Graphical abstract
Keywords: Experimental periodontitis, Morphometric analysis, Rat incisors, Nanogels, Triclosan, Flurbiprofen
Abstract
Purpose
To evaluate therapeutic effectiveness of antibacterial triclosan (TCS) and anti-inflammatory flurbiprofen (FLB)-loaded nanogels system in ligature-induced experimental periodontitis in rats.
Methodology
A total of 72 Sprague–Dawley rats were used in this study. Four groups (n = 18 each) were randomly created: Group 1 – neither subjected to experimental periodontitis nor to any treatment; Group 2 – subjected to experimental periodontitis but not treated; Group 3 – subjected to experimental periodontitis and then treated with the developed nanogels; Group 4 – subjected to experimental periodontitis and then placed on a mixture of pure TCS and FLB treatment. The experimental periodontitis was induced on the lower incisors by applying a ligature which was kept for 14 days. Treatment was done for 7 days, and sampling was done at 7, 14, and 28 day of the post-induction experimental period. Morphometric analysis was conducted to assess the clinical outcomes and healing effect.
Results
The morphometric findings showed that the group treated with the developed TCS and FLB-loaded nanogels recovered better and faster than a mixture of pure TCS and FLB. At 28 day of the experimental period, there was no significant difference (p > 0.05) between the baseline control group and the nanogels treated group.
Conclusions
The developed TCS and FLB-loaded nanogels was found to be effective in the treatment of experimental periodontitis in rats. The used experimental periodontitis model was found to be simple and easily reproducible.
1. Introduction
Animal models have been used for decades to evaluate the pathogenesis of periodontitis as well as the efficacy of periodontal dosage forms (Weinberg and Bral, 1999). Non-human primates, rats, hamsters, mice, and dogs are the reported animal models commonly employed for the in vivo evaluation of periodontal drug delivery systems (Abe and Hajishengallis, 2013). Among these, rats are the most used laboratory animal model for experimental periodontitis because of their unique features (Pramod et al., 2014).
The experimental periodontitis which leads to the inflammatory destruction of the periodontium can be spontaneous or experimentally induced in most mammalian species (Weinberg and Bral, 1999). There is clear evidence of inflammation and bone loss after the induction of experimental periodontitis in rats using the following widely used models: (1) ligature placement on the teeth (Miyajima et al., 2014, Nishikawa et al., 2012, Ribeiro et al., 2012) and (2) applying causative microorganisms such as Porphyromonas gingivalis in the periodontium or by oral gavage (Alshammari and Amar, 2019, Kesavalu et al., 2007). The former model is the most commonly used, and first or second molars are the targeted teeth for receiving the thread ligature in a cervical position which serves as a retentive tool for microbial and plaque proliferation (Fontana et al., 2018, Pramod et al., 2014). However, this model has been associated with complexity due to the size and anatomical position of the molar teeth of the common experimental animals, i.e. rats, which are situated farthest to the rear and hence difficult to access. Therefore, a modification of the existing model is presented herein, which differs by placement of a ligature on the incisors that are easily accessible.
Furthermore, the available therapeutic systems for periodontitis mainly focused on the causative bacteria rather than also targeting the pro-inflammation factors. A suitable formulation of TCS (triclosan) and FLB (flurbiprofen) combination would be highly desirable for periodontal therapy, owing to the therapeutic efficacy of the drugs. TCS is a broad-spectrum antimicrobial agent that has a recognised efficacy against several plaque-forming bacteria and has been used extensively in various oral health care products for several years (Aminu et al., 2013). FLB belongs to non-steroidal anti-inflammatory drugs (NSAIDs). Studies have revealed evidence which indicated that supplementing periodontitis treatment with NSAIDs like FLB can improve the outcome of the therapy (Jeffcoat et al., 1988, Ribeiro et al., 2012). However, clinical data that reveal the effect of treatment of periodontitis with TCS and FLB combination is lacking. The present study aimed to evaluate the therapeutic effectiveness of the TCS and FLB-loaded nanogels system in a simple and easily reproducible ligature-induced experimental periodontitis in rats. The rationale for using nanogels as the drug delivery system for this study was due to its potential to penetrate the gingival sulcus and periodontal pocket, and be retained for a prolonged period to provide and maintain effective drug release (Aminu et al., 2019). Researchers have reported striking shreds of evidence for using nanomaterials and nanocarriers to improve various dental applications (Aminu et al., 2020, Aminu et al., 2018b, Aminu et al., 2017, Aminu and Toh, 2017, Khurshid et al., 2015, Raorane et al., 2019, Salama et al., 2019, Zafar et al., 2019, Zafar et al., 2017).
2. Materials and methods
2.1. Animals
Male Sprague–Dawley rats, 72 in number were used in this study. The rats were obtained from the Animal Research and Service Centre of Universiti Sains Malaysia, and they were three months old at the beginning of the study. They were housed in plastic cages, 6 rats per cage with access to standard rat pellets and tap water. The protocol of the present investigation was granted approval by Ethical Review Board of Universiti Sains Malaysia (USM/Animal Ethics Approval/2016/(103)(795)).
2.2. Grouping and protocol for treatments
The experiments were carried out by randomly assigning 18 animals per group. The experimental groups were as follows:
-
a.
Group 1 (baseline control): these are animals that were neither subjected to experimental periodontitis nor to any treatment.
-
b.
Group 2 (non-treated): these are animals subjected to experimental periodontitis but not treated.
-
c.
Group 3 (test): these are animals subjected to experimental periodontitis and then treated with the developed nanogels which were formulated to contain 0.5% of TCS and 1% of FLB. The nanogels were prepared according to our previously published method (Aminu et al., 2019, Aminu et al., 2018a, Aminu et al., 2018c).
-
d.
Group 4 (positive control): comprises animals that were subjected to experimental periodontitis and then placed on a mixture of pure TCS and FLB treatment. An amount equivalent to 5 mg and 10 mg of pure TCS and pure FLB, respectively, were dispersed in simulated saliva (10 mL). Then, the mixture was stirred for 20 min at 800 rpm.
The treatment was carried out by applying 0.2 mL of the nanogels, and a mixture of pure TCS and FLB on group 3 and 4, respectively, on the infected gingival sites, once daily for 7 days, using a 1 mL syringe.
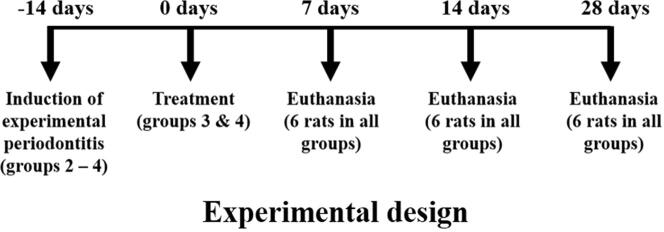
2.3. Experimental design
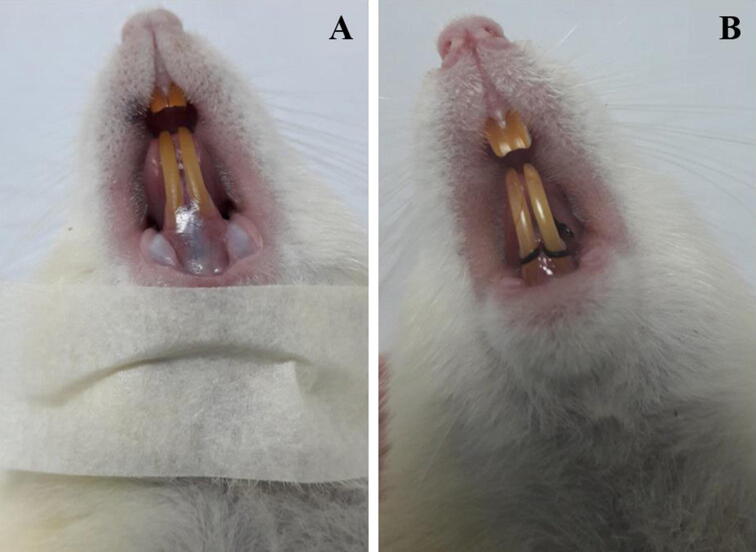
For experimental periodontitis induction, the animals were anaesthetised by intraperitoneal injection of 50 mg kg−1 of pentobarbital sodium (Dorminal 20%, Alfasan, Woerden-Holland). After achieving general anaesthesia, the animals were placed on operating table, and the mouth was opened to facilitate access to the teeth (Fig. 1A). Sterile, 3/0 non-absorbable silk thread (Vigilenz®, Malaysia) was carefully placed around the gingival sulcus of the lower incisors without damaging the nearby gingiva. This ligature was kept in place for 14 days during which it served as a gingival irritant and promoted accumulation of biofilm and subsequent development of periodontitis.
Fig. 1.
Rat on operation table, (A) showing healthy gum and incisors, just before the placement of ligature and (B) immediately after silk ligature was placed around the gingival sulcus of the lower incisors.
At the end of the two weeks of ligature placement, the thread was removed, and group 3 and 4 were treated with their respective medication for 7 days, as described in Section 2.2. Then 6 rats from each group were euthanised with a lethal dose of pentobarbital (Miyajima et al., 2014, Nishikawa et al., 2012) according to the experimental design presented in Fig. 2. The rats that were not subjected to ligature placement (group 1) were used as the baseline control. The pair of mandibles were removed, defleshed, cleaned, and air dried.
Fig. 2.
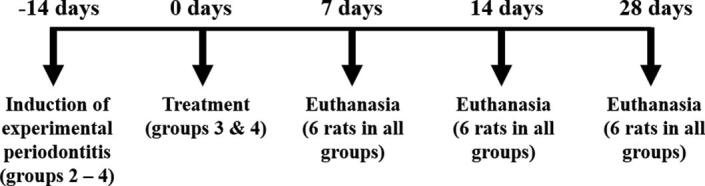
Experimental design of the periodontitis model for the present study.
2.4. Morphometric analysis
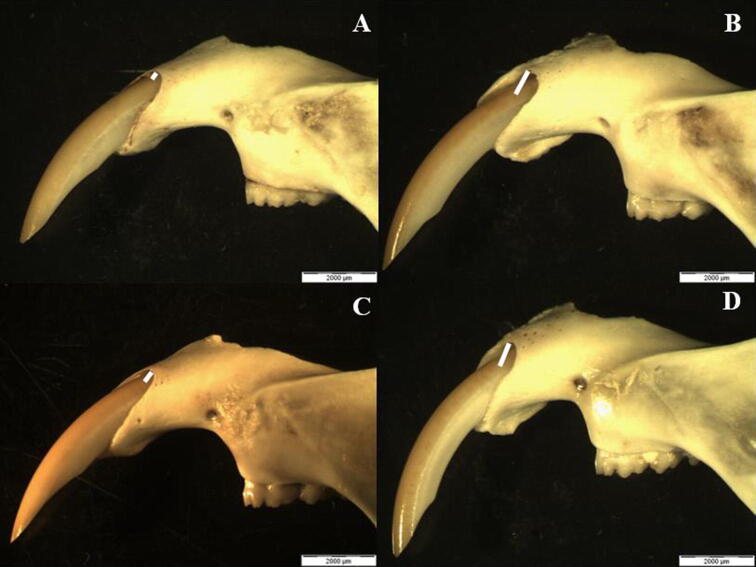
The processed mandibles were used for morphometric analysis. Alveolar bone loss was determined by measurement of the distance from the enamel to the alveolar bone crest at the ameloblastic apical region, more specifically, in the long axis of the labial surface of the lower incisors (Fig. 3). The distance measurements were estimated based on the morphology of rat incisors that was previously established by Kiukkonen et al., 2002. The analysis was carried out using a stereoscope (Olympus SZX9, Olympus Optical Co., Ltd, Japan) which was equipped with a video camera (Xcam-α, Olympus Tokyo, Japan).
Fig. 3.
Evaluated morphometric parameter of (A) group 1, (B) group 2, (C) group 3, and (D) group 4. The white lines represent the distance from the enamel to the alveolar bone in the labial surface.
2.5. Statistical analysis
Statistical significance of the data sets was assessed by two-way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test using version 7.0 Graphpad Prism® statistical software. A 95% confidence interval was considered as statistical significant.
3. Results
3.1. Induction of periodontitis
The results for the clinical outcomes of the periodontitis inducement have been published in our previous study in which we reported the preliminary findings through gingival and plaque indexes (Aminu et al., 2019). Therefore, the focus of the result for this paper is the morphometric analysis that could contribute to the knowledge of plausible therapeutics to treat periodontitis based on TCS and FLB-loaded nanogels.
3.2. Morphometric results
In order to determine the healing effect of the developed TCS and FLB-loaded nanogels on the alveolar bone, the bone loss of the treated rats was determined by measurement of the enamel–alveolar bone distance in micrometer and then compared with that of the non-treated and baseline control groups. The morphometric results are presented in Fig. 4.
Fig. 4.
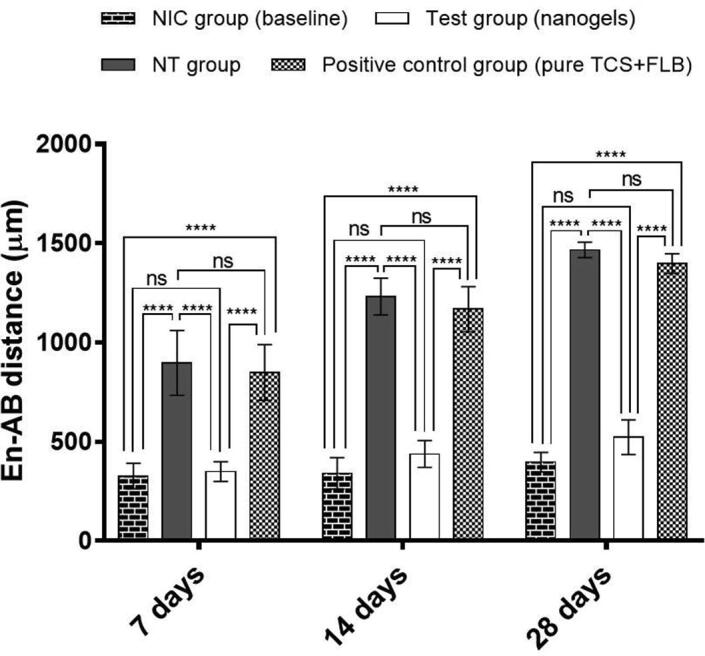
Alveolar bone loss caused by ligature-induced experimental periodontitis in rats after 7, 14, and 28 days, and effects of the 7 days treatment by the developed nanogels (test) and a mixture of pure TCS and FLB (positive control) on the reduction of the bone loss. NIC, non-induced control; NT, non-treated. The data herein are presented as means ± SD for six (n = 6) determinations. ****, p < 0.0001; ns, no significant difference.
The increase in the enamel–alveolar bone distance signifies a decrease in the alveolar bone (bone loss). Among the investigated four groups, group 2 (non-treated) and group 4 (positive control) presented the greatest enamel–alveolar bone distance, indicating significant (p < 0.0001) alveolar bone resorption when compared with group 1 (non-induced) and group 3 (nanogels). This resorption was time depended as a progressive decline in the alveolar bone level was observed over time, i.e. from 7 to 28 days (Fig. 4). On the contrary, there was no significant (p > 0.05) time-dependent increase in the enamel–alveolar bone distance observed in group 1 and 3. On the last day of the treatment, i.e., 7th day, a significant reduction (p < 0.0001) in the alveolar bone loss was noted in group 3. Within the same period, there was no significant difference (p > 0.05) in the enamel–alveolar bone distance between group 3 and the baseline control group (group 1).
4. Discussion
Studies have shown that experimental periodontitis in rats can be induced by placing silk or cotton ligatures around the lower incisors (Boşca et al., 2015, Chumakova et al., 2014, Dimitriu et al., 2019, Ionel et al., 2015), hence we used the lower incisors in this study. The used ligature-induced experimental periodontitis model was less invasive and inflicted less operative trauma on the animals. It was also found to be reproducible and straightforward when compared with the commonly used model. There were no observed changes in the animals’ eating habit and/or other general behaviours after the inducement.
It is clear that the group treated with TCS and FLB-loaded nanogels (group 3) showed better therapeutic outcomes than the group treated with a mixture of pure TCS and FLB (group 4). Multiple reasons may be responsible for the treatment failure of group 4. The first reason could be as a result of the availability of the drugs in their inherent hydrophobic crystalline forms in simulated saliva solution. Lack of suitable carrier for the drugs may restrict their access to the periodontal pockets, as they can be rapidly washed away by food substances or saliva (Mizrahi and Domb, 2008). Another reason could be as a result of the poor aqueous solubility of the drugs. Therefore, the morphometric findings implied that the developed nanogels were effective in an immediate reduction of bone loss. This effectiveness was sustained after the cessation of the treatment and up to the last day of the study period (Fig. 4). This superior effect of the developed nanogels can be explained by the immediate release of FLB from the nanogels matrix, and sustained release of TCS noted from the nanoparticles within the nanogels system, as established in our previous study (Aminu et al., 2019).
In contrast to the molars of rodents which do not grow continuously, the continuous eruption of rodent incisors may lead to difficulty in interpreting the proposed model. Another difference that may hinder better interpretation of this model is that the roots of the rodent’s molars are consistently sustained by the periodontium and linked with the alveolar bone, unlike in the case of their incisors which present a less extended area of contact in the enamel vestibular surface with periodontium and alveolar bone. However, recent advances in periodontology have helped in tackling this obstacle due to the more in-depth understanding of the evolution of rat’s incisors (Ionel et al., 2015). Therefore, the rodent's incisors are unique model for the study of processes involved in dental pathology (Goldberg et al., 2014, Ionel et al., 2015). The proposed model was employed in investigating the effects of chemical compounds on the periodontium of rats (Boşca et al., 2015, Chumakova et al., 2014, Kiukkonen et al., 2002). Of note, there is still need for further optimisation of the distance measurement from the enamel to the alveolar bone crest, as like in the case of the commonly used model which has been well optimised (Abe and Hajishengallis, 2013). Secondly, the proposed model is yet to be widely adopted, hence the need for further research in this direction to provide deeper insights.
5. Conclusions
This study demonstrated that a modified ligature-induced model on the lower incisors could be a simple alternative to the molar teeth-based periodontitis model, and the treatment effectiveness of TCS and FLB-loaded nanogels in experimental periodontitis in rats. Morphometric findings revealed significant regeneration of the lost bone for the nanogels treated group. Overall, the results showed the superior healing effect of the developed nanogels system loaded with antimicrobial TCS and anti-inflammatory FLB in the treatment of periodontitis. However, as there are limited investigations on experimental periodontitis in the rat’s incisors region, further studies are warranted to corroborate our findings.
Ethical statement
The experimental protocol of the present study was approved by the Animal Ethics Committee of Universiti Sains Malaysia. The approval number was USM/Animal Ethics Approval/2016/(103)(795).
CRediT authorship contribution statement
Nafiu Aminu: Conceptualization, Writing - original draft. Mun-Fei Yam: Validation. Siok-Yee Chan: Conceptualization. Idris Bello: Resources, Software. Nura Muhammad Umar: Critical revision of the manuscript. Tanko Nuhu: Funding acquisition. Seok-Ming Toh: Project administration, Visualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Universiti Sains Malaysia (USM) for providing some facilities to conduct this work, and Usmanu Danfodiyo University, Sokoto, Nigeria for the financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abe T., Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari A., Amar S. Proposal for a novel murine model of human periodontitis using Porphyromonas gingivalis and type II collagen antibody injections. Saudi Dent. J. 2019;31:181–187. doi: 10.1016/j.sdentj.2019.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminu N., Baboota S., Pramod K., Singh M., Dang S., Ansari S.H., Sahni J.K., Ali J. Development and evaluation of triclosan loaded poly-ε-caprolactone nanoparticulate system for the treatment of periodontal infections. J. Nanopart. Res. 2013;15:2075. doi: 10.1007/s11051-013-2075-6. [DOI] [Google Scholar]
- Aminu N., Bello I., Umar N.M., Nuhu T., Aminu A., Audu M.M. The influence of nanoparticulate drug delivery systems in drug therapy. J. Drug Deliv. Sci. Technol. 2020 doi: 10.1016/j.jddst.2020.101961. [DOI] [Google Scholar]
- Aminu N., Chan S.Y., Khan N.H., Toh S.M. Concurrent determination of triclosan and flurbiprofen by high-performance liquid chromatography in simulated saliva and its application in dental nanogel formulation. Acta Chromatogr. 2018;30:219–224. doi: 10.1556/1326.2017.00286. [DOI] [Google Scholar]
- Aminu N., Chan S.Y., Toh S.M. Formulation design and optimization of triclosan loaded nanoparticles for enhanced drug delivery across gingival sulcus by Resolution IV modeling of Design-Expert®. J. Biomed. Clin. Sci. 2018;3:83–90. [Google Scholar]
- Aminu N., Chan S.Y., Toh S.M. Development and validation of a stability-indicating HPLC-UV method for the simultaneous determination of flurbiprofen and triclosan in dental nanogel formulations. J. Phys. Sci. 2018;29:1–7. doi: 10.21315/jps2018.29.s1.1. [DOI] [Google Scholar]
- Aminu N., Chan S.Y., Toh S.M. Roles of nanotechnological approaches in periodontal disease therapy. J. Appl. Pharm. Sci. 2017;7:234–242. doi: 10.7324/JAPS.2017.70735. [DOI] [Google Scholar]
- Aminu N., Chan S.Y., Yam M.Y., Toh S.M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int. J. Pharm. 2019;570:118659. doi: 10.1016/j.ijpharm.2019.118659. [DOI] [PubMed] [Google Scholar]
- Aminu N., Toh S.M. Applicability of nanoparticles-hydrogel composite in treating periodontal diseases and beyond. Asian J. Pharm. Clin. Res. 2017;10:65–70. doi: 10.22159/ajpcr.2017.v10i2.15709. [DOI] [Google Scholar]
- Boşca A.B., Dinte E., Colosi H., Ilea A., Câmpian R.S., Uifălean A., Pârvu A.E. Curcumin effect on nitro-oxidative stress in ligature-induced rat periodontitis. Rom. Biotechnol. Lett. 2015;20:10708–10717. [Google Scholar]
- Chumakova Y., Vishnevskaya A., Kakabadze A., Karalashvili L., Kakabadze Z. Clinical and biochemical analysis of ligature-induced periodontitis in rats. Georgian Med. News. 2014:63–69. [PubMed] [Google Scholar]
- Dimitriu T., Daradics Z., Suciu Ș., Armencea G., Cătoi C., Dinu C., Băciuț G., Văcăraș S., Bran S., Băciuț M. Ligature induced periodontitis causes atherosclerosis in rat descending aorta: an experimental study. Med. Pharm. Rep. 2019;92:S39–S44. doi: 10.15386/mpr-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana C.R., Grecco C., Bagnato V.S., de Freitas L.M., Boussios C.I., Soukos N.S. Molecular analyses of two bacterial sampling methods in ligature-induced periodontitis in rats. Clin. Exp. Dent. Res. 2018;4:19–24. doi: 10.1002/cre2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M., Kellermann O., Dimitrova-Nakov S., Harichane Y., Baudry A. Comparative studies between mice molars and incisors are required to draw an overview of enamel structural complexity. Front. Physiol. 2014;5:1–6. doi: 10.3389/fphys.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionel A., Lucaciu O., Moga M., Buhatel D., Ilea A., Catoi C., Berce C., Toader S., Campian R.S. Periodontal disease induced in Wistar rats – experimental study. Hum. Vet. Med. Int. J. Bioflux Soc. 2015;7:90–95. [Google Scholar]
- Jeffcoat M.K., Williams R.C., Reddy M.S., English R., Goldhaber P. Flurbiprofen treatment of human periodontitis: effect on alveolar bone height and metabolism. J. Periodontal Res. 1988;23:381–385. doi: 10.1111/j.1600-0765.1988.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Kesavalu L., Bakthavatchalu V., Rahman M.M., Su J., Raghu B., Dawson D., Fernandes G., Ebersole J.L. Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol. Immunol. 2007;22:232–239. doi: 10.1111/j.1399-302X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- Khurshid Z., Zafar M., Qasim S., Shahab S., Naseem M., AbuReqaiba A. Advances in nanotechnology for restorative dentistry. Materials (Basel) 2015;8:717–731. doi: 10.3390/ma8020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiukkonen A., Viluksela M., Sahlberg C., Alaluusua S., Tuomisto J.T., Tuomisto J., Lukinmaa P.-L. Response of the incisor tooth to 2,3,7,8-tetrachlorodibenzo-p-dioxin in a dioxin-resistant and a dioxin-sensitive rat strain. Toxicol. Sci. 2002;69:482–489. doi: 10.1093/toxsci/69.2.482. [DOI] [PubMed] [Google Scholar]
- Miyajima S., Naruse K., Kobayashi Y., Nakamura N., Nishikawa T., Adachi K., Suzuki Y., Kikuchi T., Mitani A., Mizutani M., Ohno N., Noguchi T., Matsubara T. Periodontitis-activated monocytes/macrophages cause aortic inflammation. Sci. Rep. 2014;4:1–9. doi: 10.1038/srep05171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi B., Domb A.J. Mucoadhesive polymers for delivery of drugs to the oral cavity. Recent Pat. Drug Deliv. Formul. 2008;2:108–119. doi: 10.2174/187221108784534126. [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Naruse K., Kobayashi Y., Miyajima S., Mizutani M., Kikuchi T., Soboku K., Nakamura N., Sokabe A., Tosaki T., Hata M., Ohno N., Noguchi T., Matsubara T. Involvement of nitrosative stress in experimental periodontitis in diabetic rats. J. Clin. Periodontol. 2012;39:342–349. doi: 10.1111/j.1600-051X.2011.01848.x. [DOI] [PubMed] [Google Scholar]
- Pramod K., Aminu N., Ali J. In: Biotechnology Volume 8: Novel Drug Delivery. Singh B., Katare O.P., Govil J.N., editors. Studium Press LLC; U.S.A.: 2014. Targeted drug delivery systems for the treatment of periodontal infections; pp. 97–128. [Google Scholar]
- Raorane D.V., Chaughule R.S., Pednekar S.R., Lokur A. Experimental synthesis of size-controlled TiO2 nanofillers and their possible use as composites in restorative dentistry. Saudi Dent. J. 2019;31:194–203. doi: 10.1016/j.sdentj.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro F.V., Barrella G.E., Casarin R.C.V., Cirano F.R., Foglio M.A., Pimentel S.P. Effect of crude extract and essential oil of Cordia verbenacea in experimental periodontitis in rats. Brazil. J. Oral Sci. 2012;11:42–46. [Google Scholar]
- Salama R., Khashaba M., El Rouby D. Histomorphometric evaluation of a nano-sized eggshell-containing supplement as a natural alloplast: An animal study. Saudi Dent. J. 2019;31:375–381. doi: 10.1016/j.sdentj.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg M.A., Bral M. Laboratory animal models in periodontology. J. Clin. Periodontol. 1999;26:335–340. doi: 10.1034/j.1600-051x.1999.260601.x. [DOI] [PubMed] [Google Scholar]
- Zafar M.S., Alnazzawi A.A., Alrahabi M., Fareed M.A., Najeeb S., Khurshid Z. Advanced Dental Biomaterials. Elsevier; 2019. Nanotechnology and nanomaterials in dentistry; pp. 477–505. [DOI] [Google Scholar]
- Zafar M.S., Khurshid Z., Najeeb S., Zohaib S., Rehman I.U. Nanostructures for Oral Medicine. Elsevier; 2017. Therapeutic applications of nanotechnology in dentistry; pp. 833–862. [DOI] [Google Scholar]