Abstract
Background
Biocompatibility is an essential property for any dental root repair material that may interact with the surrounding periodontal tissues. We hypothesise that the three mineral trioxide aggregate (MTA) restorative brands ProRoot MTA, MTA Flow and Harvard MTA have similar biocompatibility. To test this hypothesis, we compared the cytotoxic effects of these materials on human gingival fibroblast (GF).
Methods
MTA cements were prepared, and after completion of setting, they were incubated in Dulbecco's Modified Eagle Medium for 1 day or 4 days to obtain low and high concentrations of MTA elutes respectively. The elutes of MTA supplemented with fetal bovine serum were added to GF and incubated for 3 days at 37 °C and 5% CO2. Untreated cells were used as control. The cell viability was assessed using a 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay at 24, 48 and 72 h.
Results
After 24 h, the MTT assay showed that both 1- and 4-day elutes of MTA flow and Harvard MTA reduced cell viability significantly compared to control (P < 0.05). After 48 h, the 1-day elute of ProRoot MTA induced GF proliferation (P = 0.0136) while MTA flow and Harvard MTA were similar to control. After 72 h, the 1-day elute of ProRoot MTA and Harvard MTA induced GF proliferation, while the elute of MTA flow was comparable to control. The 4-day elute of Harvard MTA continued to be cytotoxic to GF after 24 h, 48 h, and 72 h incubation, while the 4-day elute of ProRoot MTA and MTA flow were similar to control.
Conclusion
ProRoot MTA and MTA Flow showed comparable biocompatibility. However, the 4-day elute of Harvard MTA was cytotoxic to GF. Further studied are required to assess the cell viability after direct contact with these materials versus eluent in vitro and compare these sealers in the clinical setting.
Keywords: Cytotoxicity, MTT assay, MTA, Gingival fibroblasts
1. Introduction
The ideal endodontic repair material should be radiopaque, moisture impervious, insoluble and easy to handle, as well as having sealing ability with dimensional stability. It also should be non-toxic, biocompatible and capable of inducing cementogenesis (Bodrumlu, 2008).
Mineral trioxide aggregate (MTA) was first proposed as a potential endodontic repair material in 1993 and it was approved for endodontic use by the US FDA in 1998 (Camilleri and Pitt Ford, 2006). MTA has many endodontic applications based on its capability to stimulate pulp tissue repair and promote reparative dentine formation, both of which assist in early pulp wound healing (Torabinejad and Parirokh, 2010). MTA is used as a root-end filling material (Floratos et al., 2013) and a root perforation repair material (Ford et al., 1995). MTA is also used for apexification, pulpotomy (Leye Benoist et al., 2012), pulp capping (Shahravan et al., 2011, Tziafas et al., 2002) and root resorption treatment (Jacobovitz and De Lima, 2008).
ProRoot MTA was developed as a root repair material that has dental standards (ISO 9917). The primary components of ProRoot MTA include tricalcium silicate and dicalcium silicate, bismuth oxide, tricalcium aluminate and tetracalcium aluminoferrite (Vajrabhaya et al., 2006).
MTA Flow™ is available as a powder and a gel. The constituents of this material are powder of dicalcium and tricalcium silicate, bismuth oxide and a liquid gel of water-soluble silicone. It was developed with a smaller particle size (<10 µm) that on the mixing with water results in a material of smooth consistency that is easy to be applied. MTA Flow has a short setting time of up to 15 min. MTA Flow showed marked alkalinizing activity, low solubility, high radiopacity, and ability to form calcium phosphate deposits on its surface. The physical properties of MTA Flow are the same as those of conventional MTA (Guimaraes et al., 2017). A Recent study reported that MTA Flow showed biocompatibility and was less cytotoxic compared to ProRoot MTA (Bueno et al., 2019).
Several new formulations of MTA materials have been introduced to the dental market; Harvard MTA is one of the newest brands of MTA. Harvard MTA is a new calcium silicate cement (CSC), available in the form of powder and liquid as capsule or hand mix formula, with many endodontic clinical applications. Harvard MTA has a setting time of 40 min and working time of 2 min (El-Ma’aita et al., 2013).
The root canal system is interconnected with periodontal tissues via the apical foramen and accessory canals (Dammaschke et al., 2004). The leaked components from sealer material may migrate from pulpal to periodontal tissues causing inflammation and cytotoxic effects (Geurtsen, 2001). Fibroblasts are the main cells of the gingiva and the periodontal ligament. In vitro studies have shown that the morphology and proliferation rates of gingival fibroblasts (GF) and periodontal ligament fibroblasts (PDLF) are similar. Also, PDLF and GF derived from the same patient have shown a similar pattern of protein production (Somerman et al., 1988).
The biocompatibility of an endodontic repair material is very important property because of the intimate contact of the pulp and periodontal cells with this material (Friedman, 1991, Torabinejad et al., 1995). Several studies have evaluated the cytotoxic effect of MTA on human periodontal ligament fibroblasts (Fayazi et al., 2011, Keiser et al., 2000), human osteoblasts (Camilleri et al., 2005, Michel et al., 2017, Tani-Ishii et al., 2007), human gingival fibroblasts (Michel et al., 2017) and human endothelial cells (De Deus et al., 2005). These studies concluded that MTA has the least cytotoxic effect on human cells.
Although many studies have assessed the biological effects of ProRoot MTA, only a small amount of scientific data has been reported on the cytotoxic effects of MTA Flow and Harvard MTA on the cells of periodontal and pulp tissues. We hypothesise that ProRoot MTA, MTA Flow and Harvard MTA have similar biocompatibility. To test this hypothesis, we compared the cytotoxic effects of the new MTA formulation (Harvard MTA) with MTA Flow and Pro-Root MTA on the human gingival fibroblast using a 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay.
2. Materials and methods
This study was conducted in the research laboratory at the Faculty of Dentistry, Umm Al-Qura University. The study-design and experiments were approved by the ethical committee of the Faculty of Dentistry, Umm Al-Qura University (approval number: 131-19).
2.1. Dental materials
Three MTA sealer brands are used in this study. ProRoot MTA (Dentsply Tulsa Dental Specialties, Johnson City, TN, USA), Harvard MTA (Universal, Germany) and MTA Flow (Ultradent Products Inc., South Jordan, UT, USA) were prepared according to the manufacturer's instructions. The materials were placed at the bottom of 6-well tissue culture plates and allowed to set for 48 h in the incubator at 37 °C. Dulbecco's Modified Eagle Medium (DMEM; UFC Biotech, KSA) was then added, without serum or antibiotics, to each material and incubated for either 24 h or 4 days to obtain low and high concentrations of MTA elutes respectively. After each time point, the elutes were sterilized using a 0.2 µm filter.
2.2. Cell culture
Human gingival tissues were collected from healthy adult gingiva at Umm Al-Qura University’s Dental Teaching Hospital, after obtaining written informed consent. The gingival tissue was incubated with 3 mg/ml collagenase type I and 4 mg/ml dispase (Sigma) for one hour at 37 °C. Single-cell suspensions were obtained and cultured in complete DMEM containing 10% fetal bovine serum (FBS) and 100 μg/mL Penicillin-Streptomycin (HyClone, Thermo Fisher Scientific, USA), and incubated at 37 °C and 5% CO2. Human gingival fibroblasts (GF) at passage 3 were frozen until use.
2.3. Gingival fibroblast treatment
Gingival fibroblast at passage 3 were seeded at 20,000 cells/well in 96-well plates and incubated in complete DMEM at 37 °C and 5% CO2. After 24 h’ incubation of GF, the medium was removed, and MTA elutes in complete DMEM were added to GF and incubated for three days at 37 °C and 5% CO2. Untreated cells were used as the control group. At the end of treatment of GF with MTA elutes, the cell viability was measured using MTT assay.
2.4. MTT cell viability assay
The MTT assay is a non-radioactive colorimetric assay for measuring cell proliferation and cytotoxicity (Carmichael et al., 1987, Mosmann, 1983). The MTT assay was used in this study to assess the cell viability of GF after treatment with MTA Flow, Harvard MTA and ProRoot MTA elutes on days one, two and three. At the end of treatment, 10 μl of MTT (Thermo Fisher Scientific) at a concentration of 5 mg/ml was added to the 90 μl of complete DMEM/well and incubated for three hours at 37 °C. At the end of the incubation period, the medium was removed and DMSO: isopropanol (1:1) solvent solution was added and incubated for 30 min at 37 °C to dissolve formazan crystals. The optical density was read at 570 nm by a spectrophotometric Microplate Reader (SpectroStar Nano, BMG Lab).
2.5. Statistical analysis
MTT assays were set up in duplicate and performed twice. The data was collected, tabulated and analysed with a one-way ANOVA, using GraphPad Prism 7. The level of statistical significance was set at <0.05 (P < 0.05).
3. Results
3.1. Cell viability of gingival fibroblasts after 24 h’ treatment with MTA elutes
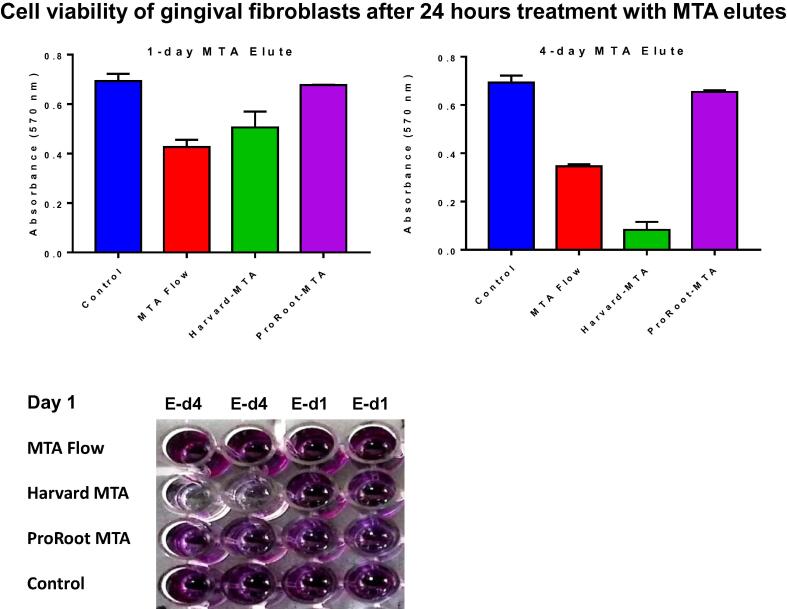
As can be seen in Fig. 1, after 24 h, the MTT assay showed that both 1- and 4-day elutes of MTA flow and Harvard MTA reduced cell viability of GF significantly compared to control (P < 0.05). However, the cell viability of GF treated with ProRoot MTA was similar to control (P > 0.05).
Fig. 1.
Cell viability of human gingival fibroblasts 24 h after treatment with MTA elutes. MTA Flow, Harvard MTA and ProRoot MTA were incubated with Dulbecco's Modified Eagle Medium and eluted on day 1 (E-d1) and day 4 (E-d4). The cells were treated with the MTA elutes to evaluate their cytotoxicity using an MTT assay compared to untreated control. MTT assays were set up in duplicate and the data are representative of two independent experiments.
3.2. Cell viability of gingival fibroblasts after 48 h’ treatment with MTA elutes
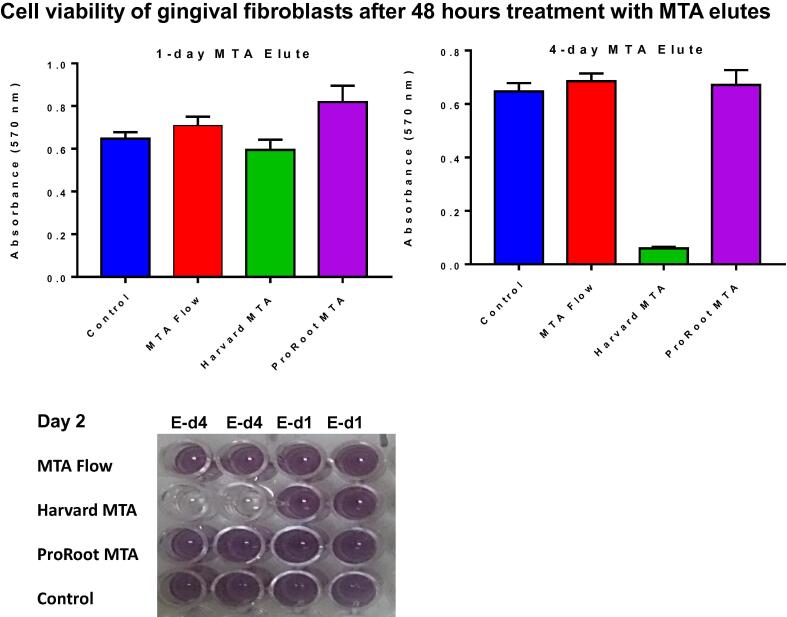
The MTT assay after 48 h (Fig. 2) showed that a 1-day elute of ProRoot MTA induced GF proliferation (P = 0.0136) at a significantly higher rate than that which occurred in the control group, while MTA flow and Harvard MTA were similar to control (P > 0.05). However, when GF was exposed to a 4-day elute of Harvard MTA, GF cell viability was reduced significantly (P < 0.0001) but the cell viability of GF exposed to a 4-day elute of ProRoot MTA and MTA flow was similar to that in the control group (P > 0.05).
Fig. 2.
Cell viability of human gingival fibroblasts 48 h after treatment with MTA elutes. MTA Flow, Harvard MTA and ProRoot MTA were incubated with Dulbecco's Modified Eagle Medium and eluted on day 1 (E-d1) and day 4 (E-d4). The cells were treated with the MTA elutes to evaluate their cytotoxicity using an MTT assay compared to untreated control. MTT assays were set up in duplicate and the data are representative of two independent experiments.
3.3. Cell viability of gingival fibroblasts after 72 h’ treatment with MTA elutes
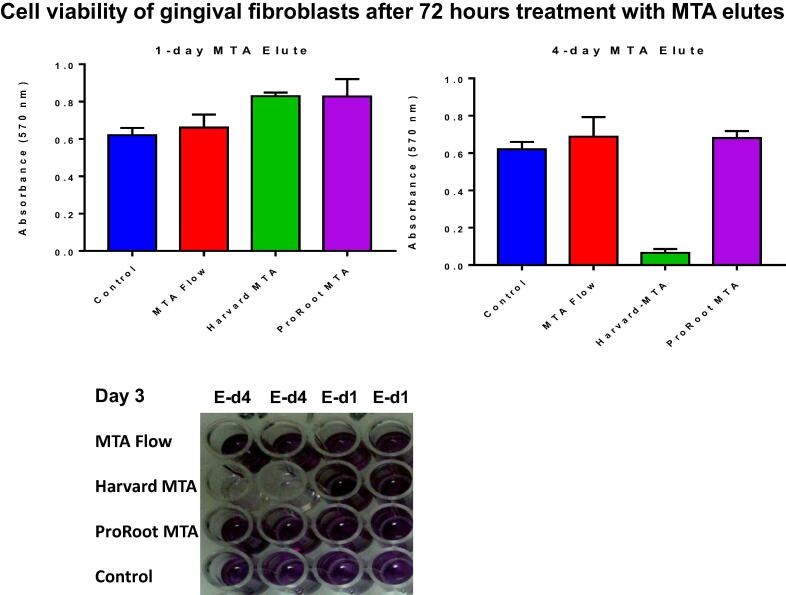
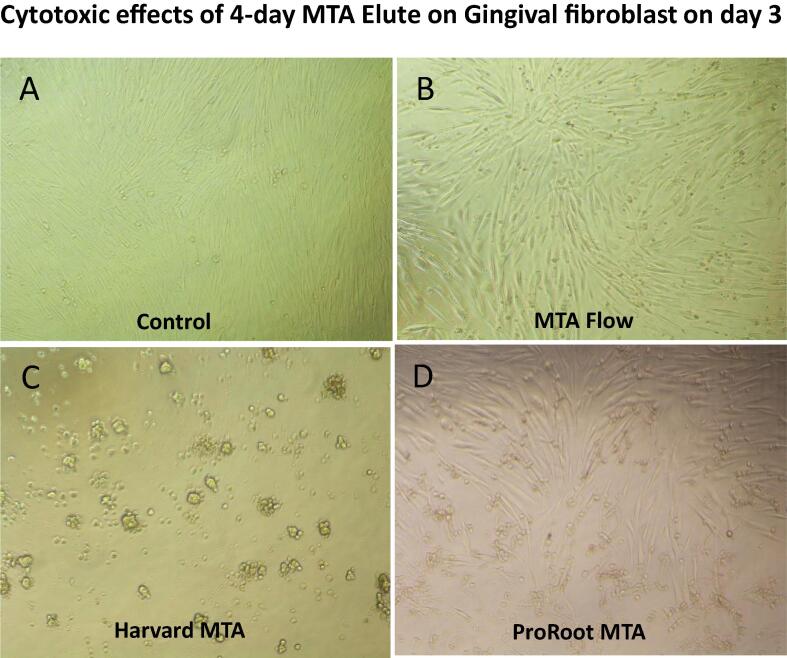
The MTT assay after 72 h (Fig. 3) showed that the 1-day elute of ProRoot MTA and Harvard MTA induced GF proliferation (P = 0.0025, P = 0.0144 respectively) while the 1-day elute of MTA flow was comparable to control. The 4-day elute of Harvard MTA was cytotoxic to GF (P < 0.0001) and the effects of the 4-day elutes of ProRoot MTA and MTA flow on GF were similar to those observed in the control group. Thus, the 4-day elute of Harvard MTA continued to be cytotoxic to GF at 24 h, 48 h and 72 h, and this cytotoxic effect was evident when we examined the cell under a microscope. The cells exposed to the 4-day elute of Harvard MTA showed pleomorphism and a reduction in cell numbers compared to the untreated control cells (Fig. 4).
Fig. 3.
Cell viability of human gingival fibroblasts 72 h after treatment with MTA elutes. MTA Flow, Harvard MTA and ProRoot MTA were incubated with Dulbecco's Modified Eagle Medium and eluted on day 1 (E-d1) and day 4 (E-d4). The cells were treated with the MTA elutes to evaluate their cytotoxicity using an MTT assay compared to untreated control. MTT assays were set up in duplicate and the data are representative of two independent experiments.
Fig. 4.
Microscopic illustrations of morphological changes of human gingival fibroblasts treated with 4-day MTA elutes. The fibroblasts were left untreated (A) or treated with a 4-day elute of MTA Flow (B) or Harvard MTA (C) or ProRoot MTA (C) for 3 days. The morphological changes of the cells were observed under a Nikon inverted microscope at a magnification of 100x.
4. Discussion
New emerging dental material should be evaluated in vitro using various tests such as cell viability assays, which calculate viable cell numbers in the presence of tested materials (Osorio et al., 1998, Paranjpe et al., 2010).
Cell viability assays evaluate and compare the clinical safety of dental materials that come into contact with the surrounding tissues (Rodríguez-Lozano et al., 2019, Tomás-Catalá et al., 2017). They also evaluate the possible changes in the cellular functions which might result in damage to cellular survival and function (Luo et al., 2014). The most commonly used viability assays include tetrazolium reduction, resazurin reduction, protease activity and luminogenic ATP assays. Although ATP assay is the fastest and the most sensitive, the tetrazolium reduction assay is a cheaper alternative with satisfactory performance. The most widely used compounds in tetrazolium reduction assays are MTT, XTT, MTS and WST-1 (Riss et al., 2004). MTT assay is simple, cheap, rapid and accurate (Chang et al., 1998).
In this study, an MTT assay was used to evaluate the cytotoxic effects of a new Harvard MTA in comparison to the effects of MTA flow and ProRoot MTA on human gingival fibroblasts. The current study found that an elute of MTA flow and a higher-concentration (4-day) elute of ProRoot MTA were not cytotoxic to GF, and comparable to control. Low-concentration (1-day) elutes of ProRoot MTA and Harvard MTA induced GF proliferation, while the 4-day elute of Harvard MTA was cytotoxic to GF.
Michel et al. (Michel et al., 2017) have reported that the cell viability of GF in the presence of discoid of ProRoot MTA or Harvard MTA were 99% and 72% respectively. Recent reports have shown that ProRoot MTA elute can induce significant proliferation of DPSCs (Jaberiansari et al., 2014) and adequate cell viability of human dermal fibroblasts (Damas et al., 2011). On the contrary, other studies have demonstrated that ProRoot MTA is cytotoxic to DPSCs (Kulan et al., 2018, Youssef et al., 2019), fibroblasts (Wadajkar et al., 2014) and endothelial cells (De Deus et al., 2005).
Cell viability is dependent on the nature of the tested materials (material itself or elute), the concentration of elute and the pH of the surrounding medium. The tested material itself may be more cytotoxic than elutes. Camilleri et al. (Camilleri, 2008) have shown that direct contact with portland cements was cytotoxic while elutes exhibited adequate cell viability.
The concentration of elutes may affect cell viability. The higher the concentration, the more cytotoxic the substance. This study found that the Harvard MTA elute at low concentration induced GF proliferation, while the high concentration was cytotoxic. This is supported by Akbulut et al.’s research, (Akbulut et al., 2018) which showed that 3-day MTA elutes were more cytotoxic than 1-day samples.
The mechanisms underlying Harvard MTA cytotoxicity are not clearly understood. A number of studies have suggested that initial release of calcium-ions, pH variations and presence of ionic and toxic components could affect the survival of cells (Gandolfi et al., 2010, Matsuya et al., 2000).
On hydration of MTA, calcium silicate hydrate and calcium hydroxide were released. Calcium hydroxide is responsible for the high pH of MTA (Camilleri, 2008, Silva et al., 2013). Different types of MTA significantly increased pH but varied in their alkalising capacities (Luczaj-Cepowicz et al., 2017). The high pH of MTA is an important factor in the mineralisation process because it activates alkaline phosphatase and neutralises the acids secreted by osteoclasts (Eriksen, 2010). However, the variability of alkaline pH could explain the cytotoxic effect of higher concentrations of Harvard MTA. In addition, MTA is a hydrophilic substance, making it more likely to release ionic components as time goes on As such, it may interfere with intracellular enzyme activities as the leached-out components increased (Schweikl and Schmalz, 1996). This could explain why Harvard MTA was more cytotoxic in 4-day elute than ProRoot MTA, MTA Flow.
This study has some potential limitations. One of these limitations is that only an MTT assay was used to assess cell viability, and we did not investigate apoptosis and necrosis. In addition, the results of this study only represent the response of gingival fibroblast without the involvement of the host surrounding environment. In addition, testing the biocompatibility of dental material in vitro using cell viability assays is only suggestive of what may happen in vivo. This is because in vitro experiments are performed on cultured cells, and the results reflect the response of these cells without the involvement of the host defence mechanism.
5. Conclusion
ProRoot MTA and MTA Flow showed comparable biocompatibility, but the high concentration elute of Harvard MTA was cytotoxic to gingival fibroblasts. Further studied are required to assess the cell viability after direct contact with these materials versus eluent in vitro and compare these root canal sealers in the clinical setting.
Acknowledgments
Acknowledgment
I’d like to thank professor Ibrahim Hamouda for his scientific assistance.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akbulut M.B., Arpaci P.U., Eldeniz A.U. Effects of four novel root-end filling materials on the viability of periodontal ligament fibroblasts. Restor. Dent. Endod. 2018;43:e24. doi: 10.5395/rde.2018.43.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrumlu E. Biocompatibility of retrograde root filling materials: a review. Aust. Endod. J. 2008;34:30–35. doi: 10.1111/j.1747-4477.2007.00085.x. [DOI] [PubMed] [Google Scholar]
- Bueno C.R.E., Vasques A.M.V., Cury M.T.S., Sivieri-Araujo G., Jacinto R.C., Gomes-Filho J.E., Cintra L.T.A., Dezan-Junior E. Biocompatibility and biomineralization assessment of mineral trioxide aggregate flow. Clin. Oral. Investig. 2019;23:169–177. doi: 10.1007/s00784-018-2423-0. [DOI] [PubMed] [Google Scholar]
- Camilleri J. The biocompatibility of modified experimental Portland cements with potential for use in dentistry. Int. Endod. J. 2008;41:1107–1114. doi: 10.1111/j.1365-2591.2008.01483.x. [DOI] [PubMed] [Google Scholar]
- Camilleri J., Montesin F.E., Di Silvio L., Pitt Ford T.R. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int. Endod. J. 2005;38:834–842. doi: 10.1111/j.1365-2591.2005.01028.x. [DOI] [PubMed] [Google Scholar]
- Camilleri J., Pitt Ford T.R. Mineral trioxide aggregate: a review of the constituents and biological properties of the material. Int. Endod. J. 2006;39:747–754. doi: 10.1111/j.1365-2591.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W.G., Gazdar A.F., Minna J.D., Mitchell J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- Chang Y.C., Huang F.M., Cheng M.H., Chou L.S., Chou M.Y. In vitro evaluation of the cytotoxicity and genotoxicity of root canal medicines on human pulp fibroblasts. J. Endod. 1998;24:604–606. doi: 10.1016/s0099-2399(98)80119-2. [DOI] [PubMed] [Google Scholar]
- Damas B.A., Wheater M.A., Bringas J.S., Hoen M.M. Cytotoxicity comparison of mineral trioxide aggregates and EndoSequence bioceramic root repair materials. J. Endod. 2011;37:372–375. doi: 10.1016/j.joen.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Dammaschke T., Witt M., Ott K., Schäfer E. Scanning electron microscopic investigation of incidence, location, and size of accessory foramina in primary and permanent molars. Quintessence Int. 2004;35:699–705. [PubMed] [Google Scholar]
- De Deus G., Ximenes R., Gurgel-Filho E.D., Plotkowski M.C., Coutinho-Filho T. Cytotoxicity of MTA and Portland cement on human ECV 304 endothelial cells. Int. Endod. J. 2005;38:604–609. doi: 10.1111/j.1365-2591.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- El-Ma’aita A.M., Qualtrough A.J., Watts D.C. The effect of smear layer on the push-out bond strength of root canal calcium silicate cements. Dent. Mater. 2013;29:797–803. doi: 10.1016/j.dental.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Eriksen E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010 doi: 10.1007/s11154-010-9153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayazi S., Ostad S.N., Razmi H. Effect of ProRoot MTA, Portland cement, and amalgam on the expression of fibronectin, collagen I, and TGFbeta by human periodontal ligament fibroblasts in vitro. Indian J. Dent Res. 2011;22:190–194. doi: 10.4103/0970-9290.84278. [DOI] [PubMed] [Google Scholar]
- Floratos S.G., Tsatsoulis I.N., Kontakiotis E.G. Apical barrier formation after incomplete orthograde MTA apical plug placement in teeth with open apex–report of two cases. Braz. Dent. J. 2013;24:163–166. doi: 10.1590/0103-6440201302163. [DOI] [PubMed] [Google Scholar]
- Ford T.R., Torabinejad M., McKendry D.J., Hong C.U., Kariyawasam S.P. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995;79:756–763. doi: 10.1016/s1079-2104(05)80313-0. [DOI] [PubMed] [Google Scholar]
- Friedman S. Retrograde approaches in endodontic therapy. Endod. Dent. Traumatol. 1991;7:97–107. doi: 10.1111/j.1600-9657.1991.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Gandolfi M.G., Ciapetti G., Taddei P., Perut F., Tinti A., Cardoso M.V., Van Meerbeek B., Prati C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent. Mater. 2010;26:974–992. doi: 10.1016/j.dental.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Geurtsen W. Biocompatibility of root canal filling materials. Aust. Endod. J. 2001;27:12–21. doi: 10.1111/j.1747-4477.2001.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Guimaraes B.M., Vivan R.R., Piazza B., Alcalde M.P., Bramante C.M., Duarte M.A.H. Chemical-physical properties and apatite-forming ability of mineral trioxide aggregate flow. J. Endod. 2017;43:1692–1696. doi: 10.1016/j.joen.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Jaberiansari Z., Naderi S., Tabatabaei F.S. Cytotoxic effects of various mineral trioxide aggregate formulations, calcium-enriched mixture and a new cement on human pulp stem cells. Iran Endod. J. 2014;9:271–276. [PMC free article] [PubMed] [Google Scholar]
- Jacobovitz M., De Lima R.K.P. Treatment of inflammatory internal root resorption with mineral trioxide aggregate: a case report. Int. Endod. J. 2008;41:905–912. doi: 10.1111/j.1365-2591.2008.01412.x. [DOI] [PubMed] [Google Scholar]
- Keiser K., Johnson C.C., Tipton D.A. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J. Endod. 2000;26:288–291. doi: 10.1097/00004770-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Kulan P., Karabiyik O., Kose G.T., Kargul B. The effect of accelerated mineral trioxide aggregate on odontoblastic differentiation in dental pulp stem cell niches. Int. Endod. J. 2018;51:758–766. doi: 10.1111/iej.12747. [DOI] [PubMed] [Google Scholar]
- Leye Benoist F., Gaye Ndiaye F., Kane A.W., Benoist H.M., Farge P. Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal((R))) in the formation of a dentine bridge: a randomised controlled trial. Int. Dent. J. 2012;62:33–39. doi: 10.1111/j.1875-595X.2011.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczaj-Cepowicz E., Marczuk-Kolada G., Pawinska M., Obidzinska M., Holownia A. Evaluation of cytotoxicity and pH changes generated by various dental pulp capping materials – an in vitro study. Folia Histochem. Cytobiol. 2017;55:86–93. doi: 10.5603/FHC.a2017.0008. [DOI] [PubMed] [Google Scholar]
- Luo Z., Kohli M.R., Yu Q., Kim S., Qu T., He W.X. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J. Endod. 2014;40:937–942. doi: 10.1016/j.joen.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Matsuya S., Takagi S., Chow L.C. Effect of mixing ratio and pH on the reaction between Ca4(PO4)2O and CaHPO4. J. Mater. Sci. – Mater. Med. 2000;11:305–311. doi: 10.1023/a:1008961314500. [DOI] [PubMed] [Google Scholar]
- Michel A., Erber R., Frese C., Gehrig H., Saure D., Mente J. In vitro evaluation of different dental materials used for the treatment of extensive cervical root defects using human periodontal cells. Clin. Oral. Investig. 2017;21:753–761. doi: 10.1007/s00784-016-1830-3. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983 doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Osorio R.M., Hefti A., Vertucci F.J., Shawley A.L. Cytotoxicity of endodontic materials. J. Endod. 1998;24:91–96. doi: 10.1016/s0099-2399(98)80084-8. [DOI] [PubMed] [Google Scholar]
- Paranjpe A., Zhang H., Johnson J.D. Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J. Endod. 2010;36:1042–1047. doi: 10.1016/j.joen.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Riss, T.L., Moravec, R.A., Niles, A.L., Duellman, S., Benink, H.A., Worzella, T.J., Minor, L., 2004. Cell Viability Assays, in: Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Bejcek, B., Caaveiro, J.M.M., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., Devanaryan, V., Foley, T.L., Glicksman, M., Hall, M.D., Haas, J. V, Hoare, S.R.J., Inglese, J., Iversen, P.W., Kahl, S.D., Kales, S.C., Kirshner, S., Lal-Nag, M., Li, Z., McGee, J., McManus, O., Riss, T., Saradjian, P., Trask Jr., O.J., Weidner, J.R., Wildey, M.J., Xia, M., Xu, X. (Eds.), Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda (MD).
- Rodríguez-Lozano F.J., Collado-González M., Tomás-Catalá C.J., García-Bernal D., López S., Oñate-Sánchez R.E., Moraleda J.M., Murcia L. GuttaFlow Bioseal promotes spontaneous differentiation of human periodontal ligament stem cells into cementoblast-like cells. Dent. Mater. 2019;35:114–124. doi: 10.1016/j.dental.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Schweikl H., Schmalz G. Toxicity parameters for cytotoxicity testing of dental materials in two different mammalian cell lines. Eur. J. Oral Sci. 1996;104:292–299. doi: 10.1111/j.1600-0722.1996.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Shahravan A., Jalali S.P., Torabi M., Haghdoost A.A., Gorjestani H. A histological study of pulp reaction to various water/powder ratios of white mineral trioxide aggregate as pulp-capping material in human teeth: a double-blinded, randomized controlled trial. Int. Endod. J. 2011;44:1029–1033. doi: 10.1111/j.1365-2591.2011.01916.x. [DOI] [PubMed] [Google Scholar]
- Silva E.J.N.L., Rosa T.P., Herrera D.R., Jacinto R.C., Gomes B.P.F.A., Zaia A.A. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J. Endod. 2013;39:274–277. doi: 10.1016/j.joen.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Somerman M.J., Archer S.Y., Imm G.R., Foster R.A. A comparative study of human periodontal ligament cells and gingival fibroblasts in vitro. J. Dent. Res. 1988;67:66–70. doi: 10.1177/00220345880670011301. [DOI] [PubMed] [Google Scholar]
- Tani-Ishii N., Hamada N., Watanabe K., Tujimoto Y., Teranaka T., Umemoto T. Expression of bone extracellular matrix proteins on osteoblast cells in the presence of mineral trioxide. J. Endod. 2007;33:836–839. doi: 10.1016/j.joen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Tomás-Catalá C.J., Collado-González M., García-Bernal D., Oñate-Sánchez R.E., Forner L., Llena C., Lozano A., Castelo-Baz P., Moraleda J.M., Rodríguez-Lozano F.J. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int. Endod. J. 2017;50:e63–e72. doi: 10.1111/iej.12859. [DOI] [PubMed] [Google Scholar]
- Torabinejad M., Hong C.U., Lee S.J., Monsef M., Pitt Ford T.R. Investigation of mineral trioxide aggregate for root-end filling in dogs. J. Endod. 1995;21:603–608. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- Torabinejad M., Parirokh M. Mineral trioxide aggregate: a comprehensive literature review-Part II: Leakage and biocompatibility investigations. J. Endod. 2010 doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Tziafas D., Pantelidou O., Alvanou A., Belibasakis G., Papadimitriou S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int. Endod. J. 2002;35:245–254. doi: 10.1046/j.1365-2591.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- Vajrabhaya L.O., Korsuwannawong S., Jantarat J., Korre S. Biocompatibility of furcal perforation repair material using cell culture technique: Ketac Molar versus ProRoot MTA. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;102:e48–e50. doi: 10.1016/j.tripleo.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Wadajkar A.S., Ahn C., Nguyen K.T., Zhu Q., Komabayashi T. In vitro cytotoxicity evaluation of four vital pulp therapy materials on l929 fibroblasts. ISRN Dent. 2014;2014:191068. doi: 10.1155/2014/191068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef A.R., Emara R., Taher M.M., Al-Allaf F.A., Almalki M., Almasri M.A., Siddiqui S.S. Effects of mineral trioxide aggregate, calcium hydroxide, biodentine and Emdogain on osteogenesis, Odontogenesis, angiogenesis and cell viability of dental pulp stem cells. BMC Oral. Health. 2019;19:133. doi: 10.1186/s12903-019-0827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]