Introduction
The kidney is affected by a wide range of disorders, most attributable to acquired disease. The rapid advancement and increased accessibility of genetic sequencing technologies has enabled diagnostic precision beyond traditional clinical and biopsy-based diagnosis in patients with otherwise uncertain hereditary kidney diseases, often in adulthood, with reported diagnostic yield ranges between 24% and 37% using whole exome sequencing.1,2 We report a family pedigree with at least 2 affected members (Figure 1a) carrying a novel missense mutation in the MYH9 gene, mimicking Alport syndrome at first glance.
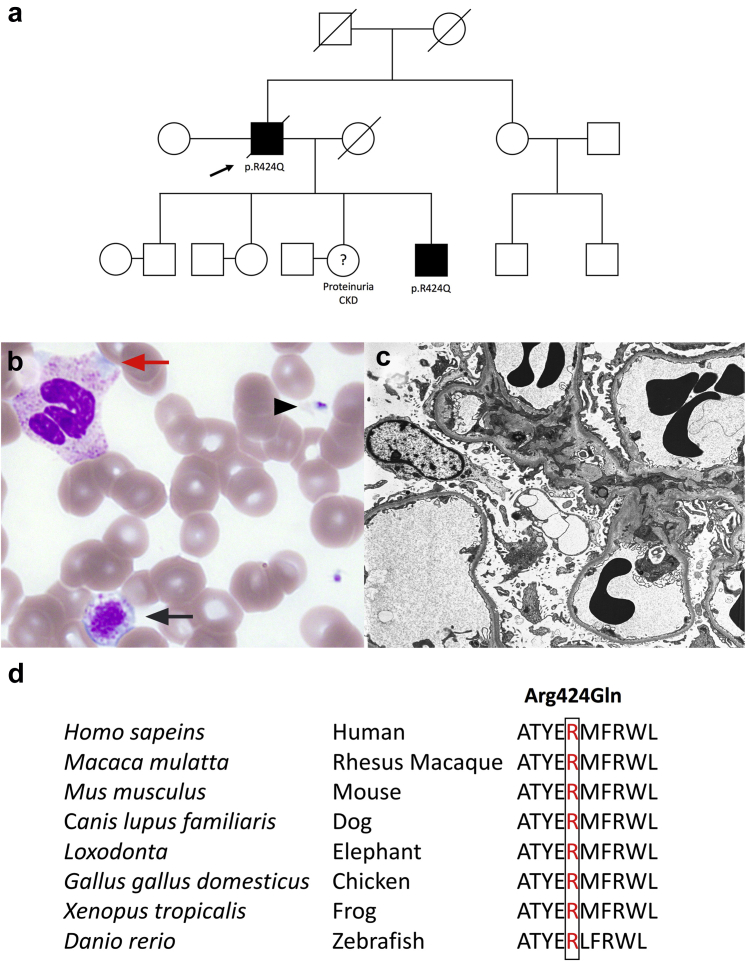
Figure 1.
Identification of the p.Arg424Gln mutation. (a) Family pedigree. Solid black symbol indicates affected disease status. The proband is indicated by the black arrow. (b) Normal platelet (black arrow head), giant platelet (black arrow), and Döhle-like cytoplasmic inclusion bodies (red arrow) in neutrophil are seen on peripheral blood smear. (c) Electron microscopy of kidney biopsy showed thinning of basement membrane and segmental podocyte foot processes effacement. (d) Amino acid conservation at Arg424 across different species (UCSC Genome Browser on Human Dec 2013).
Case Presentation
The proband, a 61-year-old Caucasian man, developed chronic kidney disease with nephrotic-range proteinuria 30 years before commencing dialysis. He was treated with a renin–angiotensin–aldosterone system inhibitor but did not have a kidney biopsy at the time of presentation. At age 50, he was found to have bilateral sensorineural hearing loss (SNHL) and mild, trilineage cytopenia (hemoglobin 119 g/l; neutrophil count 1.34 × 109/l and platelets 99 × 109/l) without a clinically evident bleeding tendency. Bone marrow biopsy and several peripheral blood smears were unremarkable, although a recent Wright-Giemsa stain of peripheral blood showed giant platelets and neutrophilic Döhle-like inclusion bodies (Figure 1b). His liver enzymes were mildly elevated (see Supplementary Table S1).
The proband’s son was also found to have nephrotic-range proteinuria (5.8 g/d) without hematuria, a serum creatinine level of 157 μmol/l, bilateral SNHL, and mild liver dysfunction. His blood film was normal. His initial kidney biopsy (at age 23) showed focal segmental glomerulosclerosis and a thin basement membrane. Progressive focal sclerosis and basement membrane thinning were found in 50% of the glomeruli on a repeat kidney biopsy 11 years later at age 34 (Figure 1c). With the supportive family history, SNHL, and thin basement membrane, a targeted-gene panel (COL4A3, COL4A4, and COL4A5) testing to search for Alport-related variants was performed but returned negative. A whole exome sequencing analysis was subsequently carried out, and both father and son were found to be heterozygous for a novel missense mutation in exon 12 of MYH9 (c.1271G>A; classified as a likely pathogenic variant according to the American College of Medical Genetics and Genomics Guidelines), resulting in the substitution of arginine for glutamine at amino acid position 424 (p.Arg424Gln). The lack of hematuria in both individuals, presence of familial glomerular kidney disease, and extrarenal manifestations, as well as the identification of a novel, likely pathogenic variant in the MYH9 gene, are all consistent with a diagnosis of MYH9-related disease (MYH9-RD). Other individuals in the family have been referred to clinical geneticists for formal evaluation.
Discussion
MYH9-RD is a rare autosomal dominant disorder caused by mutations in MYH9, which encodes for the heavy chain of nonmuscle myosin IIA (NMHC-IIA), a cytoskeletal contractile protein that is expressed in all hematopoietic cells as well as the kidneys, eyes, and ears. MYH9 is a well-conserved gene across different species (Figure 1d). NMHC-IIA plays important roles in cytokinesis, signal transduction, maintenance of cell shape, cell migration, polarization, and adhesion. Mature platelets and leukocytes exclusively rely on NMHC-IIA and are therefore most affected by its dysfunction.3 Until recently, 4 major autosomal dominant macrothrombocytopaenia syndromes were recognized: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome; each is distinguished through clinical and laboratory features. Mutations in the MYH9 gene have since been identified in all 4 syndromes, which are now considered to be a part of MYH9RD. Affected individuals display a wide clinical spectrum, varying from mild macrothrombocytopenia with leukocyte inclusions to a more severe form complicated by SNHL, presenile cataracts, kidney disease (manifesting as glomerular nephropathy), and liver enzyme abnormalities.
As of April 2021, 113 MYH9 variants are listed in the Human Gene Mutation Database,4 comprising 84 missense/nonsense mutations, 4 splicing substitutions, and 25 deletions/insertions. Several genotype–phenotype correlation studies in MYH9-RD patients have reported that variants in the head domain of NMHCII-A are associated with more severe thrombocytopenia, a higher frequency and more rapidly progressive nephropathy, and deafness compared with variants in the tail domain: the amino acid substitution p.Arg702Cys appearing to produce the most severe phenotype.5 However, Bury and colleagues recently found that 39% of patients with severe thrombocytopenia (defined as a platelet count <50 × 109/l) have a variant in the head domain while 61% of individuals have a variant in the coiled coil domain instead.6 The diagnosis for MYH9-RD remains challenging in clinical practice, due to heterogeneity in the syndromic manifestations, a low index of clinical awareness, inaccurate assessment of the degree of thrombocytopenia (due to the abnormal platelet size), easily overlooked Döhle-like inclusion bodies on Wright-Giemsa staining, and the inaccessibility of immunofluorescence testing for NMHC-IIA aggregates in most diagnostic laboratories, despite being recognized as the gold standard test.7 Therefore, it has been suggested that high-throughput sequencing might represent an alternative, comprehensive, and cost-effective diagnostic strategy in such cases.6,8
The kidney manifestations of MYH9-RD are highly heterogeneous, even within individuals carrying the same mutation. The spectrum of pathological findings in kidney biopsy on light microscopy include focal segmental glomerulosclerosis, mesangial expansion/proliferation with no immune deposits, interstitial fibrosis, and tubular atrophy and electron microscopy may further reveal focal foot process effacement and glomerular basement membrane abnormalities suggestive of Alport syndrome, such as irregular thinning and thickening of glomerular basement membrane with lamellated and basket-weave appearance,9 as in the proband’s son. Table 1 summarizes a list of differential diagnoses of oto-renal syndromes, together with the genes involved. Most of these syndromes have an early onset of clinical presentation, and specifically, Alport syndrome, Alström syndrome, branchio-oto-renal syndrome, Fabry disease and MYH9-RD are associated with glomerular disease. Alport syndrome shares several clinical features with MYH9-RD (nephritis, cataracts, and hearing impairment). However, these 2 syndromes can be differentiated by the mode of inheritance: Alport syndrome is most frequently associated with X-linked inheritance, whereas MYH9-RD is autosomal dominant. However, it is difficult to distinguish clinically between MYH9-RD and the autosomal dominant form of Alport syndrome. Importantly, the absence of thrombocytopenia does not exclude MYH9-RD. Patients could also be misdiagnosed with primary focal segmental glomerulosclerosis, often leading to unnecessary treatment with immunosuppressive agents. The renal prognosis is greatly variable, and the standard prognostic markers of chronic kidney disease progression (e.g., baseline kidney function, severity of proteinuria, and the presence of hypertension) are not useful in patients with MYH9-RD.9
Table 1.
Teaching points (S1–S3)
| Clinical diagnosis |
|---|
| A family history compatible with autosomal dominant transmission of the illness, the presence of giant platelets (macrothrombocytopenia), and/or Döhle-like inclusion bodies, sensorineural hearing loss, cataracts, or chronic kidney disease should raise suspicion for MYH9-related disease. |
| Differential diagnoses for oto-renal syndromes | |||
|---|---|---|---|
| Clinical syndrome | Manifestations |
Gene | |
| Ear | Kidney | ||
| Alport syndrome | Sensorineural hearing loss | Hematuria; kidney failure; ultrastructural changes of the glomerular basement membrane | COL4A3, COL4A4, COL4A5 |
| Alström syndrome | Sensorineural and conductive hearing loss | Glomerulosclerosis, tubular atrophy and interstitial fibrosis; nephrocalcinosis; recurrent urinary tract infections; urethral dysnergia | ALMS1 |
| Autosomal recessive distal renal tubular acidosis | Sensorineural hearing loss | Hypokalaemic hyperchloraemic metabolic acidosis | ATP6V1B1, ATP6V0A4 |
| Bartter syndrome type 4A (or 4B) | Sensorineural hearing loss | Diabetes insipidus; renal salt wasting; kidney failure | BSND (both CLCNKA and CLCNKB) |
| Branchio-oto-renal (BOR) syndrome | Hearing loss; preauricular pits; auricular malformations; atresia to stenosis of the external auditory canal; underdeveloped cochlea and semicircular canals | Duplications of collecting system; renal hypoplasia; cystic dysplasia and agenesis; hydronephrosis; ureteropelvic junction obstruction; vesicoureteral reflux; basement membrane splitting and mesangial proliferation | EYA1, SIX5, SIX1 |
| Fabry disease | Hearing loss | Glycolipid deposits in glomerular, tubular epithelial and vascular cells; segmental and global glomerulosclerosis; tubular atrophy and interstitial fibrosis; kidney failure | GLA |
| Hypoparathyroidism, sensorineural deafness, and renal anomalies syndrome (Barakat syndrome) | Sensorineural hearing loss | Congenital anomalies of the kidney and urinary tract (cystic, dysplastic, hypoplastic or aplastic kidneys, pelvicalyceal deformity, vesicoureteral reflux) | GATA3 |
| Kallmann syndrome | Hearing loss | Renal agenesis | ANOS1, CHD7, FGF8, FGFR1, PROK2, PROKR2 |
| Mitochondrial encephalopathy, lactic acidosis, stroke-like episodes (MELAS) | Hearing loss | Fanconi syndrome; focal segmental glomerulosclerosis; kidney failure | mtDNA point mutations |
| MYH9-related disease | Sensorineural hearing loss | Hematuria; proteinuria; kidney failure; focal segmental glomerulosclerosis; irregular thinning and thickening of glomerular basement membrane with lamellated and basket-weave appearance | MYH9 |
| Pendred syndrome | Sensorineural hearing loss; enlarged vestibular aqueduct | Acid–base disturbances | SLC26A4 |
| Townes-Brocks syndrome | External ear anomalies; hearing loss | Dysplastic kidneys or agenesis; horseshoe kidney; multicystic kidney; posterior urethral valves; vesicoureteral reflux; kidney failure | SALL1 |
| X-linked hypophosphatemia | Hearing loss | Hypophosphatemia; kidney stone | PHEX |
This case highlights that the combination of thrombocytopenia, chronic kidney disease, deafness, and a supportive family history should raise clinical suspicions for rare hereditary kidney diseases other than Alport syndrome. A targeted-gene panel can be a cost-effective first-line test; however, this technique could also potentially miss a genetic diagnosis. Both whole exome and genome sequencing provide unbiased screening of variants, with high diagnostic sensitivity. They are extremely useful for reanalysis but are more time-consuming and challenging with respect to the interpretation of findings. It is important to establish a genetic diagnosis because it has profound implications on the provision of care for patients affected by chronic kidney disease. It informs prognosis with disease-focused surveillance, avoids inappropriate management by sparing unnecessary immunosuppressive treatment or invasive diagnostic procedures (such as kidney biopsy), guides family planning and genetic counseling, enables screening of biologically related living kidney donors to guide donor selection for transplantation, and may inform the risk of disease recurrence following kidney transplantation.
Disclosure
All the authors declared no competing interests.
Patient Consent
The authors obtained consent from the patients discussed in the report.
Acknowledgment
L.W. is supported by the 2021 RACP Jacquot Research Establishment Fellowship (2021REF00021) and the APH Philanthropic Foundation Research and Innovation Grant.
Footnotes
Supplementary Material
Table S1. Clinical and laboratory findings.
Supplementary References
References
- 1.Connaughton D.M., Kennedy C., Shril S., et al. Monogenic causes of chronic kidney disease in adults. Kidney Int. 2019;95:914–928. doi: 10.1016/j.kint.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lata S., Marasa M., Li Y., et al. Whole-exome sequencing in adults with chronic kidney disease: A pilot study. Ann Intern Med. 2018;168:100–109. doi: 10.7326/M17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pecci A., Ma X., Savoia A., et al. Myh9: structure, functions and role of non-muscle myosin IIa in human disease. Gene. 2018;664:152–167. doi: 10.1016/j.gene.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenson P.D., Mort M., Ball E.V., et al. The human gene mutation database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133:1–9. doi: 10.1007/s00439-013-1358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saposnik B., Binard S., Fenneteau O., et al. Mutation spectrum and genotype-phenotype correlations in a large French cohort of MYH9-related disorders. Mol Genet Genomic Med. 2014;2:297–312. doi: 10.1002/mgg3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bury L., Megy K., Stephens J.C., et al. Next-generation sequencing for the diagnosis of MYH9-RD: predicting pathogenic variants. Hum Mutat. 2020;41(1):277–290. doi: 10.1002/humu.23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunishima S., Matsushita T., Kojima T., et al. Immunofluorescence analysis of neutrophil nonmuscle myosin heavy chain-a in MYH9 disorders: association of subcellular localization with MYH9 mutations. Lab Invest. 2003;83:115–122. doi: 10.1097/01.lab.0000050960.48774.17. [DOI] [PubMed] [Google Scholar]
- 8.Rabbolini D.J., Chun Y., Latimer M., et al. Diagnosis and treatment of MYH9-RD in an Australasian cohort with thrombocytopenia. Platelets. 2018;29(8):793–800. doi: 10.1080/09537104.2017.1356920. [DOI] [PubMed] [Google Scholar]
- 9.Tabibzadeh N., Fleury D., Labatut D., et al. MYH9-related disorders display heterogeneous kidney involvement and outcome. Clin Kidney J. 2019;12:494–502. doi: 10.1093/ckj/sfy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.