Purpose
The use of bioactive materials is a recent proposal in the treatment of dentin hypersensitivity (DH) due to the ability to stimulate the neoformation of a barrier on dentin surface. Questions have arisen about the effectiveness of the materials to reduce DH when compared to the control groups (placebo or non-bioactive substance). Thus, the aim of this systematic review was to evaluate the randomized controlled trials in adult patients for DH treatment with a dentifrice containing bioactive glass, applied either at-home or in-office. Methods: The study was registered in PROSPERO and followed PRISMA guidelines. Searches were carried out in four databases (Pubmed/Medline, CENTRAL, Wbb of Science, LILACS) spanning from February 2020 to March 2020, with no language or publication date restrictions. A supplementary hand-search was performed by checking the list of references. The so-called gray literature of the national and international databases for theses and dissertations, as well as unfinished, in progress and unpublished studies were also searched. Results: After reading the titles and abstracts, articles that were duplicated (74 records) or unrelated to the systematic review (76 records) were excluded. Fifteen studies were evaluated considering seven at low risk of bias, four at high risk and four at moderate risk. Conclusion: The bioactive compounds at low concentrations (2.5–7.5%) can be used as treatment of DH both at-home and in-office.
Keywords: Dentin sensitivity, Clinical trial, Dentin desensitizing agents, Bioactive glass
1. Introduction
Dentin hypersensitivity (DH) is characterized by short or transient sharp pain arising from exposed dentin in response to thermal, physical, osmotic, or chemical elements (Holland et al., 1997, West et al., 2011). According to the hydrodynamic theory, hypersensitivity results from the rapid movement of the fluid within the dentinal tubules (Brannstrom et al., 1968). It is imperative to the efficacy of the DH treatment to address the etiological factors of the condition (Femiano et al., 2017).
Desensitizing therapies consist of the use of chemical substances, laser irradiation, restorative treatments, periodontal surgeries which aim basically to control the hydrodynamic mechanisms of pain. However, these treatments do not achieve predictable results in all patients and may lose their effect over time (Cartwright, 2014, Zhu et al., 2015, Solé-Magdalena et al., 2017). Bioactive glass materials have been proposed for the treatment of DH (David, 2010, Curtis et al., 2010, Barry et al., 2011, Sauro et al., 2011, Cartwright, 2014, Hall et al., 2017, Bansal and Mahajan, 2017).
The mechanism of action of bioactive glass starts when the material is exposed to an aqueous environment. The sodium ions (Na+) in the particles immediately are switched with hydrogen cations (H+ or H3O+). This rapid release of ions allows the calcium ions (Ca+2) in the particle structure, as well as the phosphate ions (PO4-3), to be released from the glass particles. The initial reactions occur in seconds of exposure and the release of the Ca+2 and PO4-3 ions continues as long as the particles are exposed to an aqueous environment. A localized and transient increase in pH occurs during the initial material exposure due to the release of Na+. This increase in pH helps to precipitate the Ca+2 and PO4-3 ions of the particle to form a layer of calcium phosphate Ca(PO4)2. As particle reactions and the deposition of calcium and phosphorus complexes are maintened, the layer crystallizes into hydroxycarbonate apatite which is chemically and structurally equal to biological apatite. The combination of residual particles and the newly formed apatite layer lead to physically occluding the dentin tubules, resulting in remission or pain reduction (Zhong et al., 2002, Zanotto et al., 2004, Hench, 2006).
The maintenance of this layer on the dentin surface is a major challenge for the materials existing in the market, besides knowing the effectiveness of them for the treatment of DH. Studies have shown the absence of parameters such as: patient follow-up time, control groups, assessment scale for degree of pain, DH diagnostic methods and form of application of the product (Matranga et al., 2017, Hannigan and Lynch, 2013, Kim et al., 2011, Pandis et al., 2011, Fleming et al., 2013).
The use of bioactive materials is a promising proposal for DH due to the the neoformation of a bioactive barrier on dentin surface (Bansal and Mahajan, 2017). They showed the ability to occlude the dentinal tubules and form a mechanically strong layer of hydroxyapatite on the dentin surface, which can resist degradation by repeated acid challenges (Du et al., 2008).
Thus, the aim of this systematic review was to assess randomized controlled trials (RCT) carried out in the permanent teeth of adult patients in the DH treatment with bioactive dentrifices, either at-home or in-office.
2. Materials and methods
2.1. Registration and protocol
The study has been registered on the International Prospective Register of Systematic Reviews (PROSPERO - CRD42016036985), and followed the guidelines of the Statement for Reporting Systematic Reviews and Meta-Analyses of Studies (PRISMA).
2.2. Inclusion criteria
-
•
Only randomized clinical trials (RCT) that followed the Consort recommendations.
-
•
Patients: adult participants (aged 18 or older) with DH symptoms.
-
•
Intervention: the group was treated for DH pain using bioactive glass toothpaste.
-
•
Control: the group has used a placebo or a desensitizing agent without bioactive glass.
-
•
Results: clinical methods for DH diagnosis included thermal, physical or chemical tests on exposed dentin (Holland et al., 1997), examining all teeth in the area where the patient claimed dentin pain (Gillam and Orchardson, 2006). Severity or degree of pain was quantified according to a categorical scale (i.e., mild, moderate, or severe pain) or a visual analog scale (VAS) (Holland et al., 1997, Canadian Advisory Board on Dentin Hypersensitivity, 2003). Other methods used were the Schiff Cold Air Sensitivity Scale (Schiff et al., 1994) or a calibrated Yeaple Probe (200A, XiniX Research, Portsmouth, NH, USA) (Gillam et al., 1992).
2.3. Exclusion criteria
Studies on the postoperative sensitivity in composite restoration, studies without follow-up of patients and studies with conflict of interest were excluded.
2.4. Search strategies
Searches were carried out in four different databases since February 2020 to March 2020, with no language or publication date restrictions of the articles: The electronic Medline/Pubmed, CENTRAL (Cochrane Central Register of Controlled Trials), Web of Science and LILACS (Latin American and Caribbean Health Sciences Literature/Virtual Healthy Library). The search strategy and keywords are seen in Table 1. The supplementary hand-search was performed by checking the list of references of studies included in the review or in previous reviews. The so-called grey literature was researched in the bank for theses of the University of São Paulo, Portal of Journals of the Coordination for the Improvement of Higher Education Personnel, made available by the Ministry of Education and the Brazilian Institute of Science and Technology. The search was also carried out in international databases for theses and dissertations: ProQuest Dissertations & Theses Database.
Table 1.
Electronic database and search strategy.
| Medline via PubMed | |
|---|---|
| #1 | “dentin sensitivity” [MeSH] |
| #2 | “dentin sensitivity” OR “dentine sensitivity” [Title/Abstract] |
| #3 | “dentin hypersensitivity” OR “dentin* hypersensitivity” [Title/Abstract] |
| #4 | “sensitivity” OR “Hypersensitivity” OR “desensitizing” [Title/Abstract] |
| #5 | #1 or #2 or #3 or #4 |
| #6 | bioglass OR bio-glass OR “bioactive glass” OR 45S5 [Title/Abstract] |
| #7 | #5 AND #6 |
| #8 | “randomized controlled trials” OR “random allocation” OR “clinical trial” |
| #9 | #7 AND #8 |
| CENTRAL | |
| #1 | MESH descriptor: [Dentin Sensitivity] |
| #2 | “dentin sensitivity” OR “dentin hypersensitivity” |
| #3 | “sensitivity” OR “hypersensitivity” OR “desensitizing” |
| #4 | #1 or #2 or #3 |
| #5 | “bioglass” OR bio-glass OR “bioactive glass” OR “45S5” OR “Novamin” |
| #6 | “calcium sodium phosphosilicate” |
| #7 | #5 OR #6 |
| #8 | #4 AND #7 |
| #9 | randomized controlled trials OR random allocation OR clinical trial |
| #10 | #8 AND #9 |
| Lilacs | |
| (tw:(dentin sensitivity)) AND (tw:(clinical trial)) AND (tw:(dentin desensitizing agents)) AND (tw:(glass)) | |
| Web of Science | |
| #1 | Topic: (dentin sensitivity) OR Topic: (dentin sensitivity) |
| #2 | Topic: (dentin hypersensitivity) OR Topic: (dentin hypersensitivity) |
| #3 | Topic: (sensitivity) OR Topic: (hypersensitivity) OR Topic: (densensitive) |
| #4 | #1 or #2 or #3 |
| #5 | Topic: (bioglass) OR Topic: (bio-glass) OR Topic: (bioactive glass) OR Topic: (45S5) OR Topic: (Novamin) OR Topic: (calcium sodium phosphosilicate) |
| #6 | #4 AND #5 |
| #7 | randomized controlled trials OR random allocation OR clinical trial |
| #8 | #6 AND #7 |
| ClinicalTrials.gov | |
| #1 | “dentin sensitivity” OR “dentin hypersensitivity” |
| #2 | sensitivity OR hypersensitivity OR desensitive |
| #3 | #1 OR #2 |
| #4 | bioglass OR bio-glass OR “bioactive glass” OR 45S5 OR Novamin OR “calcium sodium phosphosilicate” |
| #5 | #3 AND #4 |
The unfinished, unpublished, or ongoing studies were found at: Clinical Trials (www.clinicaltrials.gov), the National Research Register, OpenGrey, the World Health Organization's International Clinical Trials Registry Platform, and at the Brazilian Registry of Clinical Trials (www.ensaiosclinicos.gov.br).
The search strategy has been adapted for each database and assessed by two reviewers (SAAF, NMAO) to identify the eligible studies.
2.5. Selection of studies
The articles were screened by two independent reviewers (SAAF, JB) according to the inclusion and exclusion criteria. Titles and abstracts were initially evaluated and the studies appearing to meet the inclusion criteria, the full reports were obtained and independently assessed. Disagreement was checked by third reviewer (JLG). During the selection and evaluation of the quality of the studies, any differences between the reviewers were discussed and, if necessary, a fourth reviewer (SFCS) was consulted. Reviewers were calibrated through a pilot study with a sample of articles to ensure that the criteria were consistent with the research question.
2.6. Data extraction
Data were extracted from each study: authors and year of publication, treatment of choice, number, and ages of participants, the interventions and control groups, follow-up period, the assessment methods and main results (Table 2).
Table 2.
Extraction of data from studies.
| Authors, year | Sample (participants) | Age (years) | Interventions | Controls | Supervision time | Method of assessment | Results |
|---|---|---|---|---|---|---|---|
| Acharya et al., 2013 | 20 | 18–65 | Dentifrice containing CSPS | Dentifrice containing potassium nitrate. | Baseline, 2, 4 and 8 weeks | EVA | CSPS showcased a higher reduction in DH in comparison to potassium nitrate. |
| Bansal and Mahajan, 2017 | 45 | 20–50 | Dentifrice containing 5% NovaMin | Dentifrice 8% (Arginine) Herbal dentifrice | Baseline, 2 and 4 weeks | EVA | Toothpaste may be an effective and inexpensive option in the treatment of DH. |
| Bevilacqua et al., 2016 | 30 | 18–60 | Biosilicate | Fluorine gel and nanostructured desensitizer | Baseline; 1, 2 and 12 weeks | EVA | Regardless of the assessment period, there was no statistical difference between the treatments in pain reduction. Both were effective in reducing DH. |
| Du et al., 2008 | 71 | 21–56 | Dentifrice containing 5% NovaMin | Dentifrice containing SrCl2 | Baseline, 2 and 6 weeks | EVA | NovaMin® more effective in reducing DH compared to control. |
| Gopinath et al., 2015 | 36 | 18–60 | Dentifrice containing CSPS | Nano-HAP dentifrice | Baseline and 4 weeks | EVA | Both groups reduced sensitivity. |
| Hall et al., 2017 | 135 | 18–60 | Dentifrice containing 5% CSPS | Colgate Pro Alivium (8% Arginine) Colgate Triple Protection | Baseline, 1, 2, 4, 6 and 11 weeks | EVA | Dentrifices containing 5% CSPS was as effective as positive control. |
| Narongdej et al., 2010 | 60 | 26–70 | Novamin power in dentifrice containing NovaMin | Placebo powder in dentifrice. Placebo powder with dental cream containing NovaMin® | Baseline, 1, 2 and 4 weeks | EVA | The use of NovaMin® powder and toothpaste containing NovaMin is more effective than the other groups. |
| Neuhaus et al., 2013 | 151 | 18–70 | Toothpaste with 15% NovaMin with 2.7% sodium fluoride; toothpaste with 15% NovaMin without fluoride. | Paste without NovaMin® and without fluorine | Baseline and 4 weeks | Yeaple Probe | The CSPS was able to reduce the DH and this effect was independent of the presence of fluorine. |
| Pradeep and Sharma, 2010 | 110 | 20–60 | Dentifrice containing CSPS | Placebo dentifrice containing NaNO3 and KNO3 | Baseline, 2 and 6 weeks | EVA | The CSPS group was significantly better at reducing DH. |
| Rajesh et al., 2012 | 30 | 18–65 | Dentifrice containing 5% NovaMin | Placebo | Baseline, 2, 6 and 8 weeks | EVA | Novamin® significantly reduced DH when compared to a placebo dentifrice. |
| Salian et al., 2010 | 30 | 20–50 | Dentifrice containing 5% NovaMin Dentifrice with 5% potassium nitrate | Non-desensitizing dentifrice | Baseline, 2 and 4 weeks | EVA | The dentrifice containing 5% NovaMin provides rapid and significantly greater relief from DH compared to the other groups. |
| Samuel et al., 2015 | 56 | 18–65 | Dentifrice containing 5% NovaMin | ProArgin™ (8% arginine) Gluma® | Baseline, 2 and 4 weeks | EVA | The three groups showed significant reductions in DH. |
| Sharma et al., 2010 | 120 | 20–50 | Dentrifice containing 7,5% calcium phosphate sodium (NovaMin) | Dentifrice with 5% potassium nitrate; dentifrice with 0,4% tin fluoride | Baseline, 2, 4 and 12 weeks | EVA | NovaMin® provided significant improvements in the early stages compared to the other groups. |
| Shashirekha and Jena, 2015 | 45 | 18–50 | 5% NovaMin | 8% arginine, 15% n-hp | Baseline, 1 and 4 weeks | EVA | Toothpaste containing 15% n-HA was more effective in reducing DH followed by 8% arginine and 5% novamin toothpastes. |
| Tirapelli et al., 2011 | 160 | 18–70 | 1% biosilicate gel Biosilicate® in distilled water at 10% | Sensi Kill® Dentrifice Sensodyne® | Baseline and 4 weeks | EVA | Biosilicate, gel or mixed with distilled water is efficient in the treatment of DH. |
2.7. Assessment of the quality of studies
Based on the design of the studies, their quality was assessed following the recommendation by Cochrane Reviewers Handbook 5.1.0 (Higgins and Green, 2011). There were adopted as the main aspects: random allocation, blinded to the patient, blinded to the examiners, and reported loss to follow up the treatment. Studies were defined as high risk of bias if at least two criteria were judged, and the risk was applied for the whole study. The results were reported using the SPSS (Statistical Package for the Social Sciences, version 13.0).
3. Results
3.1. Selection of studies
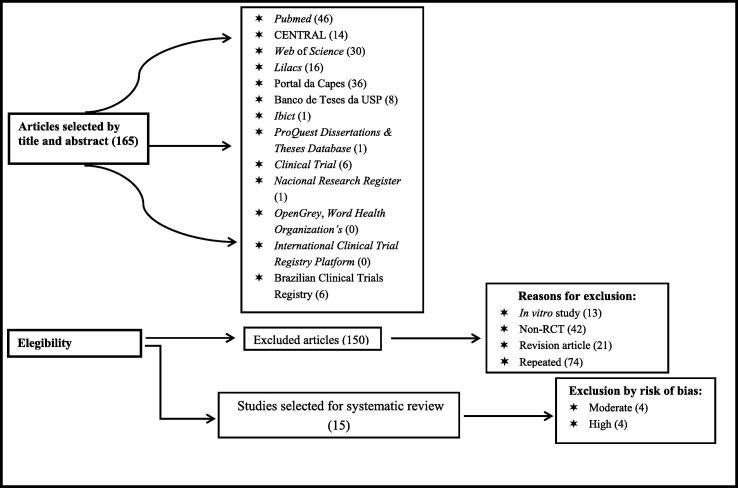
The initial search identified 165 papers. After reading the titles and abstracts, articles that were duplicated (74 records) or unrelated to the systematic review (76 records) were excluded. Fifteen articles were included in the review. The flow chart of the screening and selection process is shown in Fig. 1.
Fig. 1.
Flow chart of study selection.
3.2. Risk of bias
The assessment of the risk of bias (Table 3) revealed that seven studies were at low risk (Pradeep and Sharma, 2010, Salian et al., 2010, Tirapelli et al., 2011, Neuhaus et al., 2013, Gopinath et al., 2015, Shashirekha and Jena, 2015, Hall et al., 2017) and four showed a high risk (Rajesh et al., 2012, Acharya et al., 2013, Bevilacqua et al., 2016, Bansal and Mahajan, 2017). Other studies had moderate risk (Du et al., 2008, Sharma et al., 2010, Samuel et al., 2015, Narongdej et al., 2010).
Table 3.
Assessment of studies with risk of bias.
| Author | Random sequence generation? | Allocation concealment? | Blindness of participants and professionals? | Blindness of outcome assessors? | Incomplete outcomes? | Report of a selective outcome? | General evaluation of bias |
|---|---|---|---|---|---|---|---|
| [1] Acharya et al., 2013 |  |
 |
 |
 |
 |
 |
High |
| [2] Bansal and Mahajan, 2017 |  |
 |
 |
 |
 |
 |
High |
| [3] Bevilacqua et al., 2016 |  |
 |
 |
 |
 |
 |
High |
| [4] Du et al., 2008 |  |
 |
 |
 |
 |
 |
Moderate |
| [5] Gopinath et al., 2015 |  |
 |
 |
 |
 |
 |
Low |
| [6] Hall et al., 2017 |  |
 |
 |
 |
 |
 |
Low |
| [7] Narongdej et al., 2010 |  |
 |
 |
 |
 |
 |
Moderate |
| [8] Neuhaus et al., 2013 |  |
 |
 |
 |
 |
 |
Low |
| [9] Pradeep and Sharma, 2010 |  |
 |
 |
 |
 |
 |
Low |
| [10] Rajesh et al., 2012 |  |
 |
 |
 |
 |
 |
High |
| [11] Salian et al., 2010 |  |
 |
 |
 |
 |
 |
Low |
| [12] Samuel et al., 2015 |  |
 |
 |
 |
 |
 |
Moderate |
| [13] Sharma et al., 2010 |  |
 |
 |
 |
 |
 |
Moderate |
| [14] Shashirekha and Jena, 2015 |  |
 |
 |
 |
 |
 |
Low |
| [15] Tirapelli et al., 2011 |  |
 |
 |
 |
 |
 |
Low |
Legend: GREEN – low. YELLOW – moderate. RED: - high.
3.3. Treatment modalities
All clinical trials compared bioactive glass to a control and/or placebo group and used EVA to assess pain symptons. A study has used Yeaple Probe for assessment of DH (Neuhaus et al., 2013).
Differences were found in the concentrations of the bioactive materials: Biosilicate from 1 to 10% (Tirapelli et al., 2011, Bevilacqua et al., 2016) and Novamin (45S5/Bioglass) from 5 to 15% (Du et al., 2008, Pradeep and Sharma, 2010, Salian et al., 2010, Sharma et al., 2010, Narongdej et al., 2010, Rajesh et al., 2012, Acharya et al., 2013, Neuhaus et al., 2013, Gopinath et al., 2015, Samuel et al., 2015, Shashirekha and Jena, 2015, Bansal and Mahajan, 2017, Hall et al., 2017). The 45S5 in low concentrations (2.5–7.5%) seems to be effective in the treatment of DH either at-home or in-office (Du et al., 2008, Salian et al., 2010, Sharma et al., 2010, Rajesh et al., 2012, Neuhaus et al., 2013, Samuel et al., 2015, Shashirekha and Jena, 2015, Bansal and Mahajan, 2017).
Concerning product containing biosilicate, there was a slight reduction in pain symptoms when compared to commercially avaliable products (Tirapelli et al., 2011, Bevilacqua et al., 2016). A toothpaste containing bioglass 45S5 (5% NovaMin) had better results when compared to the control groups (placebos and/or dentrifices containing potassium nitrate, tin fluoride, gel fluoride, 8% arginine or strontium chloride) in all variations of supervision time (Du et al., 2008, Pradeep and Sharma, 2010, Salian et al., 2010, Sharma et al., 2010, Narongdej et al., 2010, Rajesh et al., 2012, Neuhaus et al., 2013, Acharya et al., 2013, Gopinath et al., 2015). However, a study has shown that dentrifice containing 5% Novamin was less effective in reducing DH symptoms compared to dentifrices containing 15% nano-hydroxyapatite (Shashirekha and Jena, 2015).
4. Discussion
There was evidence that the treatment of DH with bioactive materials was effective. The assessment of pain was subjective and the patients' responses to the various stimuli have differed (Pradeep and Sharma, 2010, Salian et al., 2010, Tirapelli et al., 2011, Neuhaus et al., 2013, Gopinath et al., 2015, Shashirekha and Jena, 2015, Hall et al., 2017, Rajesh et al., 2012, Acharya et al., 2013, Bevilacqua et al., 2016, Bansal and Mahajan, 2017, Du et al., 2008, Sharma et al., 2010, Samuel et al., 2015, Narongdej et al., 2010). To avoid interference or bias, it was required recovery time from the odontoblastic processes, but studies had no standardization in the follow up periodranged from 1 week (Narongdej et al., 2010, Bevilacqua et al., 2016) to 24 weeks (Tirapelli et al., 2011).
The lack of standardization makes it difficult for the professional the clinical decision for the best treatment and follow up period. It is essential that the RCT dealing with DH be carried out following recommendations (Consort and Canadian Advisory Board on Dentin Hypersensitivity 2003).
Another challenge was the fact that there were different scales for the assessment of pain. In several studies, the 10 cm VAS was the most commonly used instrument for measuring the magnitude of painful response (Du et al., 2008, Pradeep and Sharma, 2010, Salian et al., 2010, Sharma et al., 2010, Narongdej et al., 2010, Tirapelli et al., 2011, Rajesh et al., 2012, Acharya et al., 2013, Shashirekha and Jena, 2015, Gopinath et al., 2015, Samuel et al., 2015, Bevilacqua et al., 2016, Bansal and Mahajan, 2017, Hall et al., 2017)., Another study has used the Yeaple Probe (Neuhaus et al., 2013). It was included even though a different tool was used to assess pain symptoms, due to its low risk of bias.
All studies in this systematic review were RCT comparing bioactive glass to a control and/or placebo group. The following bioactive materials were found in the RCT: an experimental glass called Biosilicate and the Novamin toothpaste, which features the consecrated bioactive glass 45S5 (Bioaglass) developed by Larry Hench (2006). Another relevant variable was the form of application of the bioactive materials as powder, paste, gel or prophylaxis applied by the professional. Differences were also found in the concentrations: Biosilicate from 1 to 10% (Tirapelli et al., 2011, Bevilacqua et al., 2016) and Novamin (45S5/Bioglass) 5 to 15% (Du et. al, 2008; Pradeep and Sharma, 2010, Salian et al., 2010, Sharma et al., 2010, Narongdej et al., 2010, Rajesh et al., 2012, Acharya et al., 2013, Neuhaus et al., 2013, Gopinath et al., 2015, Samuel et al., 2015, Shashirekha and Jena, 2015, Bansal and Mahajan, 2017, Hall et al., 2017).
Findings on the use of Biosilicate are controversial. It has found success in the treatment of DH using gel or mixed with distilled water when compared to dentifrices containing sensitizing agents, namely Sensodyne (potassium nitrate) and Sensi Kill (potassium phosphate/calcium chloride and sodium fluoride) (Tirapelli et al., 2011). Instead, there was no significant difference between the treatment with Biosilicate and fluoride gel and desensitizer Nano P (potassium nitrate and sodium fluoride) regardless of the patient evaluation period (Bevilacqua et al., 2016). There was a slight reduction in pain with no statistically significant results of Biosilicate compared to commercially avalialable products (Sensodyne, Sensi Kill, Pro Argin, Gluma, Colgate Pro Alivium). Thus, it is suggested that the biosilicate toothpastes still need RCT to be able to prove their effectiveness. Novamin (45S5) proved to be higher in reducing DH compared to placebo or the commercially available toothpaste (Bevilacqua et al., 2016).
Toothpaste containing bioglass 45S5 (5% NovaMin) had better results when compared to the control groups (placebos and/or dentrifices containing potassium nitrate, tin fluoride, gel fluoride, 8% arginine or strontium chloride) in all variations of supervision time (Du et al., 2008, Pradeep and Sharma, 2010, Salian et al., 2010, Sharma et al., 2010, Narongdej et al., 2010, Rajesh et al., 2012, Neuhaus et al., 2013, Acharya et al., 2013, Gopinath et al., 2015).
Three studies revealed that NovaMin was as effectively as the control groups (Gopinath et al., 2015), ProArgin (calcium carbonate), Gluma Desensitizer (Samuel et al., 2015) and the dentifrice Sensitive Pro-Alivio (Hall et al., 2017). A study has reported that dentifrices containing 15% nano-hydroxyapatite were more effective in reducing pain, followed by 8% arginine, and dentrifice containing 5% Novamin (Shashirekha and Jena, 2015).
This systematic review has shown some limitations due to the many variables that point to a lack of standardization of the studies. It was found that: 1) even if there was a guide to the implementation of RCT, most of the studies did not contain all the requirements necessary, and 2) toothpastes and desensitizers were applied in different ways which makes it even harder to achieve data standardization as well as an accurate comparison. Therefore, high quality RCT not supported by the industry should be conducted.
NovaMin showed greater reduction of DH when compared to control groups. It has demonstrated long-term effectiveness throughout the variations in supervision times. The 45S5 in low concentrations (2.5–7.5%) seemed to be effective in the treatment of DH both at-home and in-office (Du et al., 2008, Salian et al., 2010, Sharma et al., 2010, Rajesh et al., 2012, Neuhaus et al., 2013, Samuel et al., 2015, Shashirekha and Jena, 2015, Bansal and Mahajan, 2017). The main aspect of this study was based on seeking evidence about the real effect of toothpaste containing bioactive glass in RCT.
5. Conclusions
The findings appear to provide clinical success in managing DH with concentrations from 2.5% to 7.5% of toothpaste containing 45S5.
Ethical Statement
For this type of publication (Systematic Review) does not apply.
Funding
The authors thank the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico [CNPq – 426145/2018-6], Fundação de Amparo a Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão [FAPEMA – Pronem 01628/14] and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) Finance Code 001.
CRediT authorship contribution statement
Samantha Ariadne Alves de Freitas: Conceptualization, Methodology Data curation, Formal analysis. Neurinéia Margarida Alves de Oliveira: Conceptualization, Methodology. Juliana Larocca de Geus: Data curation, Writing - original draft. Soraia de Fátima Carvalho Souza: Data curation, Writing - original draft. Adriana de Fátima Vasconcelos Pereira: Visualization, Supervision, Investigation. José Bauer Funding: Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Acharya A.B., Surve S.M., Thakur S.L. A clinical study of the effect of calcium sodium phosphosilicat on dentin hypersensitivity. J. Clin. Exp. Dent. 2013;5(1):e18–e22. doi: 10.4317/jced.50955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D., Mahajan M. Comparative evaluation of effectiveness of three desensitizing toothpastes for relief in the dentinal hypersensitivity. Contemp. Clin. Dent. 2017;8(2):195–199. doi: 10.4103/ccd.ccd_135_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A.S., Takahashi H., Otsuki M., Yamashita K., Tagami J. CO2 laser improves 45S5 bioglass interaction with dentin. J. Dent. Res. 2011;90(2):246–250. doi: 10.1177/0022034510387793. [DOI] [PubMed] [Google Scholar]
- Bevilacqua F.M., Catelan A., Araújo G., Coury C.H., Cézar J.E.C. Efficacy of a bioactive material and nanostructured desensitizing on dentin hypersensitivity treatment. Rev. Odontol. UNESP. 2016;45(3):127–131. [Google Scholar]
- Brannstrom M., Linden L.A., Johnson G. Movement of dentinal and pulpal fluid caused by clinical procedures. J. Dent. Res. 1968;47(5):679–682. doi: 10.1177/00220345680470050201. [DOI] [PubMed] [Google Scholar]
- Canadian Advisory Board on Dentin Hypersensitivity Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J. Can. Dent. Assoc. 2003;69(4):221–226. [PubMed] [Google Scholar]
- Cartwright R.B. Dentinal hypersensitivity: a narrative review. Community Dent. Health. 2014;31(1):15–20. [PubMed] [Google Scholar]
- Curtis A.R., West N.X., Su B. Synthesis osnanobioglass and formation os apatite rods to occlude exposed dentine tubules and eliminate hypersensitivity. Acta Biomater. 2010;6(9):3740–3746. doi: 10.1016/j.actbio.2010.02.045. [DOI] [PubMed] [Google Scholar]
- David C. NovaMin® and Tooth Sensitivity-An Overview. J. Clin. Dent. 2010;21(3):61–65. [PubMed] [Google Scholar]
- Du M.O., Bian Z., Jiang H., Greenspan D.C., Burwell A.K., Zhong J.P., et al. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (Novamin®) for the treatment of dentin hypersensitivity. Am. J. Dent. 2008;21(4):210–214. [PubMed] [Google Scholar]
- Femiano F., Femiano R., Lanza A., Lanza M., Perillo L. Effectiveness on oral pain of 808-nm diode laser used prior to composite restoration for symptomatic non-carious cervical lesions unresponsive to desensitizing agents. Lasers Med. Sci. 2017;32(1):67–71. doi: 10.1007/s10103-016-2087-4. [DOI] [PubMed] [Google Scholar]
- Fleming P.S., Koletsi D., Polychronopoulou A., Eliades T., Pandis N. Are clustering effects accounted for in statistical analysis in leading dental speciality journals? J. Dent. 2013;41(3):265–270. doi: 10.1016/j.jdent.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Gillam D.G., Newman H.N., Davies E.H., Bulman J.S. Clinical efficacy of a low abrasive dentifrice for the relief of cervical dentinal hypersensitivity. J. Clin. Periodontol. 1992;19:197–201. doi: 10.1111/j.1600-051x.1992.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Gillam D.G., Orchardson R. Advances in the treatment of root dentin sensitivity: Mechanisms and treatment principles. Endod. Topics. 2006;13:13–33. [Google Scholar]
- Gopinath N.M., John J., Nagappan N., Prabhu S., Kumar E.S. Evaluation of dentifrice containing nano-hydroxyapatite for dentinal hypersensitivity: A randomized controlled trial. J. Int. Oral Health. 2015;7(8):118–122. [PMC free article] [PubMed] [Google Scholar]
- Hall C., Mason S., Cooke J. Exploratory randomised controlled clinical study to evaluate the comparative efficacy of two occluding toothpastes - a 5% calcium sodium phosphosilicate toothpaste and an 8% arginine/calcium carbonate toothpaste - for the longer-term relief of dentine hypersensitivity. J. Dent. 2017;60(5):36–43. doi: 10.1016/j.jdent.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Hannigan A., Lynch C.D. Statistical methodology in oral and dental research: pitfalls and recommendations. J. Dent. 2013;41(5):385–392. doi: 10.1016/j.jdent.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Hench L.L. The story of bioglass. J. Mater. Sci. Mater. Med. 2006;17(11):967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- Higgins, J.P.T., Green, S., editors., 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. London, UK.
- Holland G.R., Narhi M.N., Addy M., Gangarosa L., Orchardson R. Guidelines for design and conduct of clinical trials on dentin hypersensibility. J. Clin. Periodontol. 1997;24(11):808–813. doi: 10.1111/j.1600-051x.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Kim D.K., Hong S.J. Assessment of errors and misused statistics in dental research. Int. Dent. J. 2011;61(3):163–167. doi: 10.1111/j.1875-595X.2011.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga D., Matera F., Pizzo G. Evaluating the statistical methodology of randomized trials on dentin hypersensitivity management. J. Oral Sci. 2017;59(4):461–468. doi: 10.2334/josnusd.16-0663. [DOI] [PubMed] [Google Scholar]
- Neuhaus K.W., Milleman J.L., Milleman K.R., Mongiello K.A., Simonton T.C., Clark C.E., et al. Effectiveness of a calcium sodium phosphosilicate containing prophylaxis paste in reducing dentine hypersensitivity immediately and 4 weeks after a single application: a double-blind randomized controlled trial. J. Clin. Periodontol. 2013;40(4):349–357. doi: 10.1111/jcpe.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandis N., Polychronopoulou A., Madianos P., Makou M., Eliades T. Reporting of research quality characteristics of studies published in 6 major clinical dental specialty journals. J. Evid. Based Dent. Pract. 2011;11(2):75–83. doi: 10.1016/j.jebdp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Sharma A. Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: A randomized clinical trial. J. Periodontol. 2010;81(8):1167–1173. doi: 10.1902/jop.2010.100056. [DOI] [PubMed] [Google Scholar]
- Rajesh K.S., Hedge S., Arun K.M.S., Shetty D.G. Evaluation of the efficacy of a 5% calcium sodium phosphosilicate (Novamin®) containing dentifrice for the relief of dentinal hypersensitivity: A clinical study. Indian J. Dent. Res. 2012;23(3):363–367. doi: 10.4103/0970-9290.102228. [DOI] [PubMed] [Google Scholar]
- Salian S., Thakur S., Kulkarni S., LaTorre G. A randomized controlled clinical study evaluating the efficacy of two desensitizing dentifrices. J. Clin. Dent. 2010;21(3):82–87. [PubMed] [Google Scholar]
- Samuel, S.R., Khatri, S.G., Acharya, S., Patil, S.T., 2015. Evaluation of instant desensitization after a single topical application over 30 days: a randomized trial. Aust. Dent. J. 60(3):336-342. [DOI] [PubMed]
- Narongdej T, Sakoolnamarka R, Boonroung T., 2010. The effectiveness of a calcium sodium phosphosilicate desensitizer in reducing cervical dentin hypersensitivity: a pilot study. J. Am. Dent. Assoc. 141(8): 995–999. [DOI] [PubMed]
- Sauro S., Thompson I., Watson T.F. Effects of common dental materials used in preventive or operative dentistry on dentin permeability and remineralization. Oper. Dent. 2011;36(2):222–230. doi: 10.2341/10-225-L. [DOI] [PubMed] [Google Scholar]
- Schiff T., Dotson M., Cohen S., De Vizio W., McCool J., Volpe A. Efficacy of a dentifrice containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: A twelve-week clinical study. J. Clin. Dent. 1994;5:87–92. [PubMed] [Google Scholar]
- Sharma N., Roy S., Kakar A., Greenspan D.C., Scott R. A clinical study comparing oral formulations containing 7.5% calcium sodium phosphosilicate (NovaMin), 5% potassium nitrate, and 0.4% stannous fluoride for the management of dentin hypersensitivity. J. Clin. Dent. 2010;21(3):88–92. [PubMed] [Google Scholar]
- Shashirekha G., Jena A. Comparison of efficacy of three different desensitizing agents for in-office relief of dentin hypersensitivity: A 4-week clinical study. J. Conserv. Dent. 2015;18(5):389–393. doi: 10.4103/0972-0707.164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Magdalena A., Martínez-Alonso M., Coronado C.A., Junquera L.M., Cobo J., Vega J.A. Molecular basis of dental sensitivity: The odontoblasts are multisensory cells and express multifunctional ion channels. Ann Anat. 2017;215(24):20–29. doi: 10.1016/j.aanat.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Tirapelli C., Panzeri H., Lara E.H., Soares R.G., Peitl O., Zanotto E.D. The effect of a novel crystallised bioactive glass Ceramic powder on dentine hypersensitivity: a long-term clinical study. J. Oral Rehabil. 2011;38(4):253–262. doi: 10.1111/j.1365-2842.2010.02157.x. [DOI] [PubMed] [Google Scholar]
- West N.X., Hooper S.M., O'Sullivan D., Hughes N., North M., Macdonald E.L., et al. In situ randomised trial investigating abrasive effects of two desensitising toothpastes on dentine with acidic challenge prior to brushing. J. Dent. 2011;40(1):77–85. doi: 10.1016/j.jdent.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Zanotto, E.D., Ravagnani, C., Peitl, O., Panzeri, H., Lara, E.H., 2004. Process and compositions for preparing particulate, bioactive or resorbable biosilicates for use in the treatment of oral ailments, Patent WO2004 074199, Fundação Universidade Federal de São Carlos; Universidade de São Paulo.
- Zhong J.P., Feng J.W., Greenspan D.C. A Microstructural Examination of Apatite Induced By Bioglass In-Vitro. J Mater Sci: Mater. In Med. 2002;13(3):321–326. doi: 10.1023/a:1014075320987. [DOI] [PubMed] [Google Scholar]
- Zhu, M., Li, J., Chen, B., Mei, L., Yao, L., et al., 2015. The Effect of Calcium Sodium Phosphosilicate on Dentin Hypersensitivity: A Systematic Review and Meta-Analysis. 10(11): e0140176. [DOI] [PMC free article] [PubMed]