Abstract
Objective
Among other regulatory functions, vitamin D has a role in modulating the inflammatory process of periodontal disease. Therefore, this retrospective study aimed to assess the relationship between vitamin D levels and periodontal health in dental patients from the Eastern Province of Saudi Arabia.
Methods
Radiographs and serum vitamin D levels of patients seeking dental treatment were collected. Exclusion criteria were systemic disease, smoking, recent vitamin D supplementation, and previous periodontal surgery. Gender, age, and alveolar crest height (ACH) were recorded. A total of 67 patients were categorized into three groups according to their serum vitamin D level (<10, <20, and > 20 ng/mL) and their bone loss compared.
Results
Differences in means were compared by t-test. ANOVA was used to compare vitamin D groups and the corresponding ACH, as well as the correlation (p < .05). Patients with vitamin D levels > 20 ng/mL demonstrated a mean ACH of 1.6 mm. The mean ACH was 3.1 mm for those with vitamin D levels < 20 ng/mL, and 4.6 mm for vitamin D levels < 10 ng/mL. A weak negative correlation was found between vitamin D and ACH in all groups (r = −0.055, p = .7).
Conclusion
Serum vitamin D level seems to be an important factor that influences oral health, especially the periodontal condition, of both male and female patients.
Keywords: Vitamin D, Vitamin D deficiency, Periodontal disease
1. Introduction
Vitamin D (VitD) is a fat-soluble vitamin, mainly obtained from sunlight and diet (Jagelavičienė et al., 2018). Given climate variations and inadequate exposure to sun, VitD deficiency is a common global health problem (Schwalfenberg, 2011). 25(OH)D is used to assess VitD level, since it is present in the circulation for 20 days, unlike 1,25 dihydroxy VitD, which is present only for four hours.
No consensus exists on the optimal VitD level, but VitD < 20 ng/mL is considered insufficient. Some studies have considered VitD of 30 ng/mL an acceptable essential level for health (Bikle, 2012, Vieth, 2011).
1,25(OH)2D works through the VitD nuclear receptor (VDR), which functions as a transcription factor. It modulates multiple biological processes and functions, including inflammation, cell-mediated immunity, calcium and bone homeostasis, and apoptosis (Hiremath et al., 2013). The VDR/1,25(OH)2D pathway could facilitate antibacterial, antiviral, and anti-inflammatory action. This pathway influences bone remodeling mechanisms by triggering differentiation of osteoclasts and osteoblasts. Consequently, VitD deficiency affects bone density, leading to pathological fracture, osteomalacia, and osteoporosis (Hiremath et al., 2013, Schwalfenberg, 2011).
Recently, the role of VitD in periodontal disease pathogenesis has been established as affecting bone mineral density and bone resorption (Abreu et al., 2016, Anbarcioglu et al., 2019, Hiremath et al., 2013, Laky et al., 2017). Consequently, VitD deficiency has been predicted to influence the initiation and progression of periodontal disease (Laky et al., 2017). Chronic periodontitis is an inflammatory disease caused by bacterial plaque, and its progression can cause bone loss, which ultimately leads to tooth loss.
Approximately 50% of the adult population in the United States (Boggess et al., 2011), and approximately 84% of the adult population of the Kingdom of Saudi Arabia (Alshammari and Wahi, 2019, Guile, 1992) experience periodontal disease.
Previous studies have shown that VitD deficiency increases the risk of periodontitis (Dietrich et al., 2004, Dietrich and Garcia, 2005, Miley et al., 2009). Further, the adjunctive use of VitD has been observed to help in maintaining periodontal health by increasing bone density and decreasing alveolar bone resorption (Alshouibi et al., 2013, Garcia et al., 2011, Hildebolt, 2005). Bashutski et al. (2011) reported that periodontal surgery had better treatment outcomes in patients with adequate VitD than those with VitD deficiency. Previous longitudinal studies have also concluded that VitD deficiency can be considered a risk factor for tooth loss (Jimenez et al., 2014, Zhan et al., 2014). So far, published articles have given conflicting reports about the relationship between VitD deficiency and periodontal disease. Almost 60% of cross-sectional studies have observed a strong association between poor periodontal findings and decreased VitD level (Perić et al., 2018, Pinto et al., 2018). Other studies have not proven this association, and recommended further investigations (Antonoglou et al., 2015, Bonnet et al., 2019, Eshghi et al., 2016, Garcia et al., 2011).
Therefore, this study aimed to assess the relationship between VitD level and periodontal disease in dental patients from the Eastern Province of Saudi Arabia.
2. Materials and methods
2.1. Study sample
Patients seeking treatment in dental clinics of Imam Abdulrahman Bin Faisal University, Saudi Arabia, during a six-month period (September 2019 to February 2020) were involved in this study. Inclusion criteria were no systemic diseases, age 20 to 45 years, non-smokers, no previous VitD supplementation, no missing teeth, and no bony lesions or history of previous periodontal surgery. A total of 67 patients were included in the study (mean age 23 years), of whom 74% (n = 49) were female and 26% (n = 18) were male. Patients’ medical documentation, radiographs, and serum VitD levels were analyzed according to the Declaration of Helsinki recommendations. College of Dentistry Institutional Review Board approval was obtained (IRP 2018024).
2.2. Clinical examination
Gender, age, and alveolar crest height (ACH) of the patients enrolled were recorded. All orthopantomography was taken with a PM 2002 CC Proline (Planmeca OY, Helsinki, Finland). ACH was measured on patients’ digital panoramic radiographs using computer software (MiPACS Dental Enterprise Viewer 3.1.1404, USA). To decrease the impact of panoramic magnification on the results, the magnification ratio was set to 1.2, according to the manufacturer instruction. The distance from the alveolar crest to the cement–enamel junction, both mesially and distally to every tooth, was measured, excluding the canines and third molars. The total of 48 sites were measured, then the mean ACH was calculated from the ACH of all sites. Means and standard deviations of radiographic bone loss, VitD, and age were calculated.
2.3. Assessment of plasma vitamin D
The serum VitD was measured from 10-mL venous blood samples, collected and analyzed in the same laboratory. VitD levels of the patients were categorized into three groups (<10, <20, and > 20 ng/mL) and compared with bone loss. VitD levels in plasma were measured using the LIAISON 25-hydroxyVitDTOTAL chemiluminescence assay (DiaSorin, Ltd., Stillwater, MN, USA).
2.4. Statistical analysis
Differences in means were compared using t-tests. Correlation of VitD level and ACH among the three groups was calculated and the results were compared using ANOVA, with significance set at p < .05.
3. Results
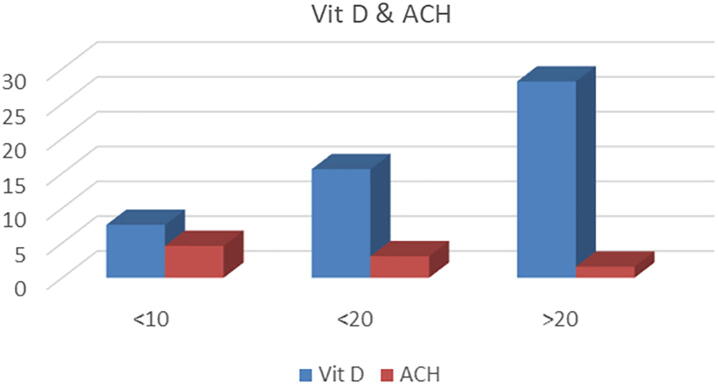
Of the 67 patients, 74% were female (mean age 24 ± 5.3 years) and 26% were male (mean age 22 ± 4.3 years). No significant difference was observed in gender distribution (t = 1.40, p = 0.164). The mean VitD levels were 14.7 ± 6.2 ng/mL and 13 ± 3.4 ng/mL for female and male patients, respectively, with no significant difference observed (t = 1.21, p = 0.11). Of the patients, 80% had VitD < 20 ng/mL. The mean ACH was 1.89 ± 0.74 mm for men and 2.4 ± 0.6 mm for women, a significant difference (t = 2.89, p = 0.002). Patients with VitD levels > 20 ng/mL demonstrated a mean ACH of 1.6 mm. A mean ACH of 3.1 mm was observed in those with VitD levels < 20 ng/mL, and 4.6 mm for VitD levels < 10 ng/mL (Fig. 1). A weak negative correlation was found between VitD and ACH in all groups (r = −0.055, p = .7; Fig. 2).
Fig. 1.
The mean alveolar crest height of the patients in relation to vitamin D.
Fig. 2.
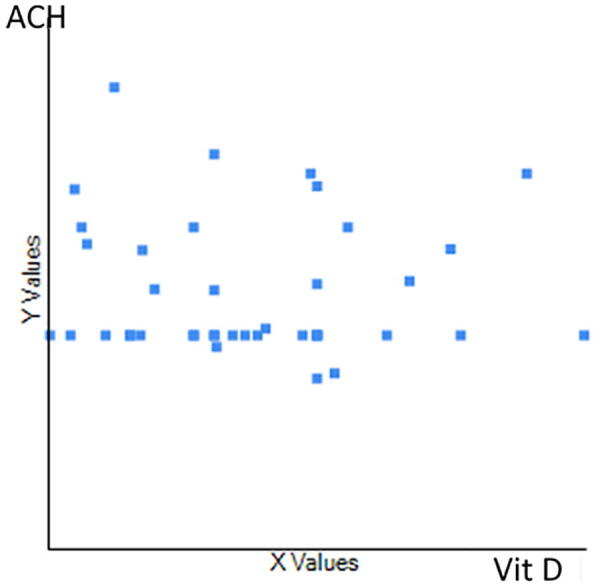
The association between vitamin D and alveolar crest height in all groups (r = -0.055, p = .7).
4. Discussion
Periodontitis is a chronic inflammatory condition caused by periodontopathic bacteria that leads to destruction of the supporting tissues of teeth. Unless promptly treated, periodontitis eventually initiates gingival detachment and further alveolar bone destruction. Worldwide, periodontitis is the main cause of teeth loss in adults; thus, emphasis should be given to prevention rather than therapy of this disease.
The immunoregulatory and anti-inflammatory roles of VitD have been reported previously (Bonnet et al., 2019, Stein et al., 2014). A correlation between VitD insufficiency (<20 ng/mL) and several inflammatory and infectious conditions, including periodontal disease, has been observed (Holick, 2007, Zhan et al., 2014). Therefore, the risk of gingivitis and chronic periodontitis can be decreased by adequate consumption of VitD (Dietrich and Garcia, 2005, Krall et al., 2001, Miley et al., 2009). Dietrich et al. (2004) reported a negative correlation between VitD level and periodontal disease. Our results showed that average bone loss (3.5 mm) was increased in patients with VitD < 10 ng/mL, while those with VitD level ˃20 ng/mL showed decreased bone loss (1.6 mm). Similar to our findings, a previous study conducted on Canadian adults revealed a relationship between low VitD and periodontal disease manifesting as alveolar bone loss (Bonnet et al., 2019).
The relation between periodontal disease and VitD has been investigated in many studies (Anbarcioglu et al., 2019, Dietrich and Garcia, 2005, Miley et al., 2009). Boggess et al. (2011) found that pregnant patients with periodontitis had VitD insufficiency; moreover, the adjunctive use of VitD was shown to be able to improve maternal oral health.
Similarly, Miley et al. (2009) observed improved periodontal health after VitD supplements. However, Hildebolt (2005) recommended further controlled clinical trials to address the adjunctive role of calcium and VitD supplements for treating periodontal disease. Anbarcioglu et al. (2019) observed an increased prevalence of VitD deficiency in patients with aggressive periodontitis, suggesting that decreased VitD level could be a risk factor, and screening is recommended where deficiency is suspected.
Radiographic assessment permits accurate estimation of alveolar bone level, osseous defect pattern, and furcation involvement (Gomes-Filho et al., 2007, Hausmann, 2000). Panoramic radiography is commonly used for screening and diagnosis, but its limitations are distortion and magnification (Rockenbach et al., 2003). Previous studies have demonstrated that linear measurements made on panoramic radiographs were underestimated when compared to actual measurements (Langlois et al., 2011). Persson et al. (2003) evaluated the correlation between intraoral and panoramic radiographs in assessing the distance from the alveolar crest to the cement–enamel junction. They concluded that the mean differences between these measurements were up to 0.04 mm, and both were in close agreement. In our study, all images were taken using the same radiographic apparatus and settings. Further, for all linear measurements, the magnification factor recommended by the manufacturer was used.
Alshouibi et al. (2013) investigated periodontal health in older men with moderate bone loss and a maintenance VitD dose of ≥ 800 IU. They suggested that this daily dose could inhibit periodontal disease progression. Ketharanathan et al. (2019) assessed radiographic bone loss and VitD levels in periodontitis patients of two ethnic groups compared with healthy controls. They observed a substantial decrease in radiographic bone loss with a limited increase in serum VitD.
Worldwide, one billion people are likely experiencing VitD deficiency due to insufficient calcium and VitD dietary intake (Glerup et al., 2000, Holick, 2006). VitD levels were expected to be sufficient in Saudi patients due to the sunny weather present most of the year. However, VitD deficiency was reported in almost half of studied samples of healthy Saudi Arabian adults (Al-Daghri, 2018, Alsuwadia et al., 2013) This could be partially due to low dietary and supplemental VitD intake, traditional dress that reduces the area of exposed skin, genetic factors, and individual preference to avoid hot sunny weather (Alsuwadia et al., 2013).
The current recommended daily calcium and VitD intakes for adults are 400–600 IU and 1,000–1,200 mg, respectively (Moore et al., 2004). Thus, increasing calcium and VitD intake through natural sources and oral supplementation has been shown to be essential for bone and oral health (Dixon et al., 2009, Heaney, 2002). However, prolonged intake of megadose VitD supplements without medical consultation could lead to a risk of VitD toxicity (VDT; Dudenkov et al., 2015). Exogenous VDT is diagnosed by a 25(OH)D level above 150 ng/mL, associated with severe hypercalcemia and hypercalciuria and significantly decreased parathyroid hormone activity (Holick, 2015).
A previous study demonstrated that older age and smoking were confounders that could severely influence the relationship between VitD and periodontal disease (Khammissa et al., 2018). In our study, non-smokers aged 20 to 45 years were selected on purpose to avoid the possible effects of age and hormonal changes on the results. Further, patients in this study were randomly selected from those seeking dental treatment; thus, panoramic radiography was used as the most common tool for patient screening. Comparing radiographic findings, as well as periodontal clinical parameters, with serum VitD level should be addressed in future studies.
The small sample size is the main limitation of this study, as well as its evaluation of the effect of VitD on ACH only. Therefore, additional randomized clinical trials with larger sample sizes are recommended. Further research should ideally evaluate the impact of VitD on attachment loss and periodontal pocket depth in patients with periodontal disease over time using intraoral radiographs.
5. Conclusion
VitD had a crucial role in the periodontal health of younger Saudi men and women. Thus, maintaining an adequate VitD level seems to be consistent with good periodontal health.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Imam Abdulrahman Bin Faisal University Institutional Fund IF-2020-006-Dent.
CRediT authorship contribution statement
Marwa Madi: Conceptualization, Methodology, Formal analysis, Writing - review & editing, Visualization, Supervision. Verica Pavlic: Methodology, Writing - review & editing, Visualization, Supervision. Shahad Mongith Alammar: Validation, Investigation, Data curation, Writing - original draft. Leenah Mohammad Alsulaimi: Validation, Investigation. Reema Shaker Alotaibi: Validation, Investigation. Ghadah Mohammed AlOtaibi: Investigation. Osama Zakaria: Methodology, Formal analysis, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
The association between vitamin D level and periodontal disease: a preliminary study
Peer review under responsibility of King Saud University.
References
- Abreu O.J., Tatakis D.N., Elias-Boneta A.R., Del Valle L.L., Hernandez R., Pousa M.S., Palacios C. Low vitamin D status strongly associated with periodontitis in Puerto Rican adults. BMC Oral Health. 2016;16(1):89–94. doi: 10.1186/s12903-016-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daghri N.M. Vitamin D in Saudi Arabia: prevalence, distribution and disease associations. J. Steroid Biochem. Mol. Biol. 2018;175:102–107. doi: 10.1016/j.jsbmb.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Alshammari A.K.S., Wahi M.M. A Narrative Review of the Prevalence of Periodontitis in Saudi Arabia: A Proposal for a National Oral Health Research Agenda for Vision 2030. TODENTJ. 2019;13(1):171–176. doi: 10.2174/1874210601913010171. [DOI] [Google Scholar]
- Alshouibi E.N., Kaye E.K., Cabral H.J., Leone C.W., Garcia R.I. Vitamin D and periodontal health in older men. J. Dent. Res. 2013;92:689–693. doi: 10.1177/0022034513495239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsuwadia A.O., Farag Y.M., Al Sayyari A.A., Mousa D.H., Alhejaili F.F., Al-Harbi A.S., Housawi A.A., Mittal B.V., Singh A.K. Prevalence of vitamin D deficiency in Saudi adults. Saudi Med J. 2013;34:814–818. [PubMed] [Google Scholar]
- Anbarcioglu E., Kirtiloglu T., Öztürk A., Kolbakir F., Acıkgöz G., Colak R. Vitamin D deficiency in patients with aggressive periodontitis. Oral Dis. 2019;25:242–249. doi: 10.1111/odi.12968. [DOI] [PubMed] [Google Scholar]
- Antonoglou G.N., Suominen A.L., Knuuttila M., Ylöstalo P., Ojala M., Männistö S., Marniemi J., Lundqvist A., Tervonen T. Associations between serum 25-hydroxyvitamin D and periodontal pocketing and gingival bleeding: results of a study in a non-smoking population in Finland. J. Periodontol. 2015;86:755–765. doi: 10.1902/jop.2015.140262. [DOI] [PubMed] [Google Scholar]
- Bashutski J.D., Eber R.M., Kinney J.S., Benavides E., Maitra S., Braun T.M., Giannobile W.V., McCauley L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011;90:1007–1012. doi: 10.1177/0022034511407771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012;10:151–159. doi: 10.1007/s11914-012-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess K.A., Espinola J.A., Moss K., Beck J., Offenbacher S., Camargo C.A., Jr Vitamin D status and periodontal disease among pregnant women. J. Periodontol. 2011;82:195–200. doi: 10.1902/jop.2010.100384. [DOI] [PubMed] [Google Scholar]
- Bonnet C., Rabbani R., Moffatt M.E.K., Kelekis-Cholakis A., Schroth R.J. The Relation Between Periodontal Disease and Vitamin D. J. Can Dent. Assoc. 2019;85:1488–2159. [PubMed] [Google Scholar]
- Dietrich T., Garcia R.I. Associations between periodontal disease and systemic disease: evaluating the strength of the evidence. J. Periodontol. 2005;76:2175–2184. doi: 10.1902/jop.2005.76.11-S.2175. [DOI] [PubMed] [Google Scholar]
- Dietrich T., Joshipura K.J., Dawson-Hughes B., Bischoff-Ferrari H.A. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004;80:108–113. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- Dixon D., Hildebolt C.F., Miley D.D., Garcia M.N., Pilgram T.K., Couture R., Spearie C.A., Civitelli R. Calcium and vitamin D use among adults in periodontal disease maintenance programmes. Br. Dent. J. 2009;206(12):617–627. doi: 10.1038/sj.bdj.2009.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudenkov D.V., Yawn B.P., Oberhelman S.S., Fischer P.R., Singh R.J., Cha S.S., Maxson J.A., Quigg S.M., Thacher T.D. Mayo Clinic Proceedings. Elsevier; 2015. Changing incidence of serum 25-hydroxyvitamin D values above 50 ng/mL: a 10-year population-based study; pp. 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshghi R., Rashidi Maybodi F., Khabazian A., Shahhosseini S. Association between serum levels of vitamin D and chronic periodontitis in premenopausal women in Yazd. Casp. J. Dent. Res. 2016;5:47–51. [Google Scholar]
- Garcia M.N., Hildebolt C.F., Miley D.D., Dixon D.A., Couture R.A., Anderson Spearie C.L., Langenwalter E.M., Shannon W.D., Deych E., Mueller C. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J. Periodontol. 2011;82:25–32. doi: 10.1902/jop.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glerup H., Mikkelsen K., Poulsen L., Hass E., Overbeck S., Thomsen J., Charles P., Eriksen E.F. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J. Intern. Med. 2000;247:260–268. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- Gomes-Filho I.S., Sarmento V.A., De Castro M.S., Da Costa N.P., Da Cruz S.S., Trindade S.C., de Freitas C.O.T., de Santana Passos J. Radiographic features of periodontal bone defects: evaluation of digitized images. Dentomaxillofacial Radiol. 2007;36:256–262. doi: 10.1259/dmfr/25386411. [DOI] [PubMed] [Google Scholar]
- Guile E.E. Periodontal status of adults in central Saudi Arabia. Community Dent. Oral Epidemiol. 1992;20:159–160. doi: 10.1111/j.1600-0528.1992.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Hausmann E. Radiographic and digital imaging in periodontal practice. J. Periodontol. 2000;71:497–503. doi: 10.1902/jop.2000.71.3.497. [DOI] [PubMed] [Google Scholar]
- Heaney R.P. The importance of calcium intake for lifelong skeletal health. Calcif. Tissue Int. 2002;70(2):70–73.. doi: 10.1007/s00223-001-0032-3. [DOI] [PubMed] [Google Scholar]
- Hildebolt C.F. Effect of vitamin D and calcium on periodontitis. J. Periodontol. 2005;76:1576–1587. doi: 10.1902/jop.2005.76.9.1576. [DOI] [PubMed] [Google Scholar]
- Hiremath V., Rao C., Naiak V., Prasad K.V.V. Anti-inflammatory effect of vitamin D on gingivitis: a dose response randomised controlled trial. Indian J. Public Health. 2013;57:29–32. doi: 10.4103/0019-557X.111365. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Mayo Clinic Proceedings. Elsevier; 2015. Vitamin D is not as toxic as was once thought: a historical and an up-to-date perspective; pp. 561–564. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagelavičienė E., Vaitkevičienė I., Šilingaitė D., Šinkūnaitė E., Daugėlaitė G. The relationship between vitamin D and periodontal pathology. Medicina (B. Aires) 2018;54:45–53. doi: 10.3390/medicina54030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M., Giovannucci E., Kaye E.K., Joshipura K.J., Dietrich T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014;17:844–852. doi: 10.1017/S1368980013000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketharanathan V., Torgersen G.R., Petrovski B.É., Preus H.R. Radiographic alveolar bone level and levels of serum 25-OH-Vitamin D 3 in ethnic Norwegian and Tamil periodontitis patients and their periodontally healthy controls. BMC Oral Health. 2019;19(1):83–90. doi: 10.1186/s12903-019-0769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khammissa R.A.G., Ballyram R., Jadwat Y., Fourie J., Lemmer J., Feller L. Vitamin D Deficiency as It Relates to Oral Immunity and Chronic Periodontitis. Int. J. Dentistry. 2018;2018:1–9. doi: 10.1155/2018/7315797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall E.A., Wehler C., Garcia R.I., Harris S.S., Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am. J. Med. 2001;111:452–456. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- Laky M., Bertl K., Haririan H., Andrukhov O., Seemann R., Volf I., Assinger A., Gruber R., Moritz A., Rausch-Fan X. Serum levels of 25-hydroxyvitamin D are associated with periodontal disease. Clin. Oral Investig. 2017;21:1553–1558. doi: 10.1007/s00784-016-1965-2. [DOI] [PubMed] [Google Scholar]
- Langlois, C. de O., SaMpaiO, M.C.C., Silva, A.E.R., Costa, N.P. da, Rockenbach, M.I.B., 2011. Accuracy of linear measurements before and after digitizing periapical and panoramic radiography images. Braz. Dent. J. 22, 404–409. [DOI] [PubMed]
- Miley D.D., Garcia M.N., Hildebolt C.F., Shannon W.D., Couture R.A., Anderson Spearie C.L., Dixon D.A., Langenwalter E.M., Mueller C., Civitelli R. Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J. Periodontol. 2009;80:1433–1439. doi: 10.1902/jop.2009.090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C., Murphy M.M., Keast D.R., Holick M.F. Vitamin D intake in the United States. J. Am. Diet. Assoc. 2004;104:980–983. doi: 10.1016/j.jada.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Perić M., Cavalier E., Toma S., Lasserre J.F. Serum vitamin D levels and chronic periodontitis in adult, Caucasian population—a systematic review. J. Periodontal Res. 2018;53:645–656. doi: 10.1111/jre.12560. [DOI] [PubMed] [Google Scholar]
- Persson R.E., Tzannetou S., Feloutzis A.G., Brägger U., Persson G.R., Lang N.P. Comparison between panoramic and intra-oral radiographs for the assessment of alveolar bone levels in a periodontal maintenance population. J. Clin. Periodontol. 2003;30:833–839. doi: 10.1034/j.1600-051x.2003.00379.x. [DOI] [PubMed] [Google Scholar]
- Pinto J., Goergen J., Muniz F., Haas A.N. Vitamin D levels and risk for periodontal disease: A systematic review. J. Periodontal Res. 2018;53:298–305. doi: 10.1111/jre.12531. [DOI] [PubMed] [Google Scholar]
- Rockenbach M.I.B., Sampaio M.C.C., da Costa L.J., da Costa N.P. Evaluation of mandibular implant sites: correlation between panoramic and linear tomography. Braz. Dent. J. 2003;14:209–213. doi: 10.1590/s0103-64402003000300013. [DOI] [PubMed] [Google Scholar]
- Schwalfenberg G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011;55:96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- Stein S.H., Livada R., Tipton D.A. Re-evaluating the role of vitamin D in the periodontium. J. Periodontal Res. 2014;49:545–553. doi: 10.1111/jre.12149. [DOI] [PubMed] [Google Scholar]
- Vieth R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/ml) Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:681–691. doi: 10.1016/j.beem.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Zhan Y., Samietz S., Holtfreter B., Hannemann A., Meisel P., Nauck M., Völzke H., Wallaschofski H., Dietrich T., Kocher T. Prospective study of serum 25-hydroxy vitamin D and tooth loss. J. Dent. Res. 2014;93:639–644. doi: 10.1177/0022034514534985. [DOI] [PMC free article] [PubMed] [Google Scholar]