Abstract
Incomplete hippocampal inversion (IHI) is an anatomical variant of the human brain resulting from an arrest in brain development, especially prevalent in the left hemisphere. We hypothesized that IHI is more common in schizophrenia and contributes to the well-known hippocampal structural differences. We studied 199 schizophrenia patients and 161 healthy control participants with 3 T MRI to establish IHI prevalence and the relationship of IHI with hippocampal volume and asymmetry. IHI was more prevalent (left hemisphere: 15% of healthy control participants, 27% of schizophrenia patients; right hemisphere: 4% of healthy control participants, 10% of schizophrenia patients) and more severe in schizophrenia patients compared to healthy control participants. Severe IHI cases were associated with a higher rate of automated segmentation failure. IHI contributed to smaller hippocampal volume and increased R > L volume asymmetry in schizophrenia. The increased prevalence and severity of IHI supports the neurodevelopmental model of schizophrenia. The impact of this developmental variant deserves further exploration in studies of the hippocampus in schizophrenia.
Subject terms: Neuroscience, Schizophrenia
Introduction
Hippocampal structure is abnormal in schizophrenia [1–4]. Neuroimaging studies indicate that hippocampal volume is reduced [4, 5], right > left hippocampal volume asymmetry is altered [6, 7], and hippocampal shape is deformed [8, 9] in schizophrenia. The timing of these hippocampal changes is not known. The neurodevelopmental hypothesis of schizophrenia locates the origin of these abnormalities in the prenatal period [10, 11]. Therefore, identifying and characterizing markers of atypical hippocampal development will advance our understanding of schizophrenia.
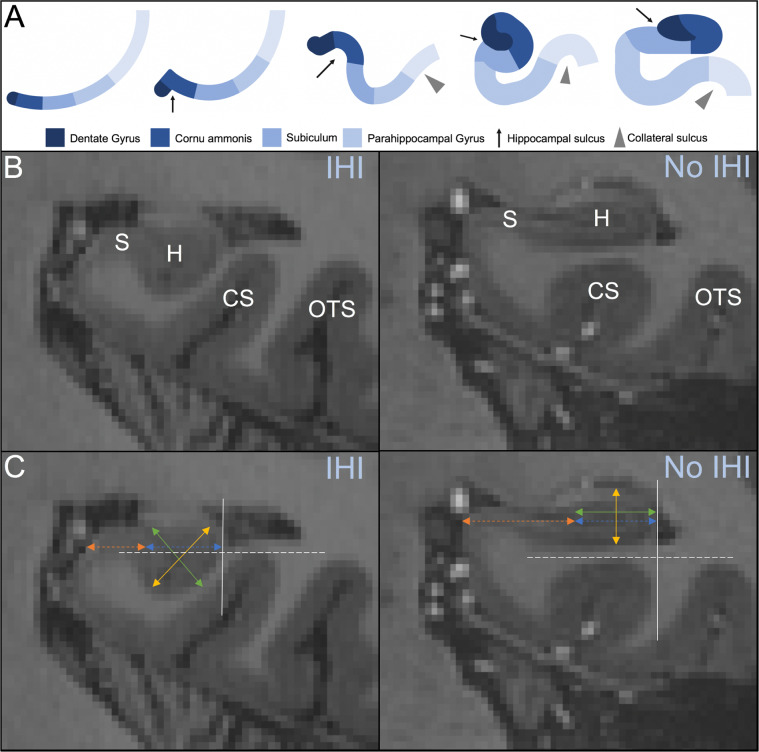
The complex development of the human hippocampus begins at gestational week (GW) 8. During GWs 10–20, the dentate gyrus and cornu ammonis are situated in the posteromedial wall of the lateral ventricles and move from the frontal to temporal lobe to surround the hippocampal sulcus [12, 13]. Between GWs 20–30, the dentate gyrus and cornu ammonis undergo a morphologic inversion around the hippocampal sulcus [14] (Fig. 1A). Failure to complete this inversion process results in an incomplete hippocampal inversion (IHI), an anatomic variant [15] characterized by a round, verticalized, medially positioned hippocampal body in the coronal plane and a deep collateral sulcus [16–20]. Prevalence of IHI is high in preterm neonates and decreases to rates comparable to adult populations by GW 25 [21]. Healthy individuals with IHI demonstrate altered sulcal patterns outside the medial temporal lobe [20]. IHI is also associated with several genetic abnormalities and developmental anomalies such as 22q11.2 deletion syndrome [22, 23] and corpus callosum agenesis [24]. A recent genome-wide association study of IHI identified a genome-wide significant locus and revealed that IHI has high heritability [25]. Taken together, IHI is of interest in the study of neuropsychiatric disorders with a neurodevelopmental profile, including schizophrenia [26].
Fig. 1. Incomplete hippocampal inversion.
A Developmental process of the hippocampus from one layer of cortical mantle through early inversion (rounded, verticalized hippocampus with deep collateral sulcus) and late inversion (flat, horizontal hippocampus with shallow collateral sulcus). Arrest in hippocampal development (at Step 4) results in IHI. B 7 T MRI coronal view of an incomplete (left) and complete (right) hippocampal inversion. The hippocampus (H), subiculum (S), collateral sulcus (CS), and occipitotemporal sulcus (OTS) are used as anatomical landmarks to identify IHI. C Criterion 1 is evaluated by comparing the width of the hippocampus (green, solid line) with the height of the hippocampal body (yellow, solid line). The gray, solid line indicates the lateral limit of the hippocampus, which is used for criterion 2. Criterion 3 is measured by comparing the length of the subiculum not covered by the dentate gyrus (orange, dotted line) with the ventral part of the cornu ammonis/subiculum that is covered by the dentate gyrus (blue, dotted line). Criterion 4 is measured using the thickness of the subiculum. The gray, dotted line located at the deepest portion of the CS or OTS is used to evaluate criterion 5.
Prevalence estimates of IHI have varied greatly in previous IHI studies [16–19, 24, 27–35], largely due to inconsistency in defining hippocampal features that constitute an IHI. Recently, Cury et al. established a clear set of quantitative IHI criteria in a large healthy cohort [20]. Subsequent studies using these criteria reported consistent prevalence estimates and findings [36, 37]. Employing the Cury criteria, the prevalence of IHI can now be reliably assessed in neuropsychiatric disorders such as schizophrenia.
Two previous studies have investigated an incomplete development of the hippocampus in schizophrenia. The first reported an increased prevalence of moderate and severe forms in familial schizophrenia patients when compared to control participants [38]. Importantly, this study reported only a qualitative anomaly (hippocampus appeared rounder or more pyramidal). The second study employed Cury’s IHI criteria in a schizophrenia cohort and reported that patients with visual hallucinations possessed more IHI-specific morphological patterns than patients with only auditory hallucinations and healthy control participants [26]. However, the authors only measured a single IHI criterion (i.e., criterion 1, hippocampal flatness) in a small patient sample. Therefore, the prevalence of IHI in schizophrenia is unknown.
Whether IHI affects hippocampal volumes in the healthy brain or contributes to volume differences between healthy control participants and patient groups (e.g., schizophrenia) needs to be explored further. Two studies have investigated the effect of IHI on hippocampal volume using the Cury criteria. The first study characterized IHI in 60 participants with a major depressive episode and 60 matched healthy control participants and reported no significant volume differences between groups [36]. In addition, the hippocampal volumes of participants with IHI did not differ from those without the variant. In a second study analyzing a healthy, aging cohort, the authors reported that participants with IHI do not differ in whole hippocampal volume, but show decreased subfield volumes, namely CA1 [37]. However, IHI lowers the accuracy of automated segmentation protocols, making it difficult to assess the effect of IHI on subfield volume estimates [39]. Cury’s IHI criteria consistently indicate that IHI is more frequent in the left (17%) than the right (6%) hemisphere [20] in healthy control participants. The left hippocampus develops more slowly than the right hippocampus, making it more likely for a developmental arrest to result in a unilateral left IHI [40]. The increased prevalence of IHI in the left hemisphere may be related to some of the hemispheric asymmetries in the human brain [19, 41], including the right > left hippocampal volume difference that is most prominent in the anterior hippocampus [42–44].
In this study, we examined IHI in a large cohort of patients with schizophrenia spectrum disorders and healthy control participants, using comprehensive and quantitative criteria. We aimed to answer two questions. First, is the prevalence of IHI increased in schizophrenia? Second, does IHI contribute to hippocampal volume differences in schizophrenia, including R > L volume asymmetry?
Methods
Participants
Participants in this study included 199 patients with schizophrenia spectrum disorder diagnoses (86 schizophreniform disorder, 77 schizophrenia, 36 schizoaffective disorder; referred to here as schizophrenia patients) and 161 healthy control participants matched to patients on age, sex, race, and parental education (Table 1). Schizophrenia patients were recruited from the inpatient unit and outpatient clinics of the Vanderbilt University Medical Center Psychotic Disorders Program as part of an ongoing data repository, the Psychiatric Phenotype/Genotype Project (PGPP) (NCT00762866). Healthy control participants were recruited from the local community via advertisement. The study was approved by the Vanderbilt University Institutional Review Board. All participants provided written informed consent and were compensated for their time. The Structured Clinical Interview for DSM-IV was used for diagnostic assessment [45]. Participant exclusion criteria include significant medical or neurological illness, age under 16 or over 65, pregnancy, head injury, or meeting criteria for substance abuse within the past month. Healthy control participants were excluded if they had a current or past psychiatric illness, a first degree relative with a psychotic illness, or psychotropic drug use. All participants with a T1-weighted MRI scan without motion artifact were selected from the PGPP repository for inclusion in this study.
Table 1.
Participant demographics and clinical characteristics.
| Healthy control participants | Schizophrenia patients | Healthy control participants < schizophrenia patients | ||||
|---|---|---|---|---|---|---|
| n = 161 | n = 199 | |||||
| Mean | SD | Mean | SD | Statistic (t) | p | |
| Age (yrs) | 33.59 | 11.35 | 35.45 | 13.29 | 1.41 | 0.16 |
| Parental education (yrs) | 14.45 | 2.35 | 14.44 | 2.76 | −0.01 | 0.99 |
| WTAR | 111.02 | 11.30 | 98.94 | 16.28 | −7.84 | <0.001 |
| CPZ equivalents | 418.48 | 258.22 | ||||
| Duration of illness (yrs) | 7.70 | 11.35 | ||||
| n | % | n | % | Statistic (X2) | p | |
|---|---|---|---|---|---|---|
| Sex (male) | 106 | 66 | 143 | 72 | 1.51 | 0.22 |
| Race (White) | 114 | 71 | 126 | 63 | 2.25 | 0.13 |
| Tobacco use | ||||||
| No | 130 | 81 | 89 | 45 | ||
| Yes, continued | 16 | 10 | 81 | 41 | ||
| Yes, quit | 14 | 9 | 29 | 15 | ||
| Missing | 1 | <1 | 0 | 0 | ||
| Diagnosis | ||||||
| Schizophreniform DO | 86 | 43 | ||||
| Schizophrenia | 77 | 39 | ||||
| Schizoaffective DO | 36 | 18 | ||||
yrs years, WTAR Wechsler test of adult reading, CPZ chlorpromazine, DO disorder.
Bolded values indicate signifance at p < 0.05 level.
MRI acquisition and processing
Structural MRI acquisition was completed on a 3 T Philips Intera Achieva scanner at the Vanderbilt University Institute of Imaging Sciences (Philips Healthcare, Inc.). Each participant received a 3D T1-weighted scan (voxel resolution: 1 mm3; field of view = 2562; number of slices = 170; TE = 3.7 ms; TR = 8.0 ms). Each image was visually inspected and determined to be free from motion or other artifacts prior to inclusion in the analysis. All images were reoriented toward the MNI152 atlas using FSL rigid body transformation [46].
Each image was processed using the FreeSurfer 6 [47, 48] hippocampal subfield module [49] with standard parameters. Segmentations were visually inspected to correct those with tissue labeled outside the hippocampus or incomplete labeling of the hippocampus. Failed automated segmentation was comprised of two major errors: (1) segmentation of the hippocampus extending beyond the hippocampal border into surrounding structures and (2) segmentation of the amygdala extending into the hippocampal head. Failed automated segmentations were corrected for inclusion in this study by manually deleting segmented voxels that extended outside of the hippocampal head, body, or tail into surrounding structures or by manually replacing amygdala segmentation voxels with hippocampal head segmentation voxels at the amygdala–hippocampal border. Manual voxel correction was completed using ITK-SNAP version 3.8.0 [50]. Volume data from all participants were included in a previous study [51].
Assessment of IHIs
The criteria used to determine the severity of IHI in this study were validated in a study by Cury et al. in 2089 participants. The IHI score for each hippocampus ranges from 0 to 10. IHI presence was defined based on a score of ≥3.75 [20]. IHI was assessed by two observers (MJR and SH) after training. Both observers were blinded to the subject group (schizophrenia patients, healthy control participants) when assessing IHI. MR evaluated IHI for all 360 participants included in the study. Ten of these individuals were randomly selected to evaluate intra- and interobserver reproducibility using the kappa statistic [52]. Cohen’s kappa indicated very strong (kappa = 0.88) intraobserver and substantial (kappa = 0.76) interobserver reliability, consistent with the findings of Cury et al. [20]. All criteria were obtained in the coronal view for both the left and right hippocampus using ITK-SNAP (Version 3.8.0). Criteria are summarized in Supplementary Text (Fig. 1B, C).
Statistical analyses
A χ2 test was performed on the IHI threshold score (≥3.75) for each hemisphere to test whether IHI is more prevalent in schizophrenia patients than healthy control participants. A t-test was performed on the continuous IHI score (0–10) for each hemisphere to test whether IHI is more severe (i.e., the total IHI score is greater) in schizophrenia patients than in healthy control participants. A subanalysis of individual criteria was assessed with t-tests in each hemisphere, and corrected for multiple comparisons using the Bonferroni method, to test which criterion differed between schizophrenia patients and healthy control participants. These statistical tests were replicated to examine differences between participants with successful and failed automated segmentations. For all statistical methods in this study, tests were two sided and the significance level was defined as alpha < 0.05. For some IHI criteria, there was a significant difference between the variance of groups being compared. Therefore, we used Welch’s unequal variance t-test, due to its reliability for samples with unequal variances or unequal sample sizes, for all t-tests completed in this study.
To examine the effect of IHI on hippocampal volume and asymmetry group differences, a two-step sensitivity analysis was conducted using linear mixed models in R (R Core Team, 2019) with the packages lme4 [53], emmeans [54], car [55], and MuMIn [56]. In Step 1, models comparing schizophrenia patient and healthy control groups were fitted by adjusting for estimated intracranial volume (ICV), age, and sex, with participant as a random effect. To test whether there is a difference in hippocampal volume in the schizophrenia patient cohort versus healthy participant cohort, a model was constructed with volume as the dependent variable and interaction between Group (schizophrenia patient, healthy control participant) and Hemisphere (left, right) as fixed effects (Volume Model, Step 1: Volume ∼ Group × Hemisphere + Age + Sex + ICV + (1|Participant)). The Group by Hemisphere interaction and main effect of Group were tested using type 2 sum of squares. To test the hypothesis that there is an asymmetry difference in the anterior or posterior regions of the hippocampus in the schizophrenia patient cohort, a separate model was constructed with asymmetry index as the dependent variable and the interaction between Group (schizophrenia patient, healthy control participant) and Region (anterior, posterior) as fixed effects (Asymmetry Index Model, Step 1: Asymmetry Index ∼ Group × Region + Age + Sex + ICV + (1|Participant)). The Group by Region interaction and main effect of Group were tested using type 2 sum of squares. The volume asymmetry index was calculated using the equation: asymmetry index = (R − L)/(0.5 × (R + L)).
In Step 2, IHI was added as a fixed effect to both models to test whether IHI contributes to volume or the asymmetry index (Volume Model, Step 2: Volume ∼ Group × Hemisphere + IHI + Age + Sex + ICV + (1|Participant); Asymmetry Index Model, Step 2: Asymmetry Index ∼ Group × Region + IHI + Age + Sex + ICV + (1|Participant)). Exploratory analyses including a Group by IHI interaction were conducted to investigate whether IHI has a different effect on Volume or Asymmetry Index in each group (Volume Model: Volume ∼ Group × Hemisphere + Group × IHI + Age + Sex + ICV + (1|Participant); Asymmetry Index Model: Asymmetry Index ∼ Group × Region + Group × IHI + Age + Sex + ICV + (1|Participant)). Significance tests were conducted on the fixed effects using analysis of variance with type 2 sum of squares so that the main effects were tested in the absence of the interaction terms. Significant interactions were followed up with contrasts adjusted for multiple comparisons using Bonferroni correction. Marginal R2 and AIC were calculated for models in Step 1 and Step 2 to assess model fit.
Results
IHI prevalence and severity
In our sample of 360 individuals (199 schizophrenia patients, 161 healthy control participants), we found 57 (16% of the total sample) unilateral left IHI (38 schizophrenia patients, 19 healthy control participants), 21 (6%) bilateral IHI (16 schizophrenia patients, 5 healthy control participants), and 6 (2%) unilateral right IHI (4 schizophrenia patients, 2 healthy control participants) cases. The remaining 276 participants (77%) showed no IHI.
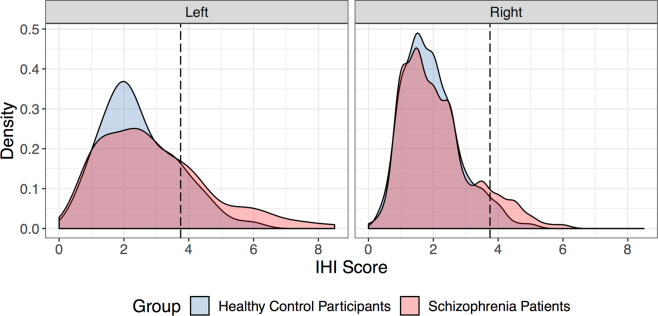
Schizophrenia patients had more frequent IHI in both the left (χ2 = 7.84, p < 0.01) and right (χ2 = 4.17, p = 0.04) hemisphere (Fig. 2, Table 2). IHI severity, as measured by the total IHI score, was significantly greater in the left (t351 = 3.00, p < 0.01) but not right (t358 = 1.60, p = 0.11) hemisphere in schizophrenia. Criterion 3 scores, which reflect the medial positioning of the hippocampus, were significantly greater for schizophrenia patients in the left hemisphere (t343 = 3.28, p < 0.01) after correction for multiple comparisons. No other criteria differed between groups in either hemisphere (Table 2).
Fig. 2. Distribution of IHI scores based on laterality of the hippocampus in both schizophrenia patients and healthy control participants.
The density indicates the percent of total hippocampi that have a specific score attributed to them. The dashed line at a score of 3.75 designates the threshold used to define an IHI based on Cury et al. [20]. The prevalence of IHI in schizophrenia patients compared to healthy control participants is greater in the left hemisphere (left panel; χ2 = 7.84, p < 0.01) and right hemisphere (right panel, χ2 = 4.17, p = 0.04).
Table 2.
IHI prevalence and severity.
| Healthy control participants | Schizophrenia patients | Healthy control participants < schizophrenia patients | ||||
|---|---|---|---|---|---|---|
| n = 161 | n = 199 | |||||
| n | % | n | % | Statistic (X2) | p | |
| Left IHI | 24 | 15 | 54 | 27 | 7.84 | 0.005 |
| Right IHI | 7 | 4 | 20 | 10 | 4.17 | 0.04 |
| Mean | SD | Mean | SD | Statistic (t) | p | |
|---|---|---|---|---|---|---|
| Left IHI score | 2.46 | 1.17 | 2.91 | 1.68 | 3.00 | 0.003 |
| C1 | 0.45 | 0.38 | 0.56 | 0.51 | 2.40 | 0.08a |
| C2 | 1.02 | 0.56 | 1.06 | 0.58 | 0.65 | 1.00a |
| C3 | 0.51 | 0.39 | 0.68 | 0.60 | 3.28 | 0.006a |
| C4 | 0.00 | 0.00 | 0.04 | 0.28 | 2.02 | 0.23a |
| C5 | 0.48 | 0.63 | 0.57 | 0.68 | 1.28 | 1.00a |
| Right IHI score | 1.95 | 0.87 | 2.11 | 1.10 | 1.60 | 0.11 |
| C1 | 0.38 | 0.31 | 0.37 | 0.36 | −0.25 | 1.00a |
| C2 | 0.95 | 0.51 | 0.95 | 0.51 | 0.05 | 1.00a |
| C3 | 0.46 | 0.39 | 0.57 | 0.53 | 2.23 | 0.13a |
| C4 | 0.00 | 0.00 | 0.00 | 0.00 | N/A | N/A |
| C5 | 0.16 | 0.37 | 0.23 | 0.49 | 1.43 | 0.77a |
IHI incomplete hippocampal inversion.
Bolded values indicate signifance at p < 0.05 level.
ap value adjusted for multiple comparisons using Bonferroni method.
Hippocampal segmentation
Automated segmentation of the hippocampus failed in 48 individuals (30 schizophrenia patients, 18 healthy control participants), of which 30 participants had IHI (16 unilateral left, 11 bilateral, 3 unilateral right) (Supplementary Table 1). The prevalence of IHI in segmentation failure was significantly greater in both the left (χ2 = 39.03, p < 0.001) and right (χ2 = 37.48, p < 0.001) hemispheres (Supplementary Table 2). Participants with failed segmentations had higher total left (t52 = 6.09, p < 0.001) and right IHI scores (t55 = 4.08, p < 0.001) than participants with successful segmentations. Multiple criteria contributed to the total score group differences. Criterion 1 (t58 = 2.74, p = 0.04), 2 (t65 = 3.99, p < 0.001), 3 (t54 = 5.56, p < 0.001), and 5 (t58 = 5.08, p < 0.001) significantly differed between segmentation successes and failures in the left hemisphere. Criterion 3 (t55 = 4.25, p < 0.001) and 5 (t54 = 3.20, p = 0.01) differed in the right hemisphere. Participants with failed segmentations also had higher parental education (t61 = 2.34, p = 0.02) and higher estimated premorbid intellectual functioning (t66 = 2.37, p = 0.02), but did not differ with respect to diagnosis (i.e., schizophrenia patient versus healthy control participant) (χ2 = 1.17, p = 0.28), age (t66 = −1.39, p = 0.17), sex (χ2 = 3.79, p = 0.05), or race (χ2 = 0.11, p = 0.74). All failed segmentations were manually corrected for inclusion in the following volumetric analyses.
Volumetric analyses
We used a linear mixed model to test whether IHI contributes to the well-known hippocampal volume differences in schizophrenia (Table 3). Without IHI in the model, we found overall smaller hippocampal volume in schizophrenia patients (main effect of Group: F1,355 = 13.72, p < 0.001). Including IHI in the model showed that volume decreases with overall IHI severity (main effect of IHI: F1,560 = 50.81, p < 0.001) and revealed a Group by Hemisphere interaction (Group × Hemisphere interaction: F1,358 = 4.42, p = 0.04). Follow-up tests showed a significantly greater effect of IHI on volume reductions of the right (t443 = −3.79, p < 0.001) than the left (t448 = −2.37, p = 0.04) hippocampus in schizophrenia patients. Including IHI as a fixed effect improved the Volume Model fit (Step 1: R2 = 0.47, AIC = 9777.27; Step 2: R2 = 0.49, AIC = 9724.85) (estimated marginal means of hippocampal volume are in Supplementary Fig. 1A). Our exploratory analysis investigating whether IHI has a different effect on volume in each group did not find evidence for an interaction (Group × IHI interaction: F1,560 = 1.21, p = 0.27).
Table 3.
Sensitivity analysis of hippocampal volume and asymmetry index.
| Volume (mm3) | |||||||
|---|---|---|---|---|---|---|---|
| Predictor | Coefficient | Coeff SD | df | F | p value | R2 | AIC |
| Step 1: model without IHI | 0.47 | 9777.3 | |||||
| Group | −86.89 | 28.96 | 1, 355 | 13.72 | <0.001 | ||
| Hemisphere | 234.31 | 14.83 | 1, 358 | 487.49 | <0.001 | ||
| ICV | 0.00 | 0.00 | 1, 355 | 188.41 | <0.001 | ||
| Age | −0.25 | 1.13 | 1, 355 | 0.05 | 0.82 | ||
| Sex | −30.62 | 36.47 | 1, 355 | 0.70 | 0.40 | ||
| Group:Hemisphere | −27.64 | 19.95 | 1, 358 | 1.92 | 0.17 | ||
| Step 2: model with IHI | 0.49 | 9724.9 | |||||
| Group | −67.40 | 28.49 | 1, 357 | 10.74 | 0.001 | ||
| Hemisphere | 211.85 | 14.46 | 1, 385 | 342.62 | <0.001 | ||
| IHI | −43.57 | 6.10 | 1, 560 | 50.81 | <0.001 | ||
| ICV | 1.37 | 0.10 | 1, 355 | 201.52 | <0.001 | ||
| Age | −0.28 | 1.11 | 1, 355 | 0.06 | 0.80 | ||
| Sex | −29.66 | 35.85 | 1, 355 | 0.68 | 0.41 | ||
| Group:Hemisphere | −40.10 | 19.06 | 1, 358 | 4.42 | 0.04 | ||
| Asymmetry index (R > L) | |||||||
|---|---|---|---|---|---|---|---|
| Predictor | Coefficient | Coeff SD | df | F | p value | R2 | AIC |
| Step 1: model without IHI | 0.13 | −1629.3 | |||||
| Group | −2.01E−02 | 7.99E−03 | 1, 355 | 0.54 | 0.46 | ||
| Region | −6.65E−02 | 7.05E−03 | 1, 355 | 110.35 | <0.001 | ||
| ICV | 3.52E−08 | 2.33E−08 | 1, 355 | 2.29 | 0.13 | ||
| Age | −8.67E−04 | 2.68E−04 | 1, 355 | 10.48 | 0.001 | ||
| Sex | −5.51E−03 | 8.63E−03 | 1, 355 | 0.41 | 0.52 | ||
| Group:Region | 3.07E−02 | 9.48E−03 | 1, 358 | 10.49 | 0.001 | ||
| Step 2: model with IHI | 0.17 | −1646.2 | |||||
| Group | −2.53E−02 | 7.85E−02 | 1, 354 | 2.54 | 0.11 | ||
| Region | −6.65E−02 | 7.05E−03 | 1, 358 | 110.35 | <0.001 | ||
| IHI | 1.15E−02 | 2.09E−03 | 1, 354 | 30.52 | <0.001 | ||
| ICV | 2.35E−08 | 2.24E−08 | 1, 354 | 1.10 | 0.30 | ||
| Age | −8.65E−04 | 2.57E−04 | 1, 354 | 11.29 | <0.001 | ||
| Sex | −3.26E−03 | 8.30E−03 | 1, 354 | 0.15 | 0.69 | ||
| Group:Region | 3.07E−02 | 9.48E-03 | 1, 358 | 10.49 | 0.001 | ||
AIC Akaike information criterion, ICV intracranial volume, IHI incomplete hippocampal inversion.
Bolded values indicate signifance at p < 0.05 level.
We conducted a similar analysis for hippocampal R > L volume asymmetry (Table 3). Without IHI in the model, we found volume asymmetry to be greater in healthy control participants (main effect of Group: F1,355 = 0.54, p = 0.46), but only in the anterior region (Group × Region interaction: F1,358 = 10.49, p < 0.01; anterior region: t654 = −2.51, p = 0.02; posterior region: t654 = 1.33, p = 0.37). Including IHI did not change these effects. Asymmetry index increases with overall IHI severity (main effect of IHI: F1,354 = 30.52, p < 0.001) and including IHI as a fixed effect improved the Asymmetry Index model (Step 1: R2 = 0.13, AIC = −1629.31; Step 2: R2 = 0.17, AIC = −1646.16) (estimated marginal means of hippocampal asymmetry index are in Supplementary Fig. 1B). Our exploratory analysis investigating whether IHI has a different effect on asymmetry index in each group did not find evidence for an interaction (Group × IHI interaction: F1,353 = 1.25, p = 0.26).
Discussion
Our study of 360 participants shows that IHI is significantly more prevalent and severe in schizophrenia. In addition, we demonstrate that IHI is strongly correlated with hippocampal volume, increases the R > L anterior hippocampal volume asymmetry, and contributes to hippocampal volume differences between schizophrenia patients and healthy control participants, particularly in the left hemisphere. To our knowledge, this is the first analysis of IHI in schizophrenia patients using a comprehensive set of quantitative and validated criteria [20].
IHI was more prevalent in schizophrenia patients in both the left and right hemisphere. Since IHI is the result of arrested brain development [40], our finding is consistent with the neurodevelopmental hypothesis of schizophrenia [57, 58]. The right hippocampus completes inversion before the left hippocampus during GW 20–30 [40]. Therefore, our finding of left > right IHI in schizophrenia is consistent with previous IHI studies [20, 36, 37] and maps the changes in schizophrenia to a later stage in hippocampal development.
After establishing increased prevalence of IHI in schizophrenia, we explored whether this variant could contribute to the well-established hippocampal structural differences in schizophrenia [4, 5, 44]. In our patient sample, we replicate findings of reduced left and right hippocampal volumes and demonstrate a reduced asymmetry index in the anterior hippocampus. Including IHI as a fixed effect in our statistical model revealed that it significantly contributes to the reduced hippocampal volume and increased R > L volume asymmetry in schizophrenia. Furthermore, including IHI as a fixed effect improved the quality of our models and accounted for more variability in our data. Lastly, inclusion of IHI in our model predicting hippocampal volume revealed a Group by Hemisphere interaction. Follow-up t-tests indicated that the inclusion of IHI decreased the between-group differences in the left but not the right hippocampus. Taken together, IHI is a significant contributor to both overall and R > L hippocampal volume in healthy control participants and schizophrenia patients.
Investigating the prevalence of IHI and its impact on hippocampal volume and asymmetry in the same study reveals an important linkage between these findings. Our results indicate IHI is more frequently found in the left hemisphere and contributes to volume reductions of both schizophrenia patients and healthy control participants; therefore, it is logical to presume that IHI should increase the R > L asymmetry index of the hippocampus. Here, we use a linear mixed model to confirm this presumption and establish IHI’s contribution to the asymmetry index for the first time. Future studies investigating the hippocampal asymmetry index should account for the prevalence of IHI in their sample.
IHI was more severe in the left hemisphere of schizophrenia patients. We found that criterion 3, i.e., the medial positioning of the hippocampus, differed most significantly between the two groups. This finding suggests a reduction in the width of the hippocampal body in schizophrenia. Numerous shape analyses conducted on the hippocampus of schizophrenia patients have described inward displacements on the medial or lateral surfaces of the left hippocampal head [6, 9, 59], body [59–61], and tail [61, 62]; however, whether IHI contributes to these findings is unknown. Future studies are needed to investigate the impact of IHI on shape differences observed between healthy control participants and schizophrenia patients.
It is important to note that of the 360 participants in this study, automated hippocampal segmentation failed in 48 participants. Automated segmentation was more likely to fail with hippocampi that met more severe IHI criteria. In addition, partitioning individual IHI criteria revealed that participants with failed segmentations have hippocampi with widespread IHI-like features. These findings indicate automated segmentation can successfully segment mild IHI, but fails when there are extensive changes to hippocampal structure. In studies using automated hippocampal segmentation to measure hippocampal volume, IHI can be a potential confound [39]. Specifically, if IHI is more severe or more frequent in a patient population and individuals with failed segmentations are excluded from the analysis, then the mean volume estimates may be biased and may underestimate the true volume difference. Here, we manually corrected automated segmentation failures, which allowed us to include all IHI cases, as we investigated the relationship of this anatomical variant with hippocampal structure.
Our study has several limitations. First, IHI criteria were only measured in the coronal view of the hippocampal body; therefore, IHI patterns located in the hippocampal head cannot be captured using the established protocol we used here [20]. Second, a recent study has shown a smaller CA1 volume in healthy participants with IHI [37], but our segmentation methods did not allow us to investigate hippocampal volumes at the subfield level because of manual corrections to the segmentations. Third, we did not examine the prevalence of other anatomic variants within the hippocampus [63] or in other brain structures associated with IHI [20, 24]. Future studies investigating the co-occurrence of IHI with other morphological variants will provide additional evidence that IHI is a marker of atypical development in schizophrenia and help determine the timing of developmental disruption. Lastly, we did not assess whether participants had a history of obstetric complications (i.e., a trigger for aberrant in utero development). Evidence suggests that obstetric complications may mediate hippocampal volume reductions in both healthy control participants and schizophrenia patients [64] and increase schizophrenia susceptibility [65]. Future studies should collect obstetric information to better elucidate the relationship between IHI, hippocampal volumes, and obstetric complications.
In conclusion, our finding of more prevalent and more severe IHI supports the neurodevelopmental hypothesis of schizophrenia. The impact of this developmental variant deserves further exploration in studies of the hippocampus in schizophrenia.
Supplementary information
Acknowledgements
Research reported in this publication was supported by the Charlotte and Donald Test Fund, the National Institute of Health (NIMH) grants R01-MH70560 (SH), R01-MH102266 (NDW), and R01-MH123563 (SV), Jack Martin, MD Research Professor in Psychopharmacology (JUB), the Vanderbilt Psychiatric Genotype/Phenotype Project, and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH) and National Institute of Health (NIH) grant T32-GM007347 (MJR).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41380-020-01010-z) contains supplementary material, which is available to authorized users.
References
- 1.Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J Neural Transm. 2002;109:891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haukvik UK, Tamnes CK, Söderman E, Agartz I. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:217–26. doi: 10.1016/j.jpsychires.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Roeske MJ, Konradi C, Heckers S, Lewis AS. Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: a systematic review and meta-analysis of postmortem studies. Mol Psychiatry. 2020;1–12. [DOI] [PMC free article] [PubMed]
- 4.Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74:1104–11. doi: 10.1001/jamapsychiatry.2017.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenton ME, Gerig G, McCarley RW, Székely G, Kikinis R. Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry Res. 2002;115:15–35. doi: 10.1016/s0925-4927(02)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Joshi SC, Miller MI, Csernansky JG. Statistical analysis of hippocampal asymmetry in schizophrenia. Neuroimage. 2001;14:531–45. doi: 10.1006/nimg.2001.0830. [DOI] [PubMed] [Google Scholar]
- 8.Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA. 1998;95:11406–11. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–6. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 10.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 11.Murray RM, Lewis SW, Lecturer L. Is schizophrenia a neurodevelopmental disorder? Br Med J. 1987;295:681–2. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphrey T. The development of the human hippocampal fissure. J Anat. 1967;101:655–76. [PMC free article] [PubMed] [Google Scholar]
- 13.Kier EL, Kim JH, Fulbright RK, Bronen RA. Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. Am J Neuroradiol. 1997;18:525–32. [PMC free article] [PubMed] [Google Scholar]
- 14.Raininko R, Bajic D. “Hippocampal malrotation”: no real malrotation and not rare. Am J Neuroradiol. 2010;31:3174. doi: 10.3174/ajnr.A2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennekam RC, Biesecker LG, Allanson JE, Hall JG, Opitz JM, Temple IK, et al. Elements of morphology: general terms for congenital anomalies. Am J Med Genet Part A. 2013;161:2726–33. doi: 10.1002/ajmg.a.36249. [DOI] [PubMed] [Google Scholar]
- 16.Baker LL, Barkovich AJ. The large temporal horn: MR analysis in developmental brain anomalies versus hydrocephalus. Am J Neuroradiol. 1992;13:115–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Bajic D, Wang C, Kumlien E, Mattsson P, Lundberg S, Eeg-Olofsson O, et al. Incomplete inversion of the hippocampus—a common developmental anomaly. Eur Radiol. 2008;18:138–42. doi: 10.1007/s00330-007-0735-6. [DOI] [PubMed] [Google Scholar]
- 18.Baulac M, De Grissac N, Hasboun D, Oppenheim C, Adam C, Arzimanoglou A, et al. Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol. 1998;44:223–33. doi: 10.1002/ana.410440213. [DOI] [PubMed] [Google Scholar]
- 19.Bernasconi N, Kinay D, Andermann F, Antel S, Bernasconi A. Analysis of shape and positioning of the hippocampal formation: an MRI study in patients with partial epilepsy and healthy controls. Brain. 2005;128:2442–52. doi: 10.1093/brain/awh599. [DOI] [PubMed] [Google Scholar]
- 20.Cury C, Toro R, Cohen F, Fischer C, Mhaya A, Samper-González J, et al. Incomplete hippocampal inversion: a comprehensive MRI study of over 2000 subjects. Front Neuroanat. 2015;9:160. doi: 10.3389/fnana.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajic D, Ewald U, Raininko R. Hippocampal development at gestation weeks 23 to 36. An ultrasound study on preterm neonates. Neuroradiology. 2010;52:489–94. doi: 10.1007/s00234-010-0673-x. [DOI] [PubMed] [Google Scholar]
- 22.Andrade D, Krings T, Chow EWC, Kiehl TR, Bassett AS. Hippocampal malrotation is associated with chromosome 22q11.2 microdeletion. Can J Neurol Sci. 2013;40:652–6. doi: 10.1017/s0317167100014876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, et al. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–28. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- 24.Atlas SW, Zimmerman RA, Bilaniuk LT, Rorke L, Hackney DB, Goldberg HI, et al. Corpus callosum and limbic system: neuroanatomic MR evaluation of developmental anomalies. Radiology. 1986;160:355–62. doi: 10.1148/radiology.160.2.3726113. [DOI] [PubMed] [Google Scholar]
- 25.Cury C, Scelsi MA, Toro R, Frouin V, Artiges E, Grigis A, et al. Genome wide association study of incomplete hippocampal inversion in adolescents. PLoS ONE. 2020;15:e0227355. doi: 10.1371/journal.pone.0227355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cachia A, Cury C, Brunelin J, Plaze M, Delmaire C, Oppenheim C, et al. Deviations in early hippocampus development contribute to visual hallucinations in schizophrenia. Transl Psychiatry. 2020;10:1–7. doi: 10.1038/s41398-020-0779-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitoz S, Atasoy C, Deda G, Erden I, Akyar S. Hippocampal malrotation with normal corpus callosum in a child with Opitz syndrome. Clin Imaging. 2003;27:75–6. doi: 10.1016/s0899-7071(02)00505-3. [DOI] [PubMed] [Google Scholar]
- 28.Montenegro MA, Kinay D, Cendes F, Bernasconi A, Bernasconi N, Coan AC, et al. Patterns of hippocampal abnormalities in malformations of cortical development. J Neurol Neurosurg Psychiatry. 2006;77:367–71. doi: 10.1136/jnnp.2005.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedl SW, Müllner-Eidenböck A, Prayer D, Bernert G, Frisch H. Auxological, ophthalmological, neurological and MRI findings in 25 Austrian patients with septo-optic dysplasia (SOD) Horm Res Paediatr. 2002;58:16–9. doi: 10.1159/000066484. [DOI] [PubMed] [Google Scholar]
- 30.Sato N, Hatakeyama S, Shimizu N, Hikima A, Aoki J, Endo K. MR evaluation of the hippocampus in patients with congenital malformations of the brain. Am J Neuroradiol. 2001;22:389–93. [PMC free article] [PubMed] [Google Scholar]
- 31.Barsi P, Kenéz J, Solymosi D, Kulin Á, Halász P, Rásonyi G, et al. Hippocampal malrotation with normal corpus callosum: a new entity? Neuroradiology. 2000;42:339–45. doi: 10.1007/s002340050895. [DOI] [PubMed] [Google Scholar]
- 32.Bajic D, Kumlien E, Mattsson P, Lundberg S, Wang C, Raininko R. Incomplete hippocampal inversion—is there a relation to epilepsy? Eur Radiol. 2009;19:2544–50. doi: 10.1007/s00330-009-1438-y. [DOI] [PubMed] [Google Scholar]
- 33.Lehericy S, Dormont D, Semah F, Clemenceau S, Granat O, Marsault C, et al. Developmental abnormalities of the medial temporal lobe in patients with temporal lobe epilepsy. Am J Neuroradiol. 1995;16:617–26. [PMC free article] [PubMed] [Google Scholar]
- 34.Beker-Acay M, Köken R, Ünlü E, Kaçar E, Balçık Ç. Evaluation of hippocampal infolding angle and incomplete hippocampal inversion in pediatric patients with epilepsy and febrile seizures. Diagn Interv Radiol. 2017;23:326–30. doi: 10.5152/dir.2017.160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emery SC, Karpinski NC, Hansen L, Masliah E. Abnormalities in central nervous system development in osteogenesis imperfecta type II. Pediatr Dev Pathol. 1999;2:124–30. doi: 10.1007/s100249900100. [DOI] [PubMed] [Google Scholar]
- 36.Colle R, Cury C, Chupin M, Deflesselle E, Hardy P, Nasser G, et al. Hippocampal volume predicts antidepressant efficacy in depressed patients without incomplete hippocampal inversion. NeuroImage Clin. 2016;12:949–55. doi: 10.1016/j.nicl.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colenutt J, McCann B, Knight MJ, Coulthard E, Kauppinen RA. Incomplete hippocampal inversion and its relationship to hippocampal subfield volumes and aging. J Neuroimaging. 2018;28:422–8. doi: 10.1111/jon.12509. [DOI] [PubMed] [Google Scholar]
- 38.Connor SEJ, Ng V, McDonald C, Schulze K, Morgan K, Dazzan P, et al. A study of hippocampal shape anomaly in schizophrenia and in families multiply affected by schizophrenia or bipolar disorder. Neuroradiology. 2004;46:523–34. doi: 10.1007/s00234-004-1224-0. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Chupin M, Colliot O, Bernhardt BC, Bernasconi N, Bernasconi A. Automatic hippocampal segmentation in temporal lobe epilepsy: impact of developmental abnormalities. Neuroimage. 2012;59:3178–86. doi: 10.1016/j.neuroimage.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 40.Bajic D, Canto Moreira N, Wikström J, Raininko R. Asymmetric development of the hippocampal region is common: a fetal MR imaging study. Am J Neuroradiol. 2012;33:513–8. doi: 10.3174/ajnr.A2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geschwind N, Galaburda AM. Cerebral lateralization: biological mechanisms, associations, and pathology: III. A hypothesis and a program for research. Arch Neurol. 1985;42:634–54. doi: 10.1001/archneur.1985.04060070024012. [DOI] [PubMed] [Google Scholar]
- 42.Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc. 2004;10:664–78. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- 43.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: meta-analyses of MRI studies. Hippocampus. 2009;19:1055–64. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 44.Woolard AA, Heckers S. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res. 2012;201:48–53. doi: 10.1016/j.pscychresns.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition with psychotic screen (SCID-I/P W/PSY SCREEN). Biometrics Research, New York State Psychiatric Institute: New York, NY, 2002.
- 46.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 47.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 48.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 49.Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–37. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 51.McHugo M, Talati P, Woodward ND, Armstrong K, Blackford JU, Heckers S. Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. NeuroImage Clin. 2018;20:1106–14. doi: 10.1016/j.nicl.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Family Med. 2005;37:360–3. [PubMed] [Google Scholar]
- 53.Bates D, Machler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 54.Lenth R. emmeans: Estimated marginal means, aka least-squares means. R Package Version 1.4.7. 2018. Available from: https://cran.r-project.org/package=emmeans.
- 55.Fox J, Weisberg S. Package ‘car’. Companion to applied regression. 2nd ed. Thousand Oaks, CA: Sage; 2011.
- 56.Barton K. MuMIn: multi-model inference. R Package Version 1.43.17. 2020. Available from: https://CRAN.R-project.org/package=MuMIn.
- 57.Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000;12:501–27. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- 58.Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346:552–7. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- 59.Lee JM, Kim SH, Jang DP, Ha TH, Kim JJ, Kim IY, et al. Deformable model with surface registration for hippocampal shape deformity analysis in schizophrenia. Neuroimage. 2004;22:831–40. doi: 10.1016/j.neuroimage.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Kalmady SV, Shivakumar V, Arasappa R, Subramaniam A, Gautham S, Venkatasubramanian G, et al. Clinical correlates of hippocampus volume and shape in antipsychotic-naïve schizophrenia. Psychiatry Res. 2017;263:93–102. doi: 10.1016/j.pscychresns.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 61.Mamah D, Harms MP, Barch D, Styner M, Lieberman JA, Wang L. Hippocampal shape and volume changes with antipsychotics in early stage psychotic illness. Front Psychiatry. 2012;3:96. doi: 10.3389/fpsyt.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Styner M, Lieberman JA, Pantazis D, Gerig G. Boundary and medial shape analysis of the hippocampus in schizophrenia. Med Image Anal. 2004;8:197–203. doi: 10.1016/j.media.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Maller JJ, Réglade-Meslin C, Thomson RHS, Daigle M, Barr MS, Daskalakis ZJ, et al. Hippocampal sulcal cavities in depression and healthy individuals. J Affect Disord. 2013;150:785–9. doi: 10.1016/j.jad.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 64.Ho BC, Magnotta V. Hippocampal volume deficits and shape deformities in young biological relatives of schizophrenia probands. Neuroimage. 2010;49:3385–93. doi: 10.1016/j.neuroimage.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cannon TD, Van Erp TGM, Rosso IM, Huttunen M, Lönnqvist J, Pirkola T, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59:35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.