Abstract
Aim
The aim of this study was to evaluate the caries intensity and Streptococcus mutans (SM) counts in patients with Turner syndrome.
Materials and methods
Nineteen patients aged 20–40 years were clinically and cytogenetically diagnosed with Turner syndrome (45, X). The karyotype was determined by chromosome analysis of peripheral blood lymphocytes. The control group comprised 47 healthy women aged 21–40 years. Both groups included non-smokers with no specific diet, such as a vegetarian or vegan diet, who were generally healthy with good oral hygiene and periodontal condition. Patients treated with antibiotics or steroid preparations in the past 6 months or with diseases or conditions that might affect the oral mucosal environment, such as disorders of salivary secretion and diabetes, were excluded from the study. Decayed, missing, and filled teeth (DMFT) scores and SM counts in saliva were determined.
Results
No colony growth of SM was noticed in 53% of patients with Turner syndrome and 4.2% of controls (p < 0.001). Colony counts of SM ≥ 105 in saliva were observed in none of the patients with Turner syndrome but in 66% of controls (p < 0.001). The mean DMFT score was 1.63 ± 2.52 in patients with Turner syndrome and 14.49 ± 6.88 in controls. Statistically significant differences between the two groups were observed (p < 0.05).
Conclusion
Patients with genetic disorders may have different severities of caries and SM counts in saliva compared to those without genetic disorders. Further studies on saliva properties and genes located on the X chromosome could contribute to determining the effect of the X chromosome on the pathological processes in the oral cavity.
Keywords: Turner syndrome, Streptococcus Mutans, Caries
1. Introduction
Turner syndrome (TS) is a complicated genetic disorder characterised by X-chromosome aberrations. Its clinical features include somatic abnormalities, such as infantile external genitalia, short stature, cubitus valgus webbed neck (Temtamy et al., 1992, Robinson and de la Chapelle, 1996, Lopez et al., 2002), and oral abnormalities, such as microdontia (Kusiak et al., 2000, Szilagyi et al., 2000a, Szilagyi et al., 2000b, Zillberman et al., 2000, Maier et al., 2019), malocclusion (Cazzolla et al., 2018, Szilagyi et al., 2000a, Szilagyi et al., 2000b), enamel hypoplasia (Lopez et al., 2002, Kusiak et al., 2008) and irregularities in the root morphology of the mandibular teeth (Varrela et al., 1990, Lopez et al., 2002, Kusiak et al., 2005). However, the caries prevalence has been reported to be reduced in patients with TS (Takala et al., 1985, Szilagyi et al., 2000a, Szilagyi et al., 2000b). Alteration in results of saliva tests reported for patients with genetic disorders such as Down’s syndrome (Stabholz et al., 1991, Yarat et al., 1999, Chaushu et al., 2002, Siqueira et al., 2005), Papilon–Lefevre syndrome (Lundgren et al., 1996), and epidermolysis bullosa (Harris et al., 2001), warrants research on saliva properties of patients with TS.
The aim of the present study was to evaluate the caries intensity and Streptococcus mutans (SM) counts of patients with TS.
2. Materials and methods
2.1. Patients’ population
Nineteen patients aged 20–40 (26.4 ± 4.4) years with TS were studied. Both the diagnosis of TS (45,X) and karyotype evaluation were performed at the Department of Biology and Genetics of the Medical University of Gdańsk. The karyotype was determined by chromosome analysis of peripheral blood lymphocytes. The control group comprised 47 healthy women aged 21–40 (29 ± 3.2) years, who first reported to the Department of Conservative Dentistry, Medical University of Gdańsk for follow-up examinations and agreed to the examination and met the inclusion criteria for the control group. Both groups included non-smokers with nonspecific diet, such as a vegetarian or vegan diet, who were in generally healthy with good oral hygiene and healthy periodontal tissues. Patients treated with antibiotics or steroid preparations in the past 6 months or with diseases that might affect the oral mucosal environment, such as disorders of salivary secretion and diabetes, were excluded from the study.
2.2. Saliva collection
Mixed stimulated saliva was collected from each study participant. The caries intensity was studied for its prevalence evaluation. Decayed, missing, filled, teeth (DMFT) scores were calculated for all patients.
Saliva was collected into sterile silicone (Corning test tubes) in the morning 2 h after the last meal. To mechanically stimulate saliva secretion, subjects were directed to chew a paraffin cube for 1 min. Saliva secreted within 1 min of paraffin chewing was expectorated and that secreted during the next 5 min was collected into calibrated Corning test tubes, as previously described (Kusiak et al., 2011). Collected saliva was used to calculate SM counts in 1 mL of stimulated saliva.
Due to the closest reflection of teeth surfaces and tongue's microbiological status, stimulated saliva was used for microbiological evaluation. The CRT® bacterial test was used (Ivoclar–Vicadent, Liechtenstein). SM counts were read in marked surface Colony Forming Units/mL of saliva and compared to the model pattern according to the manufacturer’s guidelines. SM counts < 105 and ≥105 in 1 mL of stimulated saliva were classified as low and high, respectively.
2.3. Statistical analysis
Statistical analyses were performed using the statistical suite STATISTICA (data analysis software system), version 12.0 (StatSoft. Inc., Tulsa, OK, USA). The Yates-corrected chi-square nonparametric test for unrelated values was used in the analyses of microbiological results in the TS and control groups. The caries intensity was analysed using the Kruskal–Wallis test. In all calculations, the statistical significance level was set at p < 0.05.
3. Results
Table 1 illustrates the SM counts in the stimulated saliva of patients in the TS and control groups.
Table 1.
Quantity of Streptococcus mutans colonies in stimulated saliva of patients in the Turner syndrome and control groups.
| Total number of patients | Number of patients with a particular quantity of SM colonies in 1 mL of stimulated saliva |
||||||
|---|---|---|---|---|---|---|---|
| Karyotype | N | No growth |
SM < 105 CFU/mL |
SM ≥ 105 CFU/mL |
|||
| N | % | n | % | N | % | ||
| Turner syndrome 45, X | 19 | 10 | 53a | 9 | 47 | 0 | 0c |
| Control group | 47 | 2 | 4,2b | 14 | 29,8 | 31 | 66d |
p < 0.001 for a vs. b and c vs. d; SM: Streptococcus mutans; CFU: Colony Forming Units.
No colony growth was noticed in ten women with TS(53%) and two controls(4.2%). A colony count of SM < 105in 1 mL of stimulated saliva was observed in nine women with TS(47%) and in 14 women in the control group (29.8%). Colony counts of SM ≥ 105 in saliva were not observed in any patient with TS. However, they were present in 31 women in the control group (66%). Statistically significant differences could be identified between the TS and control groups (p < 0.05).
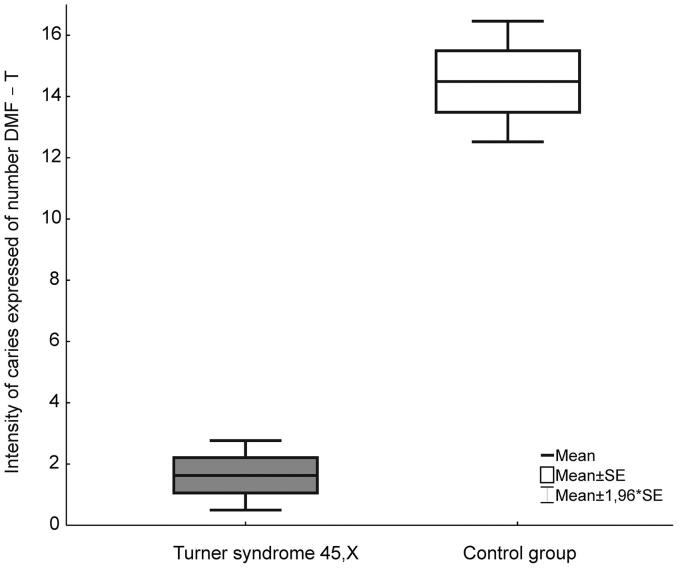
Table 2 and Fig. 1 illustrates the caries intensity expressed by the DMFT score. The mean DMFT score was 1.63 ± 2.52 in the TS group and 14.49 ± 6.88 in the control group. Statistically significant differences between the TS and control groups were observed (p < 0.05).
Table 2.
Caries intensity expressed as DMFT value in Turner syndrome and the control group.
| Karyotype | DMFT - value |
||
|---|---|---|---|
| ± SD | Range | Median | |
| Turner syndrome 45,X (N = 19) | 1.63 ± 2.52a | 0–8 | 1 |
| Control group (N = 47) |
14.49 ± 6.88b | 5–41 | 13 |
p < 0.05 for a vs. b;: mean; SD: standard deviation; N:total number of patients; DMFT: decayed, missing, filled teeth.
Fig. 1.
Intensity of caries expressed of number DMF – T in Turner syndrome and the control group.
4. Discussion
Dental caries has a complex aetiology that has not been fully elucidated to date. According to the current state of knowledge, caries is conditioned by the presence of dental plaque and cariogenic bacteria occurring in it, mainly SM, which breaks down sugars into acids and creates large amounts of extracellular and insoluble glucans, components of the plaque matrix. Other elements are the supply of carbohydrates, which are associated with oral hygiene and regular dental plaque removal, and the host's resistance (susceptibility of the enamel surface and properties of saliva). Other researchers have indicated a lower incidence of caries, which may be related to the dammonths or with diseases or conditions that mightage or absence of the X chromosome in TS (Takala et al., 1985, Szilagyi et al., 2000a, Szilagyi et al., 2000b). Takala et al. examined caries in 50 women with TS and found its low prevalence compared to the control group, particularly in relation to the premolars and molars.
There are no studies in the existing literature on the influence of saliva on oral cavity pathologies in patients with TS. Nevertheless, some authors have indicated a genetic aspect to some saliva irregularities noticed in other syndromes, such as Down syndrome (Barr-Agholme et al., 1998, Chaushu et al., 2002, Cogulu et al., 2006), Papillon-Lefévre syndrome (Lundgren et al., 1996) and epidermolysis bullosa (Harris et al., 2001).
The importance of bacterial factors in the course of caries has been pointed out by many authors (Grähn et al., 1988, Klock et al., 1990, Russell et al., 1990, Stabholz et al., 1991, Kirstilä et al., 1998, Gãbris et al., 1999, Nishikawara et al., 2006). In our study, a significant decrease in SM counts was observed in 1 mL of saliva of patients with TS compared to controls. The results obtained in the study indicated that patients with TS, whose saliva did not show increased SM counts, had a significantly lower caries intensity compared to the controls. When high colony counts of SM ≥ 105 was present, caries intensification was also significantly lower in patients with TS than in controls.
As for previous studies, Stabholz et al. (1991) reported a similar correlation in the studied population of children with Down syndrome. Decreased counts of SM in the saliva and lower caries intensity were observed in children with Down syndrome. The study group was compared with two age-matched control groups living in the same institution: a group of healthy children and another mentally retarded children without Down syndrome. The caries experience showed significantly lower mean scores for the Down syndrome group in comparison with both control groups, for whom maintaining good oral hygiene manually was similarly difficult. Among children with Down syndrome, 84% were free of caries.
Szilagyi et al., 2000a, Szilagyi et al., 2000b also observed reduced salivary SM counts and lower caries intensification in 29 patients with TS. A statistically significant correlation was found between mean DMFT scores and salivary microbiological counts. In our studies, similar results were obtained. Other properties of saliva, such as the buffering capacity, which may have an impact on caries intensity, were also altered in a study by Kusiak et al. (Kusiak et al., 2010), which revealed a significantly higher salivary buffering capacity in patients with TS compared to the control group. In the present study, the severity of caries was compared in the group of women with TS and in the control group in such a way that there were no additional disturbing factors, using strict exclusion criteria. Both groups of women were characterised by good oral hygiene and had no periodontitis, saliva secretion disorders, smoking habits or current antibiotic medication. The saliva of patients with TS had significantly lower SM counts than that of control subjects. In addition, DMFT scores were lower in women with TS than in control subjects.
The limitation of our study was the small sample size, which should be increased in the future to better assess this phenomenon.
5. Conclusion
Patients with genetic disorders may present with a different severity of caries and concentration of SM in the saliva compared to those without genetic disorders. Further studies on saliva properties and genes located on the X chromosome could contribute to determining the effect of the X chromosome on the pathological processes occurring in the oral cavity.
Acknowledgments
Acknowledgements
Statement of Ethics
The study protocol was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk. Ethical aspects of the study followed the World Medical Association Declaration of Helsinki.
Disclosure Statement
The authors declare no conflict of interest.
Funding Sources
This research received no external funding.
Footnotes
Peer review under responsibility of King Saud University.
References
- Barr-Agholme M., Dahllof M., Modéer T., Engström P.E., Enqström G.N. Periodontal conditions and salivary immunoglobulins in individuals with down syndrome. J. Periodontol. 1998;69:1119–1123. doi: 10.1902/jop.1998.69.10.1119. in this issue. [DOI] [PubMed] [Google Scholar]
- Cazzolla A.P., Lo Muzio L., Di Fede O., Lacarbonara V., Colaprico A., Testa N.F., et al. Ortopedic – orthodontic treatment of the patient with turner syndrome. Review of the literature and case report. Spec Care Dentist. 2018;38(4):239–248. doi: 10.1111/scd.12295. in this issue. [DOI] [PubMed] [Google Scholar]
- Chaushu S., Becker A., Chaushu G., Shapira J. Stimulated parotid salivary flow rate in patients with down syndrome. Spec Care Dentist. 2002;22:41–44. doi: 10.1111/j.1754-4505.2002.tb01208.x. in this issue. [DOI] [PubMed] [Google Scholar]
- Cogulu D., Sabah E., Kutukculer N., Ozkinay F. Evaluation of the relationship between caries indices and salivary secretory IgA, salivary pH, buffering capacity and flow rate in children with Downs’s syndrome. Arch. Oral. Biol. 2006;51:23–28. doi: 10.1016/j.archoralbio.2005.06.001. in this issue. [DOI] [PubMed] [Google Scholar]
- Gãbris K., Nagy G., Madlena M., Dénes Z., Márton S., Keszthelyi G., Bànóczy J. Associations between microbiological and salivary caries activity tests and caries experience in Hungarian adolescents. Caries Res. 1999;33:191–195. doi: 10.1159/000016516. in this issue. [DOI] [PubMed] [Google Scholar]
- Grähn E., Tenovuo J., Lehtonen O.P., Eeropla E., Vilija P. Antimicrobial system of human whole saliva in relation to dental caries, cariogenic bacteria and gingival inflamation in young adults. Acta Odontol. Scand. 1988;46:67–74. doi: 10.3109/00016358809004749. in this issue. [DOI] [PubMed] [Google Scholar]
- Harris J.C., Bryan R.A., Lucas V.S., Roberts G.J. Dental diseases and caries related microflora in children with dystrophic epidermolysis bullosa. Pediatr. Dent. 2001;23:438–443. in this issue. [PubMed] [Google Scholar]
- Kirstilä V., Häkkinen P., Jentsch H., Vilja P., Tenovuo J. Longitudinal analysis of the association of human salivary antimicrobial agents with caries increment and cariogenic micro-organisms: a two-year cohort study. J. Dent. Res. 1998;77:73–80. doi: 10.1177/00220345980770011101. in this issue. [DOI] [PubMed] [Google Scholar]
- Klock B., Svanberg M., Petersson L.G. Dental caries, mutans streptococci, lactobacilli and saliva secretion rate in adult. Commun. Dent. Oral. Epidemiol. 1990;18:249–252. doi: 10.1111/j.1600-0528.1990.tb00069.x. in this issue. [DOI] [PubMed] [Google Scholar]
- Kusiak A., Kochańska B., Limon J., Żółtowska A., Zedler E., Świetlik D., Kowalska- S.J. Buffering capacity and caries prevalence in Turner’s syndrome. Dent. Forum. 2010;38(2):21–25. in this issue. [Google Scholar]
- Kusiak A., Sadlak-Nowicka J., Limon J. Anomaly of morphological construction of permanent teeth in Turner syndrome with various aberration of chromosome X. Czas Stomatol. LIII. 2000:608–614. in this issue. [Google Scholar]
- Kusiak A., Sadlak-Nowicka J., Limon J., Kochańska B. Root morphology of mandibular premolars in 40 patients with Turner syndrome. Int. Endod. J. 2005;38:822–826. doi: 10.1111/j.1365-2591.2005.01023.x. [DOI] [PubMed] [Google Scholar]
- Kusiak A., Sadlak-Nowicka J., Limon J., Kochańska B. The frequency of occurrence in abnormal frenal attachment of lips and enamel defects in Turner syndrome. Oral Dis. 2008;14:158–162. doi: 10.1111/j.1601-0825.2007.01366.x. in this issue. [DOI] [PubMed] [Google Scholar]
- Kusiak A., Kochańska B., Limon J., Ochocińska J. The physico-chemical properties of saliva in Turner’s syndrome. Dent. Forum. 2011;39:19–23. in this issue. [Google Scholar]
- Lopez M.E., Bazán C., Lorca I.A., Chervonagura A. Oral and clinical characteristics of a group of patients with Turner syndrome. Oral. Surg. Oral. MedOral Pathol. Oral Radiol. Endod. 2002;94:196–294. doi: 10.1067/moe.2002.121546. in this issue. [DOI] [PubMed] [Google Scholar]
- Lundgren T., Twetman S., Johansson J., Crossner C.G., Birkhed D. Saliva composition in children and young adults with Papillon-Lefẻvre. J. Clin. Periodontol. 1996;23:1068–1072. doi: 10.1111/j.1600-051X.1996.tb01805.x. in this issue. [DOI] [PubMed] [Google Scholar]
- Maier C., Dumančić J., Brkić H., Kaić Z., Savić Pavičin I., Poje Z., et al. Tooth crown morphology in turner and Klinefelter syndrome individuals from a Croatian sample. Acta Stomatol. Croat. 2019;53(2):106–118. doi: 10.15644/asc53/2/2. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawara F., Katsumura S., Ando A., Tamaki Y., Nakamura Y., Sato K., et al. Correlation of cariogenic bacteria and dental caries in adult. J. Oral Sci. 2006;48:245–251. doi: 10.2334/josnusd.48.245. [DOI] [PubMed] [Google Scholar]
- Robinson A., de la Chapelle A. In: Principles and Practice of Medical Genetics. Rimoin D.L., Connor J.M., Pyeritz R.E., editors. Churchill Livingstone; New York: 1996. Sex chromosome Abnormalities; pp. 973–981. in this issue. [Google Scholar]
- Russell J.I., MacFarlane T.W., Aitchison T.C., Stephen K.W., Burchell C. Caries prevalence and microbiological and salivary caries activity tests in Scottish adolescents. Community Dent Oral Epidemiol. 1990;18:120. doi: 10.1111/j.1600-0528.1990.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Siqueira W.L., Bermejo P.R., Mustacchi Z., Nicolau J. Buffer capacity, pH, and flow rate in saliva of children aged 2–60 month with Down syndrome. Clin. Oral Invest. 2005;9:26–29. doi: 10.1007/s00784-004-0282-3. in this issue. [DOI] [PubMed] [Google Scholar]
- Stabholz A., Mann J., Sela M., Schurr D., Steinberg D., Shapira J. Caries experience, periodontal treatment needs, salivary pH, and Streptoccocus mutans counts in a preadolescent Down syndrome population. Spec Care Dentist. 1991;11:203–208. doi: 10.1111/j.1754-4505.1991.tb01732.x. in this issue. [DOI] [PubMed] [Google Scholar]
- Szilagyi A., Keszthelyi G., Madléna M., Tar K., Nagy G. Correlation between the prevalence of caries and the microbiologic parameters of saliva in patients with Turner syndrome. Fogory Sz. 2000;93:297–304. [PubMed] [Google Scholar]
- Szilagyi, A., Keszthelyi, G., Nagy, G., Madlena, M., 2000. Oral manifestations of patients with Turner syndrome. Oral Surg, Oral Med, Oral Pathol Oral Radiol Endod. 89, 577–584. https://doi.org/10.1067/moe.2000.104475. [DOI] [PubMed]
- Takala I., Alvesalo L., Palin-Palokas T., Paunio K., Suoranta K. Caries prevalence in Turner’s syndrome (45, X Females) J. Dent. Res. 1985;64:126–128. doi: 10.1177/00220345850640020601. in this issue. [DOI] [PubMed] [Google Scholar]
- Temtamy S.A., Ghali I., Salam M.A., Hussein F.H., Ezz E.H.A.A., Salah N. Karyotype/phenotype correlation in females with short stature. Clin. Genet. 1992;41:147–151. doi: 10.1111/j.1399-0004.1992.tb03652.x. in this issue. [DOI] [PubMed] [Google Scholar]
- Varrela J., Alvesalo L., Mayhall J. Taurodontism in 45, X Females. J. Dent Res. 1990;69:494–495. doi: 10.1177/00220345900690021501. in this issue. [DOI] [PubMed] [Google Scholar]
- Yarat A., Akyüz S., Koc L., Erdem H., Emekli N. Salivary sialic acid protein, salivary flow rate, pH, buffering capacity and caries indices in subjects with Down’s syndrome. J. Dent. 1999;27:115–118. doi: 10.1016/s0300-5712(98)00030-x. in this issue. [DOI] [PubMed] [Google Scholar]
- Zillberman U., Smith P., Alvesalo L. Crown components of mandibular molar teeth in 45, X female (Turner syndrome) Arch. Oral. Biol. 2000;45:217–225. doi: 10.1016/s0003-9969(99)00130-2. in this issue. [DOI] [PubMed] [Google Scholar]