Abstract
The deregulation of apoptosis is a key contributor to tumourigenesis as it can lead to the unwanted survival of rogue cells. Drugs known as the BH3-mimetics targeting the pro-survival members of the BCL-2 protein family to induce apoptosis in cancer cells have achieved clinical success for the treatment of haematological malignancies. However, despite our increasing knowledge of the pro-survival factors mediating the unwanted survival of solid tumour cells, and our growing BH3-mimetics armamentarium, the application of BH3-mimetic therapy in solid cancers has not reached its full potential. This is mainly attributed to the need to identify clinically safe, yet effective, combination strategies to target the multiple pro-survival proteins that typically mediate the survival of solid tumours. In this review, we discuss current and exciting new developments in the field that has the potential to unleash the full power of BH3-mimetic therapy to treat currently recalcitrant solid malignancies.
Keywords: apoptosis, BCL-2, BH3-mimetics, cancer
Introduction
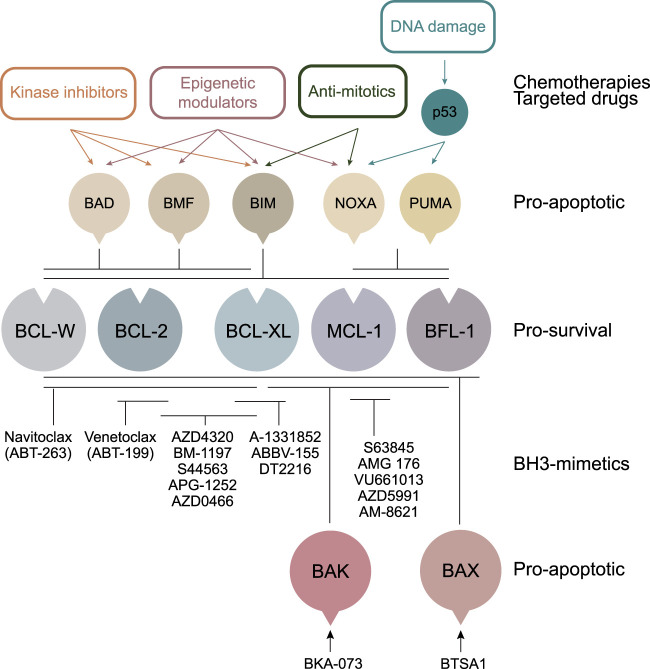
Drugs known as the ‘BH3-mimetics’ trigger cell death by antagonising the action of the pro-survival members of the BCL-2 protein family (Figure 1). These proteins, which include BCL-2, BCL-XL, MCL-1, BCL-W and BFL-1, are key negative regulators of the intrinsic apoptosis pathway which is deregulated in most, if not all cancers. Pro-survival BCL-2 proteins also contribute to resistance to standard treatments such as chemo- and radiotherapies that act through cell death induction (Figure 1). The BH3-mimetics are so-called because they mimic the natural ligands for pro-survival proteins — the pro-apoptotic BH3-only proteins. As with BH3-only proteins, binding of BH3-mimetics to their pro-survival targets leads to apoptosis induction via two mechanisms: (1) the displacement of prebound pro-apoptotic BH3-only proteins to activate BAX or BAK, and (2) through the release of activated BAX or BAK themselves [1,2]. This latter mechanism is sufficient for apoptosis induction by BH3-mimetics as the absence of BH3-only proteins in cells does not negate their killing activity [2,3]. The discovery of how these ligands bind their targets in the late 1990s inspired the development of their small molecule counterparts. However, it was not until 2016 that the first of these drugs, Venetoclax which specifically targets BCL-2, was granted FDA approval for relapsed chronic lymphocytic leukaemia (CLL) patients with 17p deletion.
Figure 1. Anti-cancer drugs often work by the induction of apoptosis.
Many anti-cancer drugs exert their cytotoxic effects by indirectly up-regulating the pro-apoptotic BH3-only proteins or by decreasing the expression of the pro-survival BCL-2 proteins. In contrast, BH3-mimetics directly antagonise the pro-survival BCL-2 proteins, bypassing defects in upstream signalling pathways such as those mediated by p53, which is commonly inactivated in cancers. Direct activators of BAX or BAK have also been developed as an alternate mechanism for inducing apoptosis.
Venetoclax has now been approved as standard-of-care treatment for CLL in combination with Rituximab or Obinutuzumab depending on the treatment status of the patient [4,5], with chemotherapies in acute myeloid leukaemia (AML) [6], and is undergoing >230 clinical trials in a variety of haematological cancers. However, the clinical use of Venetoclax and other BH3-mimetics in solid cancer treatment is not nearly as well-advanced. In this review, we primarily discuss the contributions of BCL-2 pro-survival proteins in solid cancers and the challenges that remain in the application of BH3-mimetics for their treatment.
Pro-survival protein dependencies in solid cancers: single-agent BH3-mimetic applications
BCL-XL
Prior to Venetoclax, Navitoclax was the first BH3-mimetic to enter clinical trials, binding with high affinity to BCL-2, BCL-XL and weaker to BCL-W [7]. In pre-clinical studies, Navitoclax monotherapy elicited durable tumour regression in small cell lung cancer (SCLC) comparable to clinically approved cytotoxic agents [7–9], and enhanced the in vivo activity of multiple therapeutic agents, including anti-mitotics and DNA-damaging agents in solid cancers including non-small cell lung (NSCLC), breast, mesothelioma and ovarian cancer [8,10–13]. Other BCL-XL and BCL-2 dual inhibitors developed subsequently (e.g. AZD4320, BM-1197, S44563, APG-1252) also demonstrated similar long-lasting tumour regression in SCLC, gastric and uveal melanoma xenograft models [14–16].
Further studies demonstrated that responses to Navitoclax (or its predecessor ABT-737 with an identical pro-survival binding profile) in solid cancers was mainly due to inhibition of BCL-XL and not BCL-2 [11]. This is consistent with the amplification of the chromosomal region, encompassing the BCLX gene, found in many solid tumours [17]. Further studies have now validated BCL-XL as the critical survival factor for a range of solid cancers including colorectal, bladder, pancreatic and cervical cancers [18–21].
Due to the early promise seen with targeting BCL-XL in solid cancers, a programme to develop BCL-XL-selective inhibitors was initiated. The first orally bioavailable BCL-XL-selective inhibitor A-1331852 demonstrated modest single-agent activity in NSCLC, mesothelioma, breast, ovarian and colorectal cancer xenograft models [8,11,22]. However, combinations with chemotherapies (e.g. docetaxel or irinotecan) led to significant increases in the amplitude and durability of responses [11,22].
Whilst the pre-clinical data supporting BCL-XL targeting in solid cancers is compelling, its inhibition also causes significant thrombocytopaenia due to the dependence of platelets on BCL-XL for their survival [23]. This dose-limiting toxicity resulted in several trials with Navitoclax being halted and probably underlines why A-1331852 has yet to enter the clinic. It also fuelled the development of BCL-2-selective inhibitors like Venetoclax for use in cancers reliant on BCL-2, circumventing the thrombocytopaenia arising from BCL-XL inhibition [24].
MCL-1
Like BCL-XL, focal amplification of the MCL1-containing chromosomal region occurs in ∼11% of solid cancers [17] including NSCLC [25–27] and melanoma [28]. Gene knockdown studies proved that amplified MCL-1 enabled the survival of these tumours [17,27,29]. MCL-1 is also highly expressed in most breast cancer subtypes, for example, triple-negative and HER2-amplified breast cancers [30–32], enabling tumour cell survival and chemoresistance. Its overexpression also correlates with high tumour grade and poor patient survival [33,34].
Several MCL-1 antagonists have now been successfully developed [35,36] with S63845 being the first applicable for in vivo use [37]. This was followed by several others including AMG 176, VU661013, AZD5991 and AM-8621 [38–41]. As with single-agent BCL-XL inhibition, MCL-1 inhibitors used as monotherapy are only marginally effective in solid tumour-derived cell lines [37] such as patient-derived xenograft (PDX) models of breast cancer [31] and KRAS-mutant NSCLC [40]. However, co-treatment of S63845 with oncogenic kinase inhibitors (e.g. Lapatinib, Tarceva, Trametinib) or other chemotherapeutic agents (e.g. docetaxel, trastuzumab) enhances cytotoxic responses [31,37,40], emphasising the need for prioritisation of BH3-mimetic combination therapies in the clinic, which will be discussed further below.
BCL-2
Although usually associated with haematological cancers, amplification of the BCL2 locus on chromosome 18q21 leads to BCL-2 overexpression and occurs in up to 80% of SCLC [42,43]. In pre-clinical studies, Navitoclax/ABT-737 demonstrated activity in SCLC cell lines and xenograft models [9,44] warranting entry into Phase I/II clinical trials. However, outcomes were disappointing [45,46] and this failure was attributed to the dose-limiting thrombocytopaenia from BCL-XL co-inhibition, restraining the magnitude of BCL-2 inhibition achievable. Accordingly, Venetoclax was investigated for SCLC treatment [47] as BCL-2 expression was a predictive biomarker for Venetoclax responsiveness. Venetoclax, in combination with chemotherapeutic agents, entered clinical trials for SCLC (NCT04422210, NCT04543916) but these studies were halted citing strategic prioritisation and broader development.
In addition to SCLC, ∼20% of MYCN-amplified neuroblastoma cells are highly sensitive to Venetoclax [48]. BCL-2 is expressed significantly higher in neuroblastoma cells compared with other solid cancer lines, whilst BCL-XL expression is reduced, consistent with their responsiveness to Venetoclax and Navitoclax when MYCN is amplified [48–51]. In breast cancer, BCL-2 is an estrogen-responsive gene overexpressed in ∼85% of estrogen receptor (ER)-positive breast cancers [52]. Therefore, when Venetoclax was combined with endocrine therapy central to the management of ER-positive breast cancer, marked improvements in tumour response in ER-positive PDX models were observed [53]. These findings validated BCL-2 as a therapeutic target for the treatment of ER-positive breast cancer and led to the very first clinical study with Venetoclax for a solid cancer. Critically, the combination of Veneoclax with endocrine therapy had a tolerable safety profile with notable activity in ER- and BCL-2-positive metastatic breast cancer in a Phase Ib study [54].
Whilst BCL-2 targeting has achieved remarkable clinical success in haematological malignancies, these results demonstrate the potential of targeting this eponymous member of the BCL-2 family for the treatment of solid cancers.
BCL-W and BFL-1
Whilst BCL-XL, MCL-1 and BCL-2 have received most of the attention in oncology drug development, the lesser discussed pro-survival BCL-2 proteins, BCL-W and BFL-1, also have important roles in cancer. Elevated levels of BFL-1 have been primarily described in blood cancers [55,56]. In solid tumours, aberrant BFL-1 expression has been documented in the stomach [57] and breast cancers [58,59] as well as malignant melanoma, though its requirement for melanoma cell survival varies between studies [60–66]. As with BFL-1, BCL-W is highly expressed in human B-cell lymphomas [67,68]. In solid malignancies, deregulation of BCL-W expression has been reported in bladder cancer, colorectal carcinoma and lung cancer [69–71].
Importantly, BCL-W and BFL-1 are intrinsic resistance factors to BH3-mimetics targeting BCL-XL, MCL-1 and BCL-2 [72–76]. Currently, there are no known BFL-1- or BCL-W-specific inhibitors in development despite programmes that initially set out to achieve this [77,78]. In the absence of such direct inhibitors, molecules that indirectly modulate their levels could potentially be applied instead [73].
Targeting multiple pro-survival proteins with BH3-mimetics for solid cancer treatment
Although rare, acute sensitivity to single-agent BH3-mimetics does occur, especially in haematological cancers and some solid cancers, as discussed above. However, most solid tumours are dependent on more than one pro-survival protein which dictates BH3-mimetic efficacy. These co-dependencies are discussed below.
BCL-XL and MCL-1 co-targeting
Systematic studies using BH3-mimetic ‘parsing’ defined the pro-survival protein dependency of a large panel of cancer cell lines from ten tissues of origin and showed a striking co-dependency of ∼90% of these on BCL-XL and MCL-1 [20]. Notably, targeting this specific combination led to synergistic activity in ∼50% of solid tumour cell lines representing melanoma, breast, colorectal, brain, ovarian and pancreatic cancers. Multiple solid tumour-specific studies have similarly highlighted the effectiveness of co-targeting BCL-XL and MCL-1. In squamous cell lung carcinoma, dual inhibition of MCL-1 and BCL-XL induced synergistic tumour cell death, and when combined with fibroblast growth factor receptor (FGFR)-targeted therapy, produced durable treatment responses in FGFR1-overexpressing lung squamous cell carcinoma [79]. Similar results were also observed in malignant pleural mesothelioma, NSCLC and colorectal cancer [8,12,19,40,80,81]. In these cancers, BCL-XL is the dominant survival factor as its sole targeting had a greater effect compared with the targeting of MCL-1 alone. In breast cancer, BCL-XL serves as a resistance factor to MCL-1-specific inhibitors, further exemplifying the need to target both these pro-survival proteins for effective killing [82–84]. In malignant melanoma, prostate, and KRAS-mutant NSCLC, no one pro-survival protein appeared to govern their survival as targeting both MCL-1 and BCL-XL were highly efficacious [40,62,85]. Gene set enrichment studies have suggested that the shifting dependence of solid tumours on both BCL-XL and MCL-1 or just BCL-XL alone is associated with epithelial-mesenchymal transition (EMT) [20] where both proteins are required for survival of epithelial cells, but mesenchymal cells become BCL-XL dependent due to induction of NOXA, an MCL-1 antagonist.
BCL-2 and MCL-1 co-targeting
Whilst BCL-XL and MCL-1 co-targeting with BH3-mimetics appears to be the ‘magic formula’ for delivering the coup de grâce to most solid cancers, the inhibition of BCL-2 and MCL-1 with BH3-mimetics has also been shown to be effective in some cases. For example, in MYCN-amplified neuroblastoma detailed previously, MCL-1 mediates resistance to Venetoclax, where sensitivity depends on MYCN-regulated NOXA expression to antagonise MCL-1 [48,49,51]. Accordingly, agents that down-regulate MCL-1 (e.g. Aurora kinase A inhibitor MLN8237, Cyclophosphamide, MYC activators) sensitise MYCN-amplified neuroblastoma cells to Venetoclax, providing durable responses in xenograft models [48,51]. These promising pre-clinical results have now led to Phase I clinical trials in this highly resistant cancer (NCT03236857, [86]).
Whilst BCL-XL and MCL-1 antagonism in melanoma leads to synergistic killing, co-inhibition of MCL-1 and BCL-2 is also effective in vitro, albeit to a lesser extent [62], and in in vivo models (e.g with Venetoclax and the clinically applicable MCL-1 inhibitor S64315) [64]. Notably, BCL-2 expression in patients with BRAFwild-type melanomas, for which there is little in the way of targeted therapies, is higher compared with BRAFmutant melanomas [87].
Circumventing toxicities associated with BH3-mimetic combinations
The use of BH3-mimetic combinations to target multiple pro-survival proteins appears to be an obvious means to combating solid cancers. However, given the key and often compensatory roles these proteins play in non-malignant cells, co-inhibition of two or more pro-survival proteins potentiates the opportunity for associated toxicities. The simultaneous disarmament of BCL-XL and MCL-1 with BH3-mimetics in mice is lethal due to acute liver toxicity [12,79] as both these proteins are critical for hepatocyte survival [88]. These pro-survival proteins are also essential for megakaryocyte survival [89]. Likewise, the loss of a single allele each of BCL2 and MCL1 in mice leads to reduced organismal size due to a reduction in body cellularity [90]. Nevertheless, co-targeting BCL-2 (with Venetoclax) and MCL-1 (with S64315 or AMG176) is in dose-finding clinical studies for the treatment of haematological malignancies (NCT03672695, NCT03797261) [91]. However, the progression of this combination will rely on the evaluation of the cardiac toxicity signal that arose in Phase I trials with AMG176/AMG397 (NCT03465540, NCT02675452).
One avenue to circumvent these toxicities is to combine BH3-mimetics with agents that indirectly modulate the expression of BCL-2 family members in a tumour-specific manner — either by up-regulating pro-apoptotic (Figure 1) or down-regulating pro-survival proteins. The role of BCL-2 proteins as mediators of the cytotoxic response to chemotherapeutic agents is well-established [92]. Given the limited efficacy of BH3-mimetic monotherapy in solid tumours, the potential of combining BH3-mimetics with standard-of-care therapeutics has already been demonstrated as described above, and multiple clinical trials of BH3-mimetics combined with anti-cancer agents are in progress for the treatment of solid malignancies (Table 1).
Table 1. Some of the clinical trials conducted with BH3-mimetics in solid cancers.
| Disease | BH3-mimetic | Target | Chemotherapeutic agent | Clinical study | Status | Relevant references |
|---|---|---|---|---|---|---|
| ER+ breast cancer | Venetoclax | BCL-2 | Tamoxifen | ISRCTN98335443 | Closed | [53,54,155] |
| ER + HER2− breast cancer | Venetoclax | BCL-2 | Fulvestrant | NCT03584009 | Completed | [156] |
| HER2+ breast cancer | Venetoclax | BCL-2 | Trastuzumab Emtansine | NCT04298918, CO41863 | Terminated, Closed | |
| Neuroblastoma | Venetoclax | BCL-2 | Cyclophosphamide | NCT03236857, M13-833 | Recruiting | [49,51,86] |
| SCLC | Venetoclax | BCL-2 | Atezolizumab, Carboplatin, Etoposide | NCT04422210 | Terminated | [47] |
| SCLC | Venetoclax | BCL-2 | Irinotecan | NCT04543916 | Withdrawn | [157] |
| SCLC | Venetoclax | BCL-2 | ABBV-075 | NCT02391480 | Completed | [137,139] |
| SCLC, solid cancers | APG-1252 | BCL-XL, BCL-2, BCL-W | — | NCT03387332 | Recruiting | [16,158] |
| Solid cancers | Navitoclax | BCL-XL, BCL-2, BCL-W | Trametinib | NCT02079740 | Recruiting | [114,159] |
| Solid cancers | Navitoclax | BCL-XL, BCL-2, BCL-W | Gemcitabine | NCT00887757 | Completed | [160] |
| Solid cancers | Navitoclax | BCL-XL, BCL-2, BCL-W | Docetaxel | NCT00888108 | Completed | [161] |
| Solid cancers | Navitoclax | BCL-XL, BCL-2, BCL-W | Etoposide, Cisplatin | NCT008878449 | Completed | [162] |
| Solid cancers | Navitoclax | BCL-XL, BCL-2, BCL-W | Sorafenib | NCT02143401 | Active, not recruiting | [163] |
| Melanoma, solid cancers | Navitoclax | BCL-XL, BCL-2, BCL-W | Dabrafenib, Trametinib | NCT01989585 | Recruiting | [164] |
| Solid cancers | AZD0466 | BCL-XL, BCL-2 | — | NCT04214093 | Completed | [147] |
| Solid cancers | ABBV-155 | BCL-XL | Taxanes | NCT03595059 | Recruiting | [149] |
| Solid cancers | DT2216 | BCL-XL | — | NCT04886622 | Not yet recruiting | [150] |
BH3-mimetics and anti-mitotic agents
Systematic analysis of Navitoclax's capacity to enhance the activity of clinically relevant agents across a spectrum of solid tumours revealed the greatest synergy with anti-mitotic drugs such as Docetaxel and Paclitaxel [10]). This combination significantly improves responses in a range of solid cancers including NSCLC, ovarian, gastric and breast cancer [10,13,93,94]. As mentioned above, the Aurora Kinase A inhibitor MLN8237 that also induces mitotic arrest, enhances Venetoclax activity in MYCN-amplified neuroblastoma [48] and of Navitoclax in pancreatic adenocarcinoma [95].
The synergy seen with anti-mitotic agents is attributed to MCL-1 phosphorylation and its proteasomal degradation following mitotic arrest [96,97]. Anti-tubulin chemotherapeutics also result in BCL-XL phosphorylation causing its functional inactivation [98,99], or the up-regulation of pro-apoptotic BIM [100,101] or NOXA [48,102]. The consequence of all these is the lowering of the apoptotic threshold, sensitising cells to direct BCL-XL or BCL-2 antagonism.
BH3-mimetics and oncogenic kinases
A major class of drugs under investigation for use in combination with BH3-mimetics are oncogenic kinase inhibitors. These include kinases along the mitogen-activated protein kinase (MAPK) pathway which serves as a critical bridge between extracellular signals and intracellular responses, or the cyclin-dependent kinases which co-ordinate cellular events including cell proliferation and survival (e.g. CDK9). Critically, such kinases regulate BCL-2 proteins to favour cellular survival. This occurs by the up-regulation/stabilisation of pro-survival proteins such as MCL-1 [103–106] or through post-translational modifications such as phosphorylation leading to functional inactivation or proteasomal degradation of pro-apoptotic proteins such as BIM [107,108], BAD [109,110] or BMF [111]. Notably, the predominant response to oncogenic kinase inhibition is cytostatic as opposed to cytotoxic as the elevated pro-survival protein levels in cancer cells buffer any induced pro-apoptotic BH3-only proteins [112]. Hence, BH3-mimetics can lower the apoptotic threshold to unleash the pro-apoptotic power of oncogenic kinase inhibitors.
Cancer-associated alterations of MAPK signalling arise from activating mutations to any of the downstream effector molecules along the pathway (e.g. RAS/RAF/MEK/ERK that converge on the PI3K/AKT pathway) or to upstream tyrosine kinase receptors (e.g. EGFR). As most solid cancers possess mutations along this signalling node [113] they are attractive targets to investigate in combination with BH3-mimetics. Such combinations result in potent efficacy in solid cancer models including NSCLC [10,40,114–117], melanoma [37,112,118], prostate [37,85] and colorectal cancers [112]. In almost all examples, kinase inhibition up-regulates pro-apoptotic BH3-only proteins such as BIM, PUMA or BMF [85,111,112,114–117,119]. In some cases, it is associated with decreased MCL-1 expression or its increased degradation [10,85]. Notably, these combinations of BH3-mimetics are well-tolerated and in clinical trials in solid tumours (NCT02520778, NCT02143401, NCT02079740, NCT01989585).
Another class of kinases being explored for use in combination with BH3-mimetics are cyclin-dependent kinase inhibitors, specifically those involved in gene transcription such as CDK9. CDK9 regulates transcription of oncogenic genes including MYC and is essential for the maintenance, growth and chemoresistance of many solid cancers including breast [120], lung [121], osteosarcoma [122], pancreatic [123] and melanoma [124] and is prognostic of worse overall and disease-free survival [122,123]. In all cases, CDK9 inhibition leads to the suppression of tumour formation and induces apoptosis via reduction of MCL-1 expression [84,125-128]. Therefore, CDK9 inhibitors offer an alternative approach to target MCL-1 activity indirectly in combination with BH3-mimetics [125,127,128]. Accordingly, the combination of Venetoclax and CDK9 inhibitors (e.g. Voruciclib, A-1592668, AZD4573, LS-007) [128–131] provides superior efficacy over monotherapy in haematological malignancies, and is well-tolerated in vivo. In solid cancers, the combination of Dinaciclib (which targets CDKs 1, 2, 5, 9) with A-1331852 or ABT-737 to target BCL-XL showed promising responses in soft-tissue sarcoma cells [132]. However, in vivo this combination led to liver toxicity linked to hepatocyte apoptosis, likely due to the broad action of Dinaciclib against CDKs in multiple cellular processes, hence specific CDK9 inhibitors will likely have more promise.
Based on pre-clinical studies, the scope for strategies based on BH3-mimetics in combination with oncogenic kinases is vast and promising. Whilst only two classes of oncogenic kinases have been described in detail here, other kinase families that contribute to the aetiology of solid tumours including the PI3K/Akt/mTOR pathway are also being explored in combination with BH3-mimetics [133].
BH3-mimetics and epigenetic modulators
Another promising approach being explored in combination with BH3-mimetics in solid cancers is the inhibition of epigenetic modulators such as bromodomain and extra-terminal (BET) family proteins or histone deacetylases (HDAC). Originally demonstrated in haematological malignancies, epigenetic modulators such as the HDAC inhibitor Vorinostat in combination with ABT-263/ABT-737 facilitated synergistic tumour cell apoptosis. Similarly, in solid cancers including rhabdomyosarcoma, breast cancer, SCLC and melanoma, synergistic killing was achieved, either through transcriptional up-regulation of pro-apoptotic BIM, NOXA, PUMA or BMF [134–139] or suppression of BCL-2 and/or BCL-XL expression [137,138,140,141]. In melanoma, the BET inhibitor JQ1 suppressed BFL-1 expression through inhibition of its transcriptional regulator, NF-kB [138]. As BFL-1 remains untargeted by BH3-mimetics, this effect of BET inhibition could be a strategy to circumvent BFL-1 dependency of tumours such as melanoma [60,63,66].
Intriguingly, treatment with JQ1 can also lead to an increased tumour cell dependence on pro-survival proteins resulting in resistance to pharmacological modulators of epigenetic regulation [142–144]. For example, in triple-negative breast cancer, gain of a superenhancer was detected at the BCLX locus and served as a mechanism of maintaining BCL-XL expression and resistance to JQ1 [143]. Accordingly, BH3-mimetics could serve to target overexpressed pro-survival protein(s) or redistribute any pro-apoptotic BH3-only proteins they sequester. Encouragingly, the synergistic killing afforded by combinations of BH3-mimetics and epigenetic regulators appears confined to cancer cells [134,138].
Other combinations
In addition to the combinations of BH3-mimetics with the targeted therapies discussed above, combinations with less specific DNA-damaging chemotherapies have also been explored in solid cancers. Like the drugs described above, such agents can enhance the anti-tumour activity of BH3-mimetics in multiple solid cancers including ovarian cancer [10,94], mesothelioma [8] and sarcomas [145,146] where chemotherapy is often the mainstay treatment, though the mechanism(s)-of-action for the enhanced killing is not well explored. However, it would be reasonable to assume that this is likely mediated by up-regulation of BH3-only proteins such as BIM or the p53-responsive genes PUMA and NOXA, following DNA damage [92].
The future of BH3-mimetic therapy for solid cancers
Clinical susceptibility to single-agent BH3-mimetic therapy has been mostly limited to haematological cancers. To broaden the range of cancers that can be tackled with BH3-mimetics, strategies that safely achieve the antagonism of multiple pro-survival proteins must be considered. Whilst combination therapies with agents that preferentially impact cancer cells is being investigated, another exciting approach being explored are technologies that enable direct delivery of BH3-mimetics to tumour cells.
A Phase I global clinical trial to evaluate the safety and tolerability of the dendrimer-based nanoparticle formulation of a dual BCL-2/BCL-XL inhibitor, AZD0466, in haematological and solid cancers is currently underway (NCT04214093) [147]. No combination trials of AZD0466 with either ‘unconjugated’ BH3-mimetics targeting for example MCL-1, or chemotherapeutic agents have yet been announced. However, pre-clinical studies investigating the co-administration of AZD0466 and Cisplatin in a mesothelioma xenograft model demonstrate improved tumour killing with minimal thrombocytopaenia associated with BCL-XL targeting [80]. Another approach that directs BH3-mimetics to tumour cells is to conjugate them with antibodies targeting unique or overexpressed antigens preferentially found on cancer cells. One such antigen is B7-H3, an immune regulator protein widely expressed by solid tumours including melanoma and NSCLC [148]. The compound ABBV-155, which is a first-in-class antibody drug-conjugate comprising a BCL-XL inhibitor conjugated to an anti-B7H3 antibody, is now in clinical trials either as monotherapy or in combination with taxanes for the treatment of relapsed/refractory solid tumours including NSCLC, SCLC and breast cancer (NCT03595059). The results to date demonstrate a tolerable safety profile and anti-tumour activity [149].
In addition to strategies exploiting antigens or microenvironmental properties unique to cancer cells, another approach to reduce toxicities is to apply proteolysis-targeting chimeras (PROTACs) technology to BH3-mimetics. For example, by targeting BCL-XL to the Von Hippel-Lindau E3 ligase, which is minimally expressed in platelets, the PROTAC DT2216 resulted in reduced thrombocytopaenia and enhanced activity with chemotherapeutics in solid cancer xenograft models of SCLC, triple-negative breast cancer, prostate, colon and liver cancers compared with Navitoclax [150]. Importantly, these combinations were well-tolerated and DT2216 is now in clinical trials (NCT04886622) for use in relapsed/refractory malignancies including solid tumours.
The activation of BAX and/or BAK is the primary outcome essential to the killing activity of BH3-mimetics therapy. Whilst not a focus of this review, it would be remiss of us not to highlight the active investigation into the development of compounds to directly activate BAX or BAK [151–154]. For example, a small molecule compound, BTSA1, was developed to directly activate BAX and, promisingly, demonstrated selective killing of AML cells whilst sparing normal cells [154]. Likewise, a small molecule BAK activator, BKA-073, was shown to be effective against SCLC and NSCLC in vivo. Furthermore, consistent with the overexpression of BCL-2 in SCLC potentially blunting an apoptotic response, synergistic killing was observed when BKA-073 was used in combination with Venetoclax [152]. Therefore, whilst still in proof-of-concept development, this promising approach to induce apoptosis in cancer cells warrants further investigation.
Decades of research have yielded clinically applicable drugs targeting the pro-survival members of the BCL-2 family. Whilst the path to achieving killing efficacy is now clear, how we achieve this remains the challenge in the application of BH3-mimetic therapy for the future treatment of solid cancers. The combination strategies discussed above offer significant promise, so long as a safe therapeutic window or ‘sweet spot’ can be identified.
Perspectives
BH3-mimetics drugs that directly antagonise the pro-survival proteins of the BCL-2 family to induce apoptosis are clinically approved for the treatment of some haematological cancers. However, the use of BH3-mimetics for the treatment of solid cancers is less established.
Solid cancers are often reliant on multiple pro-survival proteins for their survival. Whilst simultaneous co-targeting of these survival factors with BH3-mimetics induces efficient killing, it can also lead to adverse effects on normal cells.
As such, combination strategies utilising BH3-mimetics with other anti-cancer agents that indirectly modulate the pro-survival function of BCL-2 proteins, are being investigated in solid cancers as an alternate and safer approach. In addition, tumour-directed BH3-mimetics are being developed to circumvent the issues arising from the targeting of normal cells.
Abbreviations
- AML
acute myeloid leukaemia
- BAD
BCL2 associated agonist of cell death
- BCL-2
b-cell lymphoma 2
- BCL-W
b-cell lymphoma w (WEHI)
- BCL-XL
b-cell lymphoma-extra large
- BET
bromodomain and extra-terminal
- BFL-1
bcl-2-related gene in foetal liver 1
- BH3
bcl-2 homology 3
- BIM
bcl-2-like protein 11
- BMF
bcl-2 modifying factor
- CDK
cyclin-dependent kinase
- CLL
chronic lymphocytic leukaemia
- EGFR
epidermal growth factor receptor
- FGFR1
fibroblast growth factor receptor 1
- HDAC
histone deacetylase
- MCL-1
myeloid cell leukaemia 1
- mTOR
mechanistic target of rapamycin
- MYCN
v-myc myelocytomatosis viral related oncogene, neuroblastoma derived
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NOXA
phorbol-12-myristate-13-acetate-induced protein 1 (latin for damage)
- NSCLC
non-small cell lung cancer
- PDX
patient-derived xenograft
- PI3K
phosphatidylinositol-3-kinase
- PROTAC
proteolysis-targeting chimaera
- PUMA
p53 up-regulated modulator of apoptosis
- SCLC
small cell lung cancer
Conflicts of Interest
W.D.F. and E.F.L. were previously employees of The Walter and Eliza Hall Institute where they were involved in collaborations with AbbVie and Genentech to develop and characterise BH3-mimetic drugs and receive payments in respect of Venetoclax. They have an on-going collaboration with Astra Zeneca studying BH3-mimetics.
Funding
W.D.F. is supported by the National Health and Medical Research Council (NHMRC) of Australia (Project Grants GNT1122829, GNT1157551). E.F.L. is a fellowship recipient from the Victorian Cancer Agency (Mid-Career Fellowship MCRF19045).
Open Access
Open access for this article was enabled by the participation of La Trobe University in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with CAUL.
Author Contributions
W.D.F. and E.F.L. conceptualised the idea for the review, as well as wrote and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
References
- 1.Certo, M., Moore V, D.G., Nishino, M., Wei, G., Korsmeyer, S., Armstrong, S.A.et al. (2006) Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 10.1016/j.ccr.2006.03.027 [DOI] [PubMed] [Google Scholar]
- 2.Dai, H., Ding, H., Meng, X.W., Peterson, K.L., Schneider, P.A., Karp, J.E.et al. (2015) Constitutive BAK activation as a determinant of drug sensitivity in malignant lymphohematopoietic cells. Genes Dev. 29, 2140–2152 10.1101/gad.267997.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill, K.L., Huang, K., Zhang, J., Chen, Y. and Luo, X. (2016) Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 30, 973–988 10.1101/gad.276725.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seymour, J.F., Kipps, T.J., Eichhorst, B., Hillmen, P., D'Rozario, J., Assouline, S.et al. (2018) Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N. Engl. J. Med. 378, 1107–1120 10.1056/NEJMoa1713976 [DOI] [PubMed] [Google Scholar]
- 5.Fischer, K., Al-Sawaf, O., Bahlo, J., Fink, A.M., Tandon, M., Dixon, M.et al. (2019) Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N. Engl. J. Med. 380, 2225–2236 10.1056/NEJMoa1815281 [DOI] [PubMed] [Google Scholar]
- 6.Daver, N., Wei, A.H., Pollyea, D.A., Fathi, A.T., Vyas, P. and DiNardo, C.D. (2020) New directions for emerging therapies in acute myeloid leukemia: the next chapter. Blood Cancer J. 10, 107 10.1038/s41408-020-00376-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tse, C., Shoemaker, A.R., Adickes, J., Anderson, M.G., Chen, J., Jin, S.et al. (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 10.1158/0008-5472.CAN-07-5836 [DOI] [PubMed] [Google Scholar]
- 8.Arulananda, S., O'Brien, M., Evangelista, M., Harris, T.J., Steinohrt, N.S., Jenkins, L.J.et al. (2020) BCL-XL is an actionable target for treatment of malignant pleural mesothelioma. Cell Death Discov. 6, 114 10.1038/s41420-020-00348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoemaker, A.R., Mitten, M.J., Adickes, J., Ackler, S., Refici, M., Ferguson, D.et al. (2008) Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin. Cancer Res. 14, 3268–3277 10.1158/1078-0432.CCR-07-4622 [DOI] [PubMed] [Google Scholar]
- 10.Chen, J., Jin, S., Abraham, V., Huang, X., Liu, B., Mitten, M.J.et al. (2011) The Bcl-2/Bcl-X(L)/Bcl-w inhibitor, navitoclax, enhances the activity of chemotherapeutic agents in vitro and in vivo. Mol. Cancer Ther. 10, 2340–2349 10.1158/1535-7163.MCT-11-0415 [DOI] [PubMed] [Google Scholar]
- 11.Leverson, J.D., Phillips, D.C., Mitten, M.J., Boghaert, E.R., Diaz, D., Tahir, S.K.et al. (2015) Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 7, 279ra40 10.1126/scitranslmed.aaa4642 [DOI] [PubMed] [Google Scholar]
- 12.Potter, D.S., Du, R., Bhola, P., Bueno, R. and Letai, A. (2021) Dynamic BH3 profiling identifies active BH3 mimetic combinations in non-small cell lung cancer. Cell Death Dis. 12, 741 10.1038/s41419-021-04029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan, N., Malek, M., Zha, J., Yue, P., Kassees, R., Berry, L.et al. (2011) Navitoclax enhances the efficacy of taxanes in non-small cell lung cancer models. Clin. Cancer Res. 17, 1394–1404 10.1158/1078-0432.CCR-10-2353 [DOI] [PubMed] [Google Scholar]
- 14.Bai, L., Chen, J., McEachern, D., Liu, L., Zhou, H., Aguilar, A.et al. (2014) BM-1197: a novel and specific Bcl-2/Bcl-xL inhibitor inducing complete and long-lasting tumor regression in vivo. PLoS One 9, e99404 10.1371/journal.pone.0099404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemati, F., de Montrion, C., Lang, G., Kraus-Berthier, L., Carita, G., Sastre-Garau, X.et al. (2014) Targeting Bcl-2/Bcl-XL induces antitumor activity in uveal melanoma patient-derived xenografts. PLoS One 9, e80836 10.1371/journal.pone.0080836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi, H., Qiu, M.Z., Yuan, L., Luo, Q., Pan, W., Zhou, S.et al. (2020) Bcl-2/Bcl-xl inhibitor APG-1252-M1 is a promising therapeutic strategy for gastric carcinoma. Cancer Med. 9, 4197–4206 10.1002/cam4.3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beroukhim, R., Mermel, C.H., Porter, D., Wei, G., Raychaudhuri, S., Donovan, J.et al. (2010) The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 10.1038/nature08822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amundson, S.A., Myers, T.G., Scudiero, D., Kitada, S., Reed, J.C. and Fornace, Jr, A.J. (2000) An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 60, 6101–6110 PMID: [PubMed] [Google Scholar]
- 19.Luo, M.J., Palmieri, M., Riffkin, C.D., Sakthianandeswaren, A., Djajawi, T.M., Hirokawa, Y.et al. (2020) Defining the susceptibility of colorectal cancers to BH3-mimetic compounds. Cell Death Dis. 11, 735 10.1038/s41419-020-02815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soderquist, R.S., Crawford, L., Liu, E., Lu, M., Agarwal, A., Anderson, G.R.et al. (2018) Systematic mapping of BCL-2 gene dependencies in cancer reveals molecular determinants of BH3 mimetic sensitivity. Nat. Commun. 9, 3513 10.1038/s41467-018-05815-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, H., Xue, J., Hessler, P., Tahir, S.K., Chen, J., Jin, S.et al. (2015) Genomic analysis and selective small molecule inhibition identifies BCL-X(L) as a critical survival factor in a subset of colorectal cancer. Mol. Cancer 14, 126 10.1186/s12943-015-0397-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, L., Doherty, G.A., Judd, A.S., Tao, Z.F., Hansen, T.M., Frey, R.R.et al. (2020) Discovery of A-1331852, a first-in-class, potent, and orally-bioavailable BCL-XL inhibitor. ACS Med. Chem. Lett. 11, 1829–1836 10.1021/acsmedchemlett.9b00568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason, K.D., Carpinelli, M.R., Fletcher, J.I., Collinge, J.E., Hilton, A.A., Ellis, S.et al. (2007) Programmed anuclear cell death delimits platelet life span. Cell 128, 1173–1186 10.1016/j.cell.2007.01.037 [DOI] [PubMed] [Google Scholar]
- 24.Souers, A.J., Leverson, J.D., Boghaert, E.R., Ackler, S.L., Catron, N.D., Chen, J.et al. (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 10.1038/nm.3048 [DOI] [PubMed] [Google Scholar]
- 25.Kendall, J., Liu, Q., Bakleh, A., Krasnitz, A., Nguyen, K.C., Lakshmi, B.et al. (2007) Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc. Natl Acad. Sci. U.S.A. 104, 16663–16668 10.1073/pnas.0708286104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weir, B.A., Woo, M.S., Getz, G., Perner, S., Ding, L., Beroukhim, R.et al. (2007) Characterizing the cancer genome in lung adenocarcinoma. Nature 450, 893–898 10.1038/nature06358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, H., Guttikonda, S., Roberts, L., Uziel, T., Semizarov, D., Elmore, S.W.et al. (2011) Mcl-1 is critical for survival in a subgroup of non-small-cell lung cancer cell lines. Oncogene 30, 1963–1968 10.1038/onc.2010.559 [DOI] [PubMed] [Google Scholar]
- 28.Lin, W.M., Baker, A.C., Beroukhim, R., Winckler, W., Feng, W., Marmion, J.M.et al. (2008) Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res. 68, 664–673 10.1158/0008-5472.CAN-07-2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modugno, M., Banfi, P., Gasparri, F., Borzilleri, R., Carter, P., Cornelius, L.et al. (2015) Mcl-1 antagonism is a potential therapeutic strategy in a subset of solid cancers. Exp. Cell Res. 332, 267–277 10.1016/j.yexcr.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 30.Goodwin, C.M., Rossanese, O.W., Olejniczak, E.T. and Fesik, S.W. (2015) Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ. 22, 2098–2106 10.1038/cdd.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merino, D., Whittle, J.R., Vaillant, F., Serrano, A., Gong, J.N., Giner, G.et al. (2017) Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci. Transl. Med. 9, eaam7049 10.1126/scitranslmed.aam7049 [DOI] [PubMed] [Google Scholar]
- 32.Young, A.I., Law, A.M., Castillo, L., Chong, S., Cullen, H.D., Koehler, M.et al. (2016) MCL-1 inhibition provides a new way to suppress breast cancer metastasis and increase sensitivity to dasatinib. Breast Cancer Res. 18, 125 10.1186/s13058-016-0781-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balko, J.M., Giltnane, J.M., Wang, K., Schwarz, L.J., Young, C.D., Cook, R.S.et al. (2014) Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 4, 232–245 10.1158/2159-8290.CD-13-0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding, Q., He, X., Xia, W., Hsu, J.M., Chen, C.T., Li, L.Y.et al. (2007) Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 67, 4564–4571 10.1158/0008-5472.CAN-06-1788 [DOI] [PubMed] [Google Scholar]
- 35.Leverson, J.D., Zhang, H., Chen, J., Tahir, S.K., Phillips, D.C., Xue, J.et al. (2015) Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 6, e1590 10.1038/cddis.2014.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelz, N.F., Bian, Z., Zhao, B., Shaw, S., Tarr, J.C., Belmar, J.et al. (2016) Discovery of 2-indole-acylsulfonamide myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods. J. Med. Chem. 59, 2054–2066 10.1021/acs.jmedchem.5b01660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotschy, A., Szlavik, Z., Murray, J., Davidson, J., Maragno, A.L., Le Toumelin-Braizat, G.et al. (2016) The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538, 477–482 10.1038/nature19830 [DOI] [PubMed] [Google Scholar]
- 38.Tron, A.E., Belmonte, M.A., Adam, A., Aquila, B.M., Boise, L.H., Chiarparin, E.et al. (2018) Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 9, 5341 10.1038/s41467-018-07551-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsey, H.E., Fischer, M.A., Lee, T., Gorska, A.E., Arrate, M.P., Fuller, L.et al. (2018) A Novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 8, 1566–1581 10.1158/2159-8290.CD-18-0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nangia, V., Siddiqui, F.M., Caenepeel, S., Timonina, D., Bilton, S.J., Phan, N.et al. (2018) Exploiting MCL1 dependency with combination MEK + MCL1 inhibitors leads to induction of apoptosis and tumor regression in KRAS-mutant non-small cell lung cancer. Cancer Discov. 8, 1598–1613 10.1158/2159-8290.CD-18-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caenepeel, S., Brown, S.P., Belmontes, B., Moody, G., Keegan, K.S., Chui, D.et al. (2018) AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov. 8, 1582–1597 10.1158/2159-8290.CD-18-0387 [DOI] [PubMed] [Google Scholar]
- 42.Ikegaki, N., Katsumata, M., Minna, J. and Tsujimoto, Y. (1994) Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res. 54, 6–8 PMID: [PubMed] [Google Scholar]
- 43.Jiang, S.X., Sato, Y., Kuwao, S. and Kameya, T. (1995) Expression of bcl-2 oncogene protein is prevalent in small cell lung carcinomas. J. Pathol. 177, 135–138 10.1002/path.1711770206 [DOI] [PubMed] [Google Scholar]
- 44.Hann, C.L., Daniel, V.C., Sugar, E.A., Dobromilskaya, I., Murphy, S.C., Cope, L.et al. (2008) Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 68, 2321–2328 10.1158/0008-5472.CAN-07-5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gandhi, L., Camidge, D.R., de Oliveira M, R., Bonomi, P., Gandara, D., Khaira, D.et al. (2011) Phase I study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J. Clin. Oncol. 29, 909–916 10.1200/JCO.2010.31.6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudin, C.M., Hann, C.L., Garon, E.B., de Oliveira M, R., Bonomi, P.D., Camidge, D.R.et al. (2012) Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 18, 3163–3169 10.1158/1078-0432.CCR-11-3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lochmann, T.L., Floros, K.V., Naseri, M., Powell, K.M., Cook, W., March, R.J.et al. (2018) Venetoclax is effective in small-cell lung cancers with high BCL-2 expression. Clin. Cancer Res. 24, 360–369 10.1158/1078-0432.CCR-17-1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ham, J., Costa, C., Sano, R., Lochmann, T.L., Sennott, E.M., Patel, N.U.et al. (2016) Exploitation of the apoptosis-primed state of MYCN-amplified neuroblastoma to develop a potent and specific targeted therapy combination. Cancer Cell 29, 159–172 10.1016/j.ccell.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bate-Eya, L.T., den Hartog, I.J., van der Ploeg, I., Schild, L., Koster, J., Santo, E.E.et al. (2016) High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition. Oncotarget 7, 27946–27958 10.18632/oncotarget.8547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamers, F., Schild, L., den Hartog, I.J., Ebus, M.E., Westerhout, E.M., Ora, I.et al. (2012) Targeted BCL2 inhibition effectively inhibits neuroblastoma tumour growth. Eur. J. Cancer 48, 3093–3103 10.1016/j.ejca.2012.01.037 [DOI] [PubMed] [Google Scholar]
- 51.Tanos, R., Karmali, D., Nalluri, S. and Goldsmith, K.C. (2016) Select Bcl-2 antagonism restores chemotherapy sensitivity in high-risk neuroblastoma. BMC Cancer 16, 97 10.1186/s12885-016-2129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dawson, S.J., Makretsov, N., Blows, F.M., Driver, K.E., Provenzano, E., Le Quesne, J.et al. (2010) BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br. J. Cancer 103, 668–675 10.1038/sj.bjc.6605736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaillant, F., Merino, D., Lee, L., Breslin, K., Pal, B., Ritchie, M.E.et al. (2013) Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell 24, 120–129 10.1016/j.ccr.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 54.Lok, S.W., Whittle, J.R., Vaillant, F., Teh, C.E., Lo, L.L., Policheni, A.N.et al. (2019) A phase Ib dose-escalation and expansion study of the BCL2 inhibitor venetoclax combined with tamoxifen in ER and BCL2-positive metastatic breast cancer. Cancer Discov. 9, 354–369 10.1158/2159-8290.CD-18-1151 [DOI] [PubMed] [Google Scholar]
- 55.Morales, A.A., Olsson, A., Celsing, F., Osterborg, A., Jondal, M. and Osorio, L.M. (2005) High expression of bfl-1 contributes to the apoptosis resistant phenotype in B-cell chronic lymphocytic leukemia. Int. J. Cancer 113, 730–737 10.1002/ijc.20614 [DOI] [PubMed] [Google Scholar]
- 56.Nagy, B., Lundan, T., Larramendy, M.L., Aalto, Y., Zhu, Y., Niini, T.et al. (2003) Abnormal expression of apoptosis-related genes in haematological malignancies: overexpression of MYC is poor prognostic sign in mantle cell lymphoma. Br. J. Haematol. 120, 434–441 10.1046/j.1365-2141.2003.04121.x [DOI] [PubMed] [Google Scholar]
- 57.Park, I.C., Lee, S.H., Whang, D.Y., Hong, W.S., Choi, S.S., Shin, H.S.et al. (1997) Expression of a novel Bcl-2 related gene, Bfl-1, in various human cancers and cancer cell lines. Anticancer Res. 17, 4619–4622 PMID: [PubMed] [Google Scholar]
- 58.Beverly, L.J. and Varmus, H.E. (2009) MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene 28, 1274–1279 10.1038/onc.2008.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon, H.S., Hong, S.H., Kang, H.J., Ko, B.K., Ahn, S.H. and Huh, J.R. (2003) Bfl-1 gene expression in breast cancer: its relationship with other prognostic factors. J. Korean Med. Sci. 18, 225–230 10.3346/jkms.2003.18.2.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haq, R., Yokoyama, S., Hawryluk, E.B., Jonsson, G.B., Frederick, D.T., McHenry, K.et al. (2013) BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc. Natl Acad. Sci. U.S.A. 110, 4321–4326 10.1073/pnas.1205575110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hind, C.K., Carter, M.J., Harris, C.L., Chan, H.T., James, S. and Cragg, M.S. (2015) Role of the pro-survival molecule Bfl-1 in melanoma. Int. J. Biochem. Cell Biol. 59, 94–102 10.1016/j.biocel.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 62.Lee, E.F., Harris, T.J., Tran, S., Evangelista, M., Arulananda, S., John, T.et al. (2019) BCL-XL and MCL-1 are the key BCL-2 family proteins in melanoma cell survival. Cell Death Dis. 10, 342 10.1038/s41419-019-1568-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucas, K.M., Mohana-Kumaran, N., Lau, D., Zhang, X.D., Hersey, P., Huang, D.C.et al. (2012) Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res. 18, 783–795 10.1158/1078-0432.CCR-11-1166 [DOI] [PubMed] [Google Scholar]
- 64.Mukherjee, N., Amato, C.M., Skees, J., Todd, K.J., Lambert, K.A., Robinson, W.A.et al. (2020) Simultaneously inhibiting BCL2 and MCL1 is a therapeutic option for patients with advanced melanoma. Cancers (Basel) 12, 2182 10.3390/cancers12082182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukherjee, N., Skees, J., Todd, K.J., West, D.A., Lambert, K.A., Robinson, W.A.et al. (2020) MCL1 inhibitors S63845/MIK665 plus Navitoclax synergistically kill difficult-to-treat melanoma cells. Cell Death Dis. 11, 443 10.1038/s41419-020-2646-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senft, D., Berking, C., Graf, S.A., Kammerbauer, C., Ruzicka, T. and Besch, R. (2012) Selective induction of cell death in melanoma cell lines through targeting of Mcl-1 and A1. PLoS One 7, e30821 10.1371/journal.pone.0030821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adams, C.M., Kim, A.S., Mitra, R., Choi, J.K., Gong, J.Z. and Eischen, C.M. (2017) BCL-W has a fundamental role in B cell survival and lymphomagenesis. J. Clin. Invest. 127, 635–650 10.1172/JCI89486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adams, C.M., Mitra, R., Gong, J.Z. and Eischen, C.M. (2017) Non-Hodgkin and Hodgkin lymphomas select for overexpression of BCLW. Clin. Cancer Res. 23, 7119–7129 10.1158/1078-0432.CCR-17-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bandres, E., Cubedo, E., Agirre, X., Malumbres, R., Zarate, R., Ramirez, N.et al. (2006) Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol. Cancer 5, 29 10.1186/1476-4598-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen, X.N., Wang, K.F., Xu, Z.Q., Li, S.J., Liu, Q., Fu, D.H.et al. (2014) MiR-133b regulates bladder cancer cell proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer Cell Int. 14, 70 10.1186/s12935-014-0070-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crawford, M., Batte, K., Yu, L., Wu, X., Nuovo, G.J., Marsh, C.B.et al. (2009) MicroRNA 133B targets pro-survival molecules MCL-1 and BCL2L2 in lung cancer. Biochem. Biophys. Res. Commun. 388, 483–489 10.1016/j.bbrc.2009.07.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bisaillon, R., Moison, C., Thiollier, C., Krosl, J., Bordeleau, M.E., Lehnertz, B.et al. (2020) Genetic characterization of ABT-199 sensitivity in human AML. Leukemia 34, 63–74 10.1038/s41375-019-0485-x [DOI] [PubMed] [Google Scholar]
- 73.Boiko, S., Proia, T., San Martin, M., Gregory, G.P., Wu, M.M., Aryal, N.et al. (2021) Targeting Bfl-1 via acute CDK9 inhibition overcomes intrinsic BH3-mimetic resistance in lymphomas. Blood 137, 2947–2957 10.1182/blood.2020008528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Esteve-Arenys, A. and Roue, G. (2018) BFL-1 expression determines the efficacy of venetoclax in MYC+/BCL2+ double hit lymphoma. Oncoscience 5, 59–61 10.18632/oncoscience.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pei, S., Pollyea, D.A., Gustafson, A., Stevens, B.M., Minhajuddin, M., Fu, R.et al. (2020) Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 10, 536–551 10.1158/2159-8290.CD-19-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yecies, D., Carlson, N.E., Deng, J. and Letai, A. (2010) Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 115, 3304–3313 10.1182/blood-2009-07-233304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lessene, G., Czabotar, P.E., Sleebs, B.E., Zobel, K., Lowes, K.N., Adams, J.M.et al. (2013) Structure-guided design of a selective BCL-X(L) inhibitor. Nat. Chem. Biol. 9, 390–397 10.1038/nchembio.1246 [DOI] [PubMed] [Google Scholar]
- 78.Li, X., Dou, J., You, Q. and Jiang, Z. (2021) Inhibitors of BCL2A1/Bfl-1 protein: potential stock in cancer therapy. Eur. J. Med. Chem. 220, 113539 10.1016/j.ejmech.2021.113539 [DOI] [PubMed] [Google Scholar]
- 79.Weeden, C.E., Ah-Cann, C., Holik, A.Z., Pasquet, J., Garnier, J.M., Merino, D.et al. (2018) Dual inhibition of BCL-XL and MCL-1 is required to induce tumour regression in lung squamous cell carcinomas sensitive to FGFR inhibition. Oncogene 37, 4475–4488 10.1038/s41388-018-0268-2 [DOI] [PubMed] [Google Scholar]
- 80.Arulananda, S., O'Brien, M., Evangelista, M., Jenkins, L.J., Poh, A.R., Walkiewicz, M.et al. (2021) A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 7, 122 10.1038/s41420-021-00505-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson, M.R., Ashton, M., Koessinger, A.L., Dick, C., Verheij, M. and Chalmers, A.J. (2020) Mesothelioma cells depend on the antiapoptotic protein Bcl-xL for survival and are sensitized to ionizing radiation by BH3-mimetics. Int. J. Radiat. Oncol. Biol. Phys. 106, 867–877 10.1016/j.ijrobp.2019.11.029 [DOI] [PubMed] [Google Scholar]
- 82.Anderson, G.R., Wardell, S.E., Cakir, M., Crawford, L., Leeds, J.C., Nussbaum, D.P.et al. (2016) PIK3CA mutations enable targeting of a breast tumor dependency through mTOR-mediated MCL-1 translation. Sci. Transl. Med. 8, 369ra175 10.1126/scitranslmed.aae0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faber, A.C., Farago, A.F., Costa, C., Dastur, A., Gomez-Caraballo, M., Robbins, R.et al. (2015) Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc. Natl Acad. Sci. U.S.A. 112, E1288–E1296 10.1073/pnas.1411848112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao, Y., Nimmer, P., Sheppard, G.S., Bruncko, M., Hessler, P., Lu, X.et al. (2015) MCL-1 is a key determinant of breast cancer cell survival: validation of MCL-1 dependency utilizing a highly selective small molecule inhibitor. Mol. Cancer Ther. 14, 1837–1847 10.1158/1535-7163.MCT-14-0928 [DOI] [PubMed] [Google Scholar]
- 85.Arai, S., Jonas, O., Whitman, M.A., Corey, E., Balk, S.P. and Chen, S. (2018) Tyrosine kinase inhibitors increase MCL1 degradation and in combination with BCLXL/BCL2 inhibitors drive prostate cancer apoptosis. Clin. Cancer Res. 24, 5458–5470 10.1158/1078-0432.CCR-18-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Place, A.E., Goldsmith, K., Bourquin, J.P., Loh, M.L., Gore, L., Morgenstern, D.A.et al. (2018) Accelerating drug development in pediatric cancer: a novel phase I study design of venetoclax in relapsed/refractory malignancies. Future Oncol. 14, 2115–2129 10.2217/fon-2018-0121 [DOI] [PubMed] [Google Scholar]
- 87.Cancer Genome Atlas N. (2015) Genomic classification of cutaneous melanoma. Cell 161, 1681–1696 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hikita, H., Takehara, T., Shimizu, S., Kodama, T., Li, W., Miyagi, T.et al. (2009) Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology 50, 1217–1226 10.1002/hep.23126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Debrincat, M.A., Josefsson, E.C., James, C., Henley, K.J., Ellis, S., Lebois, M.et al. (2012) Mcl-1 and Bcl-x(L) coordinately regulate megakaryocyte survival. Blood 119, 5850–5858 10.1182/blood-2011-12-398834 [DOI] [PubMed] [Google Scholar]
- 90.Ke, F., Lancaster, G.I., Grabow, S., Murphy, A.J. and Strasser, A. (2020) Combined reduction in the expression of MCL-1 and BCL-2 reduces organismal size in mice. Cell Death Dis. 11, 185 10.1038/s41419-020-2376-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei, A.H., Roberts, A.W., Spencer, A., Rosenberg, A.S., Siegel, D., Walter, R.B.et al. (2020) Targeting MCL-1 in hematologic malignancies: rationale and progress. Blood Rev. 44, 100672 10.1016/j.blre.2020.100672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Delbridge, A.R. and Strasser, A. (2015) The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 22, 1071–1080 10.1038/cdd.2015.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oakes, S.R., Vaillant, F., Lim, E., Lee, L., Breslin, K., Feleppa, F.et al. (2012) Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc. Natl Acad. Sci. U.S.A. 109, 2766–2771 10.1073/pnas.1104778108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wong, M., Tan, N., Zha, J., Peale, F.V., Yue, P., Fairbrother, W.J.et al. (2012) Navitoclax (ABT-263) reduces Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol. Cancer Ther. 11, 1026–1035 10.1158/1535-7163.MCT-11-0693 [DOI] [PubMed] [Google Scholar]
- 95.Duan, Z., Chinn, D., Tu, M.J., Zhang, Q.Y., Huynh, J., Chen, J.et al. (2019) Novel synergistic combination of mitotic arrest and promotion of apoptosis for treatment of pancreatic adenocarcinoma. Transl. Oncol. 12, 683–692 10.1016/j.tranon.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inuzuka, H., Shaik, S., Onoyama, I., Gao, D., Tseng, A., Maser, R.S.et al. (2011) SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471, 104–109 10.1038/nature09732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wertz, I.E. Kusam, S., Lam, C., Okamoto, T., Sandoval, W., Anderson, D.J.et al. (2011) Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471, 110–114 10.1038/nature09779 [DOI] [PubMed] [Google Scholar]
- 98.Bah, N., Maillet, L., Ryan, J., Dubreil, S., Gautier, F., Letai, A.et al. (2014) Bcl-xL controls a switch between cell death modes during mitotic arrest. Cell Death Dis. 5, e1291 10.1038/cddis.2014.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terrano, D.T., Upreti, M. and Chambers, T.C. (2010) Cyclin-dependent kinase 1-mediated Bcl-xL/Bcl-2 phosphorylation acts as a functional link coupling mitotic arrest and apoptosis. Mol. Cell. Biol. 30, 640–656 10.1128/MCB.00882-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Puthalakath, H., Huang, D.C., O'Reilly, L.A., King, S.M. and Strasser, A. (1999) The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3, 287–296 10.1016/S1097-2765(00)80456-6 [DOI] [PubMed] [Google Scholar]
- 101.Tan, T.T., Degenhardt, K., Nelson, D.A., Beaudoin, B., Nieves-Neira, W., Bouillet, P.et al. (2005) Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell 7, 227–238 10.1016/j.ccr.2005.02.008 [DOI] [PubMed] [Google Scholar]
- 102.Lohard, S., Bourgeois, N., Maillet, L., Gautier, F., Fetiveau, A., Lasla, H.et al. (2020) STING-dependent paracriny shapes apoptotic priming of breast tumors in response to anti-mitotic treatment. Nat. Commun. 11, 259 10.1038/s41467-019-13689-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Booy, E.P., Henson, E.S. and Gibson, S.B. (2011) Epidermal growth factor regulates Mcl-1 expression through the MAPK-Elk-1 signalling pathway contributing to cell survival in breast cancer. Oncogene 30, 2367–2378 10.1038/onc.2010.616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Domina, A.M., Vrana, J.A., Gregory, M.A., Hann, S.R. and Craig, R.W. (2004) MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene 23, 5301–5315 10.1038/sj.onc.1207692 [DOI] [PubMed] [Google Scholar]
- 105.Townsend, K.J., Zhou, P., Qian, L., Bieszczad, C.K., Lowrey, C.H., Yen, A.et al. (1999) Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation. J. Biol. Chem. 274, 1801–1813 10.1074/jbc.274.3.1801 [DOI] [PubMed] [Google Scholar]
- 106.Zhang, Y., Zhou, L., Leng, Y., Dai, Y., Orlowski, R.Z. and Grant, S. (2017) Positive transcription elongation factor b (P-TEFb) is a therapeutic target in human multiple myeloma. Oncotarget 8, 59476–59491 10.18632/oncotarget.19761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ley, R., Balmanno, K., Hadfield, K., Weston, C. and Cook, S.J. (2003) Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278, 18811–18816 10.1074/jbc.M301010200 [DOI] [PubMed] [Google Scholar]
- 108.Luciano, F., Jacquel, A., Colosetti, P., Herrant, M., Cagnol, S., Pages, G.et al. (2003) Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 22, 6785–6793 10.1038/sj.onc.1206792 [DOI] [PubMed] [Google Scholar]
- 109.Fang, X., Yu, S., Eder, A., Mao, M., Bast, Jr, R.C., Boyd, D.et al. (1999) Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 18, 6635–6640 10.1038/sj.onc.1203076 [DOI] [PubMed] [Google Scholar]
- 110.Scheid, M.P., Schubert, K.M. and Duronio, V. (1999) Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 274, 31108–31113 10.1074/jbc.274.43.31108 [DOI] [PubMed] [Google Scholar]
- 111.Shao, Y. and Aplin, A.E. (2012) ERK2 phosphorylation of serine 77 regulates Bmf pro-apoptotic activity. Cell Death Dis. 3, e253 10.1038/cddis.2011.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cragg, M.S., Jansen, E.S., Cook, M., Harris, C., Strasser, A. and Scott, C.L. (2008) Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J. Clin. Invest. 118, 3651–3659 10.1172/JCI35437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Braicu, C., Buse, M., Busuioc, C., Drula, R., Gulei, D., Raduly, L.et al. (2019) A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers (Basel) 11, 1618 10.3390/cancers11101618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Corcoran, R.B., Cheng, K.A., Hata, A.N., Faber, A.C., Ebi, H., Coffee, E.M.et al. (2013) Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 23, 121–128 10.1016/j.ccr.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Costa, D.B., Halmos, B., Kumar, A., Schumer, S.T., Huberman, M.S., Boggon, T.J.et al. (2007) BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 4, 1669–1679. discussion 80 10.1371/journal.pmed.0040315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cragg, M.S., Kuroda, J., Puthalakath, H., Huang, D.C. and Strasser, A. (2007) Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 4, 1681–1689. discussion 90 10.1371/journal.pmed.0040316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gong, Y., Somwar, R., Politi, K., Balak, M., Chmielecki, J., Jiang, X.et al. (2007) Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 4, e294 10.1371/journal.pmed.0040294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hartman, M.L., Gajos-Michniewicz, A., Talaj, J.A., Mielczarek-Lewandowska, A. and Czyz, M. (2021) BH3 mimetics potentiate pro-apoptotic activity of encorafenib in BRAF(V600E) melanoma cells. Cancer Lett. 499, 122–136 10.1016/j.canlet.2020.11.036 [DOI] [PubMed] [Google Scholar]
- 119.Rohrbeck, L., Gong, J.N., Lee, E.F., Kueh, A.J., Behren, A., Tai, L.et al. (2016) Hepatocyte growth factor renders BRAF mutant human melanoma cell lines resistant to PLX4032 by downregulating the pro-apoptotic BH3-only proteins PUMA and BIM. Cell Death Differ. 23, 2054–2062 10.1038/cdd.2016.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sengupta, S., Biarnes, M.C. and Jordan, V.C. (2014) Cyclin dependent kinase-9 mediated transcriptional de-regulation of cMYC as a critical determinant of endocrine-therapy resistance in breast cancers. Breast Cancer Res Treat. 143, 113–124 10.1007/s10549-013-2789-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang, X., Yu, C., Wang, C., Ma, Y., Wang, T., Li, Y.et al. (2019) Novel cyclin-dependent kinase 9 (CDK9) inhibitor with suppression of cancer stemness activity against non-small-cell lung cancer. Eur. J. Med. Chem. 181, 111535 10.1016/j.ejmech.2019.07.038 [DOI] [PubMed] [Google Scholar]
- 122.Ma, H., Seebacher, N.A., Hornicek, F.J. and Duan, Z. (2019) Cyclin-dependent kinase 9 (CDK9) is a novel prognostic marker and therapeutic target in osteosarcoma. EBioMedicine 39, 182–193 10.1016/j.ebiom.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kretz, A.L., Schaum, M., Richter, J., Kitzig, E.F., Engler, C.C., Leithauser, F.et al. (2017) CDK9 is a prognostic marker and therapeutic target in pancreatic cancer. Tumour Biol. 39, 1010428317694304 10.1177/1010428317694304 [DOI] [PubMed] [Google Scholar]
- 124.Abdullah, C., Wang, X. and Becker, D. (2011) Expression analysis and molecular targeting of cyclin-dependent kinases in advanced melanoma. Cell Cycle 10, 977–988 10.4161/cc.10.6.15079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barlaam, B., Casella, R., Cidado, J., Cook, C., De Savi, C., Dishington, A.et al. (2020) Discovery of AZD4573, a potent and selective inhibitor of CDK9 that enables short duration of target engagement for the treatment of hematological malignancies. J. Med. Chem. 63, 15564–15590 10.1021/acs.jmedchem.0c01754 [DOI] [PubMed] [Google Scholar]
- 126.Gojo, I., Zhang, B. and Fenton, R.G. (2002) The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin. Cancer Res. 8, 3527–3538 PMID: [PubMed] [Google Scholar]
- 127.Gregory, G.P., Hogg, S.J., Kats, L.M., Vidacs, E., Baker, A.J., Gilan, O.et al. (2015) CDK9 inhibition by dinaciclib potently suppresses Mcl-1 to induce durable apoptotic responses in aggressive MYC-driven B-cell lymphoma in vivo. Leukemia 29, 1437–1441 10.1038/leu.2015.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Phillips, D.C., Jin, S., Gregory, G.P., Zhang, Q., Xue, J., Zhao, X.et al. (2020) A novel CDK9 inhibitor increases the efficacy of venetoclax (ABT-199) in multiple models of hematologic malignancies. Leukemia 34, 1646–1657 10.1038/s41375-019-0652-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carter, B.Z., Mak, P.Y., Tao, W., Warmoes, M., Lorenzi, P.L., Mak, D.et al. (2020) Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and resensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica Online ahead of print 10.3324/haematol.2020.260331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dey, J., Deckwerth, T.L., Kerwin, W.S., Casalini, J.R., Merrell, A.J., Grenley, M.O.et al. (2017) Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk diffuse large B-cell lymphoma to BCL2 inhibition. Sci. Rep. 7, 18007 10.1038/s41598-017-18368-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie, S., Jiang, H., Zhai, X.W., Wei, F., Wang, S.D., Ding, J.et al. (2016) Antitumor action of CDK inhibitor LS-007 as a single agent and in combination with ABT-199 against human acute leukemia cells. Acta Pharmacol. Sin. 37, 1481–1489 10.1038/aps.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rello-Varona, S., Fuentes-Guirado, M., Lopez-Alemany, R., Contreras-Perez, A., Mulet-Margalef, N., Garcia-Monclus, S.et al. (2019) Bcl-xL inhibition enhances Dinaciclib-induced cell death in soft-tissue sarcomas. Sci. Rep. 9, 3816 10.1038/s41598-019-40106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fulda, S. (2014) Synthetic lethality by co-targeting mitochondrial apoptosis and PI3K/Akt/mTOR signaling. Mitochondrion 19, 85–87 10.1016/j.mito.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 134.Chueh, A.C., Tse, J.W.T., Dickinson, M., Ioannidis, P., Jenkins, L., Togel, L.et al. (2017) ATF3 repression of BCL-XL determines apoptotic sensitivity to HDAC inhibitors across tumor types. Clin. Cancer Res. 23, 5573–5584 10.1158/1078-0432.CCR-17-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gallagher, S.J., Mijatov, B., Gunatilake, D., Tiffen, J.C., Gowrishankar, K., Jin, L.et al. (2014) The epigenetic regulator I-BET151 induces BIM-dependent apoptosis and cell cycle arrest of human melanoma cells. J. Invest. Dermatol. 134, 2795–2805 10.1038/jid.2014.243 [DOI] [PubMed] [Google Scholar]
- 136.Heinicke, U., Haydn, T., Kehr, S., Vogler, M. and Fulda, S. (2018) BCL-2 selective inhibitor ABT-199 primes rhabdomyosarcoma cells to histone deacetylase inhibitor-induced apoptosis. Oncogene 37, 5325–5339 10.1038/s41388-018-0212-5 [DOI] [PubMed] [Google Scholar]
- 137.Lam, L.T., Lin, X., Faivre, E.J., Yang, Z., Huang, X., Wilcox, D.M.et al. (2017) Vulnerability of small-cell lung cancer to apoptosis induced by the combination of BET bromodomain proteins and BCL2 inhibitors. Mol. Cancer Ther. 16, 1511–1520 10.1158/1535-7163.MCT-16-0459 [DOI] [PubMed] [Google Scholar]
- 138.Tseng, H.Y., Dreyer, J., Emran, A.A., Gunatilake, D., Pirozyan, M., Cullinane, C.et al. (2020) Co-targeting bromodomain and extra-terminal proteins and MCL1 induces synergistic cell death in melanoma. Int. J. Cancer 147, 2176–2189 10.1002/ijc.33000 [DOI] [PubMed] [Google Scholar]
- 139.Wang, H., Hong, B., Li, X., Deng, K., Li, H., Yan Lui, V.W.et al. (2017) JQ1 synergizes with the Bcl-2 inhibitor ABT-263 against MYCN-amplified small cell lung cancer. Oncotarget 8, 86312–86324 10.18632/oncotarget.21146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dawson, M.A., Prinjha, R.K., Dittmann, A., Giotopoulos, G., Bantscheff, M., Chan, W.I.et al. (2011) Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 478, 529–533 10.1038/nature10509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Delmore, J.E., Issa, G.C., Lemieux, M.E., Rahl, P.B., Shi, J., Jacobs, H.M.et al. (2011) BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 10.1016/j.cell.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gayle, S.S., Sahni, J.M., Webb, B.M., Weber-Bonk, K.L., Shively, M.S., Spina, R.et al. (2019) Targeting BCL-xL improves the efficacy of bromodomain and extra-terminal protein inhibitors in triple-negative breast cancer by eliciting the death of senescent cells. J. Biol. Chem. 294, 875–886 10.1074/jbc.RA118.004712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shu, S., Lin, C.Y., He, H.H., Witwicki, R.M., Tabassum, D.P., Roberts, J.M.et al. (2016) Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature 529, 413–417 10.1038/nature16508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Walsh, L., Haley, K.E., Moran, B., Mooney, B., Tarrant, F., Madden, S.F.et al. (2019) BET inhibition as a rational therapeutic strategy for invasive lobular breast cancer. Clin. Cancer Res. 25, 7139–7150 10.1158/1078-0432.CCR-19-0713 [DOI] [PubMed] [Google Scholar]
- 145.Alcon, C., Manzano-Munoz, A., Prada, E., Mora, J., Soriano, A., Guillen, G.et al. (2020) Sequential combinations of chemotherapeutic agents with BH3 mimetics to treat rhabdomyosarcoma and avoid resistance. Cell Death Dis. 11, 634 10.1038/s41419-020-02887-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Faqar-Uz-Zaman, S.F., Heinicke, U., Meister, M.T., Vogler, M. and Fulda, S. (2018) BCL-xL-selective BH3 mimetic sensitizes rhabdomyosarcoma cells to chemotherapeutics by activation of the mitochondrial pathway of apoptosis. Cancer Lett. 412, 131–142 10.1016/j.canlet.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 147.Patterson, C.M., Balachander, S.B., Grant, I., Pop-Damkov, P., Kelly, B., McCoull, W.et al. (2021) Design and optimisation of dendrimer-conjugated Bcl-2/xL inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 4, 112 10.1038/s42003-020-01631-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kontos, F., Michelakos, T., Kurokawa, T., Sadagopan, A., Schwab, J.H., Ferrone, C.R.et al. (2021) B7-H3: an attractive target for antibody-based immunotherapy. Clin Cancer Res. 27, 1227–1235 10.1158/1078-0432.CCR-20-2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tolcher, A.W., Carneiro, B., Dowlati, A., Ryan, A., Razak, R., Chae, Y.K.et al. (2021) A first-in-human-study of mirzotamab clezutoclax as monotherapy and in combination with taxane therapy in relapsed/refractory solid tumours: dose escalation results. J. Clin. Oncol. 39 10.1200/JCO.2021.39.15_suppl.3015 [DOI] [Google Scholar]
- 150.Khan, S., Zhang, X., Lv, D., Zhang, Q., He, Y., Zhang, P.et al. (2019) A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 25, 1938–1947 10.1038/s41591-019-0668-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leshchiner, E.S., Braun, C.R., Bird, G.H. and Walensky, L.D. (2013) Direct activation of full-length proapoptotic BAK. Proc. Natl Acad. Sci. U.S.A. 110, E986–E995 10.1073/pnas.1214313110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park, D., Anisuzzaman, A.S.M., Magis, A.T., Chen, G., Xie, M., Zhang, G.et al. (2021) Discovery of small molecule Bak activator for lung cancer therapy. Theranostics 11, 8500–8516 10.7150/thno.60349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pritz, J.R., Wachter, F., Lee, S., Luccarelli, J., Wales, T.E., Cohen, D.T.et al. (2017) Allosteric sensitization of proapoptotic BAX. Nat. Chem. Biol. 13, 961–967 10.1038/nchembio.2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reyna, D.E., Garner, T.P., Lopez, A., Kopp, F., Choudhary, G.S., Sridharan, A.et al. (2017) Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 32, 490–505.e10 10.1016/j.ccell.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lindeman, G.J., Lok, S.W., Bergin, A.R.T., Whittle, J.R., Shackleton, K., Sherman, P.et al. (2017) Safety and efficacy of the BCL2 inhibitor venetoclax in estrogen receptor (ER) and BCL2-positive metastatic breast cancer: the mBEP study. J. Clin. Oncol. 35, 1044 10.1200/JCO.2017.35.15_suppl.1044 [DOI] [Google Scholar]
- 156.Lindeman, G.J., Bowen, R., Jerzak, K.J., Song, X., Decker, T., Boyle, F.M.et al. (2021) Results from VERONICA: a randomized, phase II study of second-/third-line venetoclax (VEN) + fulvestrant (F) versus F alone in estrogen receptor (ER)-positive, HER2-negative, locally advanced, or metastatic breast cancer (LA/MBC). J. Clin. Oncol. 39, 10.1200/JCO.2021.39.15_suppl.1004 [DOI] [Google Scholar]
- 157.Lochmann, T.L., Bouck, Y.M. and Faber, A.C. (2018) BCL-2 inhibition is a promising therapeutic strategy for small cell lung cancer. Oncoscience 5, 218–219 10.18632/oncoscience.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lakhani, N.J., Rasco, D.W., Zeng, Q., Tang, Y., Liang, Z., Wang, H.et al. (2020) First-in-human study of palcitoclax (APG-1252), a novel dual Bcl-2/Bcl-xL inhibitor, demonstrated advantages in platelet safety while maintaining anticancer effect in U.S. patients with metastatic solid tumors. J. Clin. Oncol. 38, 3509 10.1200/JCO.2020.38.15_suppl.3509 [DOI] [Google Scholar]
- 159.Corcoran, R.B., Do, K.T., Cleary, J.M., Parikh, A.R., Yeku, O.O., Weekes, C.D.et al. (2019) Phase I/II study of combined BCL-XL and MEK inhibition with navitoclax (N) and trametinib (T) in KRAS or NRAS mutant advanced solid tumours. Ann. Oncol. 30, V164 10.1093/annonc/mdz244.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cleary, J.M., Lima, C.M., Hurwitz, H.I., Montero, A.J., Franklin, C., Yang, J.et al. (2014) A phase I clinical trial of navitoclax, a targeted high-affinity Bcl-2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Invest. New Drugs 32, 937–945 10.1007/s10637-014-0110-9 [DOI] [PubMed] [Google Scholar]
- 161.Puglisi, M., Molife, L.R., de Jonge, M.J., Khan, K.H., Doorn, L.V., Forster, M.D.et al. (2021) A phase I study of the safety, pharmacokinetics and efficacy of navitoclax plus docetaxel in patients with advanced solid tumors. Future Oncol. 17, 2747–2758 10.2217/fon-2021-0140 [DOI] [PubMed] [Google Scholar]
- 162.Pietanza, M.C. and Rudin, C.M. (2012) Novel therapeutic approaches for small cell lung cancer: the future has arrived. Curr. Probl. Cancer 36, 156–173 10.1016/j.currproblcancer.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koubek, E.J., Costello, B.A., Yin, J., McGovern, R.M., Buhrow, S.A., Schoon, R.A.et al. (2020) Pharmacokinetic analysis of navitoclax in combination with sorafenib in patients with relapsed or refractory solid organ tumors. Cancer Res. 80, CT114 10.1158/1538-7445.AM2020-CT114 [DOI] [Google Scholar]
- 164.Sullivan, R.J., Mehnert, J., Tawbi, H., Lawrence, D., Flaherty, K.T., Chen, H.et al. (2018) First in human, dose escalation trial of the combination of dabrafenib, trametinib, and navitoclax in patients with BRAF mutant solid tumors. Mol. Cancer Ther. 17, LB–B30 10.1158/1535-7163.TARG-17-LB-B30 [DOI] [Google Scholar]