Abstract
Purpose
To evaluate the effect of phytic acid (IP6) on the surface roughness and microhardness of human root canal dentin and compare it to other smear layer removal agents.
Materials and methods
Fifty extracted human maxillary incisors were sectioned longitudinally into a total of 100 specimens followed by embedding in auto-polymerizing acrylic resin. The specimens were polished and then randomly divided into five groups (n = 20) according to the test solution used to condition root canal dentin: 17% ethylenediaminetetraacetic acid (EDTA); 10% citric acid (CA); 1% IP6; 37% phosphoric acid (PA); or distilled water (control group). Each specimen was treated with a total volume of 1 ml of each solution for 1 min with agitation. Each group was then divided into two subgroups of 10 specimens each. The specimens of the first subgroup were used to determine microhardness, using Vickers hardness tester, and the specimens of the second subgroup were used to measure surface roughness, using a confocal laser scanning microscope. The results were analyzed statistically using one-way ANOVA and Tukey tests, α = 0.05.
Results
All the tested groups exhibited microhardness and surface roughness values that were statistically significantly different when compared with the control group (P < 0.05). The microhardness value obtained with IP6 was significantly lower when compared to EDTA, CA, and the control group, whereas its roughness value was significantly higher compared to the aforementioned groups. However, there was no significant difference between IP6 and PA (P > 0.05).
Conclusions
IP6 and PA showed the lowest microhardness and the highest surface roughness values.
Keywords: Citric acid, EDTA, Microhardness, Phosphoric acid, Phytic acid, Roughness
1. Introduction
Mechanical instrumentation aids in the debridement of the root canal system; however, it also results in the formation of a smear layer (Şen et al., 1995). Although still controversial, the literature sides toward the removal of this layer before obturation to open the dentinal tubules, enhance the adaptation of the filling material to the root canal dentin (Shahravan et al., 2007), and improve the antimicrobial effectiveness of irrigation solutions (Morago et al., 2016).
Currently, ethylenediaminetetraacetic acid (EDTA) in a concentration range of 15–18% and a time of application of 1–5 min is considered the main chelating agent of choice used in endodontics for the purpose of smear layer removal. The final irrigation with EDTA is recommended because this solution can dissolve the organic component of the smear layer, followed with sodium hypochlorite to remove the organic part of this layer (Zehnder, 2006). Various other chelating agents have been studied and were reported as effective root canal smear layer removal agents, such as maleic acid, citric acid (CA), and phosphoric acid (PA) (Ballal et al., 2010, Prado et al., 2011). Exposure to these chelating agents might influence the chemical and physical characteristics of dentin (Zehnder, 2006). Dentin surface roughness and calcium/phosphorus (Ca/P) ratio are affected by the type of the agent used (Ari et al., 2004, Ari and Erdemir, 2005); these alterations are reflected as changes in dentin permeability, solubility, and resin-bonding ability, thus compromising the coronal seal and allowing easier bacterial ingress (Ballal et al., 2010, Eldeniz et al., 2005, Rotstein et al., 1996). Previous studies have investigated the effect of EDTA, CA, and PA on dentin, which showed reduced microhardness and increased surface roughness upon treatment with the aforementioned agents (Aguilar-Mendoza et al., 2008, Ballal et al., 2010, Cruz-Filho et al., 2011, Eldeniz et al., 2005, Marcelino et al., 2014).
Phytic acid (IP6), a naturally occurring, negatively charged molecule, has been found to have the potential to be used as a root canal chelating agent. IP6 proved to be an effective agent in removing the smear layer from flat coronal dentin surfaces (Nassar et al., 2013) and instrumented root canals, while being more biocompatible to osteoblast and pulpal cells when compared to EDTA and PA, respectively (Nassar et al., 2013, Nassar et al., 2015). It was also reported to enhance resin–dentin bond strength through a speculated collagen cross-linking effect (Kong et al., 2015, Kong et al., 2017). Nikhil et al. reported that IP6 had less effect on radicular dentin hardness compared to other chelating agents (Nikhil et al., 2016). However, the effect of this agent on dentin roughness and microhardness is not yet fully understood. To our knowledge, no study has assessed the effect of IP6 on the surface roughness of radicular dentin. Therefore, the purpose of this in vitro study was to (i) evaluate the effect of IP6 on the surface roughness and microhardness of human root canal dentin and (ii) to compare it to other smear layer removal agents. The null hypothesis tested was IP6 does not affect the microhardness or the roughness of root canal dentin.
2. Materials and methods
Fifty extracted human maxillary incisors were collected with the ethical approval of the Human Research Ethics Committee at Tokyo Medical and Dental University (no. 725). Teeth with cracks, restoration, curved canals, caries lesions, root canal therapy, root resorption, or calcification were excluded. Teeth were cleared of soft-tissue debris, followed by storage in 0.1% thymol solution at 4 °C until used.
High-speed burs under water irrigation were used to decoronate the teeth at the cementoenamel junction, and then the roots were sectioned longitudinally in a buccolingual direction from the cervical to the apical area with a low-speed diamond saw (Isomet, Buehler, Lake Bluff, IL, USA). Two specimens (buccal and lingual sections) were obtained from each root. The root sections were horizontally mounted in an auto-curing acrylic resin, leaving the root canal dentin exposed. Under water cooling, the exposed dentin surfaces of the embedded specimens were ground flat and polished using a series of silicon carbide paper of 600 to 1200 grit in ascending order, followed by a final polishing using 1 µm diamond paste on a wet grinding wheel. The specimens were randomly divided into five groups (n = 20) according to the type of chelating agent that was used to condition the dentinal surface:
Group I: 17% EDTA (pH 7.5); Group II: 10% CA (pH 1.67); Group III: 1% IP6 (pH 1.2); Group IV: 37% PA (pH < 1); and Group V: distilled water (control group). All chemicals were purchased from Wako Pure Chemical Industries, Osaka, Japan. A total volume of 1 ml of each solution was applied on each specimen for 1 min with agitation, using a microbrush. Half of the samples of each group was used to conduct the microhardness test (n = 10), and the other half (n = 10) was used to evaluate surface roughness.
2.1. Microhardness measurement
The microhardness of each conditioned root canal dentin surface was measured using a Vickers hardness tester (HM-102, Mitutoyo Corporation, Yokohama, Kanagawa, Japan). Three readings for each specimen were performed at different locations: coronal, middle, and apical thirds of the root. The indentation was placed 0.5 mm from the root canal wall at each location. Each determined location was impressed with a load of 100 g for 15 sec, using a pyramid diamond indenter tip, and each residual impression was observed with an optical microscope. The measurements were converted into a Vickers hardness number (VHN) by the monitor, using the equation: HVN = 1854 (F/d2); F is the indentation load (g), and d is the diagonal of the indentation (µm). The three values for each specimen were averaged to produce one hardness value.
2.2. Surface roughness measurement
The surface roughness (Sa, µm) of the root canal dentin was determined using a confocal laser scanning microscope (CLSM) (VK-X 150 series, Keyence Corporation, Osaka, Japan) at a 50x magnification, and measurement unit was 273 × 204 µm. According to the ISO 25178, the Sa is a surface texture parameter and expresses an absolute value of the differences in the heights of each point compared to the mean value of the surface (Kyaw et al., 2019). Three tracings of different locations in the central area of each specimen were performed. The mean Sa and standard deviation of the three Sa values were used to represent the surface roughness of the specimens.
2.3. Statistical analysis
The microhardness and surface roughness values were analyzed statistically by a statistical software package (Sigma Stat, version 15.0, SPSS, Chicago, IL, USA), using one-way analysis of variance (ANOVA), and post hoc comparisons were performed using Tukey’s multiple comparison test (alpha = 0.05).
3. Results
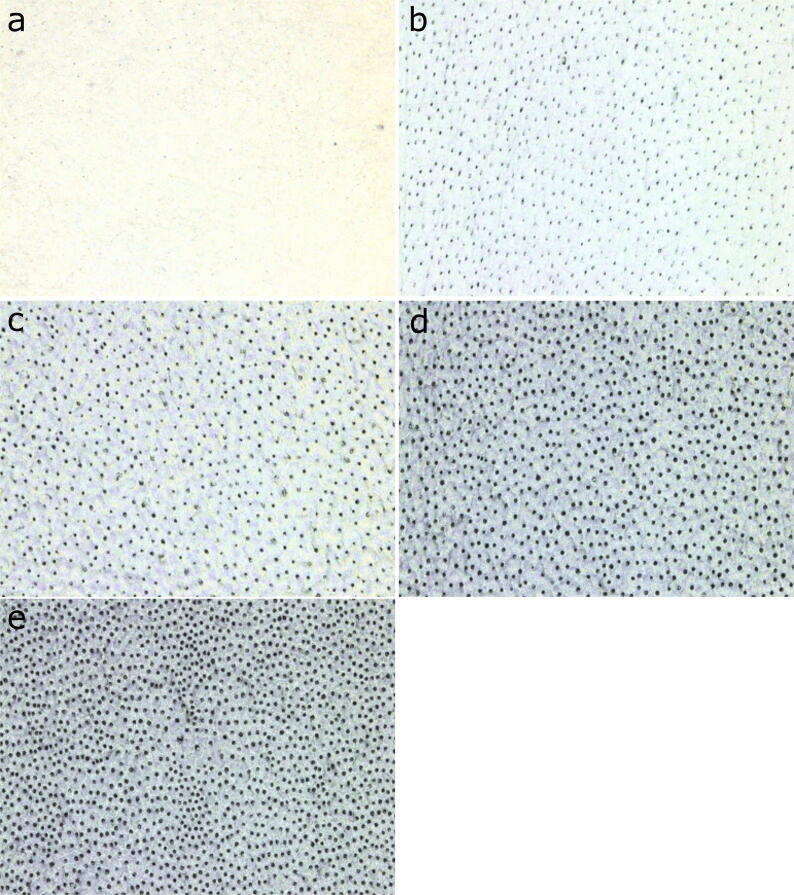
The mean values and standard deviations of root canal dentin microhardness and roughness data for all groups are listed in Table 1. All experimental groups exhibited a microhardness value that is statistically significantly lower when compared to the control group (P < 0.05). The hardness of the control group was the highest (60.29 ± 2.20) followed by EDTA, CA, IP6, and PA groups (49.00 ± 2.20, 44.46 ± 1.73, 41.34 ± 1.99, 40.52 ± 2.15); respectively. There was a significant difference among all experimental groups (P < 0.05), except for the IP6 and PA groups (P = 0.901). All experimental groups exhibited a roughness value that was significantly higher when compared to the control group (P < 0.05). The surface roughness of the control group was the lowest (0.05 ± 0.01), followed by EDTA, CA, IP6, and PA groups (0.24 ± 0.03, 0.44 ± 0.05, 0.52 ± 0.06, 0.55 ± 0.05), respectively. There was a significant difference among all the experimental groups (P < 0.05), except for the IP6 and PA groups (P = 0.915). Fig. 1 shows representative CLSM images of root dentin topography obtained in each experimental group.
Table 1.
The means and standard deviations of the root dentin microhardness (VHN) and roughness (Sa) values for experimental and control groups.
| Groups | n | Vickers Microhardness values | Roughness values |
|---|---|---|---|
| (Mean ± SD)* | (Mean ± SD)* | ||
| Control (distilled water) | 10 | 60.29 ± 2.20a | 0.05 ± 0.01A |
| EDTA | 10 | 49.00 ± 2.20b | 0.24 ± 0.03B |
| Citric acid | 10 | 44.46 ± 1.73c | 0.44 ± 0.05C |
| Phytic acid | 10 | 41.34 ± 1.99d | 0.52 ± 0.06D |
| Phosphoric acid | 10 | 40.52 ± 2.15d | 0.55 ± 0.04D |
Different letters indicate statistically significant difference at 5%.
Fig. 1.
Representative confocal laser scanning microscopy images of root canal dentin for each experimental group. (a) Control group (distilled water), (b) 17% ethylenediaminetetraacetic acid (EDTA), (c) 10% citric acid (CA), (d) 1% phytic acid (IP6), and (e) 37% phosphoric acid (PA).
4. Discussion
Chelating agents, an integral part of root canal treatment, are used to remove the inorganic part of the smear layer created on root canal surfaces during instrumentation (Torabinejad et al., 2002). However, the use of these agents might lead to alteration in the physical and chemical properties of root canal dentin that might reduce the microhardness and increase the roughness of dentin (Ari and Erdemir, 2005, Ballal et al., 2010, Dogan and Calt, 2001, Hu et al., 2010). The microhardness test is considered an indirectly suitable method to detect changes in composition and surface characteristics of dental hard tissues (Arends and ten Bosch, 1992, Ari and Erdemir, 2005). CLSM use to analyze surface topography is described in the literature and is commonly used to gauge surface texture and roughness (Rashid, 2012). In this study, different smear layer removal agents were applied to root canal dentin, and their effects on microhardness and roughness were evaluated. The results of this study revealed that all smear layer removal agents used resulted in lower dentin hardness and higher surface roughness values when compared to the control group. The descending orders of dentin microhardness and roughness values for the experimental groups were as follows: EDTA > CA > IP6 > PA and PA > IP6 > CA > EDTA, respectively. Statistically significant differences were identified among all agents used in both experiments, except for IP6 and PA. The results of this study require the rejection of the null hypothesis.
The EDTA-treated group exhibited the highest hardness and the lowest surface roughness of all experimental agents. The chelating action of EDTA mainly affects the inorganic part of dentin (Ballal et al., 2010, De-Deus et al., 2006, Poggio et al., 2012); however, due to its neutral pH, the etching and erosive potential of EDTA is thought to be minimal. The findings of this study are consistent with previously published reports in which CA decreased the dentin hardness and increased the surface roughness significantly more than EDTA did (Ballal et al., 2010, Eldeniz et al., 2005). CA, a weak organic acid used as a dentin conditioning and smear layer removal agent (Prado et al., 2011, Salama and Abdelmegid, 1994), has lower pH (1.67) compared to EDTA, resulting in deeper demineralization, more calcium loss (Hennequin et al., 1994), and increased surface roughness (Zehnder, 2006).
Results from the IP6 and PA-treated groups showed the lowest hardness and the highest roughness values of dentin. However, there was no significant difference between the aforementioned agents, but they were significantly different from CA, EDTA, and the control group. PA is a strong acid that is mostly used in adhesive dentistry and has a higher demineralizing effect when compared to EDTA and CA, resulting in enhanced smear layer removal (Prado et al., 2011), increased dentin microporosity (Pisani-Proença et al., 2011), higher reduction of microhardness (Marcelino et al., 2014), and an elevated surface roughness of dentin (Zehnder, 2006). IP6 is a highly negatively charged molecule with a high affinity to calcium. The pH of 1% IP6 solution is around 1.2; thus, its acidity, along with the chelation ability, might have contributed to the current findings. We speculate that the acidity of IP6 caused the observed significant differences from EDTA- and CA-treated groups. To the best of our knowledge, only one previous study evaluated the microhardness of root canal dentin treated with IP6 (Nikhil et al., 2016). In contrast to the results of this study, Nikhil et al. (2016) reported that 1% IP6 treatment resulted in a significantly lower reduction in microhardness than EDTA did. This might be attributed to the differences in the pH and time of application of IP6, which were 3.2 (pH) and 3 min, respectively, in the previously mentioned report. The decreased microhardness of dentin after IP6 application could facilitate the mechanical instrumentation of root canals (da Cruz-Filho et al., 2002), and the increase in surface roughness could be a benefit for micromechanical bonding of root canal resin sealers and adhesive restorative materials (Ballal et al., 2010). However, excessive demineralization may also result in the collapse of the collagen network and insufficient penetration of the adhesive or sealer, resulting in suboptimal sealing ability and higher nanoleakage (Garcia-Godoy et al., 2005).
5. Conclusions
Within the limitations of this study, all the tested agents resulted in microhardness and roughness values that were different from the control group. IP6- and PA-treated groups showed the lowest hardness and highest roughness values. However, further research is necessary to evaluate the effect of different concentrations and times of application of IP6 on these characteristics of dentin and study the sequelae on sealability and adhesion to root canal-treated teeth.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aguilar-Mendoza J.A., Rosales-Leal J.I., Rodríguez-Valverde M.A., Cabrerizo-Vílchez M.A. Effect of acid etching on dentin wettability and roughness: Self-etching primers versus phosphoric acid. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008;84B(1):277–285. doi: 10.1002/jbm.b.30871. [DOI] [PubMed] [Google Scholar]
- Arends J., ten Bosch J.J. Demineralization and Remineralization Evaluation Techniques. J. Dent. Res. 1992;71:924–928. doi: 10.1177/002203459207100S27. [DOI] [PubMed] [Google Scholar]
- Ari H., Erdemir A., Belli S. Evaluation of the effect of endodontic irrigation solutions on the microhardness and the roughness of root canal dentin. J. Endod. 2004;30(11):792–795. doi: 10.1097/01.don.0000128747.89857.59. [DOI] [PubMed] [Google Scholar]
- Ari H., Erdemir A. Effects of endodontic irrigation solutions on mineral content of root canal dentin using ICP-AES technique. J. Endod. 2005;31(3):187–189. doi: 10.1097/01.don.0000137643.54109.81. [DOI] [PubMed] [Google Scholar]
- Ballal N.V., Mala K., Bhat K.S. Evaluation of the effect of maleic acid and ethylenediaminetetraacetic acid on the microhardness and surface roughness of human root canal dentin. J. Endod. 2010;36(8):1385–1388. doi: 10.1016/j.joen.2010.04.002. [DOI] [PubMed] [Google Scholar]
- da Cruz-Filho A.M., de Paula E.A., Pécora J.D., de Sousa-Neto M.D. Effect of different EGTA concentrations on dentin microhardness. Braz. Dent. J. 2002;13(3):188–190. doi: 10.1590/s0103-64402002000300009. [DOI] [PubMed] [Google Scholar]
- Cruz-Filho A.M., Sousa-Neto M.D., Savioli R.N., Silva R.G., Vansan L.P., Pécora J.D. Effect of chelating solutions on the microhardness of root canal lumen dentin. J. Endod. 2011;37(3):358–362. doi: 10.1016/j.joen.2010.12.001. [DOI] [PubMed] [Google Scholar]
- De-Deus G., Paciornik S., Mauricio M.H.P. Evaluation of the effect of EDTA, EDTAC and citric acid on the microhardness of root dentine. Int. Endod. J. 2006;39(5):401–407. doi: 10.1111/j.1365-2591.2006.01094.x. [DOI] [PubMed] [Google Scholar]
- Dogan H., Calt S. Effects of Chelating Agents and Sodium Hypochlorite on Mineral Content of Root Dentin. J. Endod. 2001;27(9):578–580. doi: 10.1097/00004770-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Eldeniz A., Erdemir A., Belli S. Effect of EDTA and Citric Acid Solutions on the Microhardness and the Roughness of Human Root Canal Dentin. J. Endod. 2005;31(2):107–110. doi: 10.1097/01.don.0000136212.53475.ad. [DOI] [PubMed] [Google Scholar]
- Garcia-Godoy F., Loushine R.J., Itthagarun A., Weller R.N., Murrary P.E., Feilzer A., Pashley D.H., Tay F. Application of biologically-oriented dentin bonding principles to the use of endodontic irrigants. Am. J. Dent. 2005;18(4):281–290. [PubMed] [Google Scholar]
- Hennequin M., Pajot J., Avignant D. Effects of different pH values of citric acid solutions on the calcium and phosphorus contents of human root dentin. J. Endod. 1994;20(11):551–554. doi: 10.1016/S0099-2399(06)80071-3. [DOI] [PubMed] [Google Scholar]
- Hu X., Ling J., Gao Y. Effects of irrigation solutions on dentin wettability and roughness. J. Endod. 2010;36(6):1064–1067. doi: 10.1016/j.joen.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Kong K., Islam M.S., Nassar M., Hiraishi N., Otsuki M., Yiu C.K.Y., Tagami J. Effect of phytic acid etchant on the structural stability of demineralized dentine and dentine bonding. J. Mech. Behav. Biomed. Mater. 2015;48:145–152. doi: 10.1016/j.jmbbm.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Kong K., Hiraishi N., Nassar M., Otsuki M., Yiu C.K.Y., Tagami J. Effect of phytic acid etchant on resin–dentin bonding: Monomer penetration and stability of dentin collagen. J. Prosthodont. Res. 2017;61(3):251–258. doi: 10.1016/j.jpor.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Kyaw K., Otsuki M., Segarra M., Hiraishi N., Tagami J. Effect of Calcium-phosphate Desensitizers on Staining Susceptibility of Acid-eroded Enamel. Oper. Dent. 2019;44(3):281–288. doi: 10.2341/18-024-L. [DOI] [PubMed] [Google Scholar]
- Marcelino A.P.M., Bruniera J.F., Rached-junior F.A., da Silva S.R.C., Messias D.C. Impact of chemical agents for surface treatments on microhardness and flexural strength of root dentin. Braz. Oral Res. 2014;28(1):1–6. doi: 10.1590/1807-3107bor-2014.vol28.0052. [DOI] [PubMed] [Google Scholar]
- Morago A., Ordinola-Zapata R., Ferrer-Luque C.M., Baca P., Ruiz-Linares M., Arias-Moliz M.T. Influence of Smear Layer on the Antimicrobial Activity of a Sodium Hypochlorite/Etidronic Acid Irrigating Solution in Infected Dentin. J. Endod. 2016;42(11):1647–1650. doi: 10.1016/j.joen.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Nassar M., Hiraishi N., Islam M.S., Aizawa M., Tamura Y., Otsuki M., Kasugai S., Ohya K., Tagami J. Effect of phytic acid used as etchant on bond strength, smear layer, and pulpal cells. Eur. J. Oral Sci. 2013;121(5):482–487. doi: 10.1111/eos.12064. [DOI] [PubMed] [Google Scholar]
- Nassar M., Hiraishi N., Tamura Y., Otsuki M., Aoki K., Tagami J. Phytic acid: An alternative root canal chelating agent. J. Endod. 2015;41(2):242–247. doi: 10.1016/j.joen.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Nikhil V., Jaiswal S., Bansal P., Arora R., Raj S., Malhotra P. Effect of phytic acid, ethylenediaminetetraacetic acid, and chitosan solutions on microhardness of the human radicular dentin. J. Conserv. Dent. 2016;19(2):179. doi: 10.4103/0972-0707.178705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani-Proença J., Erhardt M.C.G., Amaral R., Valandro L.F., Bottino M.A., Del Castillo-Salmerón R. Influence of different surface conditioning protocols on microtensile bond strength of self-adhesive resin cements to dentin. J. Prosthet. Dent. 2011;105(4):227–235. doi: 10.1016/S0022-3913(11)60037-1. [DOI] [PubMed] [Google Scholar]
- Poggio C., Dagna A., Colombo M., Rizzardi F., Chiesa M., Scribante A., Alberti G. Decalcifying effect of different ethylenediaminetetraacetic acid irrigating solutions and tetraclean on root canal dentin. J. Endod. 2012;38(9):1239–1243. doi: 10.1016/j.joen.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Prado M., Gusman H., Gomes B.P.F.A., Simão R.A. Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with EDTA and citric acid. J. Endod. 2011;37(2):255–258. doi: 10.1016/j.joen.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Rashid H. Evaluation of the surface roughness of a standard abraded dental porcelain following different polishing techniques. J. Dent. Sci. 2012;7(2):184–198. [Google Scholar]
- Rotstein I., Dankner E., Goldman A., Heling I., Stabholz A., Zalkind M. Histochemical analysis of dental hard tissues following bleaching. J. Endod. 1996;22(1):23–26. doi: 10.1016/S0099-2399(96)80231-7. [DOI] [PubMed] [Google Scholar]
- Salama F.S., Abdelmegid F.Y. Six percent citric acid better than hydrogen peroxide in removing smear layer: an in vitro pilot study. Paediatr. Dent. 1994;16(6):424. [PubMed] [Google Scholar]
- Şen B.H., Wesselink P.R., Turkun M. The smear layer: a phenomenon in root canal therapy. Int. Endod. J. 1995;28(3):141–148. doi: 10.1111/j.1365-2591.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Shahravan A., Haghdoost A.A., Adl A., Rahimi H., Shadifar F. Effect of Smear Layer on Sealing Ability of Canal Obturation: A Systematic Review and Meta-analysis. J. Endod. 2007;33(2):96–105. doi: 10.1016/j.joen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Torabinejad M., Handysides R., Khademi A.A., Bakland L.K. Clinical implications of the smear layer in endodontics: A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002;94(6):658–666. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- Zehnder M. Root Canal Irrigants. J. Endod. 2006;32(5):389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]