Abstract
Background
Existing studies evaluating the association between schizophrenia and complications associated with pregnancy, delivery and neonatal outcomes are based on data prior to 2014 and have reported heterogeneous results. The objective of our study was to determine whether pregnant women with schizophrenia were at increased risk of pregnancy, delivery and neonatal complications compared with women without severe mental disorders.
Methods
We performed a population-based cohort study of all singleton deliveries in France between Jan. 1, 2015, and Dec. 31, 2019. We divided this population into cases (i.e., women with schizophrenia) and controls (i.e., women without a diagnosis of severe mental disorder). Cases and controls were matched (1:4) inside the same hospital and the same year by age, social deprivation, parity, smoking, alcohol and substance addictions, malnutrition, obesity, and comorbidities. Univariate and multivariate models with odds ratios and 95% confidence intervals (ORs [95% CIs]) were used to estimate the association between schizophrenia and 24 pregnancy, delivery and neonatal outcomes.
Findings
Over 5 years, 3,667,461 singleton deliveries were identified, of which 3,108 occurred in women with schizophrenia. Compared to controls, women with schizophrenia were found to be older; have more frequent smoking, alcohol and substance addictions; suffer from obesity, diabetes and chronic obstructive pulmonary disease; and often be hospitalized in tertiary maternity hospitals. Compared to matched controls, women with schizophrenia had more pregnancy complications (adjusted OR=1.41[95%CI 1.31-1.51]) (i.e., gestational diabetes, gestational hypertension, genito-urinary infection, intrauterine growth retardation and threatened preterm labour). They had more delivery complications (aOR=1.18[95%CI 1.09 1.29]) with more still births/medical abortions (aOR=2.17[95%CI 1.62-2.90]) and caesarean sections (aOR=1.15[95%CI 1.05-1.25]). Newborns of women with schizophrenia had more neonatal complications (aOR=1.38[95%CI 1.27-1.50]) with more born preterm (aOR=1.64[95%CI1.42 -1.90]), small for gestational age (aOR=1.34[95%CI 1.19-1.50]) and low birth weight (aOR=1.75[95%CI 1.53-2.00]).
Interpretation
Our results highlight the importance of health disparities between pregnant women with and without schizophrenia, as well as in their newborns. Our study calls for health policy interventions during and before pregnancy, including proportionate intensified care to the level of needs, effective case management and preventive and social determinant approaches.
Funding
No funding.
Research in context.
Evidence before this study
Previous studies have provided heterogeneous results due to healthcare evolution, country health policies, and various adjustment/matching factors. We searched PubMed for studies published in English between database inception and Oct 1, 2020, using the MeSH terms “pregnancy”, AND “schizophrenia” OR “psychotic disorder”. Most of the evidence published thus far has targeted preeclampsia, delivery type, preterm birth and birth weight, neglecting other pregnancy, delivery and neonatal outcomes. The most recent available data were from 2014. Our knowledge about pregnancy in schizophrenia is insufficient, and updated data are needed to develop further guidelines and early structured interventions.
Added value of this study
In this national population-based study, we collected a wide range of sociodemographic and health factors, and we studied 24 pregnancy, delivery and neonatal outcomes in more than 3 million women who had a singleton birth between January 1st, 2015, and December 31st, 2019. Schizophrenia was associated with increased metabolic disturbances and addictions and remained associated with still births, medical abortion, caesarean section, preterm birth, small for gestational age and low birth weight after matching and adjustment for common pregnancy risk factors. Less than one-third of pregnant women with schizophrenia received regular contacts with ambulatory psychiatric care.
Implications of all the available evidence
This study highlights the importance of health disparities between pregnant women with and without schizophrenia as well as in their newborns. Our findings suggest preliminary recommendations to pursue efforts for the prevention of metabolic risk and genito-urinary infections; to promote early smoking cessation, alcohol abstinence and substance misuse cessation; and to develop specific programmes for other unmet needs during and before pregnancy.
Alt-text: Unlabelled box
1. Introduction
Addressing inequalities that lead to and arise from mental disorders is an essential component of a worldwide mental health strategy [1]. Early interventions appear essential to improve the prognosis of mental disorders [2], prevent and/or reduce the occurrence of health-related inequalities worsened by health-risk behaviours [1] and tackle the intergenerational transmission of these inequalities [3]. Pregnancy is increasingly considered a unique opportunity to identify early serious health conditions and health-risk behaviours in young women with mental disorders and to prevent future health problems for these mothers and their children. Pregnancy unmasks chronic diseases, such as cardiovascular and metabolic diseases, that may occur later in life [4], [5], [6]. Health-risk behaviours are associated with pregnancy and neonatal complications and short- and long-term negative impacts on child health, including behavioural problems, cognitive impairment, diabetes and cardiovascular diseases [7], [8], [9], [10], [11]. Epidemiological studies have reported that approximately 20% of children live in a household with a parent with mental disorders [12,13] and that they are at significantly greater risk of poor mental and physical health than the offspring of parents without mental illness [14,15].
Among pregnant women with mental disorders, those with schizophrenia represent an extremely vulnerable and underserved population that has been neglected in research [16,17]. Schizophrenia affects around 1% of the population and contributes to a large worldwide burden with more than 12 million years of life lived with disability [18,19]. The incidence peak in schizophrenia occurs twice in women aged 20-29 and 30-39 years [20], which overlaps with the childbearing period. This is therefore a critical period for early intervention to prevent poor mental illness trajectory [2]. In recent years, several countries (e.g., the United Kingdom, Australia) have invested in programmes to improve maternal mental health care; however, most of the current perinatal mental health programmes are focused on depression and sometimes bipolar disorders but not schizophrenia [21], [22], [23], [24]. The current guidelines do not address [25,26] or poorly address [27] the therapeutic challenges for the care of pregnancy in schizophrenia. In summary, the current recommendations are to maintain (or initiate) antipsychotic treatment at the lowest dose possible [28] with an add-on of cognitive behavioural therapy if needed. These recommendations are not always adapted in daily practice. Mothers with schizophrenia may refuse antipsychotic treatments due to poor insight into illness, delusions or fear of congenital malformations. Psychotherapy is poorly accessed by schizophrenia patients due to financial difficulties and care offer disparities. Moreover, pregnant women with schizophrenia require multiple unmet needs. Women with schizophrenia are at risk of smoking, alcohol and other drug addictions with a negative impact on foetal development, leading to increased preterm birth and perinatal death [29], [30], [31]. Pregnant women with schizophrenia have a lower prenatal consultation attendance rate than women without schizophrenia [32]. However, these consultations are essential to detect obesity, gestational diabetes, hypertension and other metabolic dysfunctions that are more frequent in schizophrenia [33] and independent risk factors for perinatal complications [34,35]. Schizophrenia also has a strong impact on professional and social functioning, leading to low social status [36], which has been identified as an independent risk factor for perinatal issues [37]. To date, studies on schizophrenia and pregnancy, delivery and neonatal outcomes have reported heterogeneous results due to healthcare evolution, country health policies, and heterogeneous adjustment/matching factors [38]. Most of the evidence published thus far has targeted preeclampsia, delivery type, preterm birth and birth weight, neglecting other pregnancy, delivery and neonatal outcomes. The most recent available data are from 2014 [39].
Updated data are thus needed to propose tailored prevention and early intervention programmes for pregnant women with schizophrenia. To overcome the limitations of previous works, we carried out a population-based study exploring 24 pregnancy, delivery and neonatal outcomes and a large rank of sociodemographic and health factors in more than 3 million women who had a singleton delivery between January 1, 2015, and December 31, 2019. The objective of our study was to determine whether pregnant women with schizophrenia were at increased risk of pregnancy, delivery and neonatal complications compared with women without severe mental disorders.
2. Methods
2.1. Study design and data sources
In this population-based cohort study, we used data from Programme de Médicalisation des Systèmes d'Information (PMSI), the French national hospital database in which administrative and medical data are systematically collected for all obstetrical, acute and psychiatric hospital care facilities. The PMSI database has a system of coding with strict variable definitions which is the same for all the hospitals in France and a subset of records audited on a regular basis to avoid excessively high rates of coding errors. This database is based on diagnosis-related groups, with all diagnoses coded according to the 10th revision of the International Classification of Diseases (ICD-10). The inclusion and exclusion criteria were as follows: all women who had a singleton live birth, stillbirth or medical abortion between January 1st 2015 and December 31st 2019 were included. Women with multiple births, incorrect or missing maternal/newborn personal identification numbers and a diagnosis of severe mental disorder other than schizophrenia (i.e., bipolar disorders and recurrent major depressive disorders) were excluded.
Data in the PMSI are anonymized and can be reused for research purposes. No informed consent was necessary because all data were anonymous. A unique anonymous identifier enables to link the different inpatient stays of the patients. This study was declared to the French National Data Protection Commission in accordance with the methodological reference MR005 (declaration number: 2203797).
2.2. Procedure
For the purposes of our study, we defined two unmatched cohorts of cases and controls and two matched cohorts of cases and controls.
Cases were women who had a diagnosis of schizophrenia according to specific ICD-10 codes (i.e., F20* schizophrenia, F22* persistent delusional disorder, or F25* schizoaffective disorder) in either the obstetrical, acute or psychiatric hospital care databases in the 4 years preceding the index date (or date of delivery). The severity of the disorder is not captured in this database and all cases were included (stabilized or non-stabilized illnesses). Controls were women without severe mental disorders (who did not have a diagnosis of schizophrenia, bipolar disorders (F30*, F31*) or recurrent major depressive disorder (F33*)). To avoid the selection of ‘super-healthy’ controls, all other F* ICD-10 codes were kept in both groups (e.g., single major depressive disorder, anxiety disorders, eating disorders and personality disorders).
Then, two matched cohorts were formed using exact matching inside the same hospital and the same year. The control cohort was matched to cases in a 1:up to 4 variable matching ratio according to the following criteria: age (+/-3 years), social deprivation (more favoured, more deprived), parity (primiparous, multiparous), smoking, alcohol and substance addictions (yes, no), poor maternal nutrition (yes, no), obesity (yes, no) and comorbidities (Charlson score: 0, 1-2, ≥3).
2.3. Pregnancy, delivery and neonatal outcomes
Twenty-four outcomes were studied and were combined into three composite pregnancy, delivery and neonatal outcomes. The definitions and specific codes used for each outcome are listed in Appendix 1.
A pregnancy complication rate was computed, defined as the presence of at least one of the 14 complications recorded from the beginning of pregnancy up to delivery: gestational diabetes, gestational hypertension, preeclampsia, eclampsia, HELLP syndrome, antepartum haemorrhage, placental abruption, placental abnormalities, thrombosis, genito-urinary infection, chorioamnionitis, intrauterine growth restriction, premature rupture of membranes and threatened preterm labour.
A delivery complication rate was computed, defined as the presence of at least one of the 4 complications recorded during the index stay: foetal death (stillbirth or medical abortion), caesarean section, postpartum haemorrhage and maternal mortality.
A neonatal complication rate was computed, defined as the presence of at least one of the 6 complications recorded during the index stay: preterm birth (gestational age < 37 weeks), small for gestational age (defined as birth weight < 10th percentile), large for gestational age (defined as birth weight > 90th percentile), low birth weight (defined as birth weight < 2500 g regardless of gestational age), major congenital malformation (based on European surveillance network for congenital anomalies codes [40]) and neonatal mortality (up to 28 days after birth).
2.4. Data collected
The following sociodemographic, clinical, and hospital characteristics were collected during pregnancy due to their potential impact on pregnancy, delivery and neonatal outcomes: age, social deprivation, parity, smoking, alcohol and substance addictions, poor maternal nutrition, obesity (body mass index ≥30), somatic comorbidities, hospital category and levels of maternal care at admission. Psychiatric care (inpatient care, day-hospital or ambulatory care and no care) was collected for women with schizophrenia during pregnancy.
Social deprivation was assessed with the FDep09, an index that has been previously validated with French data and that is based on the residential code of the person's address [41]. The FDep09 index involves four socioeconomic ecological variables: the proportion of residents who graduated from high school, median household income, percentage of residents who are blue-collar workers, and unemployment rate. We classified these area data according to two categories using median threshold: more favoured vs. more deprived.
Comorbidities were assessed with the Charlson modified Comorbidity Index [42], which was computed from ICD-10 codes recorded as primary or secondary diagnoses over the course of the last year before delivery.
All the ICD-10 codes are listed in Appendix 1.
2.5. Statistical analysis
Analyses were performed in two steps.
Before matching, sociodemographic, clinical, and hospital characteristics were compared between cases and controls using standardized differences. Standardized differences (SD) use effect size methods to identify meaningful differences between groups that, unlike p-values, are not influenced by sample size [43].
After exact matching, we performed as many univariate and multivariate analyses as there were outcomes to analyse the association between cases and matched controls for each outcome. We conducted multivariable logistic regression models adjusted for the matching factors to account for any residual confounding and based on the generalized estimating equation (GEE) framework to take into account correlations within matched clusters (SAS GLIMMIX procedure). Associations were reported as adjusted odds ratios (ORs) and associated 95% confidence intervals (95% CIs).
As complementary analyses, we described psychiatric care for women with schizophrenia during pregnancy. We arbitrarily defined the optimal psychiatric care by 7 contacts or more with ambulatory psychiatric settings (consultations or day hospital) based on a monthly follow-up during pregnancy and taking into account potential preterm births. Given that pregnancy should be associated with intensive/reinforced/multi-intervention psychiatric care, this threshold was the lowest threshold to define optimal psychiatric care. Last, we compared pregnancy, delivery and neonatal outcomes between women with schizophrenia, delusional disorder and schizoaffective disorder.
P values were corrected using the false discovery rate method to take into account multiple testing [44] due to the multiplicity of outcomes. A significance of p<0•05 was used. All analyses were performed in SAS (version 9.4).
2.6. Role of the funding source
No funder had any role in the study design, data collection, data analysis, interpretation, the writing of the report or decisions on where to publish.
3. Results
Over 5 years, 3,741,016 pregnancies were identified, and 3,667,461 resulted in a singleton delivery according to the inclusion criteria (see Fig. 1 for flowchart). Overall, 3,108 women with schizophrenia were classified as cases, and 3,664,353 women without a diagnosis of severe mental disorder were classified as controls. Then, 2,659 women with schizophrenia were matched with 10,243 controls.
Fig. 1.
Flowchart.
In the unmatched analysis (Table 1), women with schizophrenia were found to be older (SD=0.20); have more frequent smoking (SD=0.40), alcohol (SD=0.17) and substance (SD=0.27) addictions, obesity (SD=0.18) and physical comorbidities (SD=0.22) (i.e., diabetes and chronic obstructive pulmonary disease); and be hospitalized more often in academic hospitals (SD=0.35) and tertiary maternity hospitals (SD=0.33) than controls.
Table 1.
Characteristics of women with schizophrenia and women without severe mental disorder in the unmatched and matched cohorts.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | Women with schizophrenia (N=3 108) N(%) | Control group (N=3 664 353) N (%) | Standardized differences | Women with schizophrenia (N=2 659) ``N (weighted %) | Control group (N=10 243) N (weighted %) | Standardized differences |
| Socio-demographic data | ||||||
| Age, mean (SD) | 31.4(5.9) | 30.3(5.3) | 0.2 | 31.4(5.8) | 31.2(5.4) | 0.04 |
| Age classes | 0.23 | 0.1 | ||||
| 15-19 years | 56(1.8) | 75348(2.1) | 40(1.5) | 117(1.2) | ||
| 20-24 years | 355(11.4) | 447400(12.2) | 300(11.3) | 1108(10.8) | ||
| 25-29 years | 758(24.4) | 1117301(30.5) | 658(24.8) | 2739(26.6) | ||
| 30-34 years | 950(30.1) | 1228074(33.5) | 827(31.1) | 3347(32.5) | ||
| ≥ 35 years | 989(31.8) | 796230(21.7) | 834(31.4) | 2932(29.0) | ||
| Social deprivation | 0.05 | 0 | ||||
| More favoured | 1408(45.3) | 1734689(47.3) | 1317(49.5) | 5134(50.0) | ||
| More deprived | 1489(47.9) | 1735819(47.4) | 1342(50.5) | 5109(50.0) | ||
| Missing | 211(6.8) | 193845(5.3) | 0(0) | 0(0) | ||
| Clinical data | ||||||
| Primiparous | 1560(50.2) | 1634234(44.6) | 0.11 | 1353(50.9) | 5179(50.9) | 0 |
| Smoking addiction | 447(14.4) | 119275(3.3) | 0.4 | 292(11.0) | 968(11.0) | 0 |
| Alcohol addiction | 51(1.6) | 1762(0.1) | 0.17 | 2(0.1) | 2(0.1) | 0 |
| Substance addiction | 127(4.1) | 7434(0.2) | 0.27 | 29(1.1) | 55(1.1) | 0 |
| Poor maternal nutrition | 10(0.3) | 3842(0.1) | 0.05 | 1(0.0) | 1(0.0) | 0 |
| Obesity | 230(7.4) | 123779(3.4) | 0.18 | 159(6.0) | 546(6.0) | 0 |
| Charlson score | 0.22 | 0 | ||||
| 0 | 2917(93.9) | 3592406(98.0) | 2561(96.3) | 9956(96.3) | ||
| 01-Feb | 166(5.3) | 65023(1.8) | 88(3.3) | 263(3.3) | ||
| ≥3 | 25(0.8) | 6924(0.2) | 10(0.4) | 24(0.4) | ||
| Charlson comorbidities | ||||||
| Renal disease | 5(0.2) | 1354(0.0) | 0.04 | 3(0.1) | 6(0.1) | 0.01 |
| Liver mild disease | 23(0.7) | 10728(0.3) | 0.06 | 11(0.4) | 44(0.6) | 0.02 |
| Liver moderate/severe disease | 0(0.0) | 356(0.0) | 0.01 | 0(0.0) | 0(0.0) | 0 |
| Peptic ulcer | 2(0.1) | 774(0.0) | 0.02 | 0(0.0) | 4(0.1) | 0.04 |
| Chronic pulmonary disease | 68(2.2) | 31744(0.9) | 0.11 | 33(1.2) | 114(1.3) | 0.01 |
| Congestive heart failure | 7(0.2) | 1165(0.0) | 0.05 | 2(0.1) | 6(0.1) | 0 |
| Myocardial infarction | 1(0.0) | 203(0.0) | 0.02 | 1(0.0) | 0(0.0) | 0.03 |
| Peripheral vascular disease | 3(0.1) | 514(0.0) | 0.04 | 2(0.1) | 4(0.0) | 0.02 |
| Cerebrovascular disease | 3(0.1) | 1962(0.1) | 0.02 | 2(0.1) | 3(0.0) | 0.02 |
| Dementia | 1(0.0) | 37(0.0) | 0.02 | 1(0.0) | 0(0.0) | 0.03 |
| Hemi/Paraplegia | 11(0.4) | 1885(0.1) | 0.07 | 6(0.2) | 4(0.0) | 0.05 |
| Rheumatic disease | 7(0.2) | 4076(0.1) | 0.03 | 4(0.2) | 25(0.4) | 0.05 |
| Metastatic solid tumour | 0(0.0) | 282(0.0) | 0.01 | 0(0.0) | 1(0.0) | 0.01 |
| Malignancy | 3(0.1) | 2271(0.1) | 0.01 | 1(0.0) | 8(0.1) | 0.02 |
| Complicated diabetes | 8(0.3) | 1756(0.1) | 0.05 | 3(0.1) | 9(0.1) | 0 |
| Non complicated diabetes | 55(1.8) | 11872(0.3) | 0.14 | 28(1.1) | 48(0.7) | 0.04 |
| AIDS/HIV | 14(0.5) | 4743(0.1) | 0.06 | 6(0.2) | 21(0.3) | 0.02 |
| Hospital data | ||||||
| Hospital type | 0.35 | 0 | ||||
| Public | 1718(55.3) | 2065583(56.4) | 1510(56.8) | 5844(56.8) | ||
| Academic | 1009(32.5) | 739276(20.2) | 811(30.5) | 3078(30.5) | ||
| Private | 381(12.3) | 859494(23.5) | 338(12.7) | 1321(12.7) | ||
| Maternity level | 0.33 | 0 | ||||
| 1 | 394(12.7) | 759585(20.7) | 349(13.1) | 1356(13.1) | ||
| 2 | 1376(44.3) | 1831649(50.0) | 1239(46.6) | 4818(46.6) | ||
| 3 | 1271(40.9) | 989030(27.0) | 1017(38.3) | 3859(38.3) | ||
| No maternity | 67(2.2) | 84089(2.3) | 54(2.0) | 210(2.0) | ||
N: effective; %: percentage; SD: standard deviation; standardized differences greater than 0.10 are clinically significant and are indicated in bold. After matching, the percentages are weighted to consider 1:4 max algorithm matching.
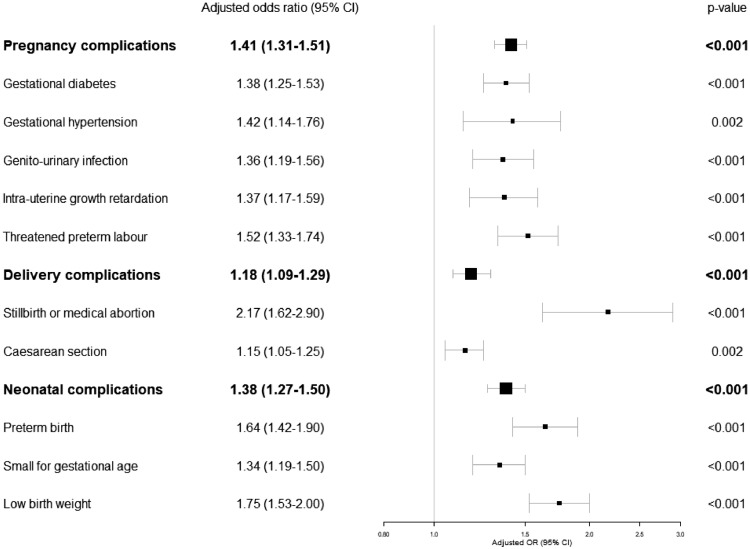
In the matched analyses (Table 2), women with schizophrenia had more pregnancy complications (adjusted OR=1.41[95% CI 1.31-1.51]) (i.e., gestational diabetes, gestational hypertension, genito-urinary infection, intrauterine growth retardation and threatened preterm labour) than controls. The admission rate in the high-risk pregnancy care unit was higher in women with schizophrenia than in controls (8.5 vs 4.6%, p <0.0001). No significant difference was found for preeclampsia (p=0.32), eclampsia (p=0.90) or HELLP syndrome (p=0.50).
Table 2.
Pregnancy, delivery and neonatal complications of women with schizophrenia and women without severe mental disorder in the matched cohorts.
| Women with schizophrenia (n=2 659)N(weighted %) | Control group (n=10 243) N(weighted %) | Unadjusted odds ratio (95% CI) | p-value | Adjusted odds ratio* (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Pregnancy complications | 1204(45.3) | 3729(37.2) | 1.40(1.31-1.50) | <0.001 | 1.41(1.31-1.51) | <0.001 |
| Gestational diabetes | 428(16.1) | 1207(12.1) | 1.39(1.26-1.53) | <0.001 | 1.38(1.25-1.53) | <0.001 |
| Gestational hypertension | 89(3.4) | 232(2.4) | 1.44(1.17-1.78) | 0.002 | 1.42(1.14-1.76) | 0.002 |
| Preeclampsia | 78(2.9) | 247(2.6) | 1.14(0.92-1.41) | 0.32 | - | - |
| Eclampsia | 3(0.1) | 10(0.1) | 1.09(0.38-3.12) | 0.9 | - | - |
| HELLP syndrome | 8(0.3) | 23(0.2) | 1.30(0.66-2.55) | 0.5 | - | - |
| Antepartum haemorrhage | 54(2.0) | 188(1.8) | 1.14(0.88-1.47) | 0.39 | - | - |
| Placental abruption | 18(0.7) | 50(0.5) | 1.39(0.88-2.20) | 0.27 | - | - |
| Placental abnormalities | 30(1.1) | 97(0.9) | 1.20(0.85-1.69) | 0.38 | - | - |
| Thrombosis | 7(0.3) | 17(0.2) | 1.65(0.76-3.58) | 0.31 | - | - |
| Genito-urinary infection | 211(7.9) | 617(6.0) | 1.35(1.18-1.55) | <0.001 | 1.36(1.19-1.56) | <0.001 |
| Chorioamnionitis | 21(0.8) | 55(0.6) | 1.43(0.93-2.19) | 0.19 | - | - |
| Intra-uterine growth retardation | 169(6.4) | 455(4.8) | 1.35(1.16-1.58) | <0.001 | 1.37(1.17-1.59) | <0.001 |
| Premature rupture of membranes | 344(12.9) | 1252(12.4) | 1.05(0.94-1.16) | 0.45 | - | - |
| Threatened preterm labour | 231(8.7) | 592(5.9) | 1.51(1.32-1.72) | <0.001 | 1.52(1.33-1.74) | <0.001 |
| Delivery complications | 731(27.5) | 2423(24.2) | 1.19(1.10-1.28) | <0.001 | 1.18(1.09-1.29) | <0.001 |
| Birth | ||||||
| Live birth | 2600(97.8) | 10137(99.0) | 1 | 1 | ||
| Stillbirth or medical abortion | 59(2.2) | 106(1.0) | 2.19(1.63-2.94) | <0.001 | 2.17(1.62-2.90) | <0.001 |
| Caesarean section | 672(25.9) | 2271(23.3) | 1.15(1.06-1.25) | 0.002 | 1.15(1.05-1.25) | 0.002 |
| Postpartum haemorrhage | 127(4.8) | 527(5.2) | 0.92(0.78-1.07) | 0.37 | - | - |
| Mortality at 42 days | 0(0.0) | 0(0.0) | - | - | - | - |
| Neonatal complications | 760(32.8) | 2158(26.2) | 1.38(1.27-1.49) | <0.001 | 1.38(1.27-1.50) | <0.001 |
| Preterm birth | 226(9.8) | 496(6.2) | 1.64(1.42-1.89) | <0.001 | 1.64(1.42-1.90) | <0.001 |
| Small for gestational age | 327(14.1) | 880(11.0) | 1.33(1.19-1.49) | <0.001 | 1.34(1.19-1.50) | <0.001 |
| Large for gestational age | 220(9.5) | 743(8.8) | 1.10(0.96-1.25) | 0.28 | - | - |
| Low birth weight | 264(11.4) | 523(6.9) | 1.73(1.51-0.98) | <0.001 | 1.75(1.53-2.00) | <0.001 |
| Congenital malformations | 63(2.7) | 186(2.2) | 1.23(0.96-1.57) | 0.18 | - | - |
| Neonatal mortality | 7(0.3) | 23(0.3) | 1.12(0.55-2.28) | 0.81 | - | - |
N: effective; %: percentage; SD: standard deviation; 95% CI: 95% confidence interval; ICU: intensive care unit; p-values< 0.05 are indicated in bold. The percentages are weighted to consider 1:4 max algorithm matching.
Multivariate models were adjusted on the following criteria: age, social deprivation, parity, smoking and substance addictions, obesity and comorbidities (Charlson score: 0, 1-2, ≥3).
Women with schizophrenia also had more delivery complications (aOR=1.18[95% CI 1.09-1.29]), with more still births/medical abortions (aOR=2.17[95% CI 1.62-2.90]) and caesarean sections (aOR=1.15[95% CI 1.05-1.25]). No significant differences were found for postpartum haemorrhage (p=0.37).
Newborns of women with schizophrenia had more neonatal complications (aOR=1.38[95% CI 1.27-1.50]) with more preterm births (aOR=1.64[95% CI 1.42-1.90]), small for gestational age (aOR=1.34[95% CI 1.19-1.50]) and low birth weight (aOR=1.75[95% CI 1.53-2.00]) than controls. No significant differences were found for congenital malformations (p=0.18) or neonatal mortality rates (p=0.81).
These findings were confirmed in the multivariate models (Fig. 2).
Fig. 2.
Pregnancy, delivery and neonatal complications of women with schizophrenia vs. women without severe mental disorder in the matched cohorts: Multivariate analyses.
Multivariate models were performed when univariate p-values were < 0.05.
Multivariate models were adjusted on the following criteria: age, social deprivation, parity, smoking and substance addictions, obesity and comorbidities (Charlson score: 0, 1-2, ≥3).
The repartition of pregnancy/delivery/neonatal complications alone or in combination is presented in Appendix 2.
The psychiatric care of women with schizophrenia during pregnancy is described in Appendices 3 and 4. Overall, 23.6% of women with schizophrenia received inpatient care during their pregnancy. Among those who did not receive inpatient care, 45.6% had at least one day of hospital and/or ambulatory care contact, but only 29.8% received 7 contacts or more with psychiatric care during their pregnancy. Delivery and neonatal complications were higher in women with inpatient care than in women with day hospital and/or ambulatory care. Regardless of psychiatric care, the rate of each complication was always higher in women with schizophrenia than in controls.
Pregnancy, delivery and neonatal complications did not differ between women with schizophrenia, delusional disorder and schizoaffective disorder (Appendix 5).
4. Discussion
In this population-based cohort study, we identified 3,108 singleton deliveries in women with schizophrenia from 2015 to 2019 in France. Women with schizophrenia were slightly older, with more frequent smoking, alcohol and substance addictions; obesity; and diabetes than controls. They had more pregnancy complications (i.e., gestational diabetes, gestational hypertension, genito-urinary infection, intrauterine growth retardation and threatened preterm labour) and delivery complications (i.e., still births, medical abortion and caesarean section) after matching and adjustment for common pregnancy risk factors. Their children also had more neonatal complications (i.e., preterm birth, small for gestational age and low birth weight).
Our results highlight the importance of health inequalities between pregnant women with and without schizophrenia as well as in their newborns.
The risk of pregnancy, delivery and neonatal complications is substantial in women with schizophrenia. Our results are in line with previous studies in other countries, suggesting that all healthcare systems experience difficulties in the clinical care of pregnant women with schizophrenia [39,[45], [46], [47], [48], [49], [50]]. We found that schizophrenia was associated with an increased risk of cardiovascular and metabolic manifestations. This risk has been poorly explored in previous studies but consistently identified in studies exploring these outcomes (2 studies for gestational diabetes [49,51] and 2 for gestational hypertension [49,52]). We also report increased risk of genito-urinary infections in pregnant women with schizophrenia consistently with one American study [53]. Genito-urinary infections are frequent and associated with a wide range of adverse perinatal and maternal outcomes [54]. Targeting these infections could specifically prevent the risk of preterm birth. The association of schizophrenia and increased risk of preterm birth, small for gestational age, low birth weight, caesarean section and still birth has been reported repeatedly in previous studies [[45], [46], [47],50] and was confirmed in our results.
These complications are all also indicative of future health inequalities for women with schizophrenia and their children. Women with adverse pregnancy cardiovascular and metabolic outcomes, preterm delivery, small for gestational age and low birth weight appear to be at increased risk of metabolic and vascular diseases later in life [6]. Their children have increased behavioural problems, cognitive impairment, diabetes and cardiovascular diseases [7], [8], [9], [10], [11]. In addition, preterm birth and small for gestational age are associated with an increased risk of a wide range of long-term poor outcomes, including slightly increased adult mortality [55], renal dysfunctions [56], lower attainment of higher education qualifications [57], less romantic partnership, sexual intercourse, or access to parenthood [58]. Caesarean section is reported to be associated with an increased risk of uterine rupture, abnormal placentation, ectopic pregnancy, stillbirth, and preterm birth in future pregnancy. In children, caesarean section is associated with a greater incidence of late childhood obesity and asthma [10]. Future studies should specifically explore the cause of caesarean section in women with schizophrenia and determine how to reduce the occurrence of unnecessary caesarean sections. Finally, perinatal risk factors for developing schizophrenia overlap those of perinatal complications in pregnant women with schizophrenia, highlighting the intergenerational transmission of the risk for schizophrenia [59,60].
These health inequalities can be explained by the existence of barriers to optimal care for pregnant women with schizophrenia.
On the patient level, schizophrenia is a specific challenge in mental health, as schizophrenia is characterized by a lack of insight into illness in approximately a half of the patients [61]. especially during the years following illness onset. This lack of insight has a strong impact on care adherence and remains paradoxically poorly explored in current research on schizophrenia pregnancy. A mother with delusions may be unaware of her mental illness, may pursue health-risk behaviours, such as tobacco smoking, and may experience pregnancy denial [62]. This phenomenon may explain the high proportion of medical abortions and still births in our results, also reported in Finland [63]. Almost one-quarter of the pregnant women with schizophrenia included in our study were hospitalized in inpatient psychiatric care during their pregnancy. This is a proxy for uncontrolled or insufficiently controlled mental illness. This rate may be an underestimation, as no contact with psychiatric care was recorded for one-third of the pregnant women with schizophrenia. These results suggest unmet needs in mental illness control, addictions and physical comorbidities in preconceptional care that could improve the outcomes of pregnancy. Women with schizophrenia may also use inconsistent nonbarrier contraception, with an increased risk of unwanted pregnancy [64,65], suggesting the need for reinforced partnership with family planning. On the opposite, it is also possible that women without contact with psychiatric services may be stabilized in a remission phase and less than an half of patients with schizophrenia has poor insight into illness [61,66]. Overall, this patient-level issue is insufficiently explored in the research field and should be specifically addressed in clinical guidelines.
On the provider/organizational level, the current French maternal health policy is based on universal health coverage and seven mandatory antenatal visits [67]. Our findings suggest that this organization is insufficient to tackle health inequalities in pregnant women with schizophrenia. A previous work performed in a universal coverage health system reported similar findings [49]. Proportionate universalism (i.e., that the intensity of care should be proportionate to the level of disadvantage and needs [68]) is an attractive principle to reduce health inequalities. The first need is to identify pregnant women with schizophrenia early and to develop highways to specialized services. In France, the first mandatory medical consultation by general practitioners (GPs), midwives or obstetricians is due before 20 weeks of pregnancy. This is an opportunity to trigger proportionate care by referring the mother to a specialist perinatal care service. Primary care pregnancy health workers should thus be provided more training to the screening, management and referring of pregnant women with schizophrenia. The next need is to reduce fragmented pathways of care that can be overcome with comprehensive patient-centred care interventions. First, the mother should receive a comprehensive multidisciplinary evaluation with a unity of time and place. We found that less than one-third of pregnant women with schizophrenia received 7 contacts or more with ambulatory psychiatric care, which seems insufficient in regard to all interventions that should be provided. Women with schizophrenia have multiple and complex needs that should be addressed, including background regimen management and information/mental illness symptom control, psychological interventions, postpartum mental health prevention, addictions, parenting abilities, nutritional care, physical activity and social/rehabilitation interventions. This early initial evaluation could help plan and coordinate care involving GPs, obstetricians, paediatricians, psychiatrists, addiction specialists, psychologists, midwives, nurses, healthcare workers and the supportive social environment of the mother. A midwife or a nurse could ensure the role of a case manager to reinforce personalized follow-up and intervention effectiveness [69]. The development of video conferences has been considerably accelerated with the COVID-19 pandemic and may be an opportunity to improve coordination and care access [70]. The question of the boundaries of the role of each healthcare professional is complex and should be clarified. The supportive environment (including fathers and caregivers) should be included in these consultation meetings as often as possible [16]. Despite limited evidence, peer support and peer support groups could also reinforce a supportive environment, which is often defective in mothers with schizophrenia [71,72].
Last, health inequalities between pregnant women with and without schizophrenia persisted after matching and adjustment for common risk factors, such as age; parity; smoking, alcohol and substance addictions; malnutrition; obesity; and comorbidities. This finding suggests that proportionate intensified care to the level of needs and effective case management would not be sufficient to compensate for these inequalities and that public health policies should consider preventive and social determinant approaches before pregnancy. Women with schizophrenia are among the most vulnerable and socially disadvantaged persons [73]. For example, modifiable risk factors found in our study, such as smoking, alcohol and substance addictions, are in part determined by material deprivation, psychosocial stress and discrimination [74]. Effective policies should thus address the underlying social conditions that make women with schizophrenia vulnerable [75].
Strengths. This study was based on exhaustive and updated national data. The size of our control group (more than 3 million deliveries) allowed us to match almost each case with up to 4 controls on multiple factors. We explored a more comprehensive rank of outcomes compared to most previous studies. To ensure comparability with the results of previous studies [76], [77], [78], multiple pregnancies were not included in the analyses.
Limits. Illness severity, psychotropic drugs, treatment observance, diet, physical activity, marital status, medical follow-up, social isolation, ethnicity and domestic violence were not available, as in most of the previous population-based studies published thus far. Women with schizophrenia have been found to be more frequently single and at risk of domestic violence victimization than those without mental disorders [79]. Singleness is associated with increased unvoluntary abortion [63], and domestic violence can lead to maternal stress and poor foetal outcomes. Ethnicity is not reported in French national databases due to ethical issues, whereas ethnicity can influence pregnancy outcomes and social behaviours [80]. Antipsychotic drugs are not available in the French national hospital database. The increased metabolic risk may be explained by second-generation antipsychotic drugs delivered to women with schizophrenia [61]. A weakness of large hospital databases is the miscoding of diagnoses during hospital stays that can underestimate patients’ comorbidities. Some data is known to be insufficiently coded in medico-administrative databases (e.g., tobacco smoking and obesity) [81]. Missing data is thus assumed as no presence of disease. Last, we used matching and adjustment based on a high number of patient characteristics and with control for confounders at hospital level to compare the cohorts of mother with and without schizophrenia. The risk of bias is to omit some potential confounders that can alter the comparability of cohorts and therefore threaten the validity of outcome measures.
5. Conclusion
This study highlights the importance of health disparities between pregnant women with and without schizophrenia as well as in their newborns. Our findings suggest preliminary recommendations to pursue efforts for the prevention of metabolic risk and genito-urinary infections; to promote early smoking cessation, alcohol abstinence and substance misuse cessation; and to develop specific programmes for other unmet needs during and before pregnancy.
Author Contributions
Concept and design: Cyprien Fabre, Laurent Boyer, Vanessa Pauly, Guillaume Fond.
Acquisition and analysis: Cyprien Fabre, Veronica Orleans, Vanessa Pauly.
Interpretation of data: Cyprien Fabre, Karine Baumstarck, Damien Etchecopar-Etchart, Pierre-Michel Llorca, Julie Blanc, Christophe Lancon, Pascal Auquier, Laurent Boyer, Guillaume Fond
Drafting of the manuscript: Cyprien Fabre, Laurent Boyer, Guillaume Fond.
Critical revision of the manuscript for important intellectual content: All the authors.
Statistical analysis: Cyprien Fabre, Vanessa Pauly.
Supervision: Laurent Boyer.
Availability of Data and Materials
The PMSI database can only be accessed by employees of French public hospitals according to the Commission Nationale de l'Informatique et des Libertés (CNIL) and is available at the following URL: https://epmsi.atih.sante.fr/welcomeEpmsi.do
Declaration of interests
We declare no competing interests.
Acknowledgements
We would like to acknowledge Fanny Romain for her proofreading and advice.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100209.
Appendix. Supplementary materials
References
- 1.Campion J, Bhugra D, Bailey S, Marmot M. Inequality and mental disorders: opportunities for action. Lancet Lond Engl. 2013;382:183–184. doi: 10.1016/S0140-6736(13)61411-7. Jul 20. [DOI] [PubMed] [Google Scholar]
- 2.McGorry PD, Mei C. Early intervention in youth mental health: progress and future directions. Evid Based Ment Health. 2018;21:182–184. doi: 10.1136/ebmental-2018-300060. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinner SA, Borschmann R. Inequality and intergenerational transmission of complex adversity. Lancet Public Health. 2017;2:e342–e343. doi: 10.1016/S2468-2667(17)30139-1. Aug 1. [DOI] [PubMed] [Google Scholar]
- 4.Neiger R. Long-Term Effects of Pregnancy Complications on Maternal Health: A Review. J Clin Med. 2017;6(8) doi: 10.3390/jcm6080076. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5575578/ [Internet]Jul 27 [cited 2021 Apr 19]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. Dec 7. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123:1485–1492. doi: 10.1542/peds.2008-1216. Jun. [DOI] [PubMed] [Google Scholar]
- 8.Banderali G, Martelli A, Landi M, Moretti F, Betti F, Radaelli G, et al. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. J Transl Med. 2015;13 doi: 10.1186/s12967-015-0690-y. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4608184/ [Internet]Oct 15 [cited 2021 Apr 19]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. Aug. [DOI] [PubMed] [Google Scholar]
- 10.Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet Lond Engl. 2018;392:1349–1357. doi: 10.1016/S0140-6736(18)31930-5. Oct 13. [DOI] [PubMed] [Google Scholar]
- 11.Hollanders JJ, Schaëfer N, van der Pal SM, Oosterlaan J, Rotteveel J, Finken MJJ, et al. Long-Term Neurodevelopmental and functional outcomes of infants born very preterm and/or with a very low birth weight. Neonatology. 2019;115:310–319. doi: 10.1159/000495133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abel KM, Hope H, Swift E, Parisi R, Ashcroft DM, Kosidou K, et al. Prevalence of maternal mental illness among children and adolescents in the UK between 2005 and 2017: a national retrospective cohort analysis. Lancet Public Health. 2019;4:e291–e300. doi: 10.1016/S2468-2667(19)30059-3. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stambaugh LF, Forman-Hoffman V, Williams J, Pemberton MR, Ringeisen H, Hedden SL, et al. Prevalence of serious mental illness among parents in the United States: results from the National Survey of Drug Use and Health, 2008-2014. Ann Epidemiol. 2017;27:222–224. doi: 10.1016/j.annepidem.2016.12.005. Mar. [DOI] [PubMed] [Google Scholar]
- 14.Leijdesdorff S, van Doesum K, Popma A, Klaassen R, van Amelsvoort T. Prevalence of psychopathology in children of parents with mental illness and/or addiction: an up to date narrative review. Curr Opin Psychiatry. 2017;30:312–317. doi: 10.1097/YCO.0000000000000341. Jul. [DOI] [PubMed] [Google Scholar]
- 15.Pierce M, Hope HF, Kolade A, Gellatly J, Osam CS, Perchard R, et al. Effects of parental mental illness on children's physical health: systematic review and meta-analysis. Br J Psychiatry J Ment Sci. 2020;217:354–363. doi: 10.1192/bjp.2019.216. Jul. [DOI] [PubMed] [Google Scholar]
- 16.Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19:313–327. doi: 10.1002/wps.20769. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campo JV, Fontanella CA, Bridge JA. Intergenerational associations of parental mental illness and child health. JAMA Pediatr. 2020;174 doi: 10.1001/jamapediatrics.2020.1755. Aug 1. [DOI] [PubMed] [Google Scholar]
- 18.He H, Liu Q, Li N, Guo L, Gao F, Bai L, et al. Trends in the incidence and DALYs of schizophrenia at the global, regional and national levels: results from the Global Burden of Disease Study 2017. Epidemiol Psychiatr Sci. 2020;29 doi: 10.1017/S2045796019000891. https://www.cambridge.org/core/journals/epidemiology-and-psychiatric-sciences/article/trends-in-the-incidence-and-dalys-of-schizophrenia-at-the-global-regional-and-national-levels-results-from-the-global-burden-of-disease-study-2017/81AD934C03A328C6BC2AE8EE36967492 [Internet]ed [cited 2021 Jul 10]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44:1195–1203. doi: 10.1093/schbul/sby058. Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Werf M, Hanssen M, Köhler S, Verkaaik M, Verhey FR, et al. RISE Investigators Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychol Med. 2014;44:9–16. doi: 10.1017/S0033291712002796. Jan. [DOI] [PubMed] [Google Scholar]
- 21.Johnson T. The Perinatal Mental Health Care Pathways: Full Implementation Guidance.:45.
- 22.Final-COPE-Perinatal-Mental-Health-Guideline.pdf [Internet]. [cited 2021 Apr 27]. Available from: https://cope.org.au/wp-content/uploads/2017/10/Final-COPE-Perinatal-Mental-Health-Guideline.pdf
- 23.National Collaborating Centre for Mental Health (UK) British Psychological Society; Leicester (UK): 2007. Antenatal and Postnatal Mental Health: The NICE Guideline on Clinical Management and Service Guidance.http://www.ncbi.nlm.nih.gov/books/NBK54487/ [Internet][cited 2021 Apr 27]. (National Institute for Health and Clinical Excellence: Guidance). Available from. [PubMed] [Google Scholar]
- 24.GOV.UK. Welcome to GOV.UK [Internet]. 2021 [cited 2021 Apr 27]. Available from: https://www.gov.uk/
- 25.NICE clinical guideline. Antenatal and postnatal mental health: clinical management and service guidance. 2014;54.
- 26.NICE clinical guideline. Psychosis and schizophrenia in adults: prevention and management. 2014;39.
- 27.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthøj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia. Part 3: Update 2015 Management of special circumstances: Depression, Suicidality, substance use disorders and pregnancy and lactation. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. 2015;16:142–170. doi: 10.3109/15622975.2015.1009163. Apr. [DOI] [PubMed] [Google Scholar]
- 28.Tosato S, Albert U, Tomassi S, Iasevoli F, Carmassi C, Ferrari S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78:e477–e489. doi: 10.4088/JCP.15r10483. May. [DOI] [PubMed] [Google Scholar]
- 29.Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2016;184:87–97. doi: 10.1093/aje/kwv301. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DEJONG K, OLYAEI A, LO JO. Alcohol use in pregnancy. Clin Obstet Gynecol. 2019;62:142–155. doi: 10.1097/GRF.0000000000000414. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham M, Alramadhan S, Iniguez C, Duijts L, Jaddoe VWV, Den Dekker HT, et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE [Internet] 2017;12(2) doi: 10.1371/journal.pone.0170946. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5322900/ Feb 23 [cited 2021 Feb 18]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennedsen BE, Mortensen PB, Olesen AV, Henriksen TB, Frydenberg M. Obstetric complications in women with schizophrenia. Schizophr Res. 2001;47:167–175. doi: 10.1016/s0920-9964(99)00234-0. Mar 1. [DOI] [PubMed] [Google Scholar]
- 33.Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry Off J World Psychiatr Assoc WPA. 2017;16:163–180. doi: 10.1002/wps.20420. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugrue R, Zera C. Pregestational diabetes in pregnancy. Obstet Gynecol Clin North Am. 2018;45:315–331. doi: 10.1016/j.ogc.2018.01.002. Jun. [DOI] [PubMed] [Google Scholar]
- 35.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. doi: 10.1136/bmj.j1. Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison G, Gunnell D, Glazebrook C, Page K, Kwiecinski R. Association between schizophrenia and social inequality at birth: case-control study. Br J Psychiatry J Ment Sci. 2001;179:346–350. doi: 10.1192/bjp.179.4.346. Oct. [DOI] [PubMed] [Google Scholar]
- 37.Lelong A, Jiroff L, Blanquet M, Mourgues C, Leymarie MC, Gerbaud L, et al. Is individual social deprivation associated with adverse perinatal outcomes? Results of a French multicentre cross-sectional survey. J Prev Med Hyg. 2015;56:E95–101. Aug 5. [PMC free article] [PubMed] [Google Scholar]
- 38.Gentile S, Fusco ML. Schizophrenia and motherhood. Psychiatry Clin Neurosci. 2019;73:376–385. doi: 10.1111/pcn.12856. Jul. [DOI] [PubMed] [Google Scholar]
- 39.Simoila L, Isometsä E, Gissler M, Suvisaari J, Halmesmäki E, Lindberg N. Obstetric and perinatal health outcomes related to schizophrenia: A national register-based follow-up study among Finnish women born between 1965 and 1980 and their offspring. Eur Psychiatry J Assoc Eur Psychiatr. 2018;52:68–75. doi: 10.1016/j.eurpsy.2018.04.001. Aug. [DOI] [PubMed] [Google Scholar]
- 40.Dolk H. EUROCAT: 25 years of European surveillance of congenital anomalies. Arch Dis Child Fetal Neonatal Ed. 2005;90:F355–F358. doi: 10.1136/adc.2004.062810. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997 –2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33. doi: 10.1186/1471-2458-9-33. Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 43.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat - Simul Comput. 2009;38:1228–1234. May 14. [Google Scholar]
- 44.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 45.Lin H-C, Chen Y-H, Lee H-C. Prenatal care and adverse pregnancy outcomes among women with schizophrenia: a nationwide population-based study in Taiwan. J Clin Psychiatry. 2009;70:1297–1303. doi: 10.4088/JCP.09m05087. Sep. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson E, Hultman CM, Cnattingius S, Olausson PO, Björk C, Lichtenstein P. Schizophrenia and offspring's risk for adverse pregnancy outcomes and infant death. Br J Psychiatry J Ment Sci. 2008;193:311–315. doi: 10.1192/bjp.bp.107.045146. Oct. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson E, Lichtenstein P, Cnattingius S, Murray RM, Hultman CM. Women with schizophrenia: pregnancy outcome and infant death among their offspring. Schizophr Res. 2002;58:221–229. doi: 10.1016/s0920-9964(01)00370-x. Dec 1. [DOI] [PubMed] [Google Scholar]
- 48.Webb RT, Pickles AR, King-Hele SA, Appleby L, Mortensen PB, Abel KM. Parental mental illness and fatal birth defects in a national birth cohort. Psychol Med. 2008;38:1495–1503. doi: 10.1017/S0033291707002280. Oct. [DOI] [PubMed] [Google Scholar]
- 49.Vigod SN, Kurdyak PA, Dennis CL, Gruneir A, Newman A, Seeman MV, et al. Maternal and newborn outcomes among women with schizophrenia: a retrospective population-based cohort study. BJOG Int J Obstet Gynaecol. 2014;121:566–574. doi: 10.1111/1471-0528.12567. Apr. [DOI] [PubMed] [Google Scholar]
- 50.Zhong Q-Y, Gelaye B, Fricchione GL, Avillach P, Karlson EW, Williams MA. Adverse obstetric and neonatal outcomes complicated by psychosis among pregnant women in the United States. BMC Pregnancy Childbirth. 2018;18:120. doi: 10.1186/s12884-018-1750-0. May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hizkiyahu R, Levy A, Sheiner E. Pregnancy outcome of patients with schizophrenia. Am J Perinatol. 2010;27:19–23. doi: 10.1055/s-0029-1225529. Jan. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen NT, Gorman M, Caughey AB. 550: Pregnancy outcomes in women with schizophrenia: a retrospective cohort study. Am J Obstet Gynecol. 2016;214:S296. Jan 1. [Google Scholar]
- 53.Heun-Johnson H, Seabury SA, Menchine M, Claudius I, Axeen S, Lakshmanan A. Association between maternal serious mental illness and adverse birth outcomes. J Perinatol Off J Calif Perinat Assoc. 2019;39:737–745. doi: 10.1038/s41372-019-0346-5. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol. 2005;32:523–559. doi: 10.1016/j.clp.2005.04.006. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crump C. Preterm birth and mortality in adulthood: a systematic review. J Perinatol Off J Calif Perinat Assoc. 2020;40:833–843. doi: 10.1038/s41372-019-0563-y. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heo JS, Lee JM. The long-term effect of preterm birth on renal function: a meta-analysis. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18062951. Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bilgin A, Mendonca M, Wolke D. Preterm birth/low birth weight and markers reflective of wealth in adulthood: a meta-analysis. Pediatrics. 2018;142 doi: 10.1542/peds.2017-3625. Jul. [DOI] [PubMed] [Google Scholar]
- 58.Mendonça M, Bilgin A, Wolke D. Association of preterm birth and low birth weight with romantic partnership, sexual intercourse, and parenthood in adulthood: a systematic review and meta-analysis. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.6961. Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies C, Segre G, Estradé A, Radua J, De Micheli A, Provenzani U, et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet Psychiatry. 2020;7:399–410. doi: 10.1016/S2215-0366(20)30057-2. May. [DOI] [PubMed] [Google Scholar]
- 60.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. Jul 1. [DOI] [PubMed] [Google Scholar]
- 61.Hartling L, Abou-Setta AM, Dursun S, Mousavi SS, Pasichnyk D, Newton AS. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157:498–511. doi: 10.7326/0003-4819-157-7-201210020-00525. Oct 2. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins A, Millar S, Robins J. Denial of pregnancy: a literature review and discussion of ethical and legal issues. J R Soc Med. 2011;104:286–291. doi: 10.1258/jrsm.2011.100376. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simoila L, Isometsä E, Gissler M, Suvisaari J, Sailas E, Halmesmäki E, et al. Schizophrenia and induced abortions: A national register-based follow-up study among Finnish women born between 1965-1980 with schizophrenia or schizoaffective disorder. Schizophr Res. 2018;192:142–147. doi: 10.1016/j.schres.2017.05.039. Feb. [DOI] [PubMed] [Google Scholar]
- 64.Gupta R, Brown HK, Barker LC, Dennis C-L, Vigod SN. Rapid repeat pregnancy in women with schizophrenia. Schizophr Res. 2019;212:86–91. doi: 10.1016/j.schres.2019.08.007. Oct. [DOI] [PubMed] [Google Scholar]
- 65.Callegari LS, Zhao X, Nelson KM, Borrero S. Contraceptive adherence among women Veterans with mental illness and substance use disorder. Contraception. 2015;91:386–392. doi: 10.1016/j.contraception.2015.01.013. May. [DOI] [PubMed] [Google Scholar]
- 66.Dickerson FB, Boronow JJ, Ringel N, Parente F. Lack of insight among outpatients with schizophrenia. Psychiatr Serv Wash DC. 1997;48:195–199. doi: 10.1176/ps.48.2.195. Feb. [DOI] [PubMed] [Google Scholar]
- 67.Rodwin VG. The health care system under French national health insurance: lessons for health reform in the United States. Am J Public Health. 2003;93:31–37. doi: 10.2105/ajph.93.1.31. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.IHE Institute of Health Equity. Fair Society Healthy Lives (The Marmot Review). 2010 [cited 2021 Apr 28]; Available from: http://www.instituteofhealthequity.org/resources-reports/fair-society-healthy-lives-the-marmot-review
- 69.Sledge WH, Astrachan B, Thompson K, Rakfeldt J, Leaf P. Case management in psychiatry: an analysis of tasks. Am J Psychiatry. 1995;152:1259–1265. doi: 10.1176/ajp.152.9.1259. Sep. [DOI] [PubMed] [Google Scholar]
- 70.Soukup T, Sevdalis N, Green JSA, Lamb BW. Quality improvement for cancer multidisciplinary teams: lessons learned from the Anglian Germ Cell Cancer Collaborative Group. Br J Cancer. 2021;124:313–314. doi: 10.1038/s41416-020-01080-4. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chien WT, Clifton AV, Zhao S, Lui S. Peer support for people with schizophrenia or other serious mental illness. Cochrane Database Syst Rev. 2019;4 doi: 10.1002/14651858.CD010880.pub2. Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castelein S, Bruggeman R, Davidson L, van der Gaag M. Creating a supportive environment: Peer Support Groups for psychotic disorders. Schizophr Bull. 2015;41:1211–1213. doi: 10.1093/schbul/sbv113. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tinland A, Boyer L, Loubière S, Greacen T, Girard V, Boucekine M, et al. Victimization and posttraumatic stress disorder in homeless women with mental illness are associated with depression, suicide, and quality of life. Neuropsychiatr Dis Treat. 2018;14:2269–2279. doi: 10.2147/NDT.S161377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Irwin A, Valentine N, Brown C, Loewenson R, Solar O, Brown H, et al. The commission on social determinants of health: Tackling the social roots of health inequities. PLOS Med. 2006;3:e106. doi: 10.1371/journal.pmed.0030106. May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marmot M. Social determinants of health inequalities. Lancet Lond Engl. 2005;365:1099–1104. doi: 10.1016/S0140-6736(05)71146-6. Mar 19. [DOI] [PubMed] [Google Scholar]
- 76.Vigod SN, Kurdyak PA, Dennis CL, Gruneir A, Newman A, Seeman MV, et al. Maternal and newborn outcomes among women with schizophrenia: a retrospective population-based cohort study. BJOG Int J Obstet Gynaecol. 2014;121:566–574. doi: 10.1111/1471-0528.12567. Apr. [DOI] [PubMed] [Google Scholar]
- 77.Simoila L, Isometsä E, Suvisaari J, Halmesmäki E, Lindberg N. Obstetric and perinatal health outcomes related to schizophrenia: A national register-based follow-up study among Finnish women born between 1965 and 1980 and their offspring. Eur Psychiatry. 2018;52:68–75. doi: 10.1016/j.eurpsy.2018.04.001. Aug. [DOI] [PubMed] [Google Scholar]
- 78.Baer RJ, Chambers CD, Bandoli G, Jelliffe-Pawlowski LL. Risk of preterm birth by subtype among Medi-Cal participants with mental illness. Am J Obstet Gynecol. 2016;215:519.e1–519.e9. doi: 10.1016/j.ajog.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 79.Suparare L, Watson SJ, Binns R, Frayne J, Galbally M. Is intimate partner violence more common in pregnant women with severe mental illness? A retrospective study. Int J Soc Psychiatry. 2020;66:225–231. doi: 10.1177/0020764019897286. May. [DOI] [PubMed] [Google Scholar]
- 80.Taylor CL, Stewart R, Ogden J, Broadbent M, Pasupathy D, Howard LM. The characteristics and health needs of pregnant women with schizophrenia compared with bipolar disorder and affective psychoses. BMC Psychiatry. 2015;15:88. doi: 10.1186/s12888-015-0451-8. Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fond G, Salas S, Pauly V, Baumstarck K, Bernard C, Orleans V, et al. End-of-life care among patients with schizophrenia and cancer: a population-based cohort study from the French national hospital database. Lancet Public Health. 2019;4:e583–e591. doi: 10.1016/S2468-2667(19)30187-2. Nov. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The PMSI database can only be accessed by employees of French public hospitals according to the Commission Nationale de l'Informatique et des Libertés (CNIL) and is available at the following URL: https://epmsi.atih.sante.fr/welcomeEpmsi.do