IL-6, IL-10, IL-4, etc.)
- LUs
leg ulcers
- MCG
modified collagen gel
- MMPs
matrix metalloproteinases
- NHS
UK National Health Services
- OFM
ovine forestomach matrix
- ORC
oxidised regenerated cellulose
- PAMPs
pathogen-associated molecular patterns
- PDGF
platelet-derived growth factor
- PHMB
polyhexamethylene biguanide
- Pus
pressure ulcers
- QHREDGS
glutamine–histidine–arginine–glutamic acid–aspartic acid–glycine–serine
- ROS
reactive oxygen species
- SDF-1α
stromal cell-derived factor-1α
- TGF-β
tissue growth factor-β
- TIMPs
tissue inhibitors of metalloproteinases
- TNF-α
tumour necrosis factor-α
- VEGF
vascular endothelial growth factor
Competing Interests
H.A.T. and A.F. are employees of 3M, and S.C. and D.V.V. received funding from 3M.
Author Contributions
D.V.V. wrote the paper with input from all authors.
References
- 1.Uluer, E.T., Vatansever, H.S. and Kurt, F.O˝. (2017) Wound healing and microenvironment. In Wound Healing (Turksen, K., ed.), pp. 67–77, John Wiley & Sons, Inc., Hoboken, NJ: 10.1002/9781119282518.ch5 [DOI] [Google Scholar]
- 2.Gabriel, A., Barrett, C., Cullen, B., Hodges, D., Lee, W., Snyder, R.et al. (2020) Infection and inflammation in the wound environment: addressing issues of delayed wound healing with advanced wound dressings. Wounds Compend. Clin. Res. Pract. 32, S1–S17 PMID: [PubMed] [Google Scholar]
- 3.Marshall, C.D., Hu, M.S., Leavitt, T., Barnes, L.A., Lorenz, H.P. and Longaker, M.T. (2018) Cutaneous scarring: basic science, current treatments, and future directions. Adv. Wound Care 7, 29–45 10.1089/wound.2016.0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Järbrink, K., Ni, G., Sönnergren, H., Schmidtchen, A., Pang, C., Bajpai, R.et al. (2017) The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst. Rev. 6, 1–7 10.1186/s13643-016-0400-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen, C.K. (2019) Human wounds and its burden: an updated compendium of estimates. Adv. Wound Care 8, 39–48 10.1089/wound.2019.0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guest, J.F., Vowden, K. and Vowden, P. (2017) The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J. Wound Care 26, 292–303 10.12968/jowc.2017.26.6.292 [DOI] [PubMed] [Google Scholar]
- 7.Guest, J.F., Fuller, G.W. and Vowden, P. (2020) Cohort study evaluating the burden of wounds to the UK's National Health Service in 2017/2018: update from 2012/2013. BMJ Open 10, 45253 10.1136/bmjopen-2020-045253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu, S., Applewhite, A.J., Niezgoda, J., Snyder, R., Shah, J., Cullen, B.et al. (2017) Oxidized regenerated cellulose/collagen dressings: review of evidence and recommendations. Adv. Skin Wound Care 30, S1–18 10.1097/01.ASW.0000525951.20270.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues, M., Kosaric, N., Bonham, C.A. and Gurtner, G.C. (2019) Wound healing: a cellular perspective. Physiol. Rev. 99, 665–706 10.1152/physrev.00067.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dattani, R. and Farouk, R. (2010) Wound classification. In Principles of Surgery Vivas for the MRCS, pp. 323–328, Cambridge University Press, Cambridge; 10.1017/cbo9780511663482.020 [DOI] [Google Scholar]
- 11.MacLeod, A.S. and Mansbridge, J.N. (2016) The innate immune system in acute and chronic wounds. Adv. Wound Care 5, 65–78 10.1089/wound.2014.0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesketh, M., Sahin, K.B., West, Z.E. and Murray, R.Z. (2017) Macrophage phenotypes regulate scar formation and chronic wound healing. Int. J. Mol. Sci. 18, 1545 10.3390/ijms18071545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krzyszczyk, P., Schloss, R., Palmer, A. and Berthiaume, F. (2018) The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 9, 419 10.3389/fphys.2018.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunnill, C., Patton, T., Brennan, J., Barrett, J., Dryden, M., Cooke, J.et al. (2017) Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 14, 89–96 10.1111/iwj.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosique, R.G., Rosique, M.J. and Farina Junior, J.A. (2015) Curbing inflammation in skin wound healing: a review. Int. J. Inflam. 2015, 316235 10.1155/2015/316235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larouche, J., Sheoran, S., Maruyama, K. and Martino, M.M. (2018) Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv. Wound Care 7, 209–231 10.1089/wound.2017.0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frykberg, R.G. and Banks, J. (2015) Challenges in the treatment of chronic wounds. Adv. Wound Care 4, 560–582 10.1089/wound.2015.0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leaper, D.J., Schultz, G., Carville, K., Fletcher, J., Swanson, T. and Drake, R. (2012) Extending the TIME concept: what have we learned in the past 10 years? Int. Wound J. 9, 1–19 10.1111/j.1742-481X.2012.01097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhivya, S., Padma, V.V. and Santhini, E. (2015) Wound dressings - a review. Biomedicine 5, 24–28 10.7603/s40681-015-0022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han, G. and Ceilley, R. (2017) Chronic wound healing: a review of current management and treatments. Adv. Ther. 34, 599–610 10.1007/s12325-017-0478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira, A., Simões, S., Ascenso, A. and Reis, C.P. (2020) Therapeutic advances in wound healing. J. Dermatol. Treat 1–21 10.1080/09546634.2020.1730296 [DOI] [PubMed] [Google Scholar]
- 22.Bourdillon, K.A., Delury, C.P. and Cullen, B.M. (2017) Biofilms and delayed healing – an in vitro evaluation of silver- and iodine-containing dressings and their effect on bacterial and human cells. Int. Wound J. 14, 1066–1075 10.1111/iwj.12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simões, D., Miguel, S.P., Ribeiro, M.P., Coutinho, P., Mendonça, A.G. and Correia, I.J. (2018) Recent advances on antimicrobial wound dressing: a review. Eur. J. Pharm. Biopharm. 127, 130–141 10.1016/j.ejpb.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 24.Sarheed, O., Ahmed, A., Shouqair, D., and Boateng, J. (2016) Antimicrobial Dressings for Improving Wound Healing. In Wound Healing: New Insights Into Ancient Challenges, InTechOpen, London, UK; 10.5772/63961 [DOI] [Google Scholar]
- 25.Vowden, P., Vowden, K. and Carville, K. (2011) Antimicrobials made easy. Wounds Int. 2, 1–6 [Google Scholar]
- 26.Skórkowska-Telichowska, K., Czemplik, M., Kulma, A. and Szopa, J. (2013) The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 68, e117–e126 10.1016/j.jaad.2011.06.028 [DOI] [PubMed] [Google Scholar]
- 27.Ghomi E, R., Khalili, S., Nouri Khorasani, S., Esmaeely Neisiany, R. and Ramakrishna, S. (2019) Wound dressings: current advances and future directions. J. Appl. Polym. Sci. 136, 47738 10.1002/app.47738 [DOI] [Google Scholar]
- 28.Zhao, R., Liang, H., Clarke, E., Jackson, C. and Xue, M. (2016) Inflammation in chronic wounds. Int. J. Mol. Sci. 17, 2085 10.3390/ijms17122085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Pont (2011) Drawtex(R) Material Safety Data Sheet. http://urgomedical.us/wp-content/uploads/2019/11/Drawtex-MSDS.pdf (Accessed 7 Jan 2020)
- 30.Beier Drawtex Healthcare (Pty) Ltd. (n.d.) How Drawtex(R) wound dressing works. https://www.drawtex.com/downloads/brochure.pdf (Accessed 7 Feb 2020)
- 31.Das, A., Abas, M., Biswas, N., Banerjee, P., Ghosh, N., Rawat, A.et al. (2019) A modified collagen dressing induces transition of inflammatory to reparative phenotype of wound macrophages. Sci. Rep. 9, 1429 10.1038/s41598-019-49435-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negron, L., Lun, S. and May, B.C. (2014) Ovine forestomach matrix biomaterial is a broad spectrum inhibitor of matrix metalloproteinases and neutrophil elastase. Int. Wound J. 11, 392–397 10.1111/j.1742-481X.2012.01106.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandla, S., Davenport Huyer, L., Wang, Y. and Radisic, M. (2019) Macrophage polarization with angiopoietin-1 peptide QHREDGS. ACS Biomater. Sci. Eng. 5, 4542–4550 10.1021/acsbiomaterials.9b00483 [DOI] [PubMed] [Google Scholar]
- 34.Long, G., Liu, D., He, X., Shen, Y., Zhao, Y., Hou, X.et al. (2020) A dual functional collagen scaffold coordinates angiogenesis and inflammation for diabetic wound healing. Biomater. Sci. 8, 6337–6349 10.1039/d0bm00999g [DOI] [PubMed] [Google Scholar]
- 35.You, C., Li, Q., Wang, X., Wu, P., Ho, J.K., Jin, R.et al. (2017) Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 7, 10489 10.1038/s41598-017-10481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suarato, G., Bertorelli, R. and Athanassiou, A. (2018) Borrowing from nature: biopolymers and biocomposites as smart wound care materials. Front. Bioeng. Biotechnol. 6, 137 10.3389/fbioe.2018.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollini, M. and Paladini, F. (2020) Bioinspired materials for wound healing application: the potential of silk fibroin. Materials (Basel) 13, 3361 10.3390/ma13153361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urciuolo, F., Casale, C., Imparato, G. and Netti, P.A. (2019) Bioengineered skin substitutes: the role of extracellular matrix and vascularization in the healing of deep wounds. J. Clin. Med. 8, 2083 10.3390/jcm8122083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olczyk, P., Mencner, Ł. and Komosinska-Vassev, K. (2014) The role of the extracellular matrix components in cutaneous wound healing. Biomed. Res. Int. 2014, 1–8 10.1155/2014/747584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ågren, M.S. and Werthén, M. (2007) The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int. J. Low Extrem Wounds 6, 82–97 10.1177/1534734607301394 [DOI] [PubMed] [Google Scholar]
- 41.Hinderer, S., Layland, S.L. and Schenke-Layland, K. (2016) ECM and ECM-like materials - biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 97, 260–269 10.1016/j.addr.2015.11.019 [DOI] [PubMed] [Google Scholar]
- 42.Center for Devices and Radiological Health. Medical devices containing materials derived from animal sources (except for in vitro diagnostic devices), guidance for FDA reviewers and industry; availability–FDA. Notice. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/medical-devices-containing-materials-derived-animal-sources-except-vitro-diagnostic-devices (accessed April 30, 2021) [PubMed]
- 43.Marieb, E.N. and Hoehn, K.N. (2015) Human Anatomy & Physiology, Global Edition, Pearson Education Limited, Harlow, UK [Google Scholar]
- 44.Shoulders, M.D. and Raines, R.T. (2009) Collagen structure and stability. Annu. Rev. Biochem. 78, 929–958 10.1146/annurev.biochem.77.032207.120833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karsdal, M.A. (2016) Introduction. In Biochemistry of Collagens, Laminins and Elastin. Structure, Function and Biomarkers, (Karsdal, M.A., ed.), pp. 197–201, Academic Press; 10.1016/B978-0-12-809847-9.02001-8 [DOI] [Google Scholar]
- 46.Pollard, T.D., Earnshaw, W.C., Lippincott-Schwartz, J. and Johnson, G.T. (2017) Extracellular matrix molecules. In Cell Biology, Cambridge, MA (US), 3rd edn, pp. 505–524, Elsevier, Amsterdam, Netherlands; 10.1016/b978-0-323-34126-4.00029-3 [DOI] [Google Scholar]
- 47.Savoji, H., Godau, B., Hassani, M.S. and Akbari, M. (2018) Skin tissue substitutes and biomaterial risk assessment and testing. Front. Bioeng. Biotechnol. 6, 86 10.3389/fbioe.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aldana, P.C. and Khachemoune, A. (2020) Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am. J. Clin. Dermatol. 21, 255–264 10.1007/s40257-019-00495-x [DOI] [PubMed] [Google Scholar]
- 49.Notodihardjo, S.C., Morimoto, N., Munisso, M.C., Le, T.M., Mitsui, T., Kakudo, N.et al. (2020) A comparison of the wound healing process after the application of three dermal substitutes with or without basic fibroblast growth factor impregnation in diabetic mice. J. Plast Reconstr. Aesthetic. Surg. 73, 1547–1555 10.1016/j.bjps.2020.01.031 [DOI] [PubMed] [Google Scholar]
- 50.De Francesco, F., Busato, A., Mannucci, S., Zingaretti, N., Cottone, G., Amendola, F.et al. (2020) Artificial dermal substitutes for tissue regeneration: comparison of the clinical outcomes and histological findings of two templates. J. Int. Med. Res. 48, 1–22 10.1177/0300060520945508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalla Paola, L., Cimaglia, P., Carone, A., Boscarino, G. and Scavone, G. (2021) Use of integra dermal regeneration template for limb salvage in diabetic patients with no-option critical limb ischemia. Int. J. Low Extrem Wounds 20, 128–134 10.1177/1534734620905741 [DOI] [PubMed] [Google Scholar]
- 52.Hicks, C.W., Zhang, G.Q., Canner, J.K., Mathioudakis, N., Coon, D., Sherman, R.L.et al. (2020) Outcomes and predictors of wound healing among patients with complex diabetic foot wounds treated with a dermal regeneration template (integra). Plast Reconstr. Surg. 146, 893–902 10.1097/PRS.0000000000007166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alnababtah, K. (2010) Management of the deeper wound with Integra® tissue regenerative products. Wounds UK 6, 72–76 [Google Scholar]
- 54.Witherel, C.E., Graney, P.L., Freytes, D.O., Weingarten, M.S. and Spiller, K.L. (2016) Response of human macrophages to wound matrices in vitro. Wound Repair Regen. 24, 514–524 10.1111/wrr.12423 [DOI] [PubMed] [Google Scholar]
- 55.Tan, G.K. and Tabata, Y. (2014) Chondroitin-6-sulfate attenuates inflammatory responses in murine macrophages via suppression of NF-κB nuclear translocation. Acta Biomater. 10, 2684–2692 10.1016/j.actbio.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 56.Moiemen, N.S., Vlachou, E., Staiano, J.J., Thawy, Y. and Frame, J.D. (2006) Reconstructive surgery with integra dermal regeneration template: histologic study, clinical evaluation, and current practice. Plast Reconstr. Surg. 117, 160S–174S 10.1097/01.prs.0000222609.40461.68 [DOI] [PubMed] [Google Scholar]
- 57.Karnik, T., Dempsey, S.G., Jerram, M.J., Nagarajan, A., Rajam, R., May, B.C.H.et al. (2019) Ionic silver functionalized ovine forestomach matrix - a non-cytotoxic antimicrobial biomaterial for tissue regeneration applications. Biomater. Res. 23. 10.1186/s40824-019-0155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capella-Monsonís, H., Tilbury, M.A., Wall, J.G. and Zeugolis, D.I. (2020) Porcine mesothelium matrix as a biomaterial for wound healing applications. Mater. Today Bio 7, 100057 10.1016/j.mtbio.2020.100057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aroa Biosurgery Ltd. (2018) Endoform Antimicrobial Dermal Template: Instructions for Use. https://aroabio.com/wp-content/uploads/2021/03/PI.9090.03-EAG-Antimicrobial-IFU.pdf (Accessed 6 Oct 2020)
- 60.Aroa Biosurgery Ltd. (2018) Endoform(R) Antimicrobial Dermal Template, Aroa Biosurgery Ltd., San Diego, CA https://aroabio.com/wp-content/uploads/2021/03/Endoform%C2%AE-AM-brochure.pdf (Accessed 6 Oct 2020)
- 61.Liden, B.A. and May, B.C.H. (2013) Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv. Skin Wound Care 26, 164–167 10.1097/01.ASW.0000428862.34294.d4 [DOI] [PubMed] [Google Scholar]
- 62.Coutts, P.M., Ryan, J. and Sibbald, R.G. (2014) Case series of lower-extremity chronic wounds managed with an antibacterial foam dressing bound with gentian violet and methylene blue. Adv. Ski Wound Care 27, 9–13 10.1097/01.ASW.0000443270.71030.71 [DOI] [PubMed] [Google Scholar]
- 63.Raizman, R., Hill, R. and Woo, K. (2020) Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv. Skin Wound Care 33, 437–444 10.1097/01.ASW.0000667052.74087.d6 [DOI] [PubMed] [Google Scholar]
- 64.Lun, S., Irvine, S.M., Johnson, K.D., Fisher, N.J., Floden, E.W., Negron, L.et al. (2010) A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials 31, 4517–4529 10.1016/j.biomaterials.2010.02.025 [DOI] [PubMed] [Google Scholar]
- 65.Hill, R. and Woo, K. (2020) Utilization of advanced antimicrobial extracellular matrix technology within the framework of antimicrobial stewardship
- 66.Samsell, B., McLean, J., Cazzell, S., Dorsch, K., Moyer, P.M. and Moore, M. (2019) Health economics for treatment of diabetic foot ulcers: a cost-effectiveness analysis of eight skin substitutes. J. Wound Care 28, S14–S26 10.12968/jowc.2019.28.Sup9.S14 [DOI] [PubMed] [Google Scholar]
- 67.Westgate, S., Cutting, K.F., DeLuca, G. and Asaad, K. (2012) Collagen dressings made easy. Wounds UK 8, 1–4 [Google Scholar]
- 68.Rangaraj, A., Harding, K. and Leaper, D. (2011) Role of collagen in wound management. Wounds UK 7, 54–63 [Google Scholar]
- 69.Pallaske, F., Pallaske, A., Herklotz, K. and Boese-Landgraf, J. (2018) The significance of collagen dressings in wound management: a review. J. Wound Care 27, 692–702 10.12968/jowc.2018.27.10.692 [DOI] [PubMed] [Google Scholar]
- 70.Gould, L.J. (2016) Topical collagen-based biomaterials for chronic wounds: rationale and clinical application. Adv. Wound Care 5, 19–31 10.1089/wound.2014.0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romanelli, M., Mulder, G., Paggi, B., Macchia, M., Panduri, S. and Dini, V. (2015) The use of a collagen matrix in hard-to-heal venous leg ulcers. J. Wound Care 24, 543–547 10.12968/jowc.2015.24.11.543 [DOI] [PubMed] [Google Scholar]
- 72.Holmes, C., Wrobel, J., Mac Eachern, M.P. and Boles, B.R. (2013) Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: a systematic review. Diabetes Metab. Syndr. Obes.: Targets Ther. 6, 17 10.2147/DMSO.S36024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chattopadhyay, S. and Raines, R.T. (2014) Review collagen-based biomaterials for wound healing. Biopolymers 101, 821–833 10.1002/bip.22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. https://www.smith-nephew.com/professional/products/advanced-wound-management/biostep/ Smith+Nephew. BIOSTEP Collagen Matrix Dressing n.d. (accessed February 9, 2021)
- 75.Finnegan, S. and Percival, S.L. (2015) EDTA: an antimicrobial and antibiofilm agent for use in wound care. Adv Wound Care 4, 415–421 10.1089/wound.2014.0577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.BSN medical Inc. (2016) Cutimed® Epiona In-Market Evaluation. BSN medical Inc., Charlotte, NC https://medical.essityusa.com/fileadmin/z-countries/United_States/PDF/Epiona_Eval_Study_Clean.pdf
- 77.BSN Medical Inc. (2015) Cutimed® Epiona Clinical Study. BSN medical Inc., Charlotte, NC https://medical.essityusa.com/fileadmin/z-countries/United_States/PDF/62460_RN_Fb_CLEAN.pdf
- 78.Gopi, S., Balakrishnan, P., Chandradhara, D., Poovathankandy, D. and Thomas, S. (2019) General scenarios of cellulose and its use in the biomedical field. Mater. Today Chem. 13, 59–78 10.1016/j.mtchem.2019.04.012 [DOI] [Google Scholar]
- 79.Sindhu, K.A., Prasanth, R. and Thakur, V.K. (2014) Medical Applications of Cellulose and its Derivatives: Present and Future. In Nanocellulose Polym. Nanocomposites (Vijay K.T., ed), vol. 9781118871904, pp. 437–477, John Wiley & Sons, Inc., Hoboken, NJ: 10.1002/9781118872246.ch16 [DOI] [Google Scholar]
- 80.Mudge, M.C. (2012) Hemostasis, surgical bleeding, and transfusion. In Equine Surgery (Jörg A.A. and John A.S., eds), pp. 35–47, Elsevier Inc., Amsterdam, The Netherlands 10.1016/B978-1-4377-0867-7.00004-1 [DOI] [Google Scholar]
- 81.Resnik, R.R. (2018) Intraoperative Complications. In Misch's Avoiding Complications in Oral Implantology (Randolph R.R. and Carl E.M., eds), pp. 267–293, Elsevier, Amsterdam, The Netherlands 10.1016/B978-0-323-37580-1.00007-X [DOI] [Google Scholar]
- 82.Cullen, B., Watt, P.W., Lundqvist, C., Silcock, D., Schmidt, R.J., Bogan, D.et al. (2002) The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int. J. Biochem. Cell Biol. 34, 1544–1556 10.1016/S1357-2725(02)00054-7 [DOI] [PubMed] [Google Scholar]
- 83.Cullen, B., Smith, R., Mcculloch, E., Silcock, D. and Morrison, L. (2002) Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 10, 16–25 10.1046/j.1524-475X.2002.10703.x [DOI] [PubMed] [Google Scholar]
- 84.Metzmacher, I., Ruth, P., Abel, M. and Friess, W. (2007) In vitro binding of matrix metalloproteinase-2 (MMP-2), MMP-9, and bacterial collagenase on collagenous wound dressings. Wound Repair Regen. 15, 549–555 10.1111/j.1524-475X.2007.00263.x [DOI] [PubMed] [Google Scholar]
- 85.Gottrup, F., Cullen, B.M., Karlsmark, T., Bischoff-Mikkelsen, M., Nisbet, L. and Gibson, M.C. (2013) Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen. 21, 216–225 10.1111/wrr.12020 [DOI] [PubMed] [Google Scholar]
- 86.Xiao, Y., Reis, L.A., Zhao, Y. and Radisic, M. (2015) Modifications of collagen-based biomaterials with immobilized growth factors or peptides. Methods 84, 44–52 10.1016/j.ymeth.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 87.Xiao, Y., Reis, L.A., Feric, N., Knee, E.J., Gu, J., Cao, S.et al. (2016) Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc. Natl Acad. Sci. U.S.A. 113, E5792–E5801 10.1073/pnas.1612277113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sparks, H.D., Sigaeva, T., Tarraf, S., Mandla, S., Pope, H., Hee, O.et al. (2020) Biomechanics of wound healing in an equine limb model: effect of location and treatment with a peptide-modified collagen–chitosan hydrogel. ACS Biomater. Sci. Eng. 7, 265–278 10.1021/acsbiomaterials.0c01431 [DOI] [PubMed] [Google Scholar]
- 89.Nardini, M., Perteghella, S., Mastracci, L., Grillo, F., Marrubini, G., Bari, E.et al. (2020) Growth factors delivery system for skin regeneration: an advanced wound dressing. Pharmaceutics 12, 120 10.3390/pharmaceutics12020120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.do Amaral, R.J.F.C., Zayed, N.M.A., Pascu, E.I., Cavanagh, B., Hobbs, C., Santarella, F.et al. (2019) Functionalising collagen-based scaffolds with platelet-rich plasma for enhanced skin wound healing potential. Front. Bioeng. Biotechnol. 7, 371 10.3389/fbioe.2019.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsang, K.K., Kwong, E.W.Y., Woo, K.Y., To, T.S.S., Chung, J.W.Y. and Wong, T.K.S. (2015) The anti-inflammatory and antibacterial action of nanocrystalline silver and manuka honey on the molecular alternation of diabetic foot ulcer: a comprehensive literature review. Evid. Based Complement Altern. Med. 2015, 218283 10.1155/2015/218283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao, D., Yu, S., Sun, B., Gao, S., Guo, S. and Zhao, K. (2018) Biomedical applications of chitosan and its derivative nanoparticles. Polymers (Basel) 10, 462 10.3390/polym10040462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kontogiannopoulos, K.N., Assimopoulou, A.N., Tsivintzelis, I., Panayiotou, C. and Papageorgiou, V.P. (2011) Electrospun fiber mats containing shikonin and derivatives with potential biomedical applications. Int. J. Pharm. 409, 216–228 10.1016/j.ijpharm.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 94.Mogoşanu, G.D. and Grumezescu, A.M. (2014) Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 463, 127–136 10.1016/j.ijpharm.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 95.Dowsett, C. (2017) Using TLC-NOSF advanced wound dressing to improve outcomes for patients with leg and diabetic foot ulcers. Wounds UK 13, 113–117 [Google Scholar]
- 96.Schmutz, J.-L., Meaume, S., Fays, S., Ourabah, Z., Guillot, B., Thirion, V.et al. (2008) Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. Int. Wound J. 5, 172–182 10.1111/j.1742-481X.2008.00453.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White, R., Cowan, T. and Glover, D. (2011) Supporting evidence based practice: clinical review of TLC healing matrix. J. Wound Care 24, S29–S36 [Google Scholar]
- 98.Augustin, M., Herberger, K., Kroeger, K., Muenter, K.C., Goepel, L. and Rychlik, R. (2016) Cost-effectiveness of treating vascular leg ulcers with UrgoStart® and UrgoCell® Contact. Int. Wound J. 13, 82–87 10.1111/iwj.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lázaro-Martínez, J.L., Edmonds, M., Rayman, G., Apelqvist, J., Van Acker, K., Hartemann, A.et al. (2019) Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of explorer. J. Wound Care 28, 358–367 10.12968/jowc.2019.28.6.358 [DOI] [PubMed] [Google Scholar]
- 100.Sigal, M.-L., Addala, A., Maillard, H., Chahim, M., Sala, F., Blaise, S.et al. (2019) Evaluation of TLC-NOSF dressing with poly-absorbent fibres in exuding leg ulcers: two multicentric, single-arm, prospective, open-label clinical trials. J. Wound Care 28, 164–175 10.12968/jowc.2019.28.3.164 [DOI] [PubMed] [Google Scholar]
- 101. https://www.nice.org.uk/guidance/mtg42/resources/urgostart-for-treating-diabetic-foot-ulcers-and-leg-ulcers-pdf-64372052418757 National Institute For Health and Clinical Excellence. Medical technologies guidance (MTG42). UrgoStart for treating diabetic foot ulcers and leg ulcers. 2019, NICE, London.
- 102.Ong, C.T., Zhang, Y., Lim, R., Samsonraj, R., Masilamani, J., Phan, T.H.H.et al. (2015) Preclinical evaluation of TegadermTM supported nanofibrous wound matrix dressing on porcine wound healing model. Adv. Wound Care 4, 110–118 10.1089/wound.2014.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leveriza-Oh, M. and Phillips, T.J. (2012) Dressings and postoperative care. In Lower Extremity Soft Tissue & Cutaneous Plastic Surgery, 2nd edn., pp. 471–488, Elsevier Ltd. 10.1016/B978-0-7020-3136-6.00032-1 [DOI] [Google Scholar]
- 104.Ahmed, E.M. (2015) Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 6, 105–121 10.1016/j.jare.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caló, E. and Khutoryanskiy V, V. (2015) Biomedical applications of hydrogels: a review of patents and commercial products. Eur. Polym. J. 65, 252–267 10.1016/j.eurpolymj.2014.11.024 [DOI] [Google Scholar]
- 106. https://bnf.nice.org.uk/wound-management/hydrocolloid-dressings.html Hydrocolloid dressings | Wound management | BNF content published by NICE n.d. (accessed January 12, 2021)
- 107.Aderibigbe, B.A. and Buyana, B. (2018) Alginate in wound dressings. Pharmaceutics 10, 42 10.3390/pharmaceutics10020042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang, M. and Zhao, X. (2020) Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 162, 1414–1428 10.1016/j.ijbiomac.2020.07.311 [DOI] [PubMed] [Google Scholar]
- 109.Ivins, N. and Naude, L. (2017) Gelling fibre dressing for moderate to highly exuding wounds shows promising exudate management - Wounds International. Wounds Int. 8, 47–51 [Google Scholar]
- 110. https://www.woundsource.com/product-category/dressings/gelling-fiber-dressings Gelling Fiber Wound Dressings | WoundSource n.d. (accessed January 26, 2021)
- 111.Probst, S., Saini, C. and Skinner, M.B. (2019) Comparison of sterile polyacrylate wound dressing with activated carbon cloth and a standard non-adhesive hydrocellular foam dressing with silver: a randomised controlled trial protocol. J. Wound Care 28, 722–728 10.12968/jowc.2019.28.11.722 [DOI] [PubMed] [Google Scholar]
- 112.Browning, P., White, R.J. and Rowell, T. (2016) Comparative evaluation of the functional properties of superabsorbent dressings and their effect on exudate management. J. Wound Care 25, 452–462 10.12968/jowc.2016.25.8.452 [DOI] [PubMed] [Google Scholar]
- 113.Barrett, S., Rippon, M. and Rogers, A.A. (2020) Treatment of 52 patients with a self-adhesive siliconised superabsorbent dressing: a multicentre observational study. J. Wound Care 29, 340–349 10.12968/jowc.2020.29.6.340 [DOI] [PubMed] [Google Scholar]
- 114.Ciecholewska-Juśko, D., Żywicka, A., Junka, A., Drozd, R., Sobolewski, P., Migdał, P.et al. (2021) Superabsorbent crosslinked bacterial cellulose biomaterials for chronic wound dressings. Carbohydr. Polym. 253, 117247 10.1016/j.carbpol.2020.117247 [DOI] [PubMed] [Google Scholar]
- 115.Beers, P.J., Adgerson, C.N. and Millan, S.B. (2016) Porcine tri-layer wound matrix for the treatment of stage IV pressure ulcers. JAAD Case Rep. 2, 122–124 10.1016/j.jdcr.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brown-Etris, M., Milne, C.T. and Hodde, J.P. (2019) An extracellular matrix graft (Oasis ® wound matrix) for treating full-thickness pressure ulcers: a randomized clinical trial. J. Tissue Viability 28, 21–26 10.1016/j.jtv.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 117.Guest, J.F., Weidlich, D., Singh, H., La Fontaine, J., Garrett, A., Abularrage, C.J.et al. (2017) Cost-effectiveness of using adjunctive porcine small intestine submucosa tri-layer matrix compared with standard care in managing diabetic foot ulcers in the US. J. Wound Care 26, S12–S24 10.12968/jowc.2017.26.Sup1.S12 [DOI] [PubMed] [Google Scholar]
- 118.Sabolinski, M.L. and Capotorto J, V. (2019) Comparative effectiveness of a human fibroblast-derived dermal substitute and a viable cryopreserved placental membrane for the treatment of diabetic foot ulcers. J. Comp. Eff. Res. 8, 1229–1238 10.2217/cer-2019-0001 [DOI] [PubMed] [Google Scholar]
- 119.Treadwell, T., Sabolinski, M.L., Skornicki, M. and Parsons, N.B. (2018) Comparative effectiveness of a bioengineered living cellular construct and cryopreserved cadaveric skin allograft for the treatment of venous leg ulcers in a real-world setting. Adv. Wound Care 7, 69–76 10.1089/wound.2017.0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zaulyanov, L. and Kirsner, R.S. (2007) A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2, 93–98 10.2147/ciia.2007.2.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stone, R.C., Stojadinovic, O., Rosa, A.M., Ramirez, H.A., Badiavas, E., Blumenberg, M.et al. (2017) A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci. Transl. Med. 9, eaaf8611 10.1126/scitranslmed.aaf8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stone, R.C., Stojadinovic, O., Sawaya, A.P., Glinos, G.D., Lindley, L.E., Pastar, I.et al. (2020) A bioengineered living cell construct activates metallothionein/zinc/MMP8 and inhibits TGFβ to stimulate remodeling of fibrotic venous leg ulcers. Wound Repair Regen. 28, 164–176 10.1111/wrr.12778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Organogenesis Inc. Apligraf® n.d.. https://apligraf.com/apligraf-overview/ (accessed February 9, 2021)
- 124.Hart, J., Silcock, D., Gunnigle, S., Cullen, B., Light, N.D. and Watt, P.W. (2002) The role of oxidised regenerated cellulose/collagen in wound repair: effects in vitro on fibroblast biology and in vivo in a model of compromised healing. Int. J. Biochem. Cell Biol. 34, 1557–1570 10.1016/S1357-2725(02)00062-6 [DOI] [PubMed] [Google Scholar]
- 125.Covalon Technologies Ltd. (2018) ColActive ®PLUS Collagen Matrix Dressings with Silver, Covalon Technologies Ltd., Missisagua, ON https://static1.squarespace.com/static/572233bd40261d210f271f97/t/5ff3723bb6dc0c1e6118384e/1609790011903/ColActive+Plus+Product+Information+CP-U-2.pdf
- 126.Salehi, H., Momeni, M., Ebrahimi, M., Fatemi, M.J., Rahbar, H., Ranjpoor, F.et al. (2018) Comparing the effect of colactive plus ag dressing versus nitrofurazone and vaseline gauze dressing in the treatment of second-degree burns. Ann. Burns Fire Disasters 31, 204–208 PMID: [PMC free article] [PubMed] [Google Scholar]
- 127.Karr, J.C., Taddei, A.R., Picchietti, S., Gambellini, G., Fausto, A.M. and Giorgi, F. (2011) A morphological and biochemical analysis comparative study of the collagen products biopad, promogram, puracol, and colactive. Adv. Skin Wound Care 24, 208–216 10.1097/01.ASW.0000397897.18003.ce [DOI] [PubMed] [Google Scholar]
- 128.Wiegand, C., Eberlein, T. and Andriessen, A. (2017) Antibacterial activity of polihexanide formulations in a co-culture of HaCaT keratinocytes and Staphylococcus aureus and at different pH levels. Wound Repair Regen. 25, 423–431 10.1111/wrr.12528 [DOI] [PubMed] [Google Scholar]
- 129.Fumarola, S., Butcher, M., Cooper, P., Gray, D., Russell, F., Stringfellow, S.et al. (2010) A clinical audit of Suprasorb® X + PHMB. Wounds UK 6(3), 78–87 [Google Scholar]
- 130.de Mattos, I.B., Holzer, J.C.J., Tuca, A.-C., Groeber-Becker, F., Funk, M., Popp, D.et al. (2019) Uptake of PHMB in a bacterial nanocellulose-based wound dressing: a feasible clinical procedure. Burns 45, 898–904 10.1016/j.burns.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 131. L&R Medical UK Ltd. Suprasorb ®X + PHMB Antimicrobial hydrobalance wound dressing. n.d.
- 132.Lenselink, E. and Andriessen, A. (2011) A cohort study on the efficacy of a polyhexanide-containing biocellulose dressing in the treatment of biofilms in wounds. J. Wound Care 20, 534–539 10.12968/jowc.2011.20.11.534 [DOI] [PubMed] [Google Scholar]
- 133.Gibbons, G.W. (2015) Grafix ®, a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Adv. Wound Care 4, 534–544 10.1089/wound.2015.0647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Duan-Arnold, Y., Gyurdieva, A., Johnson, A., Uveges, T.E., Jacobstein, D.A. and Danilkovitch, A. (2015) Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv. Wound Care 4, 523–533 10.1089/wound.2015.0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Frykberg, R.G., Gibbons, G.W., Walters, J.L., Wukich, D.K. and Milstein, F.C. (2017) A prospective, multicentre, open-label, single-arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of viable cryopreserved human placental membrane. Int. Wound J. 14, 569–577 10.1111/iwj.12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lavery, L.A., Fulmer, J., Shebetka, K.A., Regulski, M., Vayser, D., Fried, D.et al. (2014) The efficacy and safety of Grafix ® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int. Wound J. 11, 554–560 10.1111/iwj.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sood, A., Granick, M.S. and Tomaselli, N.L. (2014) Wound dressings and comparative effectiveness data. Adv. Wound Care 3, 511–529 10.1089/wound.2012.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Senet, P., Bause, R., Jørgensen, B. and Fogh, K. (2014) Clinical efficacy of a silver-releasing foam dressing in venous leg ulcer healing: a randomised controlled trial. Int. Wound J. 11, 649–655 10.1111/iwj.12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coloplast Ltd. (2015) Material Safety Data Sheet Biatain Ag https://www.coloplast.ca/Global/Canada/Documents/SDS%20Wound%20ENG/SDS%20-%20Biatain%20Ag%20Adhesive%20-%20ENG.pdf (Accessed 18 Jan 2021)
- 140.Coloplast Ltd. Silver wound dressing | Biatain® Ag Non-Adhesive n.d. https://www.coloplast.co.uk/biatain-ag-non-adhesive-en-gb.aspx#section=product-description_3 (accessed January 28, 2021)
- 141.Leaper, D., Münter, C., Meaume, S., Scalise, A., Mompó, N.B., Jakobsen, B.P.et al. (2013) The use of biatain Ag in hard-to-heal venous leg ulcers: meta-analysis of randomised controlled trials. PLoS One 8, e67083. 10.1371/journal.pone.0067083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hart, C.E., Loewen-Rodriguez, A. and Lessem, J. (2012) Dermagraft: use in the treatment of chronic wounds. Adv. Wound Care 1, 138–141 10.1089/wound.2011.0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marston, W.A., Hanft, J. and Norwood, P. (2003) The Efficacy and Safety of Dermagraft in Improving the Healing of Chronic Diabetic Foot Ulcers Results of a prospective randomized trial. Diabetes Care 26, 1701–1705 [DOI] [PubMed] [Google Scholar]
- 144.Marston, W.A. (2004) Dermagraft®, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev. Med. Devices 1, 21–31 10.1586/17434440.1.1.21 [DOI] [PubMed] [Google Scholar]
- 145.Martinson, M. and Martinson, N. (2016) Comparative analysis of skin substitutes used in the management of diabetic foot ulcers. J. Wound Care 25, S8–17 10.12968/jowc.2016.25.Sup10.S8 [DOI] [PubMed] [Google Scholar]
- 146. https://dermagraft.com/why-choose-dermagraft/ Organogenesis Inc. Dermagraft® n.d. (accessed February 9, 2021)