Abstract
Background
Oral submucous fibrosis (OSMF) is one of the common oral potentially malignant disorders that can result in severe morbidity. Depending upon the stage of disease, multiple management therapies exist which include medicinal and surgical approaches. Although the surgical approach is preferred in severe conditions, numerous studies have reported its post-surgical deteriorating outcomes including increased fibrotic changes. To reduce these post-surgical complications, Light amplification by stimulated emission of radiation (Laser) has been introduced and studied as a non-invasive technique to treat oral submucous fibrosis. However, there exists a lack of knowledge about ‘which laser shows a better post-treatment outcome’. Accordingly, this review aims to answer this question.
Materials and methods
A systematic review of the published literature was performed using an electronic search in PubMed/Medline, Science Direct, Web of Science, Embase, J- STAGE, Google Scholar, and Scopus databases, from 1952 till 2019 using keywords like, ‘Oral submucous fibrosis’, ‘Treatment’, ‘Laser’, ‘Trismus’, ‘ Fibrosis’, ‘Surgical’, ‘Non-invasive’, and ‘Postoperative results’.
Results
The search strategy revealed 20 relevant published studies in which laser had been used to treat 250 patients of OSMF. Effective results were found without any complications in all the cases after follow up.
Conclusion
Observing the current literature, it can be concluded that laser might be used as a potential non-invasive approach in the management of OSMF, however, large scale studies are required to investigate the efficacy and other effects of this technology.
Key words: Laser, Non-invasive, Oral sub mucus fibrosis, Technique, Treatment
Abbreviations: AN, Areca nut; cAMP, Cyclic adenosine monophosphate; CO2, Carbon-dioxide; CTGF/CCN2, Connective tissue growth factor; Er Cr YS GG, Erbium Chromium: Yttrium – Scandium – Gallium – Garnet; Er, YAG Erbium: Yttrium–Aluminium–Garnet; GA, General anaesthesia; GaAs, Gallium Arsenic; H2O, Water; HA, Hydroxyapatite; IF- ά, Interferon ά; KTP, Potassium titanyl phosphate; LA, Local anaesthesia; Laser, Light amplification by stimulated emission of radiation; LPLI, Low-power laser irradiation; MMP2, Matrix metalloproteinases 2; ND-YAG, Neodymium – doped: Yttrium- Aluminium Garnet; OSMF, Oral submucous fibrosis; PGs, Prostaglandins; TNF, Tumor necrosis factor; TGF- β, Transforming Growth Factor β; UUO, Unilateral ureteral obstruction; WHO, World Health Organization
1. Introduction
Oral submucous fibrosis (OSMF) is one of the common oral potentially malignant disorders (OPMDs) that can result in severe morbidity. More and Rao (2019) defined OSMF as 'a debilitating, progressive, irreversible collagen metabolic disorder induced by chronic chewing of areca nut and its commercial preparations; affecting the oral mucosa and occasionally the pharynx and oesophagus; leading to mucosal stiffness and functional morbidity; and has a potential risk of malignant transformation.'
The condition mostly affects the South East Asia region due to the increased prevalence of Areca nut (AN) chewing habit (Rao et al., 2020). According to World Health Organization (WHO), > 5 million people are affected by OSMF globally (Rao et al., 2020). It can involve any age group with the predominance of young to middle age. It has been found even in the paediatric patients and school - going children (More et al., 2020a). It has female predilection, although some studies reveal the male predominance (More et al.,2020b).
AN is the major etiological agent for OSMF and other contributory factors include smokeless tobacco, chilies, nutritional factors, genetics, autoimmunity, and infections (Shih et al., 2019).
The disease is preceded by symptoms such as burning sensation, ulceration, pain, loss of pigmentation, leathery texture of oral mucosa, blanching, marble like appearance, reduced tongue movement, progressive reduction of mouth opening, and sunken cheeks. In the more advanced stage, there is the deposition of fibrous bands in the buccal mucosa and other sites resulting in more trismus, speech and deglutition defects, shrunken uvula, and further manifests with the premalignant and malignant changes in the oral mucosa.
Histopathologically, OSMF is characterized by marked epithelial atrophy, loss of rete pegs and subepithelial hyalinization with abnormal excessive collagen deposition leading to abundant fibrosis, and muscle degeneration. Epithelium may show dysplastic changes signifying the high-risk malignant transformation of this condition. Various scholars have classified OSMF into different grades/stages depending upon clinical, functional, and histological features of this disease. The recent classification of OSMF given by Passi et al (2017) included multiple parameters (Table 1).
Table 1.
Classification system of oral submucous fibrosis proposed by Passi et al (2017) (Reference No. 45).
| Grading/staging | Clinical | Functional | Histopathological | Treatment | Prognosis |
|---|---|---|---|---|---|
| Grade I | Involvement of < 1/3rd of the oral cavity, Mild blanching, burning sensation, recurrent ulceration, and stomatitis,dryness of the mouth. |
Mouth opening up to 35 mm. |
Stage of inflammation: Fine oedematous collagen, congested blood vessels, abundant neutrophils along with lymphocytes with myxomatous changes in the subepithelial connective tissue layer of epithelium. |
Cessation of habit, nutritional support, antioxidants, topical steroid ointment. | Excellent. |
| Grade II | Involvement of 1/3rd-2/3rd of the oral cavity, blanching, mottled and marble-like appearance, palpable fibrotic bands and involvement of soft palate and premolar area. |
Mouth opening 25–35 mm, Cheek flexibility gets reduced by 33%. |
Stage of hyalinization: Juxta–epithelial collagen hyalinization, lymphocytes, eosinophils, dilated and congested blood vessels, less fibroblastic activity. granulation changes in the muscle layer with reduced inflammatory cells in the subepithelial layer. |
Habit cessation, nutritional supplements, intralesional injection of placental extracts, hyaluronidase, steroid therapy, Physiotherapy. | Good, The recurrence rate is low. |
| Grade III | Involvement of > 2/3rd of the oral cavity. Severe blanching, broad thick fibrous palpable bands at cheeks and lips and rigid mucosa, depapillated tongue and restricted tongue movement, shrunken bud-like uvula,involvement of the floor of the mouth and lymphadenopathy. |
Mouth opening 15–25 mm, Cheek flexibility gets reduced by 66%. |
Stage of fibrosis: Complete collagen hyalinization without fibroblasts and oedema, obliterated blood vessels, inflammatory infiltrate mainly plasma cells and lymphocytes, extensive fibrosis with hyalinization from subepithelial to superficial muscle layers exhibiting atrophic and degenerative changes. |
Surgical treatment including band excision and reconstruction, bilateral temporalis myotomy and coronoidectomy. | Fair, The recurrence rate is high. |
| Grade IV | Changes like leukoplakia, erythroplakia and suspicious malignant lesion. | Mouth opening < 15 mm or nil |
Stage of malignant transformation: Erythroplakia changes into squamous cell carcinoma. |
Surgical treatment and biopsy of the suspicious lesion. | Poor, Malignant transformation. |
It has been found that the derivatives of AN such as arecoline and nitrosamines can induce neoplastic changes in the oral mucosa (Arakeri et al., 2017). Due to its widespread involvement of the oral cavity and high-risk of progression towards malignancy, OSMF has become of great concern to diagnose and treat at an early stage to prevent further complications.
Till present, no specific treatment has been available for the management of OSMF. Various treatment modalities involve medicinal and surgical approaches (More et al., 2018). Although the surgical approach is preferred in severe conditions, numerous studies have reported its post-surgical deteriorating outcomes including increased fibrotic changes (Fry et al., 2014). To reduce these deteriorating complications, laser has been introduced and studied as a non-invasive approach to treat OSMF. However, there exists a lack of knowledge about which laser shows a better post-treatment outcome. Therefore, this review aims to answer this question.
2. Materials & methods
This review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Considering the nature of the current review, there was no necessity of taking any ethical approval.
2.1. Focused PICO question:
For screening the relevant studies we defined a PICO question; “Does laser therapy has effective results in the management of OSMF and which laser proves to have a better post-operative outcome?” 1. Population: OSMF patients; 2. Intervention: Laser therapy; 3. Comparison: surgical treatment/oral physiotherapy as an adjunct, or alone to laser therapy; 4. Outcome: Benefits or lack of effect on OSMF-related symptoms.
2.2. Search strategy for identification of studies (Fig. 1)
Fig. 1.
Flow chart showing study screening following PRISMA guidelines.
A systematic review of the published literature was performed using an electronic search in PubMed/Medline, Science Direct, Web of Science, Embase, J- STAGE, Google Scholar, and Scopus databases, from 1952 till 2019 using keywords like ‘Oral submucous fibrosis’, ‘Treatment’, ‘Laser’, ‘Trismus’, ‘ Fibrosis’, ‘Surgical’, ‘Non-invasive’, and ‘Postoperative results’. Journals evaluating the role of laser in the treatment of OSMF were referred for the review. A manual search of all related oral pathology, maxillofacial, dental, and medical journals was performed. The reference lists of the identified studies and relevant reviews on the subject were also checked for the possible additional studies.
2.3. Inclusion criteria
-
•
Publications reporting the cases of OSMF treated either only with laser or with medicinal and physical therapy as an adjunct.
-
•
Definitely diagnosed cases of OSMF based on clinical features.
-
•
Studies were selected beyond the restriction of limitations on parameters such as age, gender, ethnicity, or socioeconomic status etc.
-
•
Articles published in the English literature only.
2.4. Exclusion criteria
-
•
Studies characterized as review articles, editorials, conference abstracts, correspondence and letters .
-
•
Studies reporting the use of other surgical procedures for the management of OSMF.
-
•
Patients with any systemic disease or on medication that could alter the symptoms of OSMF.
-
•
In the studies dividing OSMF patients into groups, the groups treated with other therapeutic aids except laser.
-
•
Studies providing incomplete and missing information regarding the number of patients and procedures used.
2.5. Data extraction & analysis
The titles and abstracts of all reports identified through the electronic searches were read independently by the authors. For studies appearing to meet the inclusion criteria, or for which the data in the title and abstract were insufficient to make a clear decision, the full report was obtained, discussed, and resolved. The data were then extracted after careful evaluation. Data points obtained from the literature review included authors’ names and year of publication, number of patients treated with laser in each study, type of anaesthesia used, aid of treatment used, any post-operative adjunctive aids used, follow-up period, post-operative results, and any complications occurring after follow-up. Data extracted were tabulated, cross-checked and summarized (Table 2).
Table 2.
Summary of the published cases presenting the use of lasers in the management of oral submucous fibrosis (1952 to 2019).
| S. No. | Authors | Type of laser used. | Number of patients. | Under GA/LA | Treatment done. | Post-operative adjunctive aids. | Follow - up period. | Post-operative Results after follow - up. | Complications | Reference No. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shah et al (2005) | Diode | 10 | GA | Fibrotomy | • Oral Physiotherapy. | 3 months | In all cases:
|
No | 51 |
| 2 | Kameshwaran et al (2006) | KTP-532 | 15 | GA | Fibrotomy |
|
12–18 months | In all cases:
|
No | 33 |
| 3 | Talsania et al (2009) | Diode | 8 | GA | Fibrotomy | • Oral hygiene and aggressive physiotherapy with ice cream stick. | 3 months to 3 years | In all cases:
|
No | 55 |
| 4 |
Nayak et al (2009) |
KTP-532 | 9 | GA | Fibrotomy | • Oral Physiotherapy. | 1 year | In all cases:
|
No | 43 |
| 5 | Chaaya et al (2010) | CO2 | 16 | LA | Fibrotomy |
|
1 year | In all cases:
|
No | 14 |
| 6 | Cheng et al (2011) | KTP-532 | 4 | LA | Fibrotomy |
|
NA | In all cases:
|
No | 13 |
| 7 | Chaudhary et al (2011) | Er Cr: YSGG | 1 | LA | Fibrotomy |
|
6 months |
|
No | 11 |
| 8 | Garde et al (2013) | Diode | 9 | GA | Fibrotomy |
|
1 year | In all cases:
|
No | 27 |
| 9 | Chaudhary et al (2014) | Er Cr: YSGG | 16 | LA | Fibrotomy |
|
1 year | In all cases:
|
No | 12 |
| 10 | Lokesh et al (2014) | Diode | 50 | LA | Fibrotomy |
|
6 months |
|
No | 37 |
| 11 | Asnani et al. (2014) | Diode | 1 | LA | Fibrotomy |
|
6 months |
|
No | 7 |
| 12 | Tripathy et al (2014) | Diode | 5 | GA | Fibrotomy | • Oral physiotherapy. | 3 months | In all cases:
|
No | 56 |
| 13 | Agarwal et al. (2015) | Diode | 30 | GA | Fibrotomy | • Oral physiotherapy. | 6 months | In all cases:
|
No | 2 |
| 14 | Devgan et al (2015) | CO2 | 20 | LA | Fibrotomy | • Oral physiotherapy. | 6 months |
|
No | 17 |
| 15 | Mudigonda et al (2016) | Diode | 12 | LA | Fibrotomy | • Oral physiotherapy with mouth opening exercises. | 6 months |
|
No | 41 |
| 16 | Singh et al. (2017) | Diode | 20 | LA | Laser biostimulation | NA | 15 days | In all cases:
|
No | 54 |
| 17 | Kunusoth et al. (2018) | Diode | 1 | LA | Fibrotomy | • Oral Physiotherapy. | 6 months |
|
No | 36 |
| 18 | Farista et al (2018) | Diode | 2 | LA | Fibrotomy followed by LLLT. | • Oral Physiotherapy. | 1 year | In both cases:
|
No | 21 |
| 19 | Gupta et al (2018) | Diode | 30 | LA | Fibrotomy | • Oral Physiotherapy,topical corticosteroids. | 9 months | In all cases:
|
No | 30 |
| 20 | Chandra et al (2019) | Diode | 1 | LA | Photobiostimulation | • Oral Physiotherapy. | 1 month |
|
No | 10 |
| Total studies: 20; Total no. of cases: 250; NA: Not available; LLLT: Low level laser therapy. | ||||||||||
3. Results
Electronic research revealed 22 published articles in the literature from 1952 to 2019, in which the use of laser therapy on OSMF patients was presented. Out of those, one article was depicting duplicated data of some other study and in one article, full information was not provided regarding the number of patients and procedure used. So, both the articles were excluded. A manual search could not provide any additional articles related to this topic. Thus, the remaining 20 relevant studies were included for data extraction and review (Fig. 1). Total of 250 OSMF patients were treated using laser in these 20 studies (Table 2). The first case of OSMF using laser was reported in 2005, before which the use of this therapy in the treatment of OSMF could not be depicted in any published data. All the included studies evaluated the role of laser in the treatment of OSMF patients either in fibrotomy or biostimulation showing effective post-operative results except for two patients, in which laser was ineffective. In fourteen studies, the surgery was done under local anaesthesia (LA) and in six studies, general anaesthesia (GA) was used. In almost every study, oral physiotherapy was recommended to the patients as an adjunctive postoperative aid. In some of the cases, antioxidants, multivitamins, and corticosteroids were also prescribed after the surgery. Patients were encouraged to quit their habits. And all the cases were followed up. The follow-up period in different studies ranged from 15 days to 3 years. One case lost the follow-up. No complications were determined after follow-up period in any of the cases. Patients showed improvement in symptoms such as increased mouth opening, reduced burning sensation, complete healing, improved cheek flexibility, and improved tongue protrusion with variation in each case. Out of 20 studies, the most predominant laser used was diode (in 13 studies) followed by KTP- 532 (3 Studies), CO2 (2 studies), and Er Cr: YSGG (2 studies) (Table 2)
4. Discussion
Laser therapy has been used in a wide spectrum of applications in the field of medicine. The principle of this technology was first understood in 1917 when the theory of stimulated emission was explained by scientist Albert Einstein (Aoki et al., 2004). In dentistry, application of this technology was first identified in the 1960s. And since then, its usage has been increased in the dental practice in almost all specialties (David and Gupta, 2015).
4.1. Principles of laser functioning
The three key properties that make laser unique to use in the medical and dental field are monochromatism (laser beam consisting of a single wavelength), collimation (acts as a point source affecting target organ only), and coherence (being in one phase) (Gopal and George, 2019).
When the laser is applied to the targeted tissues, it involves four different types of interactions. which are reflection, transmission, scattering, and absorption (Abdulsamee, 2017, Colluzi, 2008, Imre et al., 2018).
Application of laser on the tissue results in the absorption of laser energy into the tissue that depends on laser wavelength, tissue optical properties, time of exposure, and the energy used. Wavelength is the primary factor that determines the depth of penetration and the absorption of laser energy into the tissue. Most of these wavelengths are in the visible and the infrared spectrum (Imre et al., 2018). Lasers have precise specificity for the tissue components known as chromophores which absorb light of specific wavelengths. The primary chromophores in the intraoral soft tissue are melanin, haemoglobin, and water, and in dental hard tissues are water (H2O) and hydroxyapatite (HA). In general, the longer wavelengths will have a greater affinity with H2O and HA. The shorter wavelengths are readily absorbed by the pigmented tissue and the blood elements (Verma et al., 2012). (Table 3)
Table 3.
Classification of various lasers used in dentistry.
| Type | Examples | Wavelength (in nm) | Medium | Chromatophores |
|---|---|---|---|---|
|
Soft tissues |
CO2 | 10,600 | Gas | Water |
| Argon | 458–515 | Gas | Water, Hydroxyapatite | |
| Nd: YAG | 10,064 | Solid | Pigments, Haemoglobin Melanin | |
| Diode | 850–1064 | Semiconductor | Pigments, Haemoglobin Melanin | |
| KTP | 532 | Solid | Pigments, Haemoglobin Melanin | |
| Soft & hard tissues | Er: YAG | 2940 | Solid | Water, Hydroxyapatite |
| Er: YSGG | 2970 | Solid | Water, Hydroxyapatite |
When a laser is absorbed, it elevates the temperature and produces photochemical effects depending on the water content of the tissues. At 100 °C vaporization of the water within the tissue occurs, and the process is called ablation. At 60 °C − 100 °C, proteins denaturation begins, without vaporization of the underlying tissue. Above 200 °C, the tissue is dehydrated and then burned, resulting in carbonization (Colluzi, 2008).
Laser works in two ways. One of these is the continuous mode in which the energy is emitted continuously as long as the laser works. e.g. CO2 laser, diode laser. One of its variations is the gated mode in which mechanical /electronic control is used to minimize the thermal effects on the tissue. Another is the free pulse mode in which pulses emanate at a regular threshold. e.g. Nd :YAG, Er: YAG and ErCr: YSGG laser.
4.2. Classification of lasers
Lasers can be classified into many types depending upon the medium used within laser devices such as gas, solid, liquid, dye, semiconductor, and excimers (Naik et al., 2018). In dentistry, these can be further classified into soft tissue lasers & soft and hard tissue lasers, based on the applications (Table 3).
-
1.
Soft tissue lasers: e.g. CO2, KTP-532, Diode and Nd: YAG.
-
2.
Soft and hard tissue lasers: Er: YAG and ErCr: YSGG.
4.3. Laser in OSMF
In OSMF, the center of focus is fibrosis, that is the result of various AN components and reactions of the inflammatorymediators. The alkaloids and flavonoids, from the AN, are absorbed and undergo metabolism. Arecoline undergoes nitrosation and gives rise to N nitrosamines, which might have a highly proliferative, cytotoxic and, genotoxic effect on the cultured human oral mucosal epithelial cells and fibroblasts (Shreedevi et al.,2017). Also, over some time, due to persistent habit, chronic inflammation sets in at the site and is characterized by the presence of activated T cells and macrophages which produce various chemical mediators of inflammation, especially prostaglandins (PGs), interleukin-6 (IL-6), tumor necrosis factor (TNF), interferon ά (IF- ά), transforming growth factor β (TGF-β), and connective tissue growth factor (CTGF/CCN2) (Elattar, 1985, Gao et al., 1997).
During fibrogenesis, TGF-β is the primary cytokine responsible for the increased collagen production and decreased collagen degradation in OSMF (Dyavanagoudar, 2009). CCN2 is a multifunctional heparin-binding glycoprotein that is expressed at low levels in normal tissues but reveals overexpression in fibrotic tissues (Xu et al., 2008). And this overexpression of CCN2 has been associated with fibrosis in various tissues such as pulmonary, hepatic, renal, cutaneous, and gingival (Kantarci et al., 2006).
Release and excision of fibrotic bands are essential to prevent further complications of OSMF and remains a challenging task for the clinicians. Conventional surgical procedures have resulted in more fibrosis and scarification with many post-operative complications. The present review reveals that for recent years, surgeons have been using laser as a non-invasive method to treat OSMF with successful results and their usage is becoming a common practice nowadays.
Studies have shown that low-power laser irradiation (LPLI) has been beneficial in fibrosis prevention in different damaged organs. It uses a wavelength of 660 nm mostly using diode laser. LPLI helps to relieve pain from the injured site due to inhibition of the release of inflammatory mediators such as histamine, acetylcholine, serotonin, H+, K+, etc. (Imre et al., 2018).
Fillipin et al (2005) found that LPLI using gallium–arsenic (GaAs) can reduce fibrosis in an animal experimental model of Achilles tendon injury.
Oliveira et al (2012) depicted that LPLI decreased renal interstitial fibrosis in an animal model of unilateral ureteral obstruction (UUO) by preventing tubular activation and trans differentiation.
Alves et al (2014) found that LPLI exerted a positive effect on the inflammatory process, matrix metalloproteinases 2 (MMP2) activity, and collagen organization and distribution, in the process of rat skeletal muscle repair.
Yeh et al (2017) observed that LPLI suppressed changes in arecoline-associated fibrotic marker gene expression and inhibited the transcriptional activity of CCN2 in human gingival fibroblasts, via cyclic adenosine monophosphate (cAMP) signalling pathway. Their data suggested that LPLI could be a useful therapy for managing OSMF in the long term.
4.4. Studies using diode laser for treating OSMF
The diode laser has a wavelength ranging from 805 nm to 980 nm that can be well absorbed by melanin and haemoglobin (Santosh et al., 2019) and poorly absorbed by the HA and H2O present in the enamel. It is a portable device that transmits energy in gated or continuous pulse mode delivering rays through a flexible fibreoptic cable and hence can be reached even to poorly accessible areas such as trismus in OSMF (Azma and Safavi, 2013). Its cutting depth is <0.01 mm, and thus preserves tissues beyond this depth. It gives a precise line of controlled cutting without damaging the muscles and deeper structures. Therefore, the healing is rapid even without any graft or biological dressing. The active material used is a semi-conducting crystal, usually GaAs or similar compounds (Gupta and Kumar, 2011). It has a good coagulative property, sealing the blood vessels spontaneously, allowing excellent visibility, and precision when dissecting through the tissue planes. The operating time is less and the entire procedure is carried out intraorally without leaving any extraoral scar (Shah et al., 2005). Due to the minimal morbidity associated with this procedure, better patient compliance can be experienced. Also, the procedure can be repeated if required. The limitation of this laser is its high cost.
Literature research revealed that the diode laser has been used in the maximum number of studies (13 out of 20) to treat OSMF (Table 2). The first case was reported by Shah et al (2005). In ten studies, diode laser was used for treating fibrotomy. Two studies used diode lasers for photo bio stimulation (Chandra et al., 2019, Singh et al., 2017). It is the application of laser at a low speed for a short specific interval of time affecting only the target area. This procedure is being used as an alternative to laser fibrotomy and has been proved to be a less invasive method as compared to fibrotomy. In one study, diode laser was applied for treating fibrotomy followed by LLLT (Farista et al., 2018). In all the cases, patients showed improvement in the symptoms of the disease such as trismus, burning sensation and cheek flexibility with good wound healing and no scar formation. And in most of the cases, the treatment was followed by physiotherapy (Table 2).
4.5. Studies using KTF-532 laser for OSMF
It is a solid, visible laser with a wavelength of 532 nm obtained by passing an Nd-YAG laser beam through a KTP crystal (Zeitels et al., 2006). This wavelength is selectively absorbed by blood vessels providing this laser excellent haemostatic properties. So, it can be used to excise pigmented, hyperaemic, and vascular lesions. Also, this laser can be delivered through a flexible fiber and hence can be applied to treat relatively unapproachable areas (Fornaini et al., 2012). Another major advantage of KTP-532 laser is its ability to excise the fibrotic bands precisely with minimal collateral tissue damage and a bloodless field even in the presence of trismus allowing spontaneous epithelialization without the need of surface grafting. So, affected areas are easily visible for inspection and hence any malignant change can be detected at the earliest, whereas areas covered by bulky flaps might hide malignancies under them till they are relatively large (Kameshwaran et al., 2006). The only drawback of this laser is its higher cost than the diode laser.
For its unique properties, the KFT −532 laser has been used for fibrotomy in OSMF. Published data has demonstrated three studies related to its usage in this disease (Cheng et al., 2011, Kameshwaran et al., 2006, Nayak et al., 2009). Even some of the researchers concluded that this laser had the potential to treat severe cases of paediatric OSMF for the long term management (Cheng et al., 2011, Petal et al., 2016). All patients presented with improved mouth opening and relief from trismus.
4.6. Studies using CO2 laser for treating OSMF
The CO2 laser is a water or air-cooled laser, containing a gaseous mixture with CO2 molecules. It produces a beam of infrared light having a wavelength of 10600 nm and is well absorbed by water. This wavelength provides an ease of cutting and coagulation of soft tissue, thus giving an assess to a clean operating field (Israel, 1994, Pogrel, 1989). Due to water vaporization, the temperature in the target’s environment remains in balance, not rising above 1000c. It works in continuous or pulsating mode. Due to all of these properties, this laser has been used in place of a scalpel to excise large tissue pieces, in the peeling of thin surface layers, or merely to vaporize the tissue surface. An articulated mirror arm or a hollow fiber like beam conductor used in this system makes it able to reach all sides of the oral cavity. The best properties of using this laser in surgical procedures are precision, lack of bleeding, minimum scarring and, minimum to none postoperative pain as it induces neural sealing providing local neural anaesthesia, and reduces the number of pain mediators. Few limitations are large size, high cost, inability to develop optic fiber for this system, delayed wound healing, and destructive hard tissue interactions. It results in black/brown discoloration of tissues treated which is caused by a carbon residue but becomes normal within the first 10–14 days after the procedure (Fisher et al., 1983).
Although the use of CO2 laser has been widely appreciated in the common practice to treat OSMF, during this research, we could find only two published studies related to its usage in OSMF management (Chhaya et al., 2010, Devgan et al., 2015). And both studies showed improvement in the symptoms of the disease (Table 2).
4.7. Studies using ErCr:YSGG laser for treating OSMF
ErCr :YSGG laser is used for both hard and soft tissues but has got the limited haemostatic ability as is not absorbed by Hb and has got short pulse duration. It has a wavelength of 2780 µm which has a high affinity for H2O and HA. It follows the “hydro-photonic process” in which laser energy interacts with water droplets at tissue creating water molecule excitation. This, further causes water droplet micro-expansion and propulsion that gives clean and precise hard-tissue cut (Kimmel et al., 1996). The presence of air and water spray plays a dual role; first to assist in cutting and secondly, serving as a coolant, preventing any thermal side effects (Eversole and Rizoiu, 1995).
On soft tissue, this laser works differently, utilizing a small amount of water and air as coolant. ErCr:YSGG laser energy is selectively absorbed in the target tissue via thermal–mechanical tissue ablation and may result in either a cold cut or thermal cut. It limits the amount of collagen damage to as little as 5 µm, leaving the extracellular collagen matrix unaffected (Rizoiu et al., 1996). Moreover, this laser demonstrates shallow penetration in the tissues resulting in less thermal risks to deeper tissues. The usage of this laser results in minimal post-operative pain and inflammation due to less release of histamine in the tissues.
In literature, however, only two studies have been reported which used this laser system for the management of OSMF (Chaudhry et al., 2011, 2014) and showed effective results (Table 2).
Looking at the current data and this review research, it is possible to say that the use of laser therapy has proven to provide effective long term results in the treatment of all stages of OSMF without any complications and defects that are usually demonstrated during conventional surgeries. And this technique can be used even as a chairside procedure under LA minimizing the need for a large operating field. However, different lasers used till date, have their specific properties, functioning, wavelengths, effects on tissues, benefits, and limitations. But in this review, all the studies reported favourable results regardless of the type of laser used.
One of the major point highlighted by this review is that for the successful long term effects of laser in OSMF patients, this therapy has to be followed by some post-operative adjunctive aids such as physiotherapy, cessation of habit, other nutritious supplements, and regular follow up to evaluate the improvement in oral symptoms.
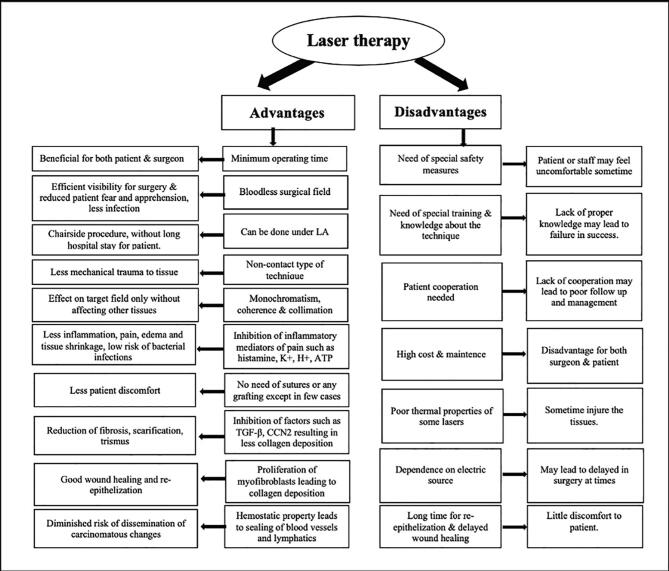
Despite a few limitations such as high cost, need for advanced training & safety measures, and delayed wound healing, (Fig. 2) laser has become indispensable in the management of OSMF.
Fig. 2.
Advantages & disadvantages of laser therapy.
5. Limitations of present study
One of the major limitations of this study is the lack of an exact scientific basis of selection and applied criteria in using lasers for the management of OSMF. Moreover, in these studies, only few specific lasers have been used, although a variety of other types are also available. So, there is also a need to evaluate their role and potential for treating OSMF.
6. Conclusion
In the modern era of dentistry, the main concern remains the bloodless surgical field, minimum operating time, least patient discomfort with effective long term postoperative results, both for the surgeon as well as the patient. Although wide varieties of surgical aids are in use in the medical and dental field, yet laser has proven to be a non-invasive, surgical technique with fewer limitations. In the premalignant conditions, such as OSMF, laser can be used as a reliable, reproducible method preventing further morbidity. It's usage must be encouraged in general practice. However, in literature, very few studies have been reported regarding the usage of laser in the treatment of OSMF with only few specific lasers, so large-scale studies are still required to investigate the higher efficacy and unknown effects of other lasers using this technology.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdulsamee N. Erbium family laser: Silent revolution in dentistry: Review. EC. Dent. Sci. 2017;13(4):168–190. [Google Scholar]
- Agarwal S., Agarwal S., Shashidhar K. Surgical management of trismus due to oral submucous fibrosis using diode laser. J. Otolaryng. Head Neck Surg. 2015;1:002. [Google Scholar]
- Alves A.N., Fernandes K.P., Melo C.A., Yamaguchi R.Y., Franca C.M., Teixeria D.F., et al. Modulating effect of low level-laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Lasers Med. Sci. 2014;29(2):813–821. doi: 10.1007/s10103-013-1428-9. [DOI] [PubMed] [Google Scholar]
- Aoki, A., Sasaki, K.M., Watanabe, H., Ishikawa, I.,2004. Lasers in nonsurgical periodontal therapy. Periodontal 2000.36,59-97. [DOI] [PubMed]
- Arakeri G., Patil S.G., Aljabab A.S., Lin K.C., Merkx M.A., Gao S., et al. Oral submucous fibrosis: an update on pathophysiology of malignant transformation. J. Oral Pathol. Med. 2017;46(6):413–417. doi: 10.1111/jop.12582. [DOI] [PubMed] [Google Scholar]
- Asnani S., Mahindra U., Oswal R. Use of diode lasers in treatment of oral submucous fibrosis: A new concept in surgical management. Int. J. Case Rep. Images. 2014;5(3):198–201. [Google Scholar]
- Azma E., Safavi N. Diode laser application in soft tissue oral surgery. J. Lasers Med. Sci. 2013;4(4):206–211. [PMC free article] [PubMed] [Google Scholar]
- Santosh B.S., Ngente Z., Daniel D., Harish A.K., Harikeerthy, Devkar A.P. Evaluation of wound healing following surgical excision of oral soft tissue lesions using diode laser (980nm) Int. J. Res. Health Allied Sci. 2019;5(1):20–27. [Google Scholar]
- Chandra S., Gujjari S.K., Sankar A.R. Biostimulation with diode lasers: A novel futuristic approach in the treatment of oral submucous fibrosis – A case report. Int. J. Med. Dent. Case Rep. 2019;6:1–4. [Google Scholar]
- Chaudhary Z., Verma M., Tandon S. Treatment of oral submucous fibrosis with Er Cr: YSGG laser. Ind. J. Dent. Res. 2011;22:472–474. doi: 10.4103/0970-9290.87073. [DOI] [PubMed] [Google Scholar]
- Chaudhary Z., Gupta S.R., Oberoi S.S. The efficacy of ErCr:YSGG laser fibrotomy in management of moderate oral submucous fibrosis: A Preliminary Study. J. Maxillofac. Oral Surg. 2014;13(3):286–294. doi: 10.1007/s12663-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L.H.H., Killick Z., Sadiq Z., Alibhai M., Quinn F. KTP laser for trismus induced by paediatric oral submucous fibrosis. British J. Oral Maxillofac. Surg. 2011;49(5):40. [Google Scholar]
- Chhaya V.A., Sinha V., Rathor R., Modi N., Rashmi G.S., Parmar V., et al. Oral submucous fibrosis - surgical treatment with CO2 laser. World Art. Ear. Nose. Throat. 2010;3:1–2. [Google Scholar]
- Colluzi D.J. Fundamentals of lasers in dentistry, basic sciences, tissue interaction and instrumentation. J Laser Dent. 2008;16:4–10. [Google Scholar]
- David C.M., Gupta P. Lasers in Dentistry: A Review. Int. J. Adv. Health Sci. 2015;2(8):7–13. [Google Scholar]
- Devgan K., Mistry P., Nagpal T. A prospective study of evaluation of methods to decrease the morbidity in oral submucous fibrosis. Int. J. Adv. Scient Res. 2015;1(01):28–32. [Google Scholar]
- Dyavanagoudar S.N. Oral Submucous Fibrosis Review on Etiopathogenesis. J. Cancer Sci. Ther. 2009;1.1:072–077. [Google Scholar]
- Elattar T.M.A. Cancer and the prostaglandins: a mini review on cancer research. J. Oral Pathol. 1985;14:511–522. doi: 10.1111/j.1600-0714.1985.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Eversole L.R., Rizoiu I.M. Preliminary investigations on the utility of an erbium, chromium: YSGG laser. J. Calif. Dent. Assoc. 1995;23:41–47. [PubMed] [Google Scholar]
- Farista S., Kalakonda B., Farista H., Iyer V.H. Diode laser-Assisted Fibrotomy in the Management of Oral Sub-Mucous Fibrosis: A New Technique in Surgical Management. J. Clin. Diag. Res. 2018;12(7):ZH01-ZH02. [Google Scholar]
- Fillipin L.I., Mauriz J.L., Vedovelli K., Moriera A.J., Zettler C.G., Lech O., et al. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg. Med. 2005;37(4):293–300. doi: 10.1002/lsm.20225. [DOI] [PubMed] [Google Scholar]
- Fisher S.E., Frame J.W., Browne R.M., Tranter R.M. A comparative histological study of wound healing following CO2 laser and conventional surgical excision of canine buccal mucosa. Arch. Oral Biol. 1983;28:287–291. doi: 10.1016/0003-9969(83)90069-9. [DOI] [PubMed] [Google Scholar]
- Fornaini C., Rocca J.P., Merigo E., Meleti M., Manfredi M., Nammour S., et al. Low energy KTP laser in oral soft tissues surgery: A 52 patients clinical study. Med. Oral Patol. Oral Cir Bucal. 2012;17(2):e287–e291. doi: 10.4317/medoral.17428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry R.R., Goyal S., Pandher P.K., Chawla J.P.S. An approach to management of oral submucous fibrosis: current status and review of literature. Int. J. Curr. Res. 2014;6(12):10598–10604. [Google Scholar]
- Gao Y., Ling T., Wu H. Expression of transforming growth factor beta 1 in keratinocytes of oral submucous fibrosis tissue. Zhonghua Kou. Qiang. Yi. Xue. Za. Zhi. (Chinese) 1997;32:239–241. [PubMed] [Google Scholar]
- Garde J.B., Dadhe D.P., Rajkumar S., Deshmukh V. Diode laser in submucous fibrosis: A case series with successful outcome. J. Dent. Lasers. 2013;7:85–86. [Google Scholar]
- Gopal S., George S. The Role of Lasers in the Treatment of Potentially Malignant Disorders of the Oral Cavity- a Review. Int. J. Curr. Adv. Res. 2019;08(05):18543–18546. [Google Scholar]
- Gupta S., Kumar S. Lasers in Dentistry - An Overview. Trends Biomater. Artif. Organs. 2011;25(3):119–123. [Google Scholar]
- Gupta S., Piyush P., Mahajan A., Mohanty S., Ghosh S., Singh K. Fibrotomy with diode laser (980 nm) and habit correlation in oral submucous fibrosis: a report of 30 cases. Lasers Med. Sci. 2018;33:1739–1745. doi: 10.1007/s10103-018-2531-8. [DOI] [PubMed] [Google Scholar]
- Imre M.M., Celibitache A., Tansu A.M.C., Pantea M., Suciu I., Parlea P. Laser’s applications in minimally invasive dental procedures – new trends in modern dentistry. Romanian Biotechnological Lett. 2018;23(6):14107–14115. [Google Scholar]
- Israel M. Use of the CO2 laser in soft tissue and periodontal surgery. Pract. Periodontics Aesthet. Dent. 1994;6:57–64. [PubMed] [Google Scholar]
- Kameshwaran M., Raghavan D., Anand Kumar R.S., Murali S. Surgical management of trismus due to oral Submucous fibrosis - Lysis of fibrotic bands with the KTP-532 laser. Ind. J. Otolaryngol. Head Neck Surg. 2006;58(3):229–231. doi: 10.1007/BF03050825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci A., Black S.A., Xydas C.E., Murawel P., Uchida Y., Yucical- Tuncer B., et al. Epithelial and connective tissue cell CTGF/CCN2 expression in gingival fibrosis. J. Pathol. 2006;210(1):59–66. doi: 10.1002/path.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A.I., Rizoiu I.M., Eversole L.R. Phase doppler particles analysis of laser energy exploding water droplets. International Laser Congress: Lasers at the Dawn of the Third Millennium. 1996 [Google Scholar]
- Kunusoth R., Alwala A.M., Prakash R., Naik P.M., Anand M. Role of diode laser in oral submucous fibrosis. J. Oral Med. Oral Surg. Oral Pathol Oral Radiol. 2018;4(4):198–200. [Google Scholar]
- Lokesh U., et al. Application of Lasers for Oral Submucous Fibrosis: An Experimental Study. Arch. Cran. OroFac Sci. 2014;1(6):81–86. [Google Scholar]
- More C.B., Gavli N., Chen Y., Rao N.R. A novel clinical protocol for therapeutic intervention in oral submucous fibrosis: An evidence based approach. J. Oral. Maxillofac. Pathol. 2018;22(3):382–391. doi: 10.4103/jomfp.JOMFP_223_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More C.B., Rao N.R. Proposed clinical definition for oral submucous fibrosis. J. Oral Biol. Craniofac Res. 2019;9(4):311–314. doi: 10.1016/j.jobcr.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More C.B., Rao N.R., Hegde R., Brahmbhatt R.M., Shrestha A., Kumar G. Oral submucous fibrosis in children and adolescents: Analysis of 36 cases. J. Indian Soc. Pedod Prev. Dent. 2020;38(2):190–199. doi: 10.4103/JISPPD.JISPPD_173_20. [DOI] [PubMed] [Google Scholar]
- More C.B., Rao N.R., More S., Johnson N.W. Reasons for Initiation of Areca Nut and Related Products in Patients with Oral Submucous Fibrosis within an Endemic Area in Gujarat, India. Subst Use Misuse. 2020;55(9):1413–1421. doi: 10.1080/10826084.2019.1660678. [DOI] [PubMed] [Google Scholar]
- Mudigonda J., Naik S., Reddy S.B., Balreddy P., Kumar G.K.P. Treatment Outcome of Diode Laser for Symptomatic Relief in Oral Sub Mucous Fibrosis: A Pilot Study. Adv. Hum Biol. 2016;6(1):37–41. [Google Scholar]
- Naik M., Dhupar V., Shetye O., Desai P. Use of laser in the treatment of common oral premalignant lesions and conditions - A review. Med. Res. Chron. 2018;5(5):398–403. [Google Scholar]
- Nayak D.R., Mahesh S.G., Aggarwal D., Pavithran P., Pujary K., Pillai S. Role of KTP-532 laser in management of oral submucous fibrosis. J. Laryngol. Otol. 2009;123(4):418–421. doi: 10.1017/S0022215108003642. [DOI] [PubMed] [Google Scholar]
- Oliveira F.A., et al. Low-level laser therapy decreases renal interstitial fibrosis. Photomed. Laser Surg. 2012;30(12):705–713. doi: 10.1089/pho.2012.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passi D., Bhanot P., Kacker D., Chahal D., Atri M., Panwar Y. Oral submucous fibrosis: Newer proposed classification with critical updates in pathogenesis and management strategies. Natl. J. Maxillofac. Surg. 2017;8:89–94. doi: 10.4103/njms.NJMS_32_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petal S., Rumani S., Patel H., Cheng L. Management of Oral Submucous Fibrosis related trismus using Potassium Titanyl Phosphate (KTP-532nm) laser. British J. Oral Maxillofac. Surg. 2016;54(10):46. [Google Scholar]
- Pogrel M.A. The carbon dioxide laser in soft tissue preprosthetic surgery. J. Prosthet. Dent. 1989;61:203–208. doi: 10.1016/0022-3913(89)90374-0. [DOI] [PubMed] [Google Scholar]
- Rao N.R., More C.B., Brahambhatt R.M., Chen Y., Ming W.K. Causal inference and directed acyclic graph: An epidemiological concept much needed for oral submucous fibrosis. J. Oral Biol. Craniofac. Res. 2020;10(4):356–360. doi: 10.1016/j.jobcr.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N.R., Villa A., More C.B., Jayasinghe R.D., Kerr A.S., Johnson N.W. Oral submucous fibrosis: a contemporary narrative review with a proposed interprofessional approach for an early diagnosis and clinical management. J. Otolaryngol. Head Neck Surg. 2020;49(3):1–10. doi: 10.1186/s40463-020-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizoiu I.M., Eversole L.R., Kimmel A.I. Effects of an erbium, chromium yttrium, scandium, gallium, garnet laser on mucocutaneous soft tissues. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996;82:386–395. doi: 10.1016/s1079-2104(96)80302-7. [DOI] [PubMed] [Google Scholar]
- Shah A., Raj S., Rasaniya V., Patel S., Vakade M. Surgical Management of OSMF with the Opus -5 Diode Laser. J. Oral Laser Appl.. 2005;5(1):37–43. [Google Scholar]
- Shih Y.H., Wang T.H., Shieh T.M., Tseng Y.S. Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy. Int. J. Mol. Sci. 2019;20:1–10. doi: 10.3390/ijms20122940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreedevi B., Shaila M., Sreejyothi H.K. Genotoxic effect of local & commercial arecanut & tobacco products- a review. Int. J. Health Sci. Res. 2017;7(3):326–331. [Google Scholar]
- Singh K., Garg A., Jain M., Uppal M.K. Role of Laser Biostimulation in Treatment of Oral Submucous Fibrosis: A Clinical Trial. Int. Healthcare Res. J. 2017;1(7):22–26. [Google Scholar]
- Talsania J.R., Shah U.B., Shah A.I., Singh N.K. Use of diode laser in oral submucous fibrosis with trismus: prospective clinical study. Ind. J. Otolaryngol. Head Neck Surg. 2009;61(Suppl 1):22–25. doi: 10.1007/s12070-009-0012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy R., Patnaik S., Acharya S.A., Akheel M. Diode Laser as a Treatment for Oral Submucous Fibrosis- A Case Report. Arch. Cran. OroFac. Sc. 2014;2(1):104–106. [Google Scholar]
- Verma S.K., Maheshwari S., Singh R.K., Chaudhari P.K. Laser in dentistry: An innovative tool in modern dental practice. Natl. J. Maxillofac. Surg. 2012;3(2):124–132. doi: 10.4103/0975-5950.111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.W., Leask A., Abraham D.J. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19(2):133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Yeh M.C., Chen K.K., Chiang M.H., Chen C.H., Chen P.H., Lee H.E., et al. Low-power laser irradiation inhibits arecoline-induced fibrosis: an in vitro study. Int. J. Oral Sci. 2017;9:38–42. doi: 10.1038/ijos.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitels S.M., Akst L.M., Burns J.A. Office- based 532-nm pulsed KTP laser treatment of glottal papillomatosis and dysplasia. Ann. Otol. Rhinol. Laryngol. 2006;115:679–685. doi: 10.1177/000348940611500905. [DOI] [PubMed] [Google Scholar]