Abstract
The objective of this article is to review the role of the dentist in the early diagnosis of pediatric obstructive sleep apnea (OSA) and to provide an in-depth review of the best evidence-based practices available to treat and/or to refer these patients for intervention.
Material and methods
A narrative review was performed using indexed data bases (PubMed, Medline, EMBASE, OVID, Scopus and Cochrane) up to year 2020, and approximately 1000 articles were reviewed. The articles included were those with the best information provided.
Results
Detailed review of the literature suggests that the role of the dentist has been redefined owing to their expertise in the orofacial region. Every patient consulting a dental practice is not merely a dental patient; he/she also requires a comprehensive medical review. The role of the dentist is pivotal in pediatric patients once diagnosed with OSA; as the patients grow, growth modification can be achieved, and future management will be easier. Initiating dental treatments during growth can benefit patients two-fold, saving them from malocclusion, and intervening in orofacial structural growth can help to avoid cumbersome treatments, such as CPAP and various surgeries. Proper diagnosis and management of systemic illnesses can prevent compromised quality of life, delays in treatment, morbidity and, in some cases, mortality.
Keywords: Pediatric, Obstructive sleep apnea (OSA), Continuous positive airway pressure (CPAP), Snoring, Apnea, Polysomnography (PSG), Rapid maxillary expansion (RME)
1. Introduction
The Mayo Clinic defines OSA as a sleep disorder in which a person transiently stops breathing while he or she is sleeping (Park et al., 2011). Snoring is a recognizable symptom of OSA in these patients (Lumeng and Chervin, 2008, Park et al., 2011, Heffernan et al., 2019). As the name implies, OSA is characterized by recurrent episodes of partial or complete obstruction of the airway at the pharyngeal level, resulting in intermittent hypoxia and cortical arousal (Ersu et al., 2004, Drager et al., 2013, Hoth et al., 2013, Jo et al., 2020). OSA manifests as a change in normal sleep patterns that includes routine snoring, sleep difficulties, agitated sleep, frequent postural changes, multiple arousals, and sleep fragmentation (multiple episodes of sleep interruptions) (Ersu et al., 2004, Chang and Chae, 2010). The older population is affected most frequently, with approximately 42% of the adult population suffering at any given time, whereas middle-aged men and women show a prevalence of 4% and 2%, respectively, and children constitute 2% to 3.5% of the total (Gislason and Benediktsdottir, 1995, Lewin et al., 2002, Shin et al., 2003, Schlaud et al., 2004, Guilleminault et al., 2005, Chang and Chae, 2010, Park et al., 2011). A large difference in gender was not reported for the prevalence of OSA in children except among adolescent boys. Higher levels of weight gain may be responsible for the increased prevalence of OSA in adolescent boys (Goodwin et al., 2003, Chang and Chae, 2010). Redline et al., suggested that an increase in body mass index (BMI) 1 kg/m2 above the mean increases the risk of developing OSA in children by 12%. Additionally, these children have larger neck circumferences than healthy children (Redline et al., 1999). Children who continued breastfeeding for more than a month showed a lower risk of witnessed sleep apnea compared than children who never breastfed or breastfed for less than a month (Brew et al., 2014).
In adult patients, male sex and obesity are the main risk factors, whereas in children, adenotonsillar hypertrophy is the leading cause (Rosen, 1999, Pirilä-Parkkinen et al., 2009, Bhattacharjee et al., 2010, Chang and Chae, 2010) (Fig. 1). However, despite a vast number of healthy children exhibiting adenotonsillar hypertrophy, only a few develop OSA (Goodwin et al., 2003, Bhattacharjee et al., 2010). Many genetic disorders and syndromes can also cause OSA, such as trisomy 21, achondroplasia, Pierre Robin anomaly, Apert syndrome, Crouzon syndrome, Treacher Collins syndrome, Turner syndrome, cleft palate, and cerebral palsy (Quo et al., 2017). Children with autism spectrum disorder (ASD) exhibit significantly more sleep disorders, including OSA and parasomnias, than pre-school children of the same age (Hirata et al., 2016). When snoring is not related to ventilatory complications, such as apnea, hypopnea, hypoxia, or hypercapnia, it is termed primary snoring (Topol and Brooks, 2001, Chang and Chae, 2010). It is very rare that primary snoring leads to OSA in children (McColley, 2005). If the child's snoring is not related to hypoxia or apnea, typically it does not require any further intervention or treatment (Rosen, 1999, McColley, 2005).
Fig. 1.
Airway Anatomy showing normal anatomy and smooth breathing on the right side and structural abnormalities on the left side illustrate that different levels of airway suggest more difficult and noisier breathing.
2. Materials and methods
The objective of this paper was to review extensively the literature on the dental diagnosis, clinical features and management of PED OSA. A narrative review was performed using indexed data bases (PubMed, Medline, EMBASE, OVID, Scopus and Cochrane) up to year 2020, and approximately 1000 articles were reviewed. The initial search was done without language and time barrier using various combinations of the following words (a) sleep apnea, (b) pediatric (c) obstructive sleep apnea (d) diagnosis + pediatric + obstructive + sleep + apnea + management (d) rapid maxillary expansion.
3. Clinical features
Clinical features of OSA vary among different pediatric age groups and can be divided into four different categories: infants (3–12 months), toddlers (1–3 years), preschool-age children and school-age children (Guilleminault et al., 2005). Most of the concerns are reported by parents, given that the children themselves can only recognize and report the complaints and symptoms at an older age. Thus, it is important to interview parents/caregivers systemically to obtain the best clinical picture of the patients (Guilleminault et al., 2005). The most common symptoms in children suffering from OSA are snoring and labored breathing (Brouillette et al., 1982, Rosen, 1999, Goodwin et al., 2003, Bhattacharjee et al., 2010). In addition to snoring, children with OSA have hypoxemia and experience hypoventilation, sleep disruption, and poor gas exchange (Rosen, 1999, Stauffer et al., 2018). Moreover, daytime fatigue and daytime sleepiness may also be seen, along with chronic snoring (Guilleminault et al., 2005, Iwasaki et al., 2009, Marcus et al., 2012). Nocturnal sweating, heavy snoring, paradoxical breathing, mouth breathing, and fragmented and agitated sleep are also reported by parents (Guilleminault et al., 2005, Chang and Chae, 2010). Drooling while sleeping, sleep terrors, sleep walking, and enuresis (involuntary urination) can also be observed in children with OSA (Guilleminault et al., 2005, Alexopoulos et al., 2014). These patients may exhibit a spectrum of behavioral changes depending on their age group. Usually, toddlers and preschool-aged children show hyperactivity, while children who are school-aged present with aggressiveness, learning difficulties, and poor academic performance (Ali et al., 1994, Lewin et al., 2002, Goodwin et al., 2003, Guilleminault et al., 2005, Chang and Chae, 2010, Behrents et al., 2019). Children with severe OSA may also show developmental delays and failure to thrive (Brouillette et al., 1982, Chan et al., 2004). Parents of children with OSA report children gasping for air, episodes of apnea and labored breathing while sleeping (Marcus et al., 2012). Morning headaches, sleep enuresis, and hyperextended neck posture while sleeping are also common findings (Marcus et al., 2012, Alexopoulos et al., 2014, Behrents et al., 2019) (Fig. 2). Children with nasal obstruction exhibit the hyponasal voice, whereas children with adenotonsillar hypertrophy demonstrate a muffled voice (Muzumdar and Arens, 2008).
Fig. 2.
Clinical features of OSA in different pediatric age groups Chang and Chae, 2010.
3.1. Dentoskeletal changes: Dentofacial and orthopedic features of children with OSA
It is imperative for pediatric dentists to look for the following dentofacial and orthopedic features in every child they are treating to enable timely diagnosis. Children diagnosed with OSA have shown distinctive features of both soft and hard tissues along with specific dental characteristics. The most common cause of OSA in children is hypertrophy of the tonsils, both palatal and lingual (Pirilä-Parkkinen et al., 2009, Cohen-Lévy et al., 2009, Seailles et al., 2009, Katyal et al., 2013). Adenotonsillar hypertrophy is followed by Mallampati deviation classes III and IV (Nuckton et al., 2006). The Mallampatti score is a score based on anatomical structures that are visualized by opening the mouth and protruding the tongue, and it is widely used as a predictor of OSA. The higher the Mallampati score, the greater the severity of OSA, provided the Mallampati score is measured accurately (Nuckton et al., 2006). Some children with OSA also exhibit an elongated soft palate (Guilleminault et al., 1996, Cakirer et al., 2001, Marino et al., 2009), a reduced airway space, a longer and thicker soft palate, a long and large tongue, and an inferior positioned hyoid bone (Tangugsorn et al., 1995, Cakirer et al., 2001, Vieira et al., 2011).
From an orthodontic point of view, there is a predominantly class II malocclusion pattern in children with OSA (Baik et al., 2002, Cozza et al., 2004, Marino et al., 2009, Pirilä-Parkkinen et al., 2009). Children with OSA present high arched and narrow palates and have long face profiles (Defabjanis, 2004, Katz and D'Ambrosio, 2008, Moré et al., 2011, Marcus et al., 2012). OSA-diagnosed children also demonstrate retrognathism of the mandible, mid-face hypoplasia or both, which ultimately force the tongue to fall back into the upper airway (Cohen-Lévy et al., 2009). The spectrum of prominent malocclusion features associated with children with OSA include greater overjet, less overbite, unilateral or bilateral open bite/cross bite and mandibular crowding. (Pirilä-Parkkinen et al., 2009, Cohen-Lévy et al., 2009, Moré et al., 2011). Deng et al., reported retrognathism of the mandible, a long lower face and a deficient/short chin as a few important causes of childhood OSA (Deng and Gao, 2012). Mandibular crowding is directly proportional to the apnea–hypopnea index (AHI) (Pirilä-Parkkinen et al., 2009). Interestingly, patients with class III malocclusion show more intraoral airway space and larger oropharyngeal airway compared than those with class I malocclusion, thus making them less prone to OSA (Iwasaki et al., 2009).
4. Diagnosis of OSA
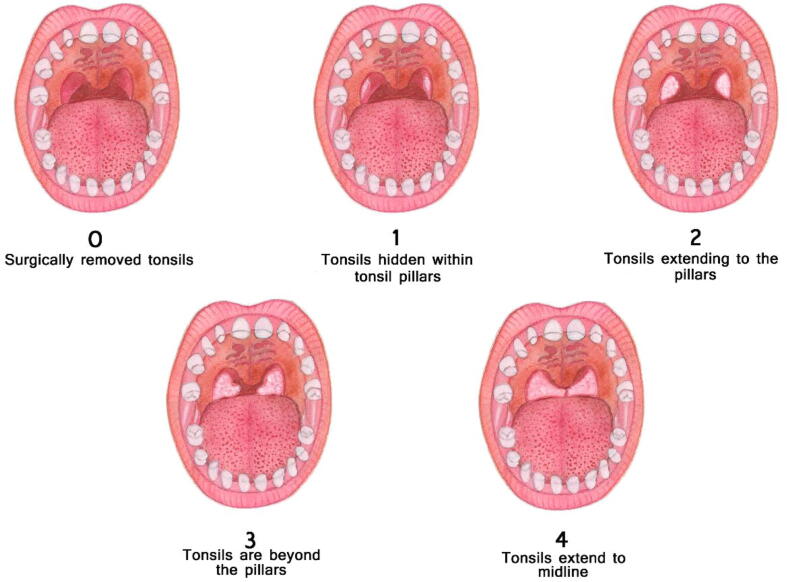
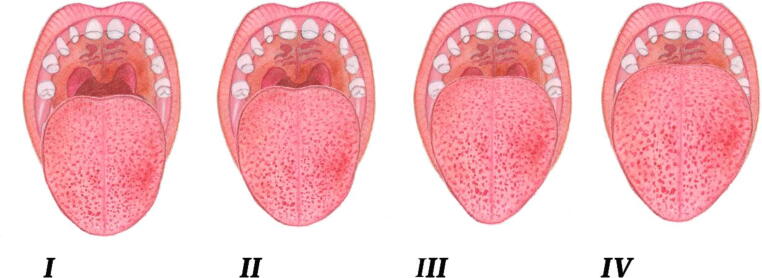
Accurate and prompt diagnosis of OSA is imperative for timely treatment and avoidance of additional complications caused by OSA. A detailed medical and sleep history as well as an oral cavity examination is needed when the patient presents in the dental clinic. Most of the parents do not volunteer information on the obvious signs and symptoms themselves. Dentists must ask them specifically about signs and symptoms such as snoring and frequent changes in sleep posture. (Marcus et al., 2012). Before ordering polysomnography (PSG), dentists should evaluate the clinical signs and symptoms and inquire about the hallmarks of OSA. Depending upon this assessment, a recommendation for a PSG or a referral to a sleep specialist should follow. The oropharyngeal structure of children with OSA should be properly evaluated (Muzumdar and Arens, 2008). During an oral cavity examination, the dentist must examine for the foremost cause and predictor of OSA, which is adenotonsillar hypertrophy followed by a Mallampati score significant enough to be an independent predictor for OSA (Friedman et al., 1999, Nuckton et al., 2006, Kumar et al., 2014). Tonsillar size is positively correlated with the severity of OSA, which means that the larger the tonsillar size is, the greater the severity of OSA (Li et al., 2002) (Fig. 3). A higher Mallampati score also increases the chance of developing OSA (Kumar et al., 2014). An increase of one point in the Mallampati score will increase the chance of developing OSA by 6-fold (Kumar et al., 2014) (Fig. 4). Children presenting with a high degree of suspicion for OSA, including a history of regular snoring, a higher grade of tonsils and/or a high Mallampati score, should be referred for a PSG (Marcus et al., 2012) to confirm diagnosis. Nocturnal oximetry is another adjunct test that can be used to evaluate OSA patients. In a cross-sectional study, Brouillette et al., suggested that positive nocturnal oximetry results have approximately 97% of the predicted value in the diagnosis of OSA (Brouillette et al., 2000). In another study, Álvarez et al., concluded that unsupervised nocturnal oximetry can be a possible substitute for PSG and at-home respiratory polygraphy (HRP) in the home setting (Álvarez et al., 2017). Early diagnosis will ensure timely intervention and management of OSA.
Fig. 3.
Friedman classification of tonsils.
Fig. 4.
The Mallampati classification.
The history of the patient should be taken in-depth, and a validated questionnaire should be used, both of which should be done before ordering a PSG for the definitive diagnosis to save on unnecessary expenses. Ahmed et al., designed a new questionnaire that was compiled from five other widely used questionnaires. Of these five questionnaires, two focused on quality of life ([i] PedsQL – Child Report, and [ii] PedsQL – Parent Report), and three focused on sleepiness and its effects ([i] Modified Epworth Sleepiness Scale, [ii] Pediatric Sleep Questionnaire, and [iii] OSA-18). The screening of OSA in children revealed a suboptimal diagnosis in each questionnaire. However, compared to all the others, the Ahmed et al., questionnaire, known as the IMP-Q, included the most informative questions (Ahmed et al., 2018) (Table 1).
Table 1.
IMP-Q Questionnaire derived from (1) PedsQL – Child Report, (2) PedsQL – Parent Report (3) Modified Epworth Sleepiness Scale, (4) Pediatric Sleep Questionnaire, (5) OSA- 18.
| Dataset | Question |
|---|---|
| CHQ | Do you have trouble sleeping? |
| CHQ | Can other kids do things you cannot? |
| SLS | Chance of Dozing or Falling Asleep: Sitting and reading |
| PSL | *Always snores? |
| PSL | *Have trouble breathing, or struggle to breath? |
| PSL | Have you ever seen your child stop breathing during the night? |
| PSL | *Tend to breathe through the mouth during the day? |
| PSL | Does your child wake up with headaches in the morning? |
| PSL | *Is your child overweight? |
| OSA-18 | **Breath holding spells or pauses in breathing at night? |
| OSA-18 | *Choking or gasping sounds while asleep? |
| OSA-18 | Mouth breathing because of nasal obstruction? |
PSG is considered to be the gold standard for the diagnosis of OSA (Rosen, 1999, Chan et al., 2004, Marcus et al., 2012, Kumar et al., 2014, Behrents et al., 2019). The PSG diagnostic cutoff values for pediatric OSA are different from those for adult OSA (Chan et al., 2004). As per the diagnostic value minimum, compared to adults, where the oxygen concentration is less than 85%, it is less than 92% in children (Fig. 5). The apnea hypopnea index (AHI) is 5 or more in adults, whereas the same index is 1 or more in children. Furthermore, the severity of OSA in children can be determined by the AHI index. If the AHI remains between 1.5 and 5, the OSA is considered mild, whereas an AHI above 5 and less than 10 is considered moderate OSA, and an AHI value greater than 10 is considered severe OSA (Haviv et al., 2014, Stauffer et al., 2018, Behrents et al., 2019). In some cases, where a proper sleep lab facility is not available, alternative diagnostic tests can be ordered, i.e., nocturnal video recording and nocturnal oximetry (Marcus et al., 2012).
Fig. 5.
A. Oxygen Saturation Cutoff values for Adults and Children. B. AHI Cut Off Values for Adults and Children.
5. Management of OSA
The American Association of Orthodontists strongly suggest that treating any underlying dental or orthopedic ailment improves, not causes or aggravates, OSA (Behrents et al., 2019). The recommendations also suggested that a definitive diagnosis should be made by the appropriate physician and recommends that the treating orthodontist should be well versed in the signs and symptoms of OSA, its risk factors, and the role of dental professionals (Behrents et al., 2019). Management of OSA can be divided into two broad categories:
-
1.
Non-Dental Treatments
-
2.
Dental- Treatments
5.1. Non-dental treatments
Non-dental treatments, including a spectrum of treatment modalities, such the surgical procedures, adenotonsillectomy, adenoidectomy and tonsillectomy, are given to the patient to address the particular underlying etiology. These different modalities may also include continuous positive airway pressure (CPAP) or even topical intranasal corticosteroids to treat residual OSA. In this paper, only dental treatments are discussed in detail. These treatments can be used alone or in combination with dental treatments per the needs of the patient.
5.2. Dental treatments
In recent years, because of better diagnostic imaging, such as cone beam computed tomography (CBCT), and the integration of dental sciences with medical sciences, there has been greater understanding of the underlying causes of OSA in the pediatric population. This understanding has helped in employing possible dental treatments for OSA in which the main cause is dentofacial deformity.
There are a variety of dental treatments available to treat pediatric OSA, which may be used as solitary regimens, as adjunctive to existing modalities such as CPAP, or to treat residual OSA following adenotonsillectomy. Most patients experience residual OSA after adenotonsillectomy. Complete resolution of OSA is reported in only 25% of patients following adenotonsillectomy (Tauman et al., 2006). These dental treatments primarily include growth modifiers of the oromandibular region (rapid maxillary expansion [RME] and mandibular growth activators), mandibular advancement appliances, and tongue retaining devices (Sacchetti and Mangiardi, 2012, Ngiam and Cistulli, 2015).
5.2.1. Rapid Maxillary Expansion (RME)
As discussed earlier, children with OSA present with a constricted maxilla, a high-arched palate, and maxillary crowding. There is also midface hypoplasia in the transverse dimension in these patients. Baratieri et al., and Ribeiro et al., showed that RME treatment resulted in an increase in the transverse width of the nasal airway (Baratieri et al., 2011, Ribeiro et al., 2012). Iwasaki et al., suggested multifaceted effects of RME, which included expansion of the pharyngeal airway space, a reduction in nasal obstruction, and an elevated tongue posture (Iwasaki et al., 2013). Several other studies also suggested a decrease in nasal airway resistance after RME (Hartgerink et al., 1987, Doruk et al., 2004, De Felippe et al., 2008). In 2013, Angelieri et al., suggested a classification-based approach for RME in the case of a specific patient (Angelieri et al., 2013). The researchers proposed a classification in the form of stages A to E in which stage A denotes no fusion of midpalatal suture and stage E is total fusion of the palatal suture (Angelieri et al., 2013). This classification can help dentists avoid possible side effects from needless surgical procedures, and it also increases the success rates of RME (Angelieri et al., 2013). Villa et al., demonstrated a considerable decrease in the AHI and a significant increase in oxygen saturation after RME treatment in their study (Villa et al., 2015, Villa et al., 2015). The researchers suggested that treatment should be started as early as possible to obtain the maximum benefit (Villa et al., 2015, Villa et al., 2015). The same group of researchers in an earlier study followed children for 24 months after a year of RME treatment and found a significant reduction in the AHI and clinical symptoms of OSA immediately after the end of a 1-year treatment period and did not observe any relapse at the 24-month follow-up (Villa et al., 2011). On the other hand, Guilleminault et al., showed that a combined therapy of adenotonsillectomy and RME is required to completely resolve OSA (Guilleminault et al., 2008). The researchers also suggested that regardless of the initial treatment modality employed (adenotonsillectomy or RME), patients eventually need both treatments to resolve OSA entirely (Guilleminault et al., 2008). Vale et al., concluded in their meta-analysis that there is a substantial reduction in the AHI after RME treatment in children and recommended RME as an alternative treatment option for OSA in children (Vale et al., 2017). In another meta-analysis on RME as a treatment option for pediatric OSA, Machado-Júnior et al., discovered in their included studies that the average AHI during follow-up was 6.86 (Machado-Júnior et al., 2016). The researchers established that RME is an effective treatment option for children with OSA (Machado-Júnior et al., 2016). Pirelli et al., in their longitudinal study of children diagnosed with OSA that is due to maxillary constriction and normal adenotonsillar tissue, observed improved and consistent PSG values post-RME and after a 12-year follow-up (Pirelli et al., 2015).
The extensive evidence appears to be in favor of RME as a treatment modality for residual OSA after adenotonsillectomy or for cases where there is normal adenotonsillar tissue and the underlying cause is maxillary constriction. In addition to correction of occlusion and correction of skeletal discrepancy, RME does have the potential to improve OSA (Behrents et al., 2019).
5.2.2. Mandibular advancement
Multiple studies suggest that retrognathism of the mandible and a class II skeletal relationship are associated with OSA (Lowe et al., 1995, Kawashima et al., 2002, Cozza et al., 2004, Cohen-Lévy et al., 2009, Pirilä-Parkkinen et al., 2009). Numerous studies have demonstrated beneficial results in treating these conditions by increasing overall airway space (Hänggi et al., 2008, Restrepo et al., 2011, Zhang et al., 2013, Ghodke et al., 2014, Temani et al., 2016). Although different studies have used a variety of treatment modalities, including different orthodontic appliances or combined orthodontic and surgical modalities, expanding airway space was a method observed in all of these studies. Zhang et al., employed a twin block appliance to correct class II and found a significant decrease in the AHI from 14.08 ± 4.25 to 3.39 ± 1.86. The researchers also reported a significant increase in airway space after a cephalometric analysis (Zhang et al., 2013). Cozza et al., showed that in children diagnosed with OSA, which was due to class II, treatment with a modified monobloc provides a significant reduction in the AHI (Cozza et al., 2004). Temani et al., evaluated the volumetric changes in the pharyngeal airway using CBCT after treating class II patients and observed a significant increase in pharyngeal airway space (Temani et al., 2016). In a 22-year-long cohort study, Hänggi et al., described an increase in the pharyngeal airway dimensions in children after using activator-headgear as a treatment (Hänggi et al., 2008). Miloro et al., and Denny et al., in two separately conducted studies on mandibular distraction osteogenesis, suggested an expansion in airway space along with a resolution of apneic signs and symptoms (Denny et al., 2001, Miloro, 2010). Villa and associates used a mandibular advancement appliance in OSA-diagnosed children with the aim of treating a retruded bite, deep bite, and cross-bite. After a 6-month trial, the researchers found a significant decrease in the AHI within the treated group (Villa et al., 2002). In another study, it was suggested that the correction of a retruded mandible in class II cases can increase the airway space and concomitantly improve nocturnal breathing (Schütz et al., 2011). Xiang et al., performed a meta-analysis to determine the efficacy of functional appliances on upper airway dimensions in growing children with class II and retruded mandibles. This study demonstrated that functional appliances can increase airway dimensions and may decrease the potential risk of OSA because of a retruded mandible (Xiang et al., 2017). Interventions performed for the advancement of the mandible can include functional appliances, surgical correction, and mandibular repositioning. Nevertheless, all these factors result in increased airway space, an improvement in apneic signs and symptoms, and a decrease in the AHI. In growing children, it is favorable that guided therapy be employed using a functional appliance so that future potential risks can be avoided or patients with established OSA can be treated. Myofunctional therapy is a treatment modality that educates the patient on how to correct the posture of their tongue and orofacial muscles (Stauffer et al., 2018). Guilleminault et al., and Villa et al., showed 50% and 62% reductions in the AHI after myofunctional therapy, respectively (Guilleminault et al., 2013, Villa et al., 2015, Villa et al., 2015).
In a systematic review and network meta-analysis conducted by Lin et al, they concluded that for the AHI as the outcome measure, the best treatment modality is surgery (Lin et al., 2020). They also suggested that RME is useful in obtaining the lowest arterial oxygen saturation (SaO2). However, none of the treatment modalities have been proven to be the only treatment that can resolve OSA completely (Lin et al., 2020).
6. Discussion
Recent advancements in sleep medicine and increasing recognition of the multiple etiological factors of OSA have enabled physicians to better understand the disease mechanism and have inspired the discovery of many novel alternative treatment options. Progress in imaging has highlighted the role of craniofacial variations in the development of OSA, which has enlightened dentists on how minor aberrations from normal anatomy, such as midface hypoplasia, cleft lip and palate, and a short lingual frenulum, can become potential risk factors for OSA (Huang et al., 2015). The role of dentists has tremendously evolved, and children seeking dental care should be examined not only in terms of teeth and teeth-related issues but also for other potential medical issues that may arise in the future. Following a comprehensive dental exam, a dentist can utilize their expertise and an appropriate degree of deliberation to predict whether the patient is susceptible to developing or currently has OSA. There are craniodental predictors that a dentist should take into account upon assessment. For instance, OSA can be suspected if there are large tonsils, a high Mallampati score, a crossbite, an overjet, an elongated soft palate, a high-arched palate, an a retruded mandible, a short lingual frenulum, and crowding. Along with an examination of these potential predictors, the dentist should thoroughly take the sleep history of the patient. On the basis of this history and examination, the dentist can more accurately identify OSA and its likelihood. If OSA is suspected, PSG is essential to diagnose OSA and to assess further intervention. The role of dentists is also essential, as they can modulate mandibular and maxillary growth in particular age groups. This growth modulation can treat OSA and may potentially avoid the need for surgical procedures or wearisome CPAP.
7. Conclusion
The role of a dentist has evolved over the years. Pediatric OSA is a serious medical condition and can have long term consequences on the overall health and quality of life of a patient. A dentist should be familiar with the signs and symptoms of OSA and should conduct a thorough history, intra/extra oral examination, questionnaires and understand the role of comorbid conditions. A timely diagnosis and management of OSA can eradicate potential long term negative effects on the health of a patient. If pediatric OSA is suspected a proper referral should be made for the definitive diagnosis and treated accordingly. An interdisciplinary treatment approach may often serve in the best interest of the patient.
Authorship Statement
All persons who are listed as authors participated sufficiently for the submitted manuscript. All the authors (Hafiz M. Moin Anwer, Hamad N. Albagieh, Mythili Kalladka, Harmeet Chiang, Sean W. McLaren, Shaima Malik and Junad Khan) participated in the concept, design, analysis, writing and revision of the manuscript.
Disclosures
The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. There is no conflict of interest on a personnel or financial level of any of the authors
Ethical Statement
-
(1)
This material has not been published in whole or in part elsewhere
-
(2)
The manuscript is not currently being considered for publication in another journal
-
(3)
All authors have been personally and actively involved in substantive work leading to the manuscript, and will hold themselves jointly and individually responsible for its content.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hafiz M. Moin Anwer, Email: anwerhm@shp.rutgers.edu.
Harmeet K. Chiang, Email: hkchiang@vcu.edu.
Shaima Malik, Email: Shaima_Malik@urmc.rochester.edu.
Sean W. McLaren, Email: Sean_McLaren@urmc.rochester.edu.
Junad Khan, Email: Junad_khan@urmc.rochester.edu.
References
- Ahmed, S., et al., An empirical study of questionnaires for the diagnosis of pediatric obstructive sleep apnea. In: 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, pp. 4097–4100. [DOI] [PubMed]
- Alexopoulos E.I. et al., Nocturnal enuresis is associated with moderate-to-severe obstructive sleep apnea in children with snoring. Pediatric Res. 2014;76(6):555–559. doi: 10.1038/pr.2014.137. [DOI] [PubMed] [Google Scholar]
- Ali N., et al. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch. Dis. Child. 1994;71(1):74–76. doi: 10.1136/adc.71.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez Daniel, et al. Automated screening of children with obstructive sleep apnea using nocturnal oximetry: an alternative to respiratory polygraphy in unattended settings. J. Clin. Sleep Med. 2017;13(5):693–702. doi: 10.5664/jcsm.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelieri F., et al. Midpalatal suture maturation: classification method for individual assessment before rapid maxillary expansion. Am. J. Orthod. Dentofacial Orthoped. 2013;144(5):759–769. doi: 10.1016/j.ajodo.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik U.B., et al. Relationship between cephalometric characteristics and obstructive sites in obstructive sleep apnea syndrome. Angle Orthod. 2002;72(2):124–134. doi: 10.1043/0003-3219(2002)072<0124:RBCCAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Baratieri C., et al. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing? Am. J. Orthod. Dentofacial Orthoped. 2011;140(2):146–156. doi: 10.1016/j.ajodo.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Behrents R.G., et al. Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White Paper. Am. J. Orthod. Dentofacial Orthoped. 2019;156(1):13–28.e11. doi: 10.1016/j.ajodo.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee R., et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children. Am. J. Respir. Crit. Care Med. 2010;182(5):676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- Brew B.K., et al. Breastfeeding and snoring: a birth cohort study. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0084956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette R.T., et al. Obstructive sleep apnea in infants and children. J. Pediat. 1982;100(1):31–40. doi: 10.1016/s0022-3476(82)80231-x. [DOI] [PubMed] [Google Scholar]
- Brouillette R.T., et al. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics. 2000;105(2):405–412. doi: 10.1542/peds.105.2.405. [DOI] [PubMed] [Google Scholar]
- Cakirer B., et al. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am. J. Respir. Crit. Care Med. 2001;163(4):947–950. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- Chan J., et al. Obstructive sleep apnea in children. Am. Fam. Physician. 2004;69(5):1147–1154. [PubMed] [Google Scholar]
- Chang S.J., Chae K.Y. Obstructive sleep apnea syndrome in children: epidemiology, pathophysiology, diagnosis and sequelae. Korean J. Pediat. 2010;53(10):863–871. doi: 10.3345/kjp.2010.53.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Lévy J., et al. Cranio-facial morphology and obstructive sleep apnea: the role of dento-facial orthopedics. J. Dentofacial Anomal. Orthod. 2009;12(3):108–120. [Google Scholar]
- Cozza P., et al. A modified monobloc for the treatment of obstructive sleep apnoea in paediatric patients. Eur. J. Orthod. 2004;26(5):523–530. doi: 10.1093/ejo/26.5.523. [DOI] [PubMed] [Google Scholar]
- De Felippe N.L.O., et al. Relationship between rapid maxillary expansion and nasal cavity size and airway resistance: short-and long-term effects. Am. J. Orthod. Dentofacial Orthop. 2008;134(3):370–382. doi: 10.1016/j.ajodo.2006.10.034. [DOI] [PubMed] [Google Scholar]
- Defabjanis P. Impact of nasal airway obstruction on dentofacial development and sleep disturbances in children: preliminary notes. J. Clin. Pediat. Dentist. 2004;27(2):95–100. doi: 10.17796/jcpd.27.2.27934221l1846711. [DOI] [PubMed] [Google Scholar]
- Deng J., Gao X. A case–control study of craniofacial features of children with obstructed sleep apnea. Sleep Breath. 2012;16(4):1219–1227. doi: 10.1007/s11325-011-0636-4. [DOI] [PubMed] [Google Scholar]
- Denny A.D., et al. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast. Reconstr. Surg. 2001;108(2):302–311. doi: 10.1097/00006534-200108000-00004. [DOI] [PubMed] [Google Scholar]
- Doruk C., et al. Evaluation of nasal airway resistance during rapid maxillary expansion using acoustic rhinometry. Eur. J. Orthod. 2004;26(4):397–401. doi: 10.1093/ejo/26.4.397. [DOI] [PubMed] [Google Scholar]
- Drager L.F., et al. Obstructive sleep apnea. J. Am. College Cardiol. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersu R., et al. Prevalence of snoring and symptoms of sleep-disordered breathing in primary school children in Istanbul. Chest J. 2004;126(1):19–24. doi: 10.1378/chest.126.1.19. [DOI] [PubMed] [Google Scholar]
- Friedman M., et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109(12):1901–1907. doi: 10.1097/00005537-199912000-00002. [DOI] [PubMed] [Google Scholar]
- Ghodke S., et al. Effects of twin-block appliance on the anatomy of pharyngeal airway passage (PAP) in class II malocclusion subjects. Prog. Orthod. 2014;15(1):68. doi: 10.1186/s40510-014-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gislason T., Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old: an epidemiologic study of lower limit of prevalence. Chest. 1995;107(4):963–966. doi: 10.1378/chest.107.4.963. [DOI] [PubMed] [Google Scholar]
- Goodwin J.L., et al. Symptoms related to sleep-disordered breathing in white and Hispanic children: the Tucson Children’s Assessment of Sleep Apnea Study. Chest. 2003;124(1):196–203. doi: 10.1378/chest.124.1.196. [DOI] [PubMed] [Google Scholar]
- Guilleminault C., et al. Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Med. 2013;14(6):518–525. doi: 10.1016/j.sleep.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Guilleminault C., et al. Pediatric obstructive sleep apnea syndrome. Arch. Pediat. Adolescent Med. 2005;159(8):775–785. doi: 10.1001/archpedi.159.8.775. [DOI] [PubMed] [Google Scholar]
- Guilleminault C., et al. Recognition of sleep-disordered breathing in children. Pediatrics. 1996;98(5):871–882. [PubMed] [Google Scholar]
- Guilleminault C., et al. Orthodontic expansion treatment and adenotonsillectomy in the treatment of obstructive sleep apnea in prepubertal children. Sleep. 2008;31(7):953–957. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hänggi M.P., et al. Long-term changes in pharyngeal airway dimensions following activator-headgear and fixed appliance treatment. Eur. J. Orthod. 2008;30(6):598–605. doi: 10.1093/ejo/cjn055. [DOI] [PubMed] [Google Scholar]
- Hartgerink D.V., et al. The effect of rapid maxillary expansion on nasal airway resistance. Am. J. Orthod. Dentofacial Orthop. 1987;92(5):381–389. doi: 10.1016/0889-5406(87)90258-7. [DOI] [PubMed] [Google Scholar]
- Haviv Y., et al. On the edge between medicine and dentistry: Review of the dentist's role in the diagnosis and treatment of snoring and sleep apnea. Quintessence Int. 2014;45(4):345–353. doi: 10.3290/j.qi.a31337. [DOI] [PubMed] [Google Scholar]
- Heffernan A., et al. Transition to adult care for obstructive sleep apnea. J. Clin. Med. 2019;8(12):2120. doi: 10.3390/jcm8122120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata I., et al. Sleep problems are more frequent and associated with problematic behaviors in preschoolers with autism spectrum disorder. Res. Dev. Disabil. 2016;49–50:86–99. doi: 10.1016/j.ridd.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Hoth K.F., et al. Obstructive sleep apnea. Sleep Breath. 2013;17(2):811–817. doi: 10.1007/s11325-012-0769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.S., et al. Short lingual frenulum and obstructive sleep apnea in children. Int. J. Pediatr. Res. 2015;1(003) [Google Scholar]
- Iwasaki, T., et al., 2009. Oropharyngeal airway in children with Class III malocclusion evaluated by cone-beam computed tomography. Am. J. Orthod. Dentofacial Orthop. 136(3), 318. e311–318. e319. [DOI] [PubMed]
- Iwasaki T., et al. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: a cone-beam computed tomography study. Am. J. Orthod. Dentofacial Orthop. 2013;143(2):235–245. doi: 10.1016/j.ajodo.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Jo J.H., et al. Quality and readability of online information on dental treatment for snoring and obstructive sleep apnea. Int. J. Med. Inf. 2020;133 doi: 10.1016/j.ijmedinf.2019.104000. [DOI] [PubMed] [Google Scholar]
- Katyal V., et al. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing and changes in quality of life with rapid maxillary expansion. Am. J. Orthod. Dentofacial Orthop. 2013;144(6):860–871. doi: 10.1016/j.ajodo.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Katz E.S., D'Ambrosio C.M. Pathophysiology of pediatric obstructive sleep apnea. Proc. Am. Thoracic Soc. 2008;5(2):253–262. doi: 10.1513/pats.200707-111MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S., et al. Craniofacial morphology in preschool children with sleep-related breathing disorder and hypertrophy of tonsils. Acta Paediatrica. 2002;91(1):71–77. doi: 10.1080/080352502753457996. [DOI] [PubMed] [Google Scholar]
- Kumar H.V.M., et al. Mallampati score and pediatric obstructive sleep apnea. J. Clin. Sleep Med.: JCSM: Off. Publ. Am. Acad. Sleep Med. 2014;10(9):985. doi: 10.5664/jcsm.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin D.S., et al. Preliminary evidence of behavioral and cognitive sequelae of obstructive sleep apnea in children. Sleep Med. 2002;3(1):5–13. doi: 10.1016/s1389-9457(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Li A., et al. Use of tonsil size in the evaluation of obstructive sleep apnoea. Arch. Dis. Child. 2002;87(2):156–159. doi: 10.1136/adc.87.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.Y., et al. Management of paediatric obstructive sleep apnoea: A systematic review and network meta-analysis. Int. J. Pediatr. Dent. 2020;30(2):156–170. doi: 10.1111/ipd.12593. [DOI] [PubMed] [Google Scholar]
- Lowe A.A., et al. Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am. J. Orthod. Dentofacial Orthop. 1995;107(6):589–595. doi: 10.1016/s0889-5406(95)70101-x. [DOI] [PubMed] [Google Scholar]
- Lumeng J.C., Chervin R.D. Epidemiology of pediatric obstructive sleep apnea. Proc. Am. Thoracic Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Júnior A.-J., et al. Rapid maxillary expansion and obstructive sleep apnea: A review and meta-analysis. Medicina Oral, Patología Oral y Cirugía Bucal. 2016;21(4):e465–e469. doi: 10.4317/medoral.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus C.L., et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- Marino A., et al. Craniofacial morphology in preschool children with obstructive sleep apnoea syndrome. Eur. J. Paediatr. Dentistry. 2009;10(4):181. [PubMed] [Google Scholar]
- McColley, S.A., 2005. Primary snoring in children. In Principles and Practice of Pediatric Sleep Medicine, pp. 263–267.
- Miloro M. Mandibular distraction osteogenesis for pediatric airway management. J. Oral Maxillofac. Surg. 2010;68(7):1512–1523. doi: 10.1016/j.joms.2009.09.099. [DOI] [PubMed] [Google Scholar]
- Moré E.E., et al. Dentofacial development abnormalities in paediatric sleep-related breathing disorders. Acta Otorrinolaringologica (English Edition) 2011;62(2):132–139. doi: 10.1016/j.otorri.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Muzumdar H., Arens R. Diagnostic issues in pediatric obstructive sleep apnea. Proc. Am. Thoracic Soc. 2008;5(2):263–273. doi: 10.1513/pats.200707-113MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngiam J., Cistulli P.A. Dental treatment for paediatric obstructive sleep apnea. Paediatr. Respir. Rev. 2015;16(3):174–181. doi: 10.1016/j.prrv.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Nuckton T.J., et al. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29(7):903–908. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- Park J.G., et al. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin. Proc. 2011;86(6):549–555. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirelli P., et al. Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: a 12-year follow-up. Sleep Med. 2015;16(8):933–935. doi: 10.1016/j.sleep.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Pirilä-Parkkinen K., et al. Dental arch morphology in children with sleep-disordered breathing. Eur. J. Orthod. 2009;31(2):160–167. doi: 10.1093/ejo/cjn061. [DOI] [PubMed] [Google Scholar]
- Quo Stacey Dagmar, Pliska Benjamin T., Huynh Nelly. Oropharyngeal growth and skeletal malformations. Principles Pract. Sleep Med. 2017:1401–1422. [Google Scholar]
- Redline S., et al. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am. J. Respir. Crit. Care Med. 1999;159(5):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- Restrepo C., et al. Oropharyngeal airway dimensions after treatment with functional appliances in class II retrognathic children. J. Oral Rehabil. 2011;38(8):588–594. doi: 10.1111/j.1365-2842.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro A.N.C., et al. Upper airway expansion after rapid maxillary expansion evaluated with cone beam computed tomography. Angle Orthod. 2012;82(3):458–463. doi: 10.2319/030411-157.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C.L. Clinical features of obstructive sleep apnea hypoventilation syndrome in otherwise healthy children. Pediatr. Pulmonol. 1999;27(6):403–409. doi: 10.1002/(sici)1099-0496(199906)27:6<403::aid-ppul7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sacchetti L.M., Mangiardi P. Nova Science Publishers; 2012. Obstructive Sleep Apnea: Causes, Treatment and Health Implications. [Google Scholar]
- Schlaud M., et al. The German study on sleep-disordered breathing in primary school children: epidemiological approach, representativeness of study sample, and preliminary screening results. Paediatr. Perinat. Epidemiol. 2004;18(6):431–440. doi: 10.1111/j.1365-3016.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- Schütz T.C.B., et al. Class II correction improves nocturnal breathing in adolescents. Angle Orthod. 2011;81(2):222–228. doi: 10.2319/052710-233.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seailles T., et al. How to detect Obstructive Sleep Apnea Syndrome (OSAS) in children. J. Dentofacial Anomal. Orthod. 2009;12(3):77–91. [Google Scholar]
- Shin C., et al. Prevalence and correlates of habitual snoring in high school students. Chest J. 2003;124(5):1709–1715. doi: 10.1378/chest.124.5.1709. [DOI] [PubMed] [Google Scholar]
- Stauffer J., et al. A review of pediatric obstructive sleep apnea and the role of the dentist. J. Dent. Sleep Med. 2018;5(4):111–130. [Google Scholar]
- Tangugsorn V., et al. Obstructive sleep apnoea: a cephalometric study. Part II. Uvulo-glossopharyngeal morphology. Eur. J. Orthod. 1995;17(1):57–67. doi: 10.1093/ejo/17.1.57. [DOI] [PubMed] [Google Scholar]
- Tauman R., et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J. Pediatr. 2006;149(6):803–808. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- Temani P., et al. Volumetric changes in pharyngeal airway in Class II division 1 patients treated with Forsus-fixed functional appliance: A three-dimensional cone-beam computed tomography study. Contemp. Clin. Dentist. 2016;7(1):31–35. doi: 10.4103/0976-237X.177100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol H.I., Brooks L.J. Follow-up of primary snoring in children. J. Pediatr. 2001;138(2):291–293. doi: 10.1067/mpd.2001.110122. [DOI] [PubMed] [Google Scholar]
- Vale F., et al. Efficacy of rapid maxillary expansion in the treatment of obstructive sleep apnea syndrome: a systematic review with meta-analysis. J. Evidence Dent. Pract. 2017;17(3):159–168. doi: 10.1016/j.jebdp.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Vieira B.B., et al. Cephalometric evaluation of facial pattern and hyoid bone position in children with obstructive sleep apnea syndrome. Int. J. Pediatr. Otorhinolaryngol. 2011;75(3):383–386. doi: 10.1016/j.ijporl.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Villa M.P., et al. Randomized controlled study of an oral jaw-positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. Am. J. Respir. Crit. Care Med. 2002;165(1):123–127. doi: 10.1164/ajrccm.165.1.2011031. [DOI] [PubMed] [Google Scholar]
- Villa M.P., et al. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep Breath. 2015;19(1):281–289. doi: 10.1007/s11325-014-1011-z. [DOI] [PubMed] [Google Scholar]
- Villa M.P., et al. Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up. Sleep Breath. 2011;15(2):179–184. doi: 10.1007/s11325-011-0505-1. [DOI] [PubMed] [Google Scholar]
- Villa M.P., et al. Rapid maxillary expansion outcomes in treatment of obstructive sleep apnea in children. Sleep Med. 2015;16(6):709–716. doi: 10.1016/j.sleep.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Xiang M., et al. Changes in airway dimensions following functional appliances in growing patients with skeletal class II malocclusion: A systematic review and meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2017;97:170–180. doi: 10.1016/j.ijporl.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Zhang C., et al. Effects of twin block appliance on obstructive sleep apnea in children: a preliminary study. Sleep Breath. 2013;17(4):1309–1314. doi: 10.1007/s11325-013-0840-5. [DOI] [PubMed] [Google Scholar]