Abstract
Background
The aim of this work is to evaluate changes following rapid maxillary expansion (RME) on breathing function in two groups of patients: mouth breathers and nasal breathers.
Materials and methods
Twenty-five oral breather patients (12 male, 13 female, mean age 15.2 ± 1.3), and 25 nasal breather patients (14 male, 11 female, mean age 15.3 ± 1.6) were treated with RME. Breathing function was evaluated by computerized spirometry. Forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), Tiffenau index (FEV1/ FVC ratio, IT%), forced expiratory flow at 25–75% of vital capacity (FEF 25–75%), and Tidal volume (TV) were assessed. Breathing function analysis was performed before RME and 6 and 12 months after RME during follow-up appointments. The Shapiro–Wilk test was used to assess whether data were normally distributed. As data were not normally distributed, Mann–Whitney U and Friedman tests were used to perform comparisons between treatment groups and within group comparisons, respectively.
Results
Oral breathers and nasal breathers showed statistically significant differences in FVC, FEF 25–75%, and TV at T0. They did not present any statistically significant difference in FEV1 and IT% at the same time point.
Statistically significant differences were noticed for all indices in the oral breather group after maxillary expansion, while the nasal breather group showed statistically significant differences only in FCV, FEF 25–75%, and TV after treatment.
There were no statistically significant differences in all indices 12 months after maxillary expansion between the oral breather and nasal breather groups.
Conclusions
RME appeared to improve breathing function in both groups. Forced vital capacity (FVC), forced expiratory flow at 25–75% of vital capacity (FEF 25–75), and Tidal volume (TV) reached similar values in both groups after treatment with RME.
Keywords: Breathing function, Rapid maxillary expansion, Spirometry
1. Introduction
Connections between breathing function and orthodontic treatment have been studied, using different methods, throughout time. Oral breathing during growth has been connected to maxillary hypoplasia. This habit can lead to severe malocclusion and aesthetic issues, greater upper airway resistances, and alteration of tongue posture (Aloufi et al., 2012, Lucchese et al., 2011, Lucchese et al., 2012a, Lucchese et al., 2012b, McNamara et al., 2015, Vale et al., 2017, Maspero et al., 2019a, Maspero et al., 2019b, Maspero et al., 2019c, Manuelli, 2012, Maspero et al., 2012).
Breathing disorders are both a cause and an effect of hypertonic tonsils, nasal septum deviation, sinonasal polyposis, and chronic rhinitis (Farronato et al., 2012a, Farronato et al., 2012b). These conditions require interdisciplinary treatment, in which pediatricians, otolaryngologists, speech therapists, and orthodontists are involved. Among several possible orthodontic treatments, the one that has shown greater impact on breathing function is rapid maxillary expansion (RME).
RME is the treatment of choice in the case of maxillary hypoplasia. Epidemiological studies have reported that between 8% and 20% of children suffer from this condition (Thilander et al., 1984). Rapid palatal expanders exert heavy forces that separate the mid-palatal suture, thus orthopedically enlarging maxilla with slight movements of anchorage teeth (Haas, 1961, Isaacson and Ingram, 1964, Haas, 1970, Wertz, 1970). At the same time, these forces also have an impact on other maxillofacial sutures, such as fronto-maxillary, zygomaticomaxillary, zygomaticotemporal, and pterygopalatine sutures (Starnbach et al., 1966).
RME is, particularly recommended in patients with obstructive pathologies of the upper airway tract, sleep disorders, and conductive hearing loss (Farronato et al., 2012a, Farronato et al., 2012b, Eichenberger and Baumgartner, 2014, Fagundes et al., 2017).
The effects of RME over nasal cavities, paranasal sinuses, and the upper respiratory tract have been investigated using bidimensional imaging (e.g., posteroanterior and lateral cephalograms) and functional tests (e.g., rhinomanometry, polysomnography, and so on) (Maspero et al., 2020a, Maspero et al., 2020b, Maspero et al., 2020c, Maspero et al., 2020d, Maspero et al., 2020e). Recently, the development of three-dimensional software to thoroughly analyze cone beam-computed tomography (CBCT) scans have allowed the measurement of changes in respiratory volumes (Farronato et al., 2019, Lanteri et al., 2020, Maspero et al., 2019a, Maspero et al., 2019b, Maspero et al., 2019c, Maspero et al., 2020a, Maspero et al., 2020b, Maspero et al., 2020c, Maspero et al., 2020d, Maspero et al., 2020e, Abate et al., 2020; Maspero, Farronato et al., 2020). Other possible exams that have been used to evaluate airway changes after RME are polysomnography (PSG) (Caprioglio et al., 2014) and magnetic resonance imaging (MRI) (Maspero et al., 2019a, Maspero et al., 2019b, Maspero et al., 2019c).
A rhinomanometric exam assesses nasal resistances to airflow and is the most-used test for proximal airway evaluation (Berretin-Felix et al., 2006, Baratieri et al., 2011). These diagnostic procedures show a significant decrease in nasal airway resistance, with consequent improvement in nasal breathing, after RME procedures (Compadretti et al., 2006, Enoki et al., 2006, Farronato et al., 2009, Maspero et al., 2009, Monini et al., 2009, Maspero et al., 2020a, Maspero et al., 2020b, Maspero et al., 2020c, Maspero et al., 2020d, Maspero et al., 2020e).
However, to our knowledge, no study has been published on the influence of RME on respiratory function, taking into account the lower airways. Pulmonary functional tests such as spirometry have been proved to be reliable tools in the evaluation of pathological respiratory conditions. These tests are aimed at assessing lower airway patency and functionality.
In this study, spirometry is used to assess whether RME could produce any change in commonly evaluated spirometric indices in two groups of young adults presenting transverse maxillary hypoplasia and either a nasal or oral breathing pattern.
2. Materials and methods
A retrospective case–control study was conducted on patients treated between 2014 and 2018 who underwent RME treatment and spirometry tests. The study was approved in the research project of year 2018O.U. N. 420/425 of Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano.
2.1. Types of participant and eligibility criteria
Records of 50 Caucasian patients with maxillary transverse hypoplasia were selected from the archives of the Orthodontics department of Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano (Italy). Informed consent for the use of their children’s records for research purposes was given by the parents of all patients.
Patients were divided into two groups: group I was composed of 25 patients (12 male and 13 female; mean age 15.2 ± 1.3 years old) with moderate to severe maxillary hypoplasia and mouth breathing disorder; group II was composed of 25 patients matched for age and sex (11 male and 14 female, mean age 14.9 ± 1.7 years old) with moderate to severe maxillary hypoplasia and a correct nasal breathing pattern. No patient suffered from any other chronic disease.
Spirometric evaluations (SpiroPro; SensorMedics, Viasys Healthcare, Inc., Yorba Linda, CA) were performed in the Pediatric department of the Policlinico Hospital of Milan. Measurements were taken with the patient both standing and laying down supine, in accordance with the American Thoracic Society guidelines (American Thoracic Society. Standardization of spirometry, 1994).
Patients performed maximal inspiration, then forced continuous expiration into the machine until residual volume was reached and then maximal inspiration again. The instrument automatically computes, given the patient’s age, sex, height, and weight (in kilograms), the predicted normal values for breathing function using regression equations.
Tight-fitting clothing were loosened and patients were instructed to keep a normal and relaxed occlusion.
The following parameters were evaluated:
-
–
Forced vital capacity (FVC), which is the maximum volume of air—expressed in milliliters (cm3)—that can be exhaled when blowing out as fast as possible;
-
–
Forced expiratory volume in the first second (FEV1) is the amount of air—expressed in liters (L)—exhaled in the first second of a forced expiration after maximal inhalation;
-
–
Tiffenau index (FEV1/ FVC ratio, IT%), which underlines the possible presence of obstructions (if the index decreases) or restrictions (if the index increases);
-
–
Forced expiratory flow at 25–75% of vital capacity (FEF 25–75); and
-
–
Tidal volume (TV), which is the amount of air—in liters—during quiet breathing (either inspiration or expiration).
For FVC and FEV1, the patients were asked to perform forced inspiration, as much as possible, and then to blow out as fast and as long as possible.
2.2. Rapid maxillary expansion
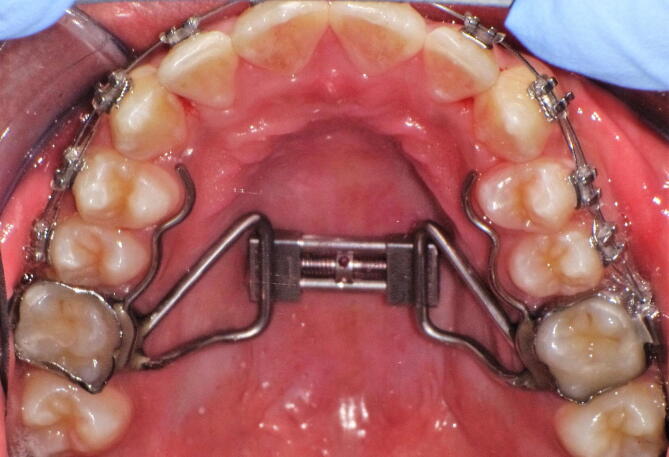
The two groups examined were treated with rapid palatal expansion using a Hyrax appliance (Fig. 1). The RME was bonded on the first permanent upper molars with glass-ionomer cement (Multi-Cure Glass Ionomer Cement; Unitek, Monrovia, CA, USA). The protocol for activation of the transversal screw consisted of four activations at the dental chair (1.0 mm of expansion). The parents of patients were instructed to perform two turns of the screw (0.5 mm) every day for the first week. Patients were visited each seven days, until desired expansion was achieved, setting the number of turns they would do each week. The expansion phase lasted between 10 and 16 days in all patients, in concordance with data from the literature (De Rossi, De Rossi et al., 2009, Maspero et al., 2009). Then, the screw was blocked with a light-cure composite resin (Premise Flowable; Kerr Corporation, Orange, CA, USA). The appliance was kept in situ for 6 months for retention.
Fig. 1.
Rapid palatal expander anchored to the permanent teeth.
Spirometry tests were performed for all patients before treatment and after treatment, during follow-up appointments 6 and 12 months after maxillary expansion, in order to evaluate changes and their stability in breathing function over time.
2.3. Statistical analysis
The statistical analysis was performed using the SPSS ® 17.00 software package for Windows (IBM Corporation, Sommers, NY).
The Shapiro–Wilk test was used to assess whether the data were normally distributed. As the data distribution appeared to be non-normal, non-parametric tests were used. Descriptive statistics are reported as mean and standard deviation.
Comparison between groups at the beginning (T0) and the end of the treatment (T2) was calculated using the Mann–Whitney U test. The Friedman test was used to verify the effect of treatment within groups and the post-hoc Wilcoxon signed-rank test with Bonferroni’s correction was used. P value < 0.05 was considered as statistically significant.
3. Results
3.1. Within-group comparisons: Oral breathers
Within-group comparison showed a significant improvement for FVC, FEV1, IT%, FEF 25–75%, and TV after treatment in oral breathers (Table 2).
Table 2.
Descriptive statistics and statistical comparison of spirometric indexes of the Oral breathers group at T0, T1 and T2. Values are expressed as Mean and Standard deviation (S.D.).
| Oral breathers group |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indicies | T0 before treatment |
T1 after 6 months |
T2 after 12 months |
Friedman Test | T0 vs T1 | T1 vs T2 | T0 vs T2 | |||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | p value | p value | p value | p value | |
| FVC | 4.25 | 0.52 | 4.65 | 0.81 | 4.67 | 0.82 | <0.001* | <0.001* | NS | <0.001* |
| FEV1 | 3.57 | 0.68 | 3.66 | 0.74 | 3.72 | 0.71 | <0.001* | NS | <0.001* | <0.001* |
| IT%(FEV1/CVF) | 78.44 | 6.18 | 78.76 | 6.78 | 79.71 | 6.88 | <0.001* | NS | <0.001* | <0.001* |
| FEF 25–75% | 95.15 | 6.06 | 100.25 | 8.34 | 102.03 | 8.13 | <0.001* | <0.001* | NS | <0.001* |
| TV | 502.54 | 59.90 | 568.33 | 61.76 | 587.50 | 67.52 | 0.002* | 0.012* | NS | 0.008* |
Abbreviations: FVC = Forced vital capacity; FEV1 = forced expiratory volume in the first second; IT% = Tiffenau index; FEF 25–75 = forced expiratory flow at 25–75% of vital capacity; TV = Tidal volume.
P value < 0.05*.
Not significant = NS.
The post-hoc Wilcoxon signed-rank test found the following results, comparing the evaluations performed at different follow-up appointments: FCV showed a statistically significant increase during the periods T0–T1 and T0–T2. No statistically significant increase from 6 to 12 months was found (Table 2).
A statistically significant difference was found for FEV1 after treatment with RME, between T1–T2 and T0–T2. No statistically significant difference was found between T0–T1 (Table 2).
The post-hoc test showed a statistically significant increase of the Tiffenau index (IT%) between T1–T2 and T0–T2. Tiffenau index (IT%) did not show any statistically significant difference between T0–T1.
FEF 25–75% showed a statistically significant increase during the periods T0–T1 and T0–T2.
No statistically significant difference was noticed in FEF 25–75% between T1–T2 (Table 2).
TV increased in a statistically significant way after maxillary expansion during the periods T0–T1 and T0–T2. From 6 to 12 months, no statistically significant difference in TV was found.
3.2. Within-group comparisons: Nasal breathers
Statistically significant differences for CVF, FEF 25–75%, and TV were found in the nasal breather group, while IT% and FEV1 showed no statistically significant difference after RME (Table 3).
Table 3.
Descriptive statistics and statistical comparison of spirometric indexes of the Nasal breathers group at T0, T1 and T2. Values are expressed as Mean and Standard deviation (S.D.).
| Nasal breathers group |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indicies | T0 before treatment |
T1 after 6 months |
T2 after 12 months |
Friedman Test | T0 vs T1 | T1 vs T2 | T0 vs T2 | |||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | p value | p value | p value | p value | |
| FVC | 4.60 | 0.79 | 4.71 | 0.77 | 4.68 | 0.79 | <0.05 | <0.001* | NS | <0.05* |
| FEV1 | 3.63 | 0.63 | 3.68 | 0.67 | 3.74 | 0.59 | NS | / | / | / |
| IT% (FEV1/CVF) | 78.95 | 6.17 | 77.83 | 4.23 | 79.79 | 3.97 | NS | / | / | / |
| FEF 25–75% | 98.95 | 6.04 | 102.33 | 8.54 | 103.87 | 7.44 | <0.001 | <0.001* | NS | <0.05* |
| TV | 538.96 | 60.61 | 579.34 | 67.65 | 584.35 | 57.04 | 0.002 | 0.012* | NS | 0.008* |
Abbreviations: FVC = Forced vital capacity; FEV1 = forced expiratory volume in the first second; IT% = Tiffenau index; FEF 25–75 = forced expiratory flow at 25–75% of vital capacity; TV = Tidal volume.
P value < 0.05 *.
Not significant = NS.
The post-hoc Wilcoxon signed-rank test found the following results, comparing spirometry indices over time: FCV showed a statistically significant increase during the periods T0–T1 and T0–T2. No statistically significant difference from 6 to 12 months was noticed in FCV (Table 3).
FEF 25–75% showed a statistically significant increase. The post-hoc test showed a significant increase during the periods T0–T1 and T0–T2 (Table 3).
A statistically significant difference was highlighted after maxillary expansion during the periods T0–T1 and T0–T2 for TV measurements. From 6 to 12 months, no statistically significant difference was noticed in TV (Table 3).
3.3. Between-groups comparison: T0 and T2
FVC, FEF 25–75%, and TV showed statistically significant differences at time T0 between the oral breather and nasal breather groups (Table 1), while FEV1 and IT% did not show any statistically significant differences between the two groups at T0.
Table 1.
Spirometric indexes with descriptive statistics and comparison with Mann – Whitney U test between oral breathers (OB group) and nasal breathers (NB group) at T0. Values are expressed as Mean and Standard deviation (S.D.)
| Oral breathers group T0 |
Nasal breathers group T0 |
||||
|---|---|---|---|---|---|
| Indicies | Mean | S.D. | Mean | S.D. | p value |
| FVC | 4.25 | 0.52 | 4.60 | 0.63 | <0.05* |
| FEV1 | 3.33 | 0.68 | 3.63 | 0.63 | NS |
| IT%(FEV1/CVF) | 78.44 | 6.18 | 78.95 | 6.17 | NS |
| FEF 25–75% | 95.15 | 6.06 | 98.95 | 6.04 | <0.05* |
| TV | 502.54 | 59.90 | 538.96 | 60.61 | <0.05* |
Abbreviations: FVC = Forced vital capacity; FEV1 = forced expiratory volume in the first second; IT% = Tiffenau index; FEF 25–75 = forced expiratory flow at 25–75% of vital capacity; TV = Tidal volume.
P value < 0.05 *.
Not significant = NS.
All spirometric indices did not show any statistically significant difference 12 months after maxillary expansion between the oral breather and nasal breather groups (Table 4).
Table 4.
Spirometric indexes with descriptive statistics and comparison with Mann-Whitney test between Oral breathers (OB group) and Nasal breathers (NB group) at T2. Values are expressed as Mean and Standard deviation (S.D.)
| Oral breathers group T2 |
Nasal breathers group T2 |
||||
|---|---|---|---|---|---|
| Indicies | Mean | S.D. | Mean | S.D. | p value |
| FVC | 4.67 | 0.82 | 4.68 | 0.79 | NS |
| FEV1 | 3.72 | 0.71 | 3.74 | 0.59 | NS |
| IT% (FEV1/CVF) | 78.44 | 6.18 | 79.79 | 3.97 | NS |
| FEF 25–75% | 102.03 | 8.13 | 103.87 | 7.44 | NS |
| TV | 587.50 | 67.52 | 584.35 | 57.04 | NS |
Abbreviations: FVC = Forced vital capacity; FEV1 = forced expiratory volume in the first second; IT% = Tiffenau index; FEF 25–75 = forced expiratory flow at 25–75% of vital capacity; TV = Tidal volume.
P value < 0.05 *.
Not significant = NS.
4. Discussion
Correlations between the skeletal effects of RME and respiratory function have been extensively studied in the literature.
Several studies have reported the positive effects of RME on the airflow and proximal airway resistances in subjects with maxillary hypoplasia (Berretin-Felix et al., 2006, Farronato et al., 2008, Smith et al., 2012, Chang et al., 2013, Maspero et al., 2014, Pereira-Filho et al., 2014; Maspero, Abate et al., 2020, Fama et al., 2020). Haas, in 1961, observed considerable changes in the nasal-maxillary complex after RME (Haas, 1961). Catar Wertz (1968) suggested that nasal stenosis located in the anterior–inferior portion of nasal chambers would improve after maxillary suture opening (Wertz, 1968). In 2014, Hakan et al., also confirmed that RME led to a significant increase in nasal passage airway volume (El and Palomo, 2014). Many studies have demonstrated that patients with maxillary constriction tend to have a higher nasal airway resistance (Farronato et al., 2011, Maspero et al., 2015). RME, by separating the external walls of nasal cavities, causes lowering of the palatal vault and straightening of the nasal septum, consequently inducing an increase in nasal volume and nasal airflow in breathing and decreasing nasal resistances (Farronato et al., 2012a, Farronato et al., 2012b, Lo Giudice et al., 2017). How these events influence breathing, however, have still not yet been investigated.
All parameters of breathing function (FVC, FEV1, FEF 25–75%) showed a statistically significant improvement after treatment in both studied groups. Increases in FEV1 and IT% were statistically significant only in the oral breather group (Table 2).
FVC value is usually reduced in restrictive disorders (Venkateshiah et al., 2008) and may be reduced due to severe airflow obstruction and air trapping (El-Helaly et al., 2012, Y Baena et al., 2013, Lucchese et al., 2011). FVC showed, as expected, a significant difference between the two groups before RME (Miller et al., 2005; Pellegrino et al., 2005). After treatment with RME, both groups showed a statistically significant increase in all variables, due to less airflow obstruction, while FVC showed no statistical difference between groups (Table 2, Table 3).
FEV1 in oral breathers is affected by mechanical obstruction of the upper and lower airways. FEV1 is the most widely used functional index in asthma follow-up, in order to assess the impact of increased respiratory resistances on breathing function (Moeller et al., 2015, Gallucci et al., 2019). Children with asthma and FEV1 < 60% have a double risk of asthma exacerbations in the following year, compared to those with FEV1 > 80%. Even though both groups analyzed started with FEV1 values of about 80% with no significant difference, FEV1 showed a statistically significant increase in the group of oral respiratory patients only. This result could be explained by considering that palatal expansion led to a greater benefit in the oral breather group, by decreasing the peripheral resistances that had previously increased due to upper airways being reduced in size. This condition was not present in the nasal breather control group. Nevertheless, no statistically significant difference in T2 was reported between the two groups.
Normal values of the Tiffenau index (IT%) are approximately 75%, depending on age, sex, height, and ethnicity.
In obstructive pulmonary pathologies, FEV1 is reduced due to the obstruction of the air coming out of the lungs; for this reason, IT% is also reduced. In the samples analyzed in the present study, IT% started with normal values in both groups. IT% and FEV1 had a statistically significant improvement only in the oral breather group. The reason for this can be traced back to the previously explanation for the FEV1 index.
According to most guidelines, FEF is recommended for subjects with severe asthma or with poor perception of airflow limitation. FEF 25–75% values are significantly correlated with nasal obstruction or nasal symptoms alone (Bateman et al., 2008; Traini et al., 2011). Both FEV1 and FEF 25–75% are usually reduced in children with moderate to severe persistent allergic rhinitis. FEF measurement can be used to document the variability of bronchial obstruction in asthma and is useful in evaluating the progression of asthma (Reddel et al., 2005, Scichilone et al., 2013). FEF 25–75% values improved statistically in the two groups after treatment, revealing that the maximal expiratory flow peak increased. No statistically meaningful difference was noted at T2 between the two groups, indicating that the group of oral breathers reached normal values, as shown in Table 4.
In this study, follow-up controls of the mouth and nasal breather sample treated with RME confirmed its tendency to increase tidal respiratory volume (TV) by improving the amount of air volume inhaled or exhaled during quiet breathing. At time T2, the two groups did not show any significant differences. Therefore, the correction of transverse maxillary hypoplasia by palatal expansion could have beneficial organismic repercussions on respiratory muscles, as well. By considering the improvement of all of the parameters considered in this study, it is possible to conclude that rapid maxillary expansion improved the expiratory capacity significantly in both groups.
Oral breathers benefited from the expansion in a more significant way than nasal breathers. Indeed, IT% and FEV1 showed a statistically significant increase only in this group. The comparison between the two groups did not show significant differences at T2 for all spirometric indices.
5. Conclusions
Treatment with rapid palatal expansion had beneficial effects in both nasal and oral breathers. Meaningful statistical differences were not found after 12 months of treatment between groups. FEV1 and IT%, among the spirometric indices, showed a significant improvement only in oral breathers. This was probably due to a starting condition with increased peripheral resistances in oral breathers. RME treatment led to a decrease in peripheral resistance with beneficial effects on lung ventilation and the respiratory act, in general. Forced vital capacity (FVC), forced expiratory flow at 25–75% of vital capacity (FEF 25–75), and Tidal volume (TV) reached values similar to those of nasal breathers after treatment with RME.
The present study should be considered a pilot study for the spirometric evaluation of the effects of RME in oral breathers. For this reason, further studies using a larger sample size are required.
Author contribution statement
A.F. and A.A. conceived the protocol and revised the manuscript. M.M, D.C. and A.F. contributed to the data acquisition and processing. D.C. and A.A. contributed to the statistical analysis. D.L. and F.A. performed the final revision. All authors read and approved the final version of the manuscript.
Ethical approval
A retrospective case control study was performed analyzing spirometric exam of oral breathers patients and comparing them with healthy controls. This study was approved by the appropriate Institutional Review Board (IRB) within the research project of the year 2018O.U.N. 420/425 of Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano. All procedures performed in this retrospective study involving human participants were in accordance with the ethical standards of the institutional and/or research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patient’s parents, have given their informed consent to allow using their medical records in anonymous form for research purposes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abate A., Cavagnetto D., Fama A., Matarese M., Bellincioni F., Assandri F. Efficacy of Operculectomy in the Treatment of 145 Cases with Unerupted Second Molars: A Retrospective Case-Control Study. Dent. J. 2020;8:65. doi: 10.3390/dj8030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloufi, F., Preston, C.B., Zawawi, K.H., 2012. Changes in the Upper and Lower Pharyngeal Airway Spaces Associated with Rapid Maxillary Expansion. ISRN Dent. Epub. 10.5402/2012/290964. [DOI] [PMC free article] [PubMed]
- Baratieri C., Alves M., De Souza M.M.G., De Souza Araújo M.T., Maia L.C. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing? Am. J. Orthod. Dentofac. Orthop. 2011;140:146–156. doi: 10.1016/j.ajodo.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Bateman E.D., Hurd S.S., Barnes P.J., Bousquet J., Drazen J.M., FitzGerald M., Gibson P., Ohta K., O’Byrne P., Pedersen S.E. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- Berretin-Felix G., Yamashita R.P.P., Nary Filho H., Gonales E.S.S., Trindade A.S., Jr, Trindade I.E.K.E.K., Filho H.N., Gonales E.S.S., Trindade A.S., Trindade I.E.K.E.K., Nary Filho H., Gonales E.S.S., Trindade A.S., Jr, Trindade I.E.K.E.K. Short- and long-term effect of surgically assisted maxillary expansion on nasal airway size. J. Craniofac. Surg. 2006;17:1045–1049. doi: 10.1097/01.scs.0000234987.84615.0c. [DOI] [PubMed] [Google Scholar]
- Caprioglio A., Meneghel M., Fastuca R., Zecca P.A., Nucera R., Nosetti L. Rapid maxillary expansion in growing patients: correspondence between 3-dimensional airway changes and polysomnography. Int. J. Pediatr. Otorhinolaryngol. 2014;78:23–27. doi: 10.1016/j.ijporl.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Chang Y., Koenig L.J., Pruszynski J.E., Bradley T.G., Bosio J.A., Liu D. Dimensional changes of upper airway after rapid maxillary expansion: a prospective cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2013;143:462–470. doi: 10.1016/j.ajodo.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Compadretti G.C., Tasca I., Bonetti G.A. Nasal airway measurements in children treated by rapid maxillary expansion. Am. J. Rhinol. 2006;20:385–393. doi: 10.2500/ajr.2006.20.2881. [DOI] [PubMed] [Google Scholar]
- De Rossi M., De Rossi A., Hallak J.E.C., Vitti M., Regalo S.C.H. Electromyographic evaluation in children having rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2009;136:355–360. doi: 10.1016/j.ajodo.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Eichenberger M., Baumgartner S. The impact of rapid palatal expansion on children’s general health: a literature review. Eur. J. Paediatr. Dent. 2014;15:67–71. [PubMed] [Google Scholar]
- El-Helaly N., Samy S.M., Ibrahim T.S., Morcos W.M., El-Hoshy H.M., Mohamed D.A. Pulmonary function changes in allergic rhinitis with or without bronchial asthma. J. Am. Sci. 2012;8:110–114. [Google Scholar]
- El H., Palomo J.M. Three-dimensional evaluation of upper airway following rapid maxillary expansion A CBCT study. Angle Orthod. 2014;84:265–273. doi: 10.2319/012313-71.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki C., Valera F.C.P., Lessa F.C.R., Elias A.M., Matsumoto M.A.N., Anselmo-Lima W.T. Effect of rapid maxillary expansion on the dimension of the nasal cavity and on nasal air resistance. Int. J. Pediatr. Otorhinolaryngol. 2006;70:1225–1230. doi: 10.1016/j.ijporl.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Fagundes N.C.F., Rabello N.M., Maia L.C., Normando D., Mello K.C.F.R. Can rapid maxillary expansion cause auditory improvement in children and adolescents with hearing loss? A systematic review. Angle Orthod. 2017;87:886–896. doi: 10.2319/021517-111.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama, A., Cavagnetto, D., Abate, A., Mainardi, E., De Filippis, A., Esposito, L., 2020. Treatment of dental dilacerations. Narrative review. Dent. Cadmos, in press.
- Farronato G., Giannini L., Galbiati G., Maspero C. Comparison of the dental and skeletal effects of two different rapid palatal expansion appliances for the correction of the maxillary asymmetric transverse discrepancies. MinervaStomatol. 2012;61:45–55. [PubMed] [Google Scholar]
- Farronato G., Giannini L., Galbiati G., Maspero C. RME: influences on the nasal septum. MinervaStomatol. 2012;61:125–134. [PubMed] [Google Scholar]
- Farronato G., Maspero C., Esposito L., Briguglio E., Farronato D., Giannini L. Rapid maxillary expansion in growing patients. Hyrax versus transverse sagittal maxillary expander: a cephalometric investigation. Eur. J. Orthod. 2011;33:185–189. doi: 10.1093/ejo/cjq051. [DOI] [PubMed] [Google Scholar]
- Farronato G., Maspero C., Farronato D., Giannini L. Modified Hyrax expander for correction of upper midline deviation. J. Clin. Orthod. 2009;43:158–160. [PubMed] [Google Scholar]
- Farronato G., Maspero C., Giannini L., Farronato D. Occlusal splint guides for presurgical orthodontic treatment. J. Clin. Orthod. 2008;42:508–512. [PubMed] [Google Scholar]
- Farronato M., Cavagnetto D., Abate A., Cressoni P., Fama A., Maspero C. Assessment of condylar volume and ramus height in JIA patients with unilateral and bilateral TMJ involvement: retrospective case-control study. Clin. Oral Investig. 2019;24:2635–2643. doi: 10.1007/s00784-019-03122-5. [DOI] [PubMed] [Google Scholar]
- Lanteri V., Farronato M., Ugolini A., Cossellu G., Gaffuri F., Parisi F.M.R., Cavagnetto D., Abate A., Maspero C. Volumetric changes in the upper airways after rapid and slow maxillary expansion in growing patients: a case-control study. Materials. 2020;13:2239. doi: 10.3390/ma13102239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci M., Carbonara P., Pacilli A.M.G., di Palmo E., Ricci G., Nava S. Use of symptoms scores, spirometry, and other pulmonary function testing for asthma monitoring. Front. Pediatr. 2019;7:54. doi: 10.3389/fped.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A.J. Palatal expansion: Just the beginning of dentofacial orthopedics. Am. J. Orthod. 1970;57:219–255. doi: 10.1016/0002-9416(70)90241-1. [DOI] [PubMed] [Google Scholar]
- Haas A.J. Rapid expansion of the maxillary dental arch and nasal cavity by opening the midpalatal suture. Angle Orthod. 1961;31:73–90. doi: 10.1043/0003-3219(1961)031<0073:REOTMD>2.0.CO;2. [DOI] [Google Scholar]
- Isaacson R.J., Ingram A.H. Forces produced by rapid maxillary expansion: II. Forces present during treatment. Angle Orthod. 1964;34:261–270. doi: 10.1043/0003-3219(1965)035<0178:FPBRME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lo Giudice A., Fastuca R., Portelli M., Militi A., Bellocchio M., Spinuzza P., Briguglio F., Caprioglio A., Nucera R. Effects of rapid vs slow maxillary expansion on nasal cavity dimensions in growing subjects: a methodological and reproducibility study. Eur. J. Paediatr. Dent. 2017;18:299–304. doi: 10.23804/ejpd.2017.18.04.07. [DOI] [PubMed] [Google Scholar]
- Lucchese A., Carinci F., Brunelli G., Monguzzi R. An in vitro study of resistance to corrosion in brazed and laser welded orthodontic. Eur. J. Inflamm. 2012;9:67–72. [Google Scholar]
- Lucchese A., Carinci F., Brunelli G., Monguzzi R. Everstick® and Ribbond® fiber reinforced composites: Scanning Electron Microscope (SEM) comparative analysis. Eur. J. Inflamm. 2011;9:73–79. [Google Scholar]
- Lucchese A., Carinci F., Brunelli G. Skeletal effects induced by twin block in therapy of class II malocclusion. Eur. J. Inflamm. 2012;10:83–87. [Google Scholar]
- Manuelli M. On line is the future. Prog. Orthod. 2012;13:201. doi: 10.1016/j.pio.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Maspero C., Abate A., Bellincioni F., Cavagnetto D., Lanteri V., Costa A., Farronato M. Comparison of a tridimensional cephalometric analysis performed on 3T-MRI compared with CBCT: a pilot study in adults. Prog. Orthod. 2019;20:40. doi: 10.1186/s40510-019-0293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maspero C., Abate A., Cavagnetto D., El Morsi M., Fama A., Farronato M. Available technologies, applications and benefits of teleorthodontics. A literature review and possible applications during the COVID-19 pandemic. J. Clin. Med. 2020;9:1891. doi: 10.3390/jcm9061891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maspero, C, Abate, A., Cavagnetto, D., Fama, A., Stabilini, A., Farronato, G., Farronato, M., 2019. Operculectomy and spontaneous eruption of impacted second molars: a retrospective study. J. Biol. Regul. Homeost. Agents 33, 1909–1912. 10.23812/19-302-L. [DOI] [PubMed]
- Maspero C., Cavagnetto D., Fama A., Giannini L., Galbiati G., Farronato M. Hyrax versus transverse sagittal maxillary expander: an assessment of arch changes on dental casts. A retrospective study. Saudi Dent. J. 2020;32:93–100. doi: 10.1016/j.sdentj.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maspero C., Cavagnetto D., Abate A., Cressoni P., Farronato M. Effects on the facial growth of rapid palatal expansion in growing patients affected by juvenile idiopathic arthritis with monolateral involvement of the temporomandibular joints: a case-control study on posteroanterior and lateral cephalograms. J. Clin. Med. 2020;9:1159. doi: 10.3390/jcm9041159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maspero C., Fama A., Cavagnetto D., Abate A., Farronato M. Treatment of dental dilacerations. J. Biol. Regul. Homeost Agents. 2019;33:1623–1628. [PubMed] [Google Scholar]
- Maspero C., Farronato M., Bellincioni F., Annibale A., Machetti J., Abate A., Cavagnetto D. Three-dimensional evaluation of maxillary sinus changes in growing subjects: a retrospective cross-sectional study. Materials (Basel) 2020;13:1007. doi: 10.3390/ma13041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maspero C., Farronato C., Bellincioni F., Abate A. Assessing mandibular body changes in growing subjects: a comparison of CBCT and reconstructed lateral cephalogram measurements. Sci. Rep. 2020;10:11722. doi: 10.1038/s41598-020-68562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maspero C., Galbiati G., Giannini L., Farronato G. Sagittal and vertical effects of transverse sagittal maxillary expander (TSME) in three different malocclusion groups. Prog. Orthod. 2015;25:16. doi: 10.1186/s40510-015-0075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maspero C., Galbiati G., Perillo L., Favero L., Giannini L. Orthopaedic treatment efficiency in skeletal Class III malocclusions in young patients: RME-face mask versus TSME. Eur. J. Paediatr. Dent. 2012;13:225–230. [PubMed] [Google Scholar]
- Maspero C., Giannini L., Riva R., Tavecchia M.G., Farronato G. Nasal cycle evaluation in 10 young patients: Rhynomanometric analysis. Mondo Ortod. 2009;34:263–268. doi: 10.1016/j.mor.2008.11.001. [DOI] [Google Scholar]
- Maspero C., Prevedello C., Giannini L., Galbiati G., Farronato G. Atypical swallowing: a review. MinervaStomatol. 2014;63:217–227. [PubMed] [Google Scholar]
- McNamara J.A., Lione R., Franchi L., Angelieri F., Cevidanes L.H.S., Darendeliler M.A., Cozza P. The role of rapid maxillary expansion in the promotion of oral and general health. Prog. Orthod. 2015;16:33. doi: 10.1186/s40510-015-0105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M.R., Hankinson, J., Brusasco, V., Burgos, F., Casaburi, R., Coates, A., Crapo, R., Enright, P. vd, Van der Grinten, C.P.M., Gustafsson, P., 2005. Standardisation of spirometry. Eur. Respir. J. 26, 319–338. [DOI] [PubMed]
- Moeller A., Carlsen K.H., Sly P.D., Baraldi E., Piacentini G., Pavord I., Lex C., Saglani S., Brand P.L.P., Eber E., Frischer T., Hedlin G., Kulkarni N., Lødrup Carlsen K.C., Mäkelä M.J., Mantzouranis E., Pijnenburg M.W., Price D., Rottier B.L., Szefler S.J., Turner S., Wooler E. Monitoring asthma in childhood: Lung function, bronchial responsiveness and inflammation. Eur. Respir. Rev. 2015;24:204–215. doi: 10.1183/16000617.00003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monini S., Malagola C., Villa M.P., Tripodi C., Tarentini S., Malagnino I., Marrone V., Lazzarino A.I., Barbara M. Rapid maxillary expansion for the treatment of nasal obstruction in children younger than 12 years. Arch. Otolaryngol. Head Neck Surg. 2009;135:22–27. doi: 10.1001/archoto.2008.521. [DOI] [PubMed] [Google Scholar]
- Traini T., Danza M., Zollino I., Altavilla R., Lucchese A., Sollazzo V., Trapella G., Brunelli G., Carinci F. Histomorphometric evaluation of an immediately loaded implant retrieved from human mandible after 2 years. Int. J. Immunopathol. Pharmacol. 2011;24:31–36. doi: 10.1177/03946320110240S207. [DOI] [PubMed] [Google Scholar]
- Pereira-Filho V.A., Monnazzi M.S., Gabrielli M.A.C., Spin-Neto R., Watanabe E.R., Gimenez C.M.M., Santos-Pinto A., Gabrielli M.F.R. Volumetric upper airway assessment in patients with transverse maxillary deficiency after surgically assisted rapid maxillary expansion. Int. J. Oral Maxillofac. Surg. 2014;43:581–586. doi: 10.1016/j.ijom.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Reddel H.K., Vincent S.D., Civitico J. The need for standardisation of peak flow charts. Thorax. 2005;60:164–167. doi: 10.1136/thx.2004.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scichilone N., Contoli M., Paleari D., Pirina P., Rossi A., Sanguinetti C.M., Santus P., Sofia M., Sverzellati N. Assessing and accessing the small airways; implications for asthma management. Pulm. Pharmacol. Ther. 2013;26:172–179. doi: 10.1016/j.pupt.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Smith T., Ghoneima A., Stewart K., Liu S., Eckert G., Halum S., Kula K. Three-dimensional computed tomography analysis of airway volume changes after rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2012;141:618–626. doi: 10.1016/j.ajodo.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Starnbach H., Bayne D., Cleall J., Subtelny J.D. Facioskeletal and dental changes resulting from rapid maxillary expansion. Angle Orthod. 1966;36:152–164. doi: 10.1043/0003-3219(1966)036<0152:FADCRF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Thilander B., Wahlund S., Lennartsson B. The effect of early interceptive treatment in children with posterior cross-bite. Eur. J. Orthod. 1984;6:25–34. doi: 10.1093/ejo/6.1.25. [DOI] [PubMed] [Google Scholar]
- Vale F., Albergaria M., Carrilho E., Francisco I., Guimarães A., Caramelo F., Maló L. Efficacy of rapid maxillary expansion in the treatment of obstructive sleep Apnea syndrome: a systematic review with meta-analysis. J. Evid. Based. Dent. Pract. 2017;17:159–168. doi: 10.1016/j.jebdp.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Venkateshiah S.B., Ioachimescu O.C., McCarthy K., Stoller J.K. The utility of spirometry in diagnosing pulmonary restriction. Lung. 2008;186:19–25. doi: 10.1007/s00408-007-9052-8. [DOI] [PubMed] [Google Scholar]
- Wertz R.A. Skeletal and dental changes accompanying rapid midpalatal suture opening. Am. J. Orthod. 1970;58:41–66. doi: 10.1016/0002-9416(70)90127-2. [DOI] [PubMed] [Google Scholar]
- Wertz R.A. Changes in nasal airflow incident to rapid maxillary expansion. Angle Orthod. 1968;38:1–11. doi: 10.1043/0003-3219(1968)038<0001:CINAIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Y Baena, R.R., Lupi, S.M., Pastorino, R., Maiorana, C., Lucchese, A., Rizzo, S., 2013. Radiographic evaluation of regenerated bone following poly(lactic-co-glycolic) acid/hydroxyapatite and deproteinized bovine bone graft in sinus lifting. J. Craniofac. Surg. 24, 845-848. 10.1097/SCS.0b013e31827ca01a. [DOI] [PubMed]