Abstract
The phenotypic and genotypic characterization of five clinical isolates of Leuconostoc pseudomesenteroides associated with nosocomially acquired urinary tract infections is described. All the strains were susceptible to chloramphenicol, clindamycin, erythromycin, gentamicin, and tetracycline; all were resistant to nalidixic acid, norfloxacin, and vancomycin; and all were intermediately affected by ampicillin and penicillin. Analysis of chromosomal DNA by pulsed-field gel electrophoresis after treatment with SmaI indicated a clonal relationship of the isolates. The results provide evidence for the possibility of nosocomial transmission of this unusual opportunistic, vancomycin-resistant pathogen.
The genus Leuconostoc is composed by catalase-negative gram-positive microorganisms with irregular coccoid morphology. These organisms may be misidentified as Lactobacillus, Streptococcus (particularly the viridans group), Pediococcus, or even Enterococcus, as all share several biochemical properties (3, 20). Unlike most other gram-positive bacteria, these microorganisms have an important physiological marker related to their intrinsic resistance to vancomycin (6, 18). Before 1985, Leuconostoc species were usually considered nonpathogenic and, therefore, of little or no importance in clinical microbiology (3, 19). Since then, increasing numbers of infections due to Leuconostoc have been reported (1, 2, 8–12, 23). Despite remaining uncommon, these pathogens are gaining importance as opportunistic agents of human infections associated with high mortality rates, mainly bacteremia (14, 15, 18). Infections due to Leuconostoc occur more frequently in patients being treated for underlying diseases with vancomycin therapy (7, 13), although Leuconostoc infections have also been documented in otherwise healthy patients (4). The present study describes the phenotypic and genotypic characterization of a cluster of five Leuconostoc pseudomesenteroides strains recovered from hospitalized patients with symptomatic urinary tract infections, providing evidence for the possible nosocomial transmission of this opportunistic vancomycin-resistant bacterium.
Five clinical isolates of catalase-negative, vancomycin-resistant, gram-positive cocci recovered from urine specimens obtained from five inpatients admitted to a University Hospital in Rio de Janeiro, Brazil, were studied. The strains were isolated within a period of 1 week (in April 1997) from patients in two units (nephrology and urology) located on the same hospital floor. Clinical manifestations of the infections included dysuria and/or fever, and the microorganisms grew in pure cultures. All five patients had been admitted to the hospital due to other medical conditions, and only one of the patients had a urinary catheter at the time the culture-positive urine was collected. The most common risk factors associated with infection acquisition are described in Table 1.
TABLE 1.
Characteristics of patients with urinary tract infections caused by L. pseudomesenteroides
| Patient no. | Age (yr), sexa | Underlying conditions | Previous therapy | Associated symptoms | Concn of organisms in urine (CFU/ml) | Clinical outcome |
|---|---|---|---|---|---|---|
| 1 | 16, M | Hydrocephalus and prolonged hospital stay | Vancomycin and cephalosporin | Fever | 5 × 105 | Death |
| 2 | 29, M | Asthma, alcoholism, and drug addiction | None | Dysuria and fever | 5 × 105 | Recovery |
| 3 | 14, F | Renal transplantation and prolonged hospital stay | Vancomycin and trimethoprim-sulfametoxazol | Dysuria | 3 × 105 | Recovery |
| 4 | 18, F | Endometriosis and prolonged hospital stay | Vancomycin and cephalosporin | Dysuria and fever | 4 × 105 | Recovery |
| 5 | 37, F | Not determined | None | Dysuria and fever | 4 × 105 | Recovery |
M, male; F, female.
Identification of the strains to the genus level was performed as described elsewhere (6) by using tests for detecting the following physiological characteristics: presence of catalase, pyrrolidonyl arylamidase and leucine aminopeptidase activities, hydrolysis of esculin in the presence of bile, growth in the presence of 6.5% NaCl, vancomycin susceptibility, and production of gas in lactobacilli De Mann, Rogosa, and Sharp (MRS; Difco Laboratories, Detroit, Mich.) broth. Additional physiological tests, including production of acids from arabinose, lactose, maltose, melibiose, salicin, sucrose, threalose, and xylose, were used for the characterization of the isolates to the species level. All five clinical isolates had similar physiological characteristics. They were negative for catalase, pyrrolidonyl arylamidase, and leucine aminopeptidase activities and did not grow in broth containing 6.5% NaCl. They all were resistant to vancomycin, were esculin-positive in bile, produced gas in MRS broth, and produced acid from arabinose, lactose, maltose, melibiose, salicin, sucrose, threalose, and xylose. On the basis of these results, the most likely identity of the isolates was L. pseudomesenteroides. The isolation of L. pseudomesenteroides from human clinical specimens is rare, and, to the best of our knowledge, there are no specific reports of its association with urinary tract infections. The majority of Leuconostoc strains associated with human infections have been identified as Leuconostoc mesenteroides, followed by Leuconostoc lactis and Leuconostoc citreum (5, 6). On the other hand, the discrimination between species of Leuconostoc is often problematic, and the description of the role of each individual species as infectious agent has possibly been hindered by the difficulty of precise identification. Differentiation of L. pseudomesenteroides and the most frequent species, L. mesenteroides, is mainly based on the results of growth in 6.5% NaCl, a test which is sometimes difficult to interpret and to reproduce (5, 6).
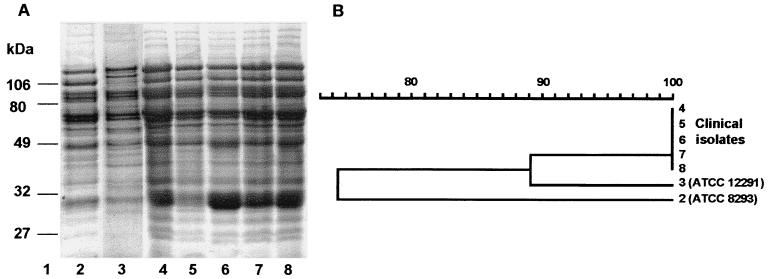
To confirm the identification of the isolates, analysis of whole-cell protein profiles using one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed as described by Merquior et al. (16). This method has been considered to be a reliable and reproducible tool for the differentiation and identification of several species of catalase-negative, gram-positive cocci, including Leuconostoc spp. (5, 21, 22). Protein profiles were compared and clustered by the unweighted pair group method with averages by using the Molecular Analyst Fingerprint Plus software of the Image Analysis System (Bio-Rad Laboratories, Richmond, Calif.). The clinical isolates had virtually indistinguishable protein profiles (Fig. 1) and had higher similarity (average similarity, 89%) with the profile of the L. pseudomesenteroides type strain (SS 1292, ATCC 12291) than with that of the L. mesenteroides type strain (SS 1238, ATCC 8293). These findings confirmed the identification based on conventional physiological tests and indicate that analysis of whole-cell protein profiles can be recommended as an additional tool for the precise identification of L. pseudomesenteroides.
FIG. 1.
(A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles of whole-cell protein extracts of Leuconostoc strains. Lane 1, molecular mass markers (in kilodaltons); lane 2, L. mesenteroides ATCC 8293; lane 3, L. pseudomesenteroides ATCC 12291; lanes 4–8, clinical isolates of L. pseudomesenteroides. (B) Dendrogram resulting from computer-assisted analysis of the protein profiles shown in panel A. The scale represents the average percentage of similarity.
MICs were determined by the microdilution method according to the recommendations of the National Committee for Clinical Laboratory Standards for Streptococcus spp. other than Streptococcus pneumoniae (17), since no criteria are specified for Leuconostoc strains. Results indicated that the clinical isolates were susceptible to chloramphenicol (MIC = 8 μg/ml), clindamycin (0.015 μg/ml), erythromycin (0.1 μg/ml), gentamicin (0.12 μg/ml), and tetracycline (4 μg/ml) and were resistant to nalidixic acid (MIC = 128 μg/ml), norfloxacin (32 μg/ml), and vancomycin (512 μg/ml). Intermediate results were obtained for ampicillin (MIC = 2 μg/ml) and penicillin (1 μg/ml). No strain-to-strain variation in the MICs was observed.
The genotypic relationship of the strains was investigated by analysis of SmaI-digested chromosomal DNA by pulsed-field gel electrophoresis (PFGE) based on the procedure recommended by Teixeira et al. (21). The following parameters for electrophoresis were used: voltage gradient, 6 V/cm; running time, 22 h; temperature, 11°C; pulse time, ramping from 2 to 25 s; and included angle, 120°. The PFGE patterns of all the isolates were found to be identical, and they were distinct from the pattern obtained for the L. pseudomesenteroides type strain (Fig. 2). These data suggested that the isolates may have originated from a common source.
FIG. 2.
PFGE patterns of chromosomal DNA of Leuconostoc strains after digestion with SmaI. Lane 1, molecular size markers (in kilobases); lane 2, L. mesenteroides ATCC 8293; lane 3, L. pseudomesenteroides ATCC 12291; lanes 4–8, clinical isolates of L. pseudomesenteroides.
In conclusion, this report presents the phenotypic and genotypic characterization of a cluster of five L. pseudomesenteroides strains associated with nosocomially acquired urinary tract infections. Taken together, the isolation, within a short period of time, of such a rare opportunistic bacterial species, in pure cultures and in significant numbers, from the urine of symptomatic patients in two related hospital units, in association with the results of genotypic characterization of the isolates, provided evidence of the outbreak potential and the risk of possible nosocomial transmission of this vancomycin-resistant bacterial species. With the increased use of vancomycin, infections due to vancomycin-resistant microorganisms, such as Leuconostoc, may be more frequently encountered, especially in debilitated individuals. The observation of the outbreak described in this paper highlights the need for clinical laboratories to be aware of the potential clinical significance of these bacteria and to be prepared to promptly and accurately identify them. The incorporation of precise procedures into the catalase-negative, gram-positive coccus identification schemes and the application of molecular tools will allow proper detection and characterization of unusual pathogens such as Leuconostoc species, thereby improving our knowledge of the epidemiological aspects of human infections caused by these microorganisms and their routes of transmission.
Acknowledgments
This investigation was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Financiadora de Estudos e Projetos (FINEP), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Ministério da Ciência e Tecnologia (MCT/PRONEX).
REFERENCES
- 1.Barreau C, Wagener G. Characterization of Leuconostoc lactis strains from human sources. J Clin Microbiol. 1990;28:1728–1733. doi: 10.1128/jcm.28.8.1728-1733.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry H, Clancy M T, Brady A, O'Higgins N. Isolation of a Leuconostoc species from a retroareolar breast abscess. J Infect. 1993;27:208–210. doi: 10.1016/0163-4453(93)95025-e. [DOI] [PubMed] [Google Scholar]
- 3.Buu-Hoi A, Branger C, Acar J F. Vancomycin-resistant streptococci or Leuconostoc sp. Antimicrob Agents Chemother. 1985;28:458–460. doi: 10.1128/aac.28.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coovadia Y M, Solwa Z, Van Den Ende J. Meningitis caused by vancomycin-resistant Leuconostoc sp. J Clin Microbiol. 1987;25:1784–1785. doi: 10.1128/jcm.25.9.1784-1785.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliot J A, Facklam R R. Identification of Leuconostoc spp. by analysis of soluble whole-cell protein patterns. J Clin Microbiol. 1993;31:1030–1033. doi: 10.1128/jcm.31.5.1030-1033.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Facklam R, Elliott J A. Identification, classification, and clinical relevance of catalase-negative, gram-positive cocci, excluding streptococci and enterococci. Clin Microbiol Rev. 1995;8:479–495. doi: 10.1128/cmr.8.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrer S, Miguel G, Domingo P, Pericas R, Prats G. Pulmonary infection due to Leuconostoc species in a patient with AIDS. Clin Infect Dis. 1995;21:225–226. doi: 10.1093/clinids/21.1.225. [DOI] [PubMed] [Google Scholar]
- 8.Friedland I R, Snipelisky M, Khoosal M. Meningitis in a neonate caused by Leuconostoc sp. J Clin Microbiol. 1990;28:2125–2126. doi: 10.1128/jcm.28.9.2125-2126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacometti A, Ranaldi R, Siquini F M, Scalise G. Leuconostoc citreum isolated from lung in AIDS patient. Lancet. 1993;342:622. doi: 10.1016/0140-6736(93)91452-r. [DOI] [PubMed] [Google Scholar]
- 10.Golledge C L. Infection due to Leuconostoc species. Rev Infect Dis. 1991;13:184–185. doi: 10.1093/clinids/12.5.184. [DOI] [PubMed] [Google Scholar]
- 11.Green M, Wadowsky R M, Barbadora K. Recovery of vancomycin-resistant gram-positive cocci from children. J Clin Microbiol. 1990;28:484–488. doi: 10.1128/jcm.28.3.484-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handwerger S, Horowitz H, Coburn K, Kolokathis A, Wormser G P. Infection due to Leuconostoc species: six cases and review. Rev Infect Dis. 1990;12:602–610. doi: 10.1093/clinids/12.4.602. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz H W, Handwerger S, van Horn K G, Wormser G P. Leuconostoc, an emerging vancomycin-resistant pathogen. Lancet. 1987;ii:1329–1330. doi: 10.1016/s0140-6736(87)91217-7. [DOI] [PubMed] [Google Scholar]
- 14.Jiménez-Mejías M E, Becerril B, Gómez-Cía T, Del Nozal M, Palomino-Nicás J. Bacteremia caused by Leuconostoc cremoris in a patient with severe burn injuries. Eur J Clin Microbiol Infect Dis. 1997;16:533–535. doi: 10.1007/BF01708238. [DOI] [PubMed] [Google Scholar]
- 15.Ling M L. Leuconostoc bacteraemia. Singapore Med J. 1992;33:241–243. [PubMed] [Google Scholar]
- 16.Merquior V L C, Peralta J M, Facklam R R, Teixeira L M. Analysis of electrophoretic whole-cell protein profiles as a toll for characterization of Enterococcus species. Curr Microbiol. 1994;28:149–153. [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Document M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 18.Quirós J C L B, Munõz P, Cercenado E, Sampelayo T H, Moreno S, Bouza E. Leuconostoc species as a cause of bacteremia: two case reports and a literature review. Eur J Clin Microbiol Infect Dis. 1991;10:505–509. doi: 10.1007/BF01963938. [DOI] [PubMed] [Google Scholar]
- 19.Rubin L G, Vellozzi E, Shapiro J, Isenberg H D. Infection with vancomycin-resistant “streptococci” due to Leuconostoc species. J Infect Dis. 1988;157:216. doi: 10.1093/infdis/157.1.216. [DOI] [PubMed] [Google Scholar]
- 20.Ruoff K L, Kuritzkes D R, Wolfson J S, Ferraro M J. Vancomycin-resistant gram-positive bacteria isolated from human sources. J Clin Microbiol. 1988;26:2064–2068. doi: 10.1128/jcm.26.10.2064-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixeira L M, Carvalho M G S, Merquior V L C, Steigerwalt A G, Brenner D J, Facklam R R. Phenotypic and genotypic characterization of Vagococcus fluvialis, including strains isolated from human sources. J Clin Microbiol. 1997;35:2778–2781. doi: 10.1128/jcm.35.11.2778-2781.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villani F, Moschetti G, Blaiotta G, Coppola S. Characterization of strains of Leuconostoc mesenteroides by analysis of soluble whole-cell protein pattern, DNA fingerprint and restriction of ribosomal DNA. J Appl Microbiol. 1997;82:578–588. doi: 10.1111/j.1365-2672.1997.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 23.Wenocur H S, Smith M A, Vellozzi E M, Shapiro J, Isenberg H D. Odontogenic infection secondary to Leuconostoc species. J Clin Microbiol. 1988;26:1893–1894. doi: 10.1128/jcm.26.9.1893-1894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]