Abstract
Introduction
Titanium (Ti) is widely accepted as a biomaterial for orthopaedic and dental implants, primarily due to its capacity to integrate directly into the bone and its superior corrosion resistance. It has been suggested that titanium–zirconium alloy (TiZr), with 13–17% of zirconium, has better mechanical properties than pure Ti, but there are very few published studies assessing the suitability of TiZr for high-load- bearing implants. This study aimed to compare the mechanical properties and microstructures of TiZr and commercially pure titanium (Ti).
Methodology
Pure Ti and TiZr alloy discs were prepared and subjected to characterisation by nanoindentation, electron dispersive spectroscopy (EDS), X-ray diffraction (XRD), and electron backscatter diffraction (EBSD).
Results
The TiZr alloy was found to have significantly lower elastic modulus value (p < 0.0001) and greater hardness than Ti (p < 0.05). The EDS results confirmed the presence of Zr (13–17%) in the TiZr alloy, with XRD and EBSD images showing microstructure with the alpha phase similar to commercially available Ti.
Conclusion
The lower elastic modulus, higher hardness, presence of alpha phase, and the finer grain size of the TiZr alloy make it more suitable for high-load-bearing implants compared to commercially available Ti and is likely to encourage a positive biological response.
Keywords: Dental implants, Titanium, Titanium-Zirconium Alloy, Mechanical properties
1. Introduction
Dental implant treatment involves the replacement of missing teeth using metal screws embedded into the jawbone to support restoration. The success of implants depends on the formation of a direct functional connection between bone and the metal surface – a process referred to as osseointegration (Albrektsson et al., 1981). Titanium and its alloys remain the most popular material of choice for the fabrication of dental implants due to their inherent properties of biocompatibility, corrosion resistance, and mechanical strength (Ikarashi et al., 2007, Niinomi, 2002, Wen et al., 2002). However, the lower mechanical/tensile strength of commercially pure titanium (Ti) and its high elastic modulus and low wear resistance are reported as causes for concern (Katou et al., 1996, Williams, 1994). Several studies have reported the appearance of increased wear debris from Ti to be associated with tissue inflammation (Jacobs et al., 1998, Wang, 1996). The wear and corrosion of Ti implants can result in the accumulation of metal particles in the peri-implant tissues (Olmedo et al., 2009, Tawse-Smith et al., 2012), which may lead to increased inflammation (Olmedo et al., 2003) and, possibly, hypersensitivity reactions (Siddiqi et al., 2011).
To improve the mechanical strength and wear resistance of Ti, various elements have been added to create new alloys from three microstructural categories: alpha-stabilisers (such as Al, O, N, and C), beta-stabilisers (such as Mo, V, Fe, Cr, Ni, and Co), and neutral stabilisers (such as Zr). The properties of Ti alloys vary according to the composition of the elements. Ti alloys with alpha and near alpha microstructures exhibit superior corrosion resistance but lower strength. On the other hand, alpha + beta and beta alloys such as Ti–6Al–4 V ELI, Ti– 5Al–2.5Fe, and Ti–6Al–7Nb have high strength and good formability but relatively low corrosion resistance (Semlitsch et al., 1985, Wang, 1996, Zwicker, 1980). Ti–6Al–4 V has gained popularity for its relatively high strength but it has been reported to have greater toxicity than pure Ti due to the presence of elements such as Al and V (Okazaki et al., 1996). Similarly, other Ti alloys such as Ti–6Al–7Nb and Ti–13Nb–13Zr have been reported to improve corrosion rate, mechanical properties, and biocompatibility (Astrand et al., 2004, Bottino et al., 2009, Cremasco et al., 2008, Jager et al., 2008, Jungner et al., 2005, Khan et al., 1999, Schupbach et al., 2005).
In the recent years, zirconium (Zr) has been commonly used as an alloying element. Zr, which belongs to the same group as TI, shows chemical and physical properties similar to Ti. The titanium–zirconium (TiZr) system is a solid solution, which makes it more resistant to corrosion than other alloys and gives it biocompatibility comparable with pure Ti but with better or comparable mechanical properties (Guglielmotti et al., 1999, Kobayashi et al., 1995, Kobayashi et al., 1998, Stojilovic et al., 2005, Tsuchiya et al., 1998). Olmedo et al. (2012) stated that “the elasticity, corrosion resistance, and other mechanical properties of zirconium and its alloys make them a suitable material for biomedical implants.”
One brand of dental implants that has been marketed commercially by the name of Roxolid® (Straumann, Switzerland) is made from a TiZr alloy. The manufacturer claims that these implants have better mechanical strength and improved biocompatibility than existing Ti alloy implants (Bernhard et al., 2009, Gottlow et al., 2012, Grandin et al., 2012, Sista et al., 2011). Preclinical testing in the developmental phase showed favourable mechanical strength and corrosion properties with biocompatibility and comparable osseointegration, as seen in animal studies (Saulacic et al., 2012, Thoma et al., 2011). Similarly, in vitro and in vivo studies by our group comparing c.p. TI and TiZr on osseointegration have shown promising results (Sharma et al., 2015, Sharma et al., 2016). However, there are very few research studies on the mechanical properties of Ti alloyed with 13–17% of Zr.
To the best of our knowledge, there is no published literature evaluating the structural and mechanical properties of TiZr with 13–17% Zr concentration. The aim of this study is to compare the TiZr alloy with pure Ti in terms of their composition by electron dispersive spectroscopy (EDS), microstructure/crystallinity by electron backscatter diffraction (EBSD), and X-ray diffraction (XRD) and their mechanical properties (elastic modulus, hardness) by nanoindentation.
2. Materials and methods
2.1. Preparation of specimens
Pure Grade-IV rod Ti was sourced from Southern Implants (South Africa), and a TiZr alloy (Zr 13–17 wt%) rod was purchased from Huizhou Applied Materials Co., Ltd. (China). Disks (n = 5) were cut from the Ti and TiZr rods (10 mm diameter × 1.5 mm thickness). Commercially available Straumann Roxolid® implants with similar Zr composition (13–17%) (3.5 mm diameter × 12 mm long) were purchased from Straumann Australia for comparison.
The Ti, TiZr, and Roxolid® implants were embedded in the conductive SiO2-filled phenolic mounting compound and sequentially ground and polished.
2.2. Elemental composition by electron dispersive spectroscopy
Elemental analysis was performed using the JEOL 2300F EDS system at an accelerating voltage of 20–25 kV. The system was calibrated before use using metal and mineral reference standards. The analysis was carried out at 25 kV. Five different areas of the samples were analysed at 1000x magnification for 100 s / area, with the location of each image determined in a semi-random manner where one was taken in the centre and the others from four corners. The results of all the groups were compared for the presence of component elemental peaks.
2.3. Nanoindentation for elastic modulus and hardness
Nanoindentation was performed using an Ultra Micro-Indentation System (UMIS-2000, CSIRO, Australia) with a calibrated Berkovich indenter and, a compliance correction of 0.0002 μm/N. Twenty-five indentations (n = 25) were performed per sample using a load of 200 mN with 50 μm spacing between indents. The Poisson’s ratio (0.37), required to calculate the hardness and elastic modulus, was standardised across the three materials based on Ti ASTM Grade 4 (Citeau et al., 2005).
2.4. X-ray diffraction
The phase composition and crystallinity of the alloys Ti, TiZr and Roxolid® (n = 5) were detected by an XRD system (Philips, The Netherlands) operated at 60 kV and 60 mA with nickel filtered CuKα radiation. The phase composition was identified by matching each characteristic peak with the corresponding International Centre for Diffraction Data files.
2.5. Electron backscatter diffraction (EBSD)
EBSD analyses were performed on three samples in each group (n = 5) using a scanning electron microscope optimised for analysis. EBSD patterns were collected at 0.1–0.5 μm step sizes (Oxford Instruments Aztec @20 kV, ~13nA). EBSD data were post-processed using HKL CHANNEL 5 software following the methods outlined by Prior et al. (2009). The crystallographic orientation of crystals is determined using Euler angles. Subsequent phase maps and grain size analyses were produced, with grains defined as cells and surrounded by boundaries with misorientations of 10° or more (Shigematsu et al., 2006).
3. Statistical analysis
The statistical package PRISM (GraphPad Prism 6, La Jolla, USA) was used for all the statistical analyses. The data were statistically analysed using analysis of variance (ANOVA) and Tukey post hoc tests for multiple comparisons between the experimental groups. The probability of p-value > 0.05 was considered to be statistically significant.
4. Results
4.1. Elemental analysis
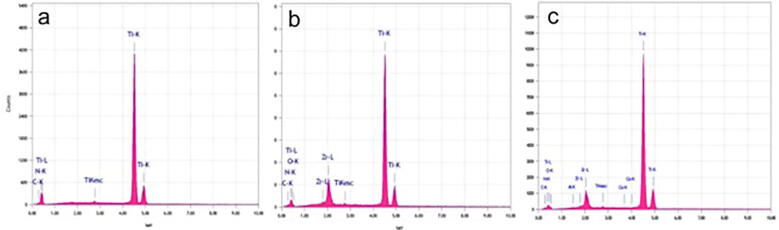
The elemental composition of the three materials is presented in Table 1. The average Zr percentage in TiZr and Roxolid® was 13.2 ± 0.45% and 13.98 ± 0.58%, respectively. The EDS peaks, as shown in Fig. 1, correspond to the elements present in Ti, TiZr, and Roxolid®, respectively. All groups showed characteristic peaks for carbon (C1s) and nitrogen (N1s), indicating only minor hydrocarbon contamination.
Table 1.
Elemental composition of materials as evaluated by electron dispersive spectroscopy (EDS) analysis.
| Titanium% | Zr % | C | N | |
|---|---|---|---|---|
| Ti | 97.6 ± 0.89 | x | 2.4 ± 0.89 | 2.4 ± 2.06 |
| TiZr | 81.25 ± 2.34 | 13.2 ± 0.45 | 2.42 ± 0.53 | 2.54 ± 0.82 |
| Roxolid® | 80.79 ± 3.21 | 13.98 ± 0.58 | 1.29 ± 0.37 | 2.71 ± 0.54 |
Fig. 1.
Electron dispersive spectroscopy (EDS) analysis showing the elemental composition. a) Ti b) TiZr and c) Roxolid® implants.
4.2. Elastic modulus
The elastic modulus of the samples is presented in Fig. 2. The mean elastic modulus of Ti was 99.27 ± 1.80 GPa, TiZr 96.42 ± 1.53 GPa, and Roxolid® was 96.12 ± 2.82 GPa. TiZr and Roxolid® were found to have significantly lower elastic modulus values than Ti (p < 0.0001, one-way ANOVA). However, no statistically significant difference was found between the TiZr and Roxolid® (p = 0.8712, ANOVA) values.
Fig. 2.
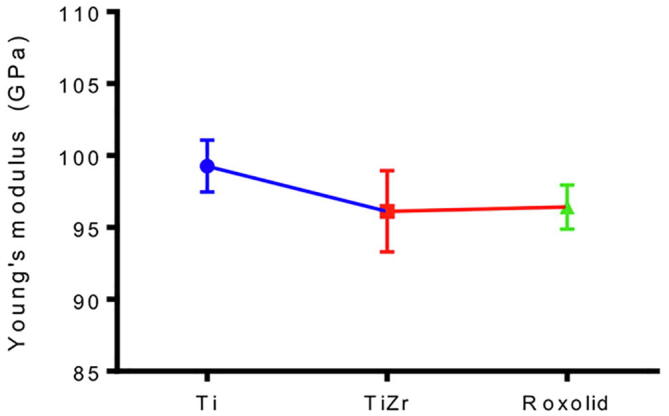
Young's modulus (Y) of Ti, TiZr and Roxolid by nanoindentation. There was a significant difference in Y between Ti compared to TiZr and Roxolid. (**** p < 0.0001, one-way ANOVA, n = 25, whereas no significant difference was observed between Y of TiZr and Roxolid [p = 0.8712, ANOVA].
4.3. Hardness
The hardness values of the Ti, TiZr, and Roxolid® samples are presented in Fig. 3. The hardness value of Ti by nanoindentation was 2.38 ± 0.13 GPa and 2.87 ± 0.28 GPa and 3.19 ± 0.09 GPa for TiZr and Roxolid®, respectively. Both TiZr alloys had significantly higher (p < 0.05) hardness than Ti. However, there was no significant difference between TiZr and Roxolid® (p > 0.05).
Fig. 3.

Hardness (H) of Ti, TiZr and Roxolid by nanoindentation. Roxolid showed significantly higher 'H' compared to Ti (**** p < 0.0001, one-way ANOVA, n = 25).
4.4. Crystallography
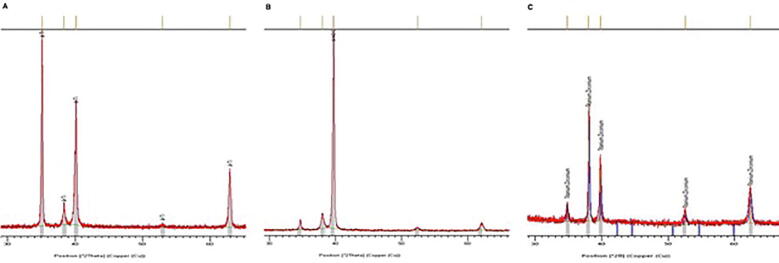
The crystal structure of the oxide layer was analysed by assessing the X-ray diffraction pattern. In this study, the Ti surface had a stronger anatase peak at the same degree as TiZr alloy and Roxolid®, as shown in Fig. 4. For all three samples, the diffraction peaks matched well with those of the α-phase.
Fig. 4.
X-ray diffraction (XRD) patterns of Ti and Ti Zr alloy. A) Ti, B) TiZr alloy and C) Roxolid implants.
4.5. Electron backscatter diffraction (EBSD)
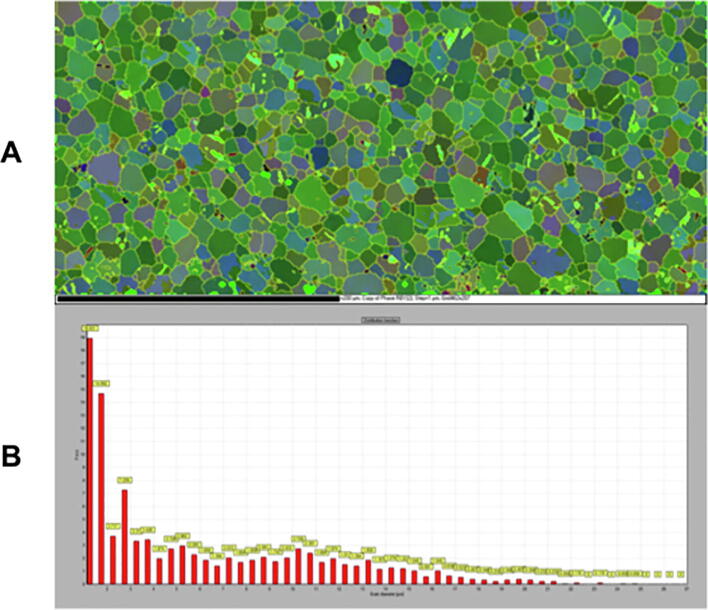
Pure Ti has a typical pure hexagonal closed-packed (hcp) phase with fine-grained microstructure, as shown in Fig. 5. The average grain size measured was 5.97 μm. The TiZr and Roxolid® specimens had grain sizes smaller than the lower resolution limits of the EBSD system (20 nm) (Maitland and Sitzman, 2007) and, therefore, were not included in the results.
Fig. 5.
Electron backscatter diffraction (EBSD). A) Representative crystal orientation map of the α-phase as evaluated using EBSD. Ti grains are Euler colour coded based on their orientation & B). Histogram of spatial grain size distribution of Ti in µm.
5. Discussion
Mechanical properties and surface characteristics of dental implants play a critical role in successful and stable osseointegration, while poor bone quality, early loading requirements, and occlusal forces can lead to the failure of implants. Ti is the material of choice for implant manufacturing because of its superior biocompatibility, high corrosion resistance, and good mechanical properties (Depprich et al., 2008). However, the lack of adequate tensile strength in load-bearing areas led to the development of newer Ti alloys with better mechanical properties and biocompatibility similar to commercially pure Ti. Amongst the newly developed Ti alloys, TiZr (13–15% Zr) implants have shown promise as a biomaterial with high strength and a biocompatible alternative to pure Ti (Bernhard et al., 2009, Gottlow et al., 2012, Kobayashi et al., 1995).
There are very few studies in the published literature evaluating the mechanical properties of TiZr with 13–17% Zr concentration. Therefore, this study was performed to assess and compare TiZr alloy (13–17% Zr) and cpTi in terms of the elemental composition, microstructure/crystallinity, and mechanical properties relating to commercially available Roxolid® implants.
The results of the elemental analysis showed that the compositions of TiZr and Roxolid® aligned with the published studies (Al-Nawas et al., 2012, Chiapasco et al., 2012, Thoma et al., 2011). When examined using EDS, the compositions of the two alloys exhibited similar percentages of zirconium, with slightly higher Zr content in Roxolid® but still within the range of 13–17%, suitable to provide the desirable properties for implant biomaterial (Bernhard et al., 2009, Gottlow et al., 2012).
The difference in elastic modulus between implant material and bone leads to a “stress shielding effect”. Higher stiffness or elastic modulus of the implant will result in a greater amount of peri-implant bone loss. A study using finite element analysis showed that lower elastic modulus helps in the distribution of stress to the surrounding bone and, therefore, promotes bone formation (Sumitomo et al., 2008).
The results of nanoindentation showed significantly lower elastic moduli for TiZr alloys (TiZr and Roxolid®) than Ti – a finding that has been evidenced in previous studies (for instance, Ho et al., 2008, Ho, 2008, Hsu et al., 2010). This is possibly because of the change in crystal size and structure after the addition of Zr (Ho et al., 2008). Nevertheless, implant materials with lower elastic moduli are beneficial as they reduce the stress-shielding effect, prevent bone atrophy, and promote bone reorganisation around the implant (Sumitomo et al., 2008).
The addition of Zr precipitates the alpha phase by complete solid solution, which, in turn, increases the hardness of the alloy, as mentioned earlier (Ho et al., 2008, Kobayashi et al., 1998). Furthermore, a study indicating the composition dependence of hardness for TiZr alloys has been published (Ho et al., 2008). The hardness of Ti increases with an increase in the Zr concentration and reaches a peak when the percentage of Zr reaches 50% (Kobayashi et al., 1995). The value is approximately 2.5 times as high as the hardness of pure Ti (Kobayashi et al., 1995). For alloys with concentrations of over 50% Zr, the hardness decreased with the Zr content. These results were independent of the alloy’s process/heat treatments (Imgram et al., 1962). The presence of martensitic microstructure in all Zr alloys – irrespective of heat treatments – reinforces the fact that the hardness of TiZr alloys is predominantly determined by alloy composition (Kobayashi et al., 1995).
As seen in the X-ray pattern of the samples, TiZr and Roxolid showed peaks in the same positions as Ti. This indicates that the addition of Zr does not change the crystalline structure of the material, corresponding to the alpha phase (hcp), which is in agreement with the results of previous studies (Ho et al., 2008). There were higher anatase peaks for Roxolid®, which may be due to the presence of the oxide layer formed. It has been reported that as the oxide layer increases, there is a predominant increase in the anatase phase (Oh et al., 2005). There was no hint of β phase peaks or any other intermediate phases. This may be because the TiZr alloy is a completely solid solution, as previously described. The TiZr alloy shows a complete solid solution for the low-temperature alpha phase as well as the high-temperature beta phase (Ho et al., 2008). For the TiZr alloy, the peaks matched those of the hcp alpha phase of Ti. This contributes to an increase in the hardness of the material.
As for Roxolid® and TiZr, the biggest difference in microstructure when compared to Ti was that the two alloys had a crystal size much smaller than that of Ti. Several attempts were made to prepare the specimens, but we could not obtain useable pattern qualities. This could be attributed to the fine grain sizes of TiZr and Roxolid®, which probably underwent some sort of grain refinement. Also, TiZr is innately difficult to prepare due to its high level of hardness (Williams et al., 2010).
The results of the study confirm that the addition of Zr at 13–15% to Ti increase the strength of the implant, which is beneficial for high-load-bearing areas and lower elastic moduli, which reduce the “stress shielding” effect and eventually lead to implant failures. The above, along with superior corrosion resistance, can be useful in designing implants of smaller diameters in narrow ridges with poor bone support, possibly eliminating the need for additional bone grafting procedures. Further mechanical properties testing, such as removal torque tests and tensile strength on cyclic loading, should be conducted. These were not done in this study due to the disc design of the samples, which is a major limitation. Further studies should be done with a larger sample size and screw-shaped implants to simulate commercially available dental implants for clinical application.
6. Conclusion
A lower elastic modulus and significantly higher hardness for TiZr (13–17% Zr) make this alloy stronger and suitable for high-load-bearing implants.
CRediT authorship contribution statement
Ajay Sharma: Conceptualization, Investigation, Visualization, Formal analysis. John N. Waddell: Resources, Methodology, Data curation, Supervision. Kai C. Li: Software, Data curation, Validation. Lavanya A Sharma: Writing - original draft, Writing - review & editing. David J. Prior: Resources, Methodology, Supervision. Warwick J. Duncan: Project administration, Supervision.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
This research was supported by Dentsply Research Fund (Australia).We wish to acknowledge the assistance of Liz Girwan, Otago Centre for Electron Microscopy, University of Otago, for her assistance in the SEM.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Nawas B., Brägger U., Meijer H.J.A., Naert I., Persson R., Perucchi A., Quirynen M., Raghoebar G.M., Reichert T.E., Romeo E., Santing H.J., Schimmel M., Storelli S., ten Bruggenkate C., Vandekerckhove B., Wagner W., Wismeijer D., Müller F. A Double-Blind Randomized Controlled Trial (RCT) of Titanium-13Zirconium versus Titanium Grade IV Small-Diameter Bone Level Implants in Edentulous Mandibles - Results from a 1-Year Observation Period. Clin. Implant Dent. Relat. Res. 2012;14:896–904. doi: 10.1111/j.1708-8208.2010.00324.x. [DOI] [PubMed] [Google Scholar]
- Albrektsson T., Brånemark P.-I., Hansson H.-A., Lindström J. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981;52:155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- Astrand P., Engquist B., Dahlgren S., Grondahl K., Engquist E., Feldmann H. Astra Tech and Branemark system implants: a 5-year prospective study of marginal bone reactions. Clin. Oral Implants Res. 2004;15:413–420. doi: 10.1111/j.1600-0501.2004.01028.x. [DOI] [PubMed] [Google Scholar]
- Bernhard N., Berner S., De Wild M., Wieland M. The binary TiZr alloy—A newly developed Ti alloy for use in dental implants. Forum Implantol. 2009:30–39. [Google Scholar]
- Bottino M.C., Coelho P.G., Henriques V.A.R., Higa O.Z., Bressiani A.H.A., Bressiani J.C. Processing, characterization, and in vitro/in vivo evaluations of powder metallurgy processed Ti-13Nb-13Zr alloys. J. Biomed. Mater. Res. - Part A. 2009;88:689–696. doi: 10.1002/jbm.a.31912. [DOI] [PubMed] [Google Scholar]
- Chiapasco M., Casentini P., Zaniboni M., Corsi E., Anello T. Titanium-zirconium alloy narrow-diameter implants (Straumann Roxolid ®) for the rehabilitation of horizontally deficient edentulous ridges: prospective study on 18 consecutive patients. Clin. Oral Implants Res. 2012;23:1136–1141. doi: 10.1111/j.1600-0501.2011.02296.x. [DOI] [PubMed] [Google Scholar]
- Citeau A., Guicheux J., Vinatier C., Layrolle P., Nguyen T.P., Pilet P., Daculsi G. In vitro biological effects of titanium rough surface obtained by calcium phosphate grid blasting. Biomaterials. 2005;26:157–165. doi: 10.1016/j.biomaterials.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Cremasco A., Osório W.R., Freire C.M.A., Garcia A., Caram R. Electrochemical corrosion behavior of a Ti–35Nb alloy for medical prostheses. Electrochim. Acta. 2008;53:4867–4874. [Google Scholar]
- Depprich R., Zipprich H., Ommerborn M., Naujoks C., Wiesmann H.-P., Kiattavorncharoen S., Lauer H.-C., Meyer U., Kübler N.R., Handschel J. Osseointegration of zirconia implants compared with titanium: an in vivo study. Head Face Med. 2008;4:30. doi: 10.1186/1746-160X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlow J., Dard M., Kjellson F., Obrecht M., Sennerby L. Evaluation of a New Titanium-Zirconium Dental Implant: A Biomechanical and Histological Comparative Study in the Mini Pig. Clin. Implant Dent. Relat. Res. 2012;14:538–545. doi: 10.1111/j.1708-8208.2010.00289.x. [DOI] [PubMed] [Google Scholar]
- Grandin H.M., Berner S., Dard M. A Review of Titanium Zirconium (TiZr) Alloys for Use in Endosseous Dental Implants. Materials. 2012;5(12):1348–1360. [Google Scholar]
- Guglielmotti M.-B., Renou S., Cabrini R.L. A histomorphometric study of tissue interface by laminar implant test in rats. Int. J. Oral Maxillofac. Implants. 1999;14(4):565–570. [PubMed] [Google Scholar]
- Ho W.-F., Chen W.-K., Wu S.-C., Hsu H.-C. Structure, mechanical properties, and grindability of dental Ti–Zr alloys. J. Mater. Sci. - Mater. Med. 2008;19:3179–3186. doi: 10.1007/s10856-008-3454-x. [DOI] [PubMed] [Google Scholar]
- Ho W.F. Effect of omega phase on mechanical properties of Ti-Mo alloys for biomedical applications. J. Med. Biol. Eng. 2008;28:47–51. [Google Scholar]
- Hsu H.C., Wu S.C., Pan C.H., Wang H.W., Ho W.F. Grindability evaluation of dental cast Ti-20Cr-X alloys. J. Med. Biol. Eng. 2010;30:73–78. [Google Scholar]
- Ikarashi Y., Toyoda K., Kobayashi E., Doi H., Yoneyama T., Hamanaka H., Tsuchiya T. Nippon Kinzoku Gakkaishi/Journal Japan Inst; Met: 2007. Improved biocompatibility of titanium-zirconium (Ti-Zr) alloy: Tissue reaction and sensitization to Ti-Zr alloy compared with pure Ti and Zr in rat implantation study. [Google Scholar]
- Imgram A.G., Williams D.N., Ogden H.R. Tensile properties of binary titanium-zirconium and titanium-hafnium alloys. J. Less Common Met. 1962;4:217–225. [Google Scholar]
- Jacobs J.J., Gilbert J.L., Urban R.M. Corrosion of Metal Orthopaedic Implants. J. Bone Jt. Surg. (American Vol) 1998;80:268–282. doi: 10.2106/00004623-199802000-00015. [DOI] [PubMed] [Google Scholar]
- Jager M., Urselmann F., Witte F., Zanger K., Li X., Ayers D.C., Krauspe R. Osteoblast differentiation onto different biometals with an endoprosthetic surface topographyin vitro. J. Biomed. Mater. Res. Part A. 2008;86A:61–75. doi: 10.1002/jbm.a.31552. [DOI] [PubMed] [Google Scholar]
- Jungner M., Lundqvist P., Lundgren S. Oxidized titanium implants (Nobel BiocareR TiUnitetm) compared with turned titanium implants (Nobel BiocareR mark IIItm) with respect to implant failure in a group of consecutive patients treated with early functional loading and two-stage protocol. Clin. Oral Implants Res. 2005;16:308–312. doi: 10.1111/j.1600-0501.2005.01101.x. [DOI] [PubMed] [Google Scholar]
- Katou F., Andoh N., Motegi K., Nagura H. Immuno-inflammatory responses in the tissue adjacent to titanium miniplates used in the treatment of mandibular fractures. J. Cranio-Maxillofacial Surg. 1996;24:155–162. doi: 10.1016/s1010-5182(96)80049-7. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Williams R.L., Williams D.F. Conjoint corrosion and wear in titanium alloys. Biomaterials. 1999;20:765–772. doi: 10.1016/s0142-9612(98)00229-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Doi H., Yoneyama T., Hamanaka H., Gibson I.R., Best S.M., Shelton J.C., Bonfield W. Influence of aging heat treatment on mechanical properties of biomedical Ti-Zr based ternary alloys containing niobium. J. Mater. Sci. - Mater. Med. 1998;9:625–630. doi: 10.1023/a:1008927407556. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Matsumoto S., Doi H., Yoneyama T., Hamanaka H. Mechanical properties of the binary titanium-zirconium alloys and their potential for biomedical materials. J. Biomed. Mater. Res. 1995;29:943–950. doi: 10.1002/jbm.820290805. [DOI] [PubMed] [Google Scholar]
- Maitland T., Sitzman S. Scanning Microsc. Nanotechnol. Tech Appl; 2007. Electron Backscatterd Diffraction (EBSD) Technique and Materials Characterization Examples. [Google Scholar]
- Niinomi M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A. 2002;33:477–486. [Google Scholar]
- Oh H.-J., Lee J.-H., Jeong Y., Kim Y.-J., Chi C.-S. Microstructural characterization of biomedical titanium oxide film fabricated by electrochemical method. Surf. Coatings Technol. 2005;198:247–252. [Google Scholar]
- Okazaki Y., Ito Y., Kyo K., Tateishi T. Corrosion resistance and corrosion fatigue strength of new titanium alloys for medical implants without V and Al. Mater. Sci. Eng., A. 1996;213:138–147. [Google Scholar]
- Olmedo D., Fernández M.M., Guglielmotti M.B., Cabrini R.L. Macrophages Related to Dental Implant Failure. Implant Dent. 2003;12:75–80. doi: 10.1097/01.id.0000041425.36813.a9. [DOI] [PubMed] [Google Scholar]
- Olmedo D.G., Tasat D.R., Duffó G., Guglielmotti M.B., Cabrini R.L. The issue of corrosion in dental implants: a review. Acta Odontol. Latinoam. 2009;22:3–9. [PubMed] [Google Scholar]
- Olmedo D., Duffó G., Cabrini R., Guglielmotti M. Pitting Corrosion. IntechOpen; United Kingdom: 2012. Systemic and local tissue response to titanium corrosion; pp. 93–118.https://www.intechopen.com/books/pitting-corrosion/systemic-and-local-tissue-response-to-titanium-corrosion (Accessed 23 March 2012). In this issue. [Google Scholar]
- Prior D.J., Mariani E., Wheeler J. Electron Backscatter Diffraction in Materials Science. Springer; US, Boston, MA: 2009. EBSD in the Earth Sciences: Applications, Common Practice, and Challenges; pp. 345–360. [Google Scholar]
- Schupbach P., Glauser R., Rocci A., Martignoni M., Sennerby L., Lundgren A., Gottlow J. The Human Bone-Oxidized Titanium Implant Interface: A Light Microscopic, Scanning Electron Microscopic, Back-Scatter Scanning Electron Microscopic, and Energy-Dispersive X-Ray Study of Clinically Retrieved Dental Implants. Clin. Implant Dent. Relat. Res. 2005;7:s36–s43. doi: 10.1111/j.1708-8208.2005.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Semlitsch M., Staub F., Weber H. Titanium-Aluminium-Niobium alloy, Development for biocompatible, high strength surgical implants. Biomed. Tech. Eng. 1985;30:334–339. doi: 10.1515/bmte.1985.30.12.334. [DOI] [PubMed] [Google Scholar]
- Sharma A., McQuillan A.J., Shibata Y., Ajay Sharma L., Waddell J.N., Duncan W.J. Histomorphometric and histologic evaluation of titanium-zirconium (aTiZr) implants with anodized surfaces. J. Material Science: Materials in Medicine. 2016;27:86. doi: 10.1007/s10856-016-5695-4. Epub 2016 Mar 12. [DOI] [PubMed] [Google Scholar]
- Sharma A., McQuillan A.J., Ajay Sharma L., Waddell J.N., Shibata Y., Duncan W.J. Spark anodization of titanium–zirconium alloy: surface characterization and bioactivity assessment. J. Mater. Sci. - Mater. Med. 2015;26:1–11. doi: 10.1007/s10856-015-5555-7. [DOI] [PubMed] [Google Scholar]
- Saulacic N., Bosshardt D., Bornstein M., Berner S., Buser D. Bone apposition to a titanium-zirconium alloy implant, as compared to two other titanium-containing implants. European cells & materials. 2012;23:273–288. doi: 10.22203/ecm.v023a21. [DOI] [PubMed] [Google Scholar]
- Shigematsu N., Prior D.J., Wheeler J. First combined electron backscatter diffraction and transmission electron microscopy study of grain boundary structure of deformed quartzite. J. Microsc. 2006;224:306–321. doi: 10.1111/j.1365-2818.2006.01697.x. [DOI] [PubMed] [Google Scholar]
- Siddiqi A., Payne A.G.T., De Silva R.K., Duncan W.J. Titanium allergy: could it affect dental implant integration? Clin. Oral Implants Res. 2011;22:673–680. doi: 10.1111/j.1600-0501.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- Sista S., Wen C., Hodgson P.D., Pande G. The influence of surface energy of titanium-zirconium alloy on osteoblast cell functions in vitro. Journal of Biomedical Materials Research - Part A. 2011;1(97A):27–36. doi: 10.1002/jbm.a.33013. [DOI] [PubMed] [Google Scholar]
- Stojilovic N., Bender E., Ramsier R. Surface chemistry of zirconium. Prog. Surf. Sci. 2005;78(3):101–184. [Google Scholar]
- Sumitomo N., Noritake K., Hattori T., Morikawa K., Niwa S., Sato K., Niinomi M. Experiment study on fracture fixation with low rigidity titanium alloy. J. Mater. Sci. - Mater. Med. 2008;19:1581–1586. doi: 10.1007/s10856-008-3372-y. [DOI] [PubMed] [Google Scholar]
- Tawse-Smith A., Ma S., Siddiqi A., Duncan W.J., Girvan L., Hussaini H.M. Titanium Particles in Peri-Implant Tissues: Surface Analysis and Histologic Response. Clin. Adv. Periodontics. 2012;2:232–238. [Google Scholar]
- Thoma D.S., Jones A.A., Dard M., Grize L., Obrecht M., Cochran D.L. Tissue Integration of a New Titanium-Zirconium Dental Implant: A Comparative Histologic and Radiographic Study in the Canine. J. Periodontol. 2011;82:1453–1461. doi: 10.1902/jop.2010.100737. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Nakamura A., Ohshima Y., Kobayashi E., Doi H., Yoneyama T., et al. Chondrogenic cellular responses to titanium and zirconium alloys in vitro. Tissue Eng. 1998;2(4):197-204. [Google Scholar]
- Wang K. The use of titanium for medical applications in the USA. Mater. Sci. Eng., A. 1996;213:134–137. [Google Scholar]
- Wen C.E., Yamada Y., Shimojima K., Chino Y., Asahina T., Mabuchi M. Processing and mechanical properties of autogenous titanium implant materials. J. Mater. Sci. - Mater. Med. 2002;13:397–401. doi: 10.1023/a:1014344819558. [DOI] [PubMed] [Google Scholar]
- Williams D. Titanium: epitome of biocompatibility or cause for concern. J. Bone Joint Surg. Br. 1994;76-B:348–349. [PubMed] [Google Scholar]
- Williams R., Genc A., Huber D., Fraser H. Sample Surface Preparation For Traditional EBSD Collection and 3D EBSD Collection. Microsc. Microanal. 2010;16:706–707. [Google Scholar]
- Zwicker, U., 1980. Mechanical properties and tissue reactions of a titanium alloy for implant materials. Titan. 80’Science Technol. AIME.