Abstract
Background
The NLST reported a significant 20% reduction in lung cancer mortality with three annual low-dose CT (LDCT) screens and the Dutch-Belgian NELSON trial indicates a similar reduction. We present the results of the UKLS trial.
Methods
From October 2011 to February 2013, we randomly allocated 4 055 participants to either a single invitation to screening with LDCT or to no screening (usual care). Eligible participants (aged 50–75) had a risk score (LLPv2) ≥ 4.5% of developing lung cancer over five years. Data were collected on lung cancer cases to 31 December 2019 and deaths to 29 February 2020 through linkage to national registries. The primary outcome was mortality due to lung cancer. We included our results in a random-effects meta-analysis to provide a synthesis of the latest randomised trial evidence.
Findings
1 987 participants in the intervention and 1 981 in the usual care arms were followed for a median of 7.3 years (IQR 7.1–7.6), 86 cancers were diagnosed in the LDCT arm and 75 in the control arm. 30 lung cancer deaths were reported in the screening arm, 46 in the control arm, (relative rate 0.65 [95% CI 0.41–1.02]; p=0.062). The meta-analysis indicated a significant reduction in lung cancer mortality with a pooled overall relative rate of 0.84 (95% CI 0.76–0.92) from nine eligible trials.
Interpretation
The UKLS trial of single LDCT indicates a reduction of lung cancer death of similar magnitude to the NELSON and NLST trials and was included in a meta-analysis of nine randomised trials which provides unequivocal support for lung cancer screening in identified risk groups.
Funding
NIHR Health Technology Assessment programme; NIHR Policy Research programme; Roy Castle Lung Cancer Foundation.
Keywords: Lung Cancer, CT Screening, Lung Cancer Mortality, Meta-analysis
Introduction
Lung cancer screening trials were initiated in the 1970s [1,2] based on chest X-rays and sputum analysis, however, there was no evidence of any mortality advantage. The first low dose computed tomography (LDCT) screening was undertaken in Japan [3] and later the potential of utilising LDCT screening was published in a landmark paper from the Early Lung Cancer Action Project (ELCAP) in 1999 [4]. Two large LDCT screening trials have provided evidence of a statistically significant reduction in lung cancer mortality in the individuals recruited in the LDCT screening arm [5,6], NLST also reported a small but significant reduction in overall mortality [5]. In the US, lung cancer screening has been recommended by the United States Preventive Services Task Force (USPSTF) [[7], [8], [9]]. Six LDCT screening trials have been undertaken in Europe, which have already published their mortality data [6,[10], [11], [12], [13], [14]], as well as programmes in Canada [15] Japan [16] and Korea [17].
Research in context.
Evidence before this study
Evidence before this study included all lung cancer RCTs and associated projects on lung cancer CT screening undertaken internationally. In this manuscript we have focused on the outcome on the participants who were randomised into the UK lung cancer screening trial (UKLS) and we have undertaken lung cancer mortality and all-cause mortality analysis. As the UKLS represents the last of the European lung cancer CT screening trials to report outcome, it is also opportune to undertake a meta-analysis of all published trials with outcome data, per the published analysis plan.
Data for this meta-analysis were identified from searches of PubMed, Medline, and references from relevant articles with all of the search terms listed in the manuscript Supplementary section. Abstracts and reports from meetings were included only when they related directly to previous published work. Only articles published in English between 2010 and 2020 were included. The quality (risk of bias) of evidence utilised in the meta-analysis, is provided in detail in the supplementary section.
The added value of this manuscript has been to enhance our knowledge of lung cancer CT screening, including effects on mortality (lung cancer mortality and all-cause mortality), as well as on stage of the disease and on pulmonary nodule classification. As the UKLS had only a single screen, this study adds considerably to knowledge of the pattern of lung cancer incidence and mortality post-screening. The associated meta-analysis, which includes the UKLS trial, provides the most up to date international view of lung cancer mortality in lung cancer CT screening studies.
The implications of the study provide a recommendation to national policy makers for the implementation of lung cancer CT screening.
Alt-text: Unlabelled box
The largest randomised trials, the US National Lung Screening Trial (NLST) and the Dutch-Belgian Lung Cancer Screening Trial (NELSON), have provided conclusive evidence that the intervention reduces lung cancer mortality, so that we should now seriously consider implementation of lung cancer CT screening in Europe and the rest of the world [[18], [19], [20]].
The UKLS trial of 4 055 individuals was undertaken from 2011 to 2013 [21,22], and in this paper, we report on incidence and mortality outcomes for 3 968 with cancer registry and mortality data available. We also undertook a meta-analysis of the randomised, controlled LDCT screening trials which have reported lung cancer mortality with at least a median of three years’ follow-up.
Methods
Study design
UKLS was a randomised controlled trial, comparing LDCT screening with usual care using the “Wald Single-Screen” design [21]. The UKLS trial is unique in its design being a single LDCT screening in a high-risk population. The UKLS is a RCT of LDCT compared with usual care, for the early detection of lung cancer. The methods for the UKLS pilot study were derived from an initial feasibility study and follow the Wald Single-Screen Design. Other screening trials have used this design, including the UK Flexisig Trial, the UK Aortic Aneurysm Screening Trial and the Singapore Breast Screening Trial [21].
The study was based in two thoracic hospitals in the United Kingdom, the Liverpool Heart and Chest Hospital, on Merseyside, and Royal Papworth Hospital, in Cambridgeshire. Ethical approval was received from the Liverpool Central Research Ethics Committee (reference 10/H1005/74). Trial registration: International Standard Randomised Controlled Trial Register (reference 78513845). Full details of the design and protocol have been described elsewhere [21].
Participants
To recruit participants with a high risk of developing lung cancer from a target population broadly representative of the UK population, an initial invitation letter, UKLS participant information sheet and questionnaire were sent to individuals aged 50-75 living in specific primary care trusts (PCTs) in the vicinity of the two hospital sites [21].
For those individuals who returned completed questionnaires, the responses were analysed to identify those at high risk of developing lung cancer over the next five years defined as a risk score of at least 4.5% as per version 2 of the validated Liverpool Lung Project risk model (LLPv2) [23,24]. Factors contributing to the LLP risk score are highlighted in Table 1. A second questionnaire was sent to these individuals and the following exclusion criteria applied: inability to give consent, or any condition precluding written informed consent; any comorbidity which would unequivocally contraindicate either screening or treatment if lung cancer were to be detected; a chest CT performed within the preceding year; inability to lie flat. Those remaining eligible were invited to attend a clinic at one of the recruitment centres, where written informed consent was obtained.
Table 1.
Baseline characteristics.
| Total n=4055 | Screen Arm (n=2028) | Control Arm (n=2027) |
|---|---|---|
| Sex# | ||
| Male | 1529 (75%) | 1507 (74%) |
| Female | 499 (25%) | 520 (26%) |
| Median age# at date of consent | 68 (IQR 65-71) | 68 (IQR 65-71) |
| Age 50-59 | 44 (2%) | 58 (3%) |
| Age 60-69 | 1295 (64%) | 1291 (64%) |
| Age 70-76 | 689 (34%) | 678 (33%) |
| Median IMD Rank | 17374 | 17704 |
| Median LLPv2 score (IQR) | 7.11 (5.58 – 10.08) | 7.35 (5.59 – 10.08) |
| Smoking history | ||
| Never smokers | 2 (0%) | 0 (0%) |
| Current smokers | 777 (38%) | 791 (39%) |
| Ex-smokers | 1249 (62%) | 1236 (61%) |
| Smoking duration ‡# | ||
| 10-19 years | 117 (6%) | 116 (6%) |
| 20+ years | 1895 (93%) | 1907 (94%) |
| Unknown | 14 (1%) | 4 (0%) |
| Asbestos exposure # | 763 (38%) | 763 (38%) |
| History of respiratory disease *# | 1056 (52%) | 1023 (50%) |
| History of solid tumour ⁎⁎# | 378 (19%) | 396 (20%) |
| Family history of lung cancer# | 498 (25%) | 554 (27%) |
| Early onset (before age 60) | 215 (11%) | 215 (11%) |
| Late onset (on or after age 60) | 283 (14%) | 339 (17%) |
All smoking duration figures refer to current- and ex-smokers combined.
bronchitis, TB, pneumonia, COPD, or emphysema.
cancers of brain, head & neck, oesophagus, breast, colon or “other”.
these factors contribute to the LLP risk score
Abbreviations: IMD = Index of Multiple Deprivation; IQR = inter quartile range.
Randomisation and masking
Eligible, consenting individuals who attended a clinic were randomised at a ratio of 1:1 into the intervention arm (LDCT) or the no screening control arm (usual care). Randomisation was stratified by trial site and took place outside of the clinic and after the individuals had left the clinic using a two-stage computer algorithm with an automated procedure. This ensured allocation concealment from the clinic staff. Further technical details are given by Field et al. [21]. Due to the nature of the intervention, blinding of the participants and screening staff thereafter was not possible, and participants were informed of which group they were in within two weeks after randomisation. However, outcomes were determined without knowledge of trial allocation, since these came from routine cancer registration and death certification (see below).
Procedures
The LDCT subjects received a baseline scan (16+ channel multi-detector CT, no contrast, 100-140 kVp) and nodules were assessed by two local radiologists (Liverpool Heart and Chest Hospital or Papworth Hospital) and placed in one of four pre-defined nodule categories: Category 4 (large), Category 3 (medium), Category 2 (small), Category 1 (other) [21,25] (Supplementary material, Fig. 2). Consensus nodule category was assigned following central reading at the Royal Brompton Hospital, with a read for arbitration when necessary. Category 4 nodules were immediately referred to participating MDT clinic for work-up and clinical management. Category 3 nodules identified in the baseline scan were reanalysed in follow-up CT scans at three and twelve months; Category 2 nodules at twelve months only. Growth of nodules was based on their characteristics and volume doubling time (VDT); i.e. VDT < 400 days or new solid component of non-solid nodule was classified as growth in the UKLS trial and these cases were referred to the trial participating MDT clinic for work-up and clinical management [21,26]. Subjects with nodules that resolved were discharged and those with stable nodules were further monitored according to local practice.
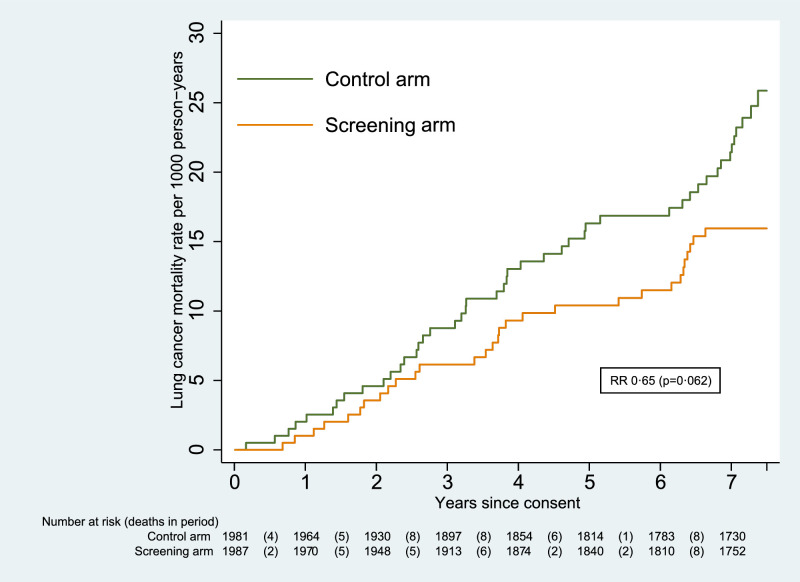
Fig. 2.
Cumulative mortality from lung cancer.
Outcomes
Outcomes from UK cancer and death registry data were provided by NHS Digital and the National Cancer Registration and Analysis Service (NCRAS) who were not aware of the participants’ allocated trial arm. The follow-up period for mortality was up to 29 February 2020 (last death recorded in ONS mortality data), and for incidence of lung cancer was up to 31 December 2019 (data from: NCRAS to March 2018; NHS Digital Cancer Registration data to Feb 2019; cause of death from ONS mortality data up to December 2019).
The primary outcome in this analysis was mortality due to lung cancer. This was defined as a death during the follow-up period where lung cancer was listed as the underlying cause of death in the UK civil registrations data provided by NHS Digital (ONS mortality data).
Secondary outcomes investigated for all participants were mortality from all causes, mortality from all cancers, mortality from causes other than lung cancer, and lung cancer incidence. Secondary outcomes for those diagnosed with lung cancer were all-cause mortality, mortality from causes other than lung cancer, and the distributions of the stage and histological type of the diagnosed cancers. Stage and histology were provided by NCRAS.
Lung cancer incidence was investigated and compared by baseline CT scan result using the pre-defined nodule category (described above) in participants in the intervention arm.
Differences between males and females were investigated for the primary outcome, for lung cancer incidence, for all-cause mortality in those diagnosed with lung cancer, and for stage distribution.
Statistical analysis
The primary outcome to be compared between intervention and control groups was lung cancer mortality. Sample size calculations, as set out in the study protocol, stated that for a relative risk of lung cancer mortality of 0.69 after three years, based on a single screen intervention, with 90% power to detect a significant difference with 2-sided testing at the 5% level, and allowing for a compliance rate of 80%, it was determined that 16,000 participants would need to be recruited into each arm. For the pilot stage, the target recruitment total was 4 000 participants (2 000 in each arm). The study did not proceed beyond the pilot stage, and hence the data presented here are not powered to detect significant mortality benefits.
Mortality data were analysed by trial arm using Poisson regression for the purposes of significance testing, and to produce estimates of relative rates and 95% confidence intervals. The Nelson-Aalen method was used to produce cumulative hazard estimates. Incidence data were analysed in the same way. Differences in stage distribution and in histological type were assessed using Pearson's chi-squared.
Analyses were carried out on an intention-to-treat basis. Differences in lung cancer mortality were also conducted on a per-protocol basis excluding those allocated to the screening arm who did not undergo CT screening.
The pilot study was not powered for a reduction in lung cancer mortality, accordingly a meta-analysis of randomised controlled trials of LDCT screening was also undertaken. This included randomised trials published up to 2nd November 2020, with at least 3 years median follow up on the basis that true underlying differences in the lung cancer mortality would be very unlikely to become apparent earlier than this due to the effects of lead time, based on Chien and Chen's publication on mean sojourn time and effectiveness of mortality reduction for lung cancer screening with computed tomography [27]. The “metan” suite of commands in Stata was used to produce a summary risk ratio of the effect of invitation to LDCT screening on the most recently published lung cancer mortality and all-cause mortality. A random effects model was assumed with heterogeneity reported using the chi-squared test and I2 statistic. The overall effect of LDCT screening on lung cancer mortality was explored by sex where data were available in the trials of LDCT screening.
The Statistical Analysis Plan for UKLS and the meta-analysis is provided in the Supplementary Appendix (pages 1–11) and was signed off prior to comparative analysis. All statistical analyses were carried out using Stata, version 16.1.
The trial was registered with the International Standard Randomised Controlled Trial Register (reference 78513845).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
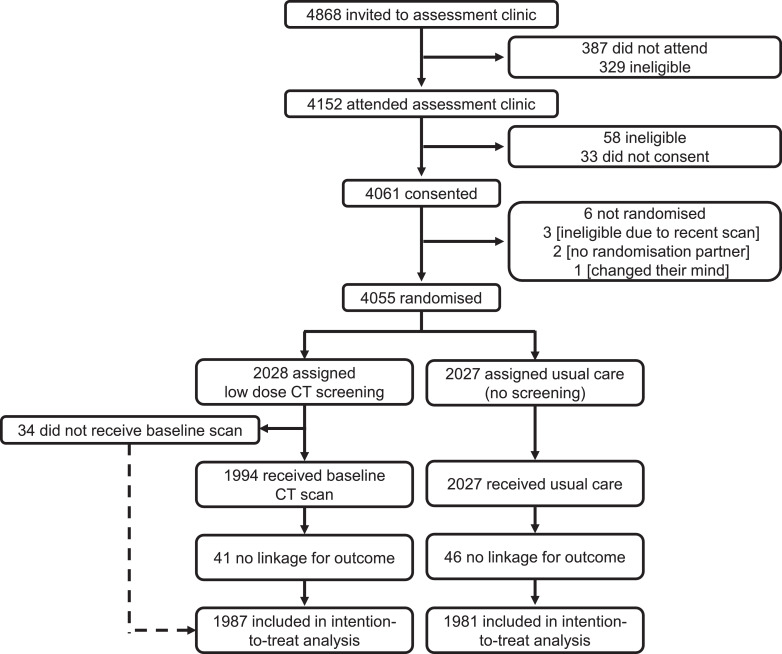
Postal invitations were sent to 247 354 individuals in two separate tranches, between August 2011 and March 2012, and between May 2012 and August 2012. Of 75 958 who responded positively, 8 729 were deemed to fall into the high-risk category. Of these, 5 967 responded positively to the second questionnaire, of whom 4 868 were invited to attend a clinic at a recruitment centre. A total of 4 152 attended a clinic between 17 October 2011 and 22 February 2013, of whom 4 061 gave written informed consent. Six consenting individuals were excluded before randomisation; of the remaining 4 055 participants, 2 028 were randomised into the intervention arm (screening) and 2 027 into the control arm (usual care).
Subsequent to randomisation, it was identified that 56 individuals (30 in the intervention arm and 26 in the control arm) had not provided consent for their data to be linked for follow-up. A further 31 (11 in the intervention arm and 20 in the control arm) were identified as having undergone censoring events before they had given consent. All 87 of these individuals were excluded from the analysis, leaving a total of 3 968 participants (1 987 in the intervention arm and 1 981 in the control arm). See Fig. 1 for further details.
Fig. 1.
Trial profile.
Baseline characteristics of the participants who were randomised were balanced as shown in Table 1. In the screening arm, 1987 are included in the intention to treat analysis (41 of 2 028 excluded with no linkage). 34 had no baseline LDCT scan (1 of which also had no linkage), hence 33 were excluded for the per-protocol analysis (n = 1 954). From 1954 subjects receiving a baseline scan, LDCT screening identified 42 cancers (Tables 2–4) from 114 subjects requiring further diagnostic investigation immediately after baseline or follow-up scans (a false positive rate of 3.6%) [21,22]. We now report 3 false negatives (defined as cancers detected within a year of their last negative UKLS LDCT scan), a false negative rate of 6.7%. Over 7.2 years follow-up lung cancers were diagnosed at a rate of 4.3% (86/1987) in the LDCT arm and 3.8% (75/1981) in the control arm. These rates are significantly lower than the median risk provided by the LLPv2 risk model (> 7%, Table 1), in keeping with the overestimation corrected in the recalibrated LLPv3 risk score [23].
Table 2.
Lung cancers by stage – number (%).
| Stage* | Screen Detected (%) | Subsequent Cancer# (screening arm) | Screening Total (%) | Control Total (%) |
|---|---|---|---|---|
| IA | 22 (52.4) | 15 (53.6) | 37 (52.9) | 8 (14.5) |
| IB | 5 (11.9) | 3 (10.7) | 8 (11.4) | 4 (7.3) |
| IIA | 7 (16.7) | _ | 7 (10.0) | 4 (7.3) |
| IIB | 1 (2.4) | 1 (3.6) | 2 (2.9) | 2 (3.6) |
| IIIA | 5 (11.9) | 2 (7.1) | 7 (10.0) | 4 (7.3) |
| IIIB | (0) | 2 (7.10 | 2 (2.9) | 6 (10.9) |
| IV | 2 (4.8) | 5 (17.9) | 7 (10.0) | 27 (49.1) |
| NA | 16 | 16 | 20 | |
| Total | 42 | 44 | 86 | 75 |
TNM version 7 staging; NA= not available from NCRAS, e.g. not staged (n=4) or during 2018-2019 (staging data not released at time of analysis, n=33); % refers to cancers with known staging only; # Subsequent cancer in screening arm are those detected subsequent to LD-CT screen(s) carried out per protocol.
Table 4.
Lung cancer incidence by baseline nodule category of subject (screening arm only).
| Baseline nodule category | Number (%) | Lung cancers (incidence) | Lung cancer detected at: |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3m FU | 12m FU | 1-2y | 2-5y | >5y | |||
| 1 | 963 (49.3) | 8 (0.8%) | - | - | - | 3 | 0 | 5 |
| 2 | 469 (24.0) | 9 (1.9%) | - | - | 1 | 1 | 3 | 4 |
| 3 | 458 (23.4) | 32 (7.0%) | - | 2 | 7 | 0 | 14 | 9 |
| 4 | 64 (3.3) | 35 (54.7%) | 32 | - | - | 2 | 1 | 0 |
| All | 1954 | 84* | 32 | 2 | 8 | 6 | 18 | 18 |
UKLS Pulmonary Nodule categories described in Supplementary materials; Note: All subjects with Category 4 nodules investigated clinically after baseline; those with Category 3 nodules had follow up scan (FU) at 3 and 12 months (m); those with Category 2 nodules had FU at 12m; Category 1 subjects (nodules < Category 2 or no nodules detected) had no FU.
Data not available for 2 cancers diagnosed in CT screening arm as amongst the 34 subjects who did not receive a LDCT scan (one at 7 months and one at 5.7 years).
Mortality data were collected until 29 February 2020 and the median follow-up was 7.3 years (interquartile range 7.1 to 7.6). The total follow-up was 14 071.4 person-years in the screening arm and 13 921.6 person-years in the control arm. For the screening arm, the median follow-up from last UKLS LDCT is 7.0 years (interquartile range 6.2 to 7.3).
In that period, 76 lung cancer deaths were recorded (30 in the screening arm and 46 in the control arm). The primary analysis showed that this difference was not statistically significant (RR 0.65 [95% CI 0.41–1.02]; p=0.062). The cumulative mortality graph is given in Fig. 2. There were no significant differences in lung cancer mortality in the male (RR 0.63 [95% CI 0.37–1.08]; p=0.091) and female (RR 0.69 [95% CI 0.28–1.69]; p=0.419) subgroups.
When analysis was repeated on a per-protocol basis, to assess the impact of the 33 individuals allocated to the screening arm who did not in fact undergo CT screening, a similar result (RR 0.67 [95% CI 0.42–1.05]; p=0.082) was observed.
There was a total of 512 deaths from any cause during the follow-up period (246 in the screening arm and 266 in the control arm), a difference which was not significant (RR 0.91 [0.77–1.09]; p=0.315). There was also no significant difference in mortality from any cancer (118 deaths in the screening arm and 125 in the control arm; RR 0.93 [0.73–1.20]; p=0.594), or in mortality from causes other than lung cancer (216 in the screening arm and 220 in the control arm; RR 0.97 [0.81–1.17]; p=0.762).
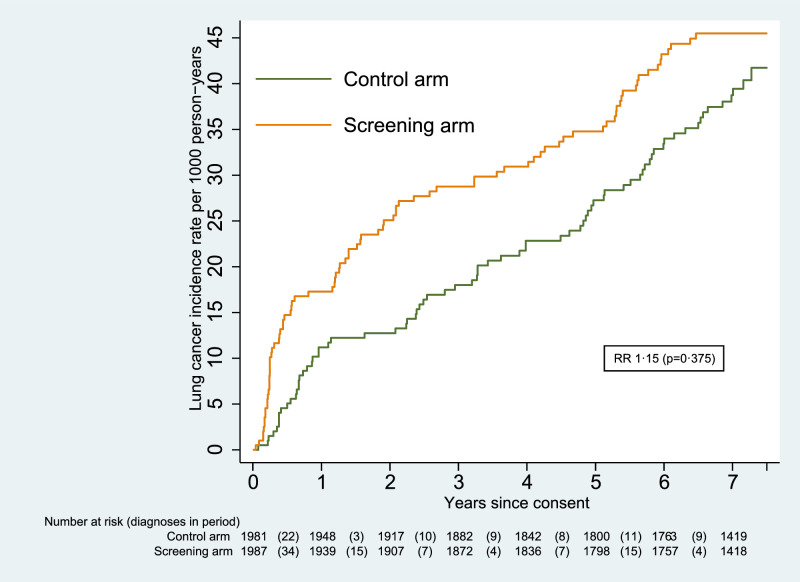
Lung cancer incidence was collected until 31 December 2019 and the median follow-up was 7.2 years (interquartile range 7.0 to 7.5). The total follow-up was 13 493.8 person-years in the screening arm, and 13 539.1 person-years in the control arm.
A total of 161 lung cancers were diagnosed in that period (86 in the screening arm and 75 in the control arm). This difference in incidence by trial arm was not statistically significant (RR 1.15 [0.84–1.57]; p=0.375). The cumulative incidence graph is given in Fig. 3. There was a non-significant increase in incidence of 7% for males (RR 1.07 [0.75–1.54]; p=0.702), and of 41% for females (RR 1.41 [0.76–2.59]; p=0.274); further results by sex are provided in Supplementary data, Table S1.
Fig. 3.
Cumulative incidence of lung cancer.
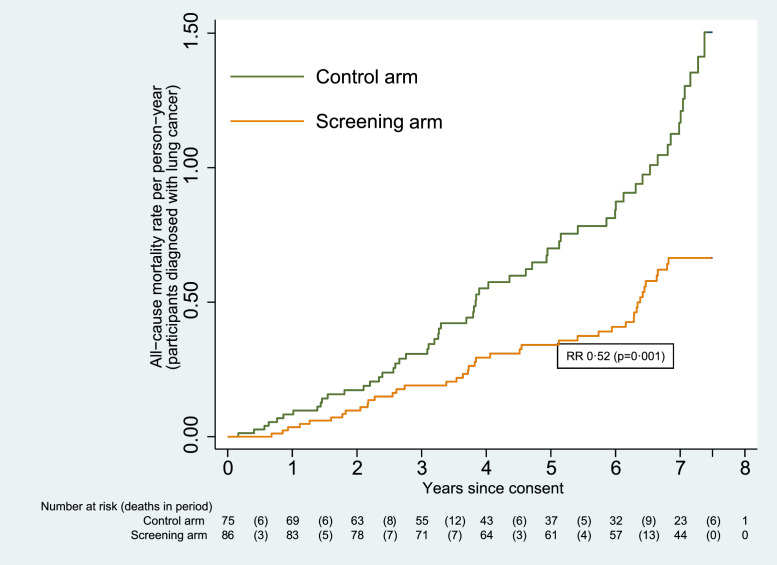
Of the 161 participants diagnosed with lung cancer, a total of 100 died (from any cause). The number of deaths among participants in the screening arm was significantly lower than in the control arm, at 42 compared to 58 (RR 0.52 [0.35–0.77]; p=0.001). The cumulative mortality graph is given in Fig. 4. The difference was also significant for males (RR 0.52 [0.32–0.82]; p=0.005), but not for females (RR 0.53 [0.24–1.16]; p=0.112).
Fig. 4.
Cumulative mortality from all causes (participants diagnosed with lung cancer).
There were 12 deaths in each arm from causes other than lung cancer, among those diagnosed with lung cancer. This difference was not significant (RR 0.72 [0.32–1.60]; p=0.416).
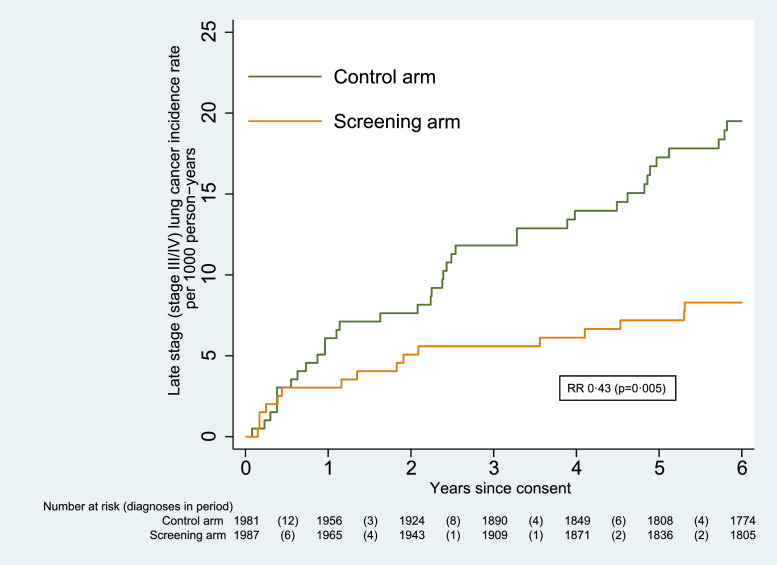
The stage distribution of lung cancers shown in Table 2, indicated a higher proportion of early-stage disease in the screening arm (Pearson's chi-squared = 30.16, p< 0.001). The odds of a cancer being diagnosed at a late stage (stage III or IV) were significantly lower in the screening arm than in the control arm (odds ratio 0.14 [95% CI 0.07–0.32]; p< 0.001). Overall, there were significantly fewer late-stage lung cancers in the screening arm compared to the control arm, at 16 vs 37 (RR 0.43 [0.24–0.77]; p=0.005). The cumulative incidence of stage III/IV lung cancers is shown as Fig. 5.
Fig. 5.
Cumulative incidence of late stage lung cancer.
Analysis was carried out to explore the histological types of the lung cancers diagnosed, with the outcomes being shown in Table 3. There was no significant difference between the two arms (Pearson's chi-squared = 8.68, p=0.070).
Table 3.
Lung cancers by histological type – number (percentage).
| Histology | Screen Detected | Subsequent Cancer (screening arm)# | Screening Total | Control |
|---|---|---|---|---|
| Adenocarcinoma | 25 (59.5) | 19 (43.2) | 44 (51.2) | 26 (34.7) |
| Squamous cell carcinoma | 12 (28.6) | 7 (15.9) | 19 (22.1) | 18 (24) |
| NSCLC NOS* | 0 (0) | 1 (2.3) | 1 (1.2) | 2 (2.7) |
| Small cell carcinoma | 3 (7.1) | 6 (13.6) | 9 (10.5) | 8 (10.7) |
| Carcinoid/ Large Cell | 1 (2.4) | 0 (0) | 1 (1.2) | 4 (5.3) |
| NOS | 1 (2.4) | 11 (25.0) | 12 (14.0) | 17 (22.7) |
| Total | 42 | 44 | 86 | 75 |
NOS = not otherwise specified; # Subsequent cancer in screening arm are those detected subsequent to LD-CT screen(s) carried out per protocol.
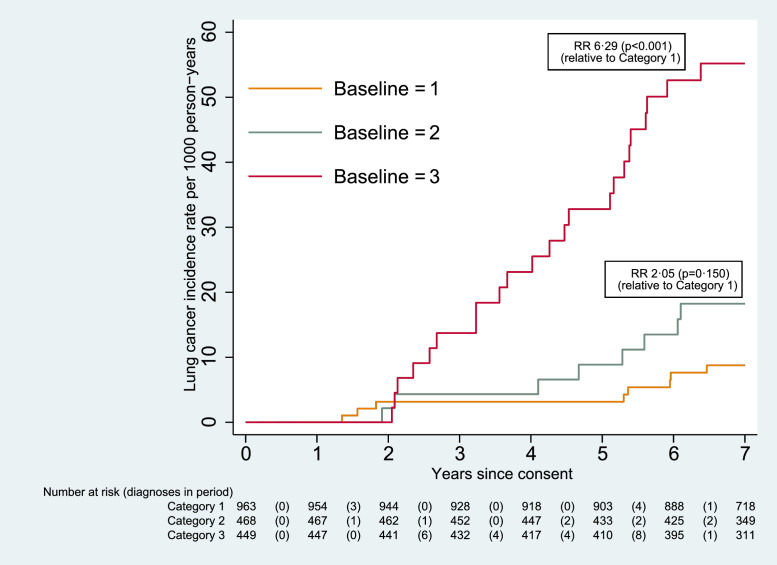
For those in the screening arm, Table 4 sets out the number of cancers diagnosed for each nodule classification category, and the time at which they were detected. Fig. 6 shows the cumulative incidence for each category, for those cancers which were not screen-detected (i.e. detected at UKLS baseline or follow-up LDCT scans). The risk for those in Category 3 was more than six times that of those in Category 1 (RR 6.29 [95% CI 2.81–14.06]; p< 0.001), and for those in Category 4 it was more than twelve times greater (RR 12.29 [3.26–46.32]; p<0.001).
Fig. 6.
Cumulative incidence of lung cancer by nodule classification category (screening arm only, excluding screen-detected cancers).
Fig. 3 suggests that the cumulative incidence in the two arms begins to converge after five years. This is also the period with regard to which the LLP risk (used as one of the eligibility criteria) is calculated. However, a post-hoc analysis showed that the difference up to that point was not significant (67 lung cancers in the screening arm compared to 52 in the control arm, RR 1.30 [0.90–1.86]; p=0.162).
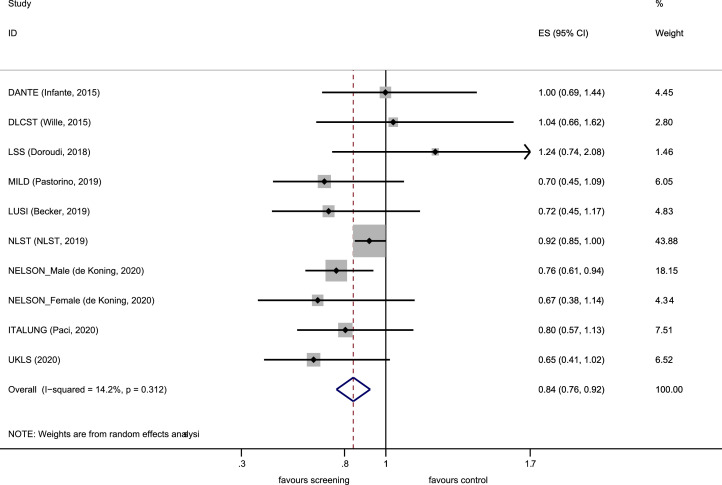
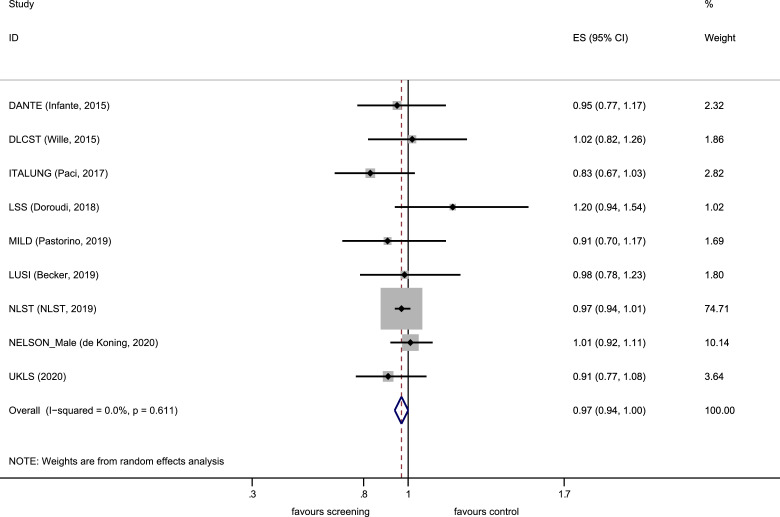
Results from nine randomised controlled trials were included in the meta-analysis (Supplementary Appendix; literature search, PRISMA flow diagram, characteristic of included studies and risk of bias assessment). LDCT screening was associated with a 16% relative reduction in lung cancer mortality when compared against a non-LDCT control arm (RR 0.84 [0.76–0.92]) with no significant heterogeneity (p= 0.31, I2=14.2%) as shown in Fig.7. A small relative reduction in all-cause mortality was observed (RR 0.97 [0.94–1.00]) with no heterogeneity (p=0.61, I2=0.0%) as shown in Fig. 8. There was no statistically significant evidence (p=0.47, Poisson regression interaction test) of a differential effect on lung cancer mortality by sex in the available, published randomised trial data.
Fig. 7.
Forest plot, lung cancer mortality.
Fig. 8.
Forest plot, mortality from all causes.
Discussion
The UKLS trial has seven years’ follow-up outcome data providing lung cancer mortality results which while not statistically significant (RR 0.65 [95% CI 0.41–1.02]; p=0.062) (Fig. 2) are consistent with the findings from other trials of a substantial reduction in lung cancer mortality. During the follow-up period, there were a total of 512 deaths from any cause (246 in the screening arm and 266 in the control arm), a difference which was not significant (RR 0.91 [0.77–1.09]).
The results from nine randomised controlled trials, including the UKLS trial, were included in the meta-analysis, which demonstrated a 16% relative reduction in lung cancer mortality, in the LDCT arm, when compared against a non-LDCT control arm (RR 0.84 [0.76–0.92]) with no significant heterogeneity (p= 0.31, I2=14.2%). A small relative reduction in all-cause mortality was also observed (RR 0.97 [0.94–1.00]).
The fundamental basis on which one undertakes lung cancer screening is to identify early lung cancer when it is still readily curable. The UKLS successfully provided evidence indicating a higher proportion of early-stage disease associated with LDCT screening (p< 0.001). No significant difference was observed by UKLS trial arm in terms of histological type (Pearson's chi-squared 8.68, p=0.070), with the majority being non-small cell lung cancer. There was an association between nodule size at baseline and relative risk of lung cancer during the follow-up period.
The UKLS results are consistent with the findings of the NLST [5] and NELSON [6] trials, however, the uniqueness of the UKLS lies in the fact that it was the only lung cancer CT RCT to utilise a formal, multivariate lung cancer risk prediction model to select high risk participants (4.5% risk over five year [23,24]).
The UKLS Wald Single Screen design resulted in diagnosis of lung cancer with 67% at stage 1 and 83% suitable for surgical intervention [21]. The intervention had no long-term psychological impact, successfully integrated smoking cessation and was considered cost effective [21]. The “Wald Single Screen” design allows us to demonstrate the continued benefits of lung cancer LDCT screening beyond the initial screen. Fig. 2 demonstrates the benefit in terms of lung cancer mortality with the difference emerging most strongly in years 3–6 after randomisation and continuing for the seven year follow-up period. Fig. 2. demonstrates the benefit of early detection is maintained beyond five years after randomisation. The UKLS trial, demonstrating long-term benefit from a single screen, provides potentially important data for inclusion in future modelling studies to optimise the screening interval.
The potential difference in effectiveness of screening between males and females is an issue of interest. The NELSON trial found a larger reduction in mortality in the small population of females, not significant at ten years follow-up, but significant at earlier time points. Similar results have been seen (albeit non-significant) in the NLST and the German LUSI Trial. Although this finding was not seen in the UKLS results, possibly due to small numbers, the differential effect by sex clearly requires further research.
Overdiagnosis is a potential issue in all cancer screening programmes, as well as in lung cancer CT screening [28]; overdiagnosis is defined as the diagnosis of cancer, histologically confirmed, as a result of screening, which would never have been diagnosed in the host's lifetime if screening had not taken place. NELSON reported 8.9% overdiagnosis [6], the NLST initially reported 18% [28], however, more recent follow-up has suggested only 3% overdiagnosis in the LDCT arm [29]. Estimates from the other trials vary considerably [12,30]. In the UKLS the absolute incidence after a median follow-up of 7.2 years (Fig. 3, 75 vs 86 cases) indicates a potential 15% excess incidence in the lung cancer screening arm, which represents an estimate of the worst-case scenario for over-diagnosis, since screening stopped after the single screen. The MISCAN lung cancer model estimated overdiagnosis to be 10% in screened populations [31].
While UKLS results are not statistically significant, there is sufficient follow-up to include in a meta-analysis together with the eight other previously reported randomised controlled trials. Our updated meta-analysis provides conclusive evidence of a reduction in lung cancer mortality with LDCT screening (0.84 [95% CI 0.76–0.92]) (Fig. 7). This meta-analysis also included the USA LSS trial, as well as utilising the NELSON male and female mortality data, and the updated NLST report [29]. The results strengthen recently reported meta-analyses of lung cancer screening indicating consistent and robust evidence overall [32,33]. Despite differences in protocols there was no significant heterogeneity amongst the trials.
Worldwide evidence searches indicate that there are a number of lung cancer CT screening trials in China [34]. Only one of these to date has published initial outcome data, but this was not included in our meta-analysis as it has not reported long-term follow-up data [35].
No one trial was designed with the intention of demonstrating a reduction in all-cause mortality. It is appreciated that over 100 000 individuals would be required to achieve such an objective. The meta-analysis here presented includes data from 94 834 individuals across the nine RCTs with a small reduction in all-cause mortality (RR 0.97 [0.94–1.00]) (Fig. 8). However, even a small reduction in all-cause mortality as shown here, does represent a large number of lives should countries around the world adopt lung cancer screening programmes. It is also consistent with the proportion of deaths from lung cancer in the trial populations.
To follow precedent and to demonstrate even-handedness, the meta-analysis used the most recent primary reported mortality results from the randomised trials. It should be noted, however, that this is conservative, since the most recent reported results include deaths from lung cancers diagnosed after the screening phase of the trials and when both trial groups were receiving usual care. While this does not affect the absolute benefit, it dilutes the relative effect of the intervention, conservatively biasing the RR of lung cancer mortality towards unity. In the breast and bowel screening trials, this bias is avoided by including only deaths (whenever they occur) from cancers diagnosed during the screening phase of the trials [36,37]. Duffy and Smith [36] show that this bias is reduced but estimates remain slightly conservative when analysis is restricted to cancers diagnosed during the screening phase. The NLST publication reporting the RR of 0.92 of deaths from lung cancer diagnosed up to 12 years after randomisation (9 years after the screening phase ended), also reported a secondary analysis including only deaths from lung cancers diagnosed up to 6 years after randomisation, giving a RR of 0.89 [29]. Thus, the relative benefit is likely to be underestimated in the meta-analysis.
The number of individuals recruited into the UKLS pilot trial is its major limitation when considering the effect on lung cancer mortality. Pragmatically, we relied on nationally curated data (ONS) rather than having a cause of death committee. This, however, does mean that the cause of death was determined in the absence of knowledge of which trial group the subjects belonged to. UKLS predates introduction of British Thoracic Society pulmonary nodule guidelines [38], but utilised similar nodule categorization to NELSON. However, the trial has provided valuable demonstration of the use of a formal risk prediction model to select high risk individuals, the identification of early-stage disease and the number of individuals suitable for surgical intervention [21]. Its contribution to the overall meta-analysis adds to the consistency of evidence internationally.
Lung cancer screening has been implemented in the USA through funding from MEDICARE but uptake has been low (~ 4%) [39]. Lung cancer CT screening implementation programmes incorporating risk model based LDCT screening are well underway in the UK; led by the Liverpool Healthy Lung Project (LHLP) [40], followed by Manchester [41], Yorkshire [42], West London [43] and now the NHS England Targeted Healthy Lung Checks Programme [44]. The targeted approach in the UK has resulted in higher participation rates (40–53%) [40,45].
In conclusion the meta-analysis incorporating the results from nine RCTs provides further support for lung cancer screening by low-dose chest CT.
Contributors
JKF, SWD provided substantial contributions to the conception and design of the UKLS trial. JKF, SWD, RG, DV, MPAD contributed to the statistical analysis plan, interpreted the data and wrote the paper. SWD, RG, DV did the statistical analysis. DRB contributed to the UKLS trial design and development of the nodule management protocol. DRB, KEB, AD, TE, JG, BG, JH, TK, KK, ML, KJL, FEM, AN, RDP, MKBP, DMR, RCR, JS, NJW, DW, DKW, PRW, GY contributed to the longstanding success of the UKLS trial. All authors reviewed and approved the manuscript.
Funding
The UKLS was funded by the Health Technology Assessment programme of the National Institute for Health Research (NIHR). Michael Davies is a Roy Castle Lung Cancer Foundation Senior Research Fellow. Daniel Vulkan's and Stephen Duffy's contribution to this research was funded by the NIHR Policy Research Programme, conducted through the Policy Research Unit in Cancer Awareness, Screening and Early Diagnosis, PR-PRU-1217-21601. Robert Rintoul was funded in part by the NIHR Cambridge Biomedical Research Centre and Cancer Research UK Cambridge Centre. The funding source had no role in the design of our analyses, its interpretation, or the decision to submit the manuscript for publication. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Declaration of Interests
JKF has received fees from AstraZeneca (Speaker's Bureau) and advisory boards of Epigenomics; NUCLEIX Ltd. AstraZeneca, iDNA; Grant Support: Janssen Research & Development, LLC. RCR is on the advisory boards of AstraZeneca and Roche. DRB has received speaker remuneration from AstraZeneca, Roche, MSD, BMS, Johnson and Johnson. KB has received personal fees from Astra Zeneca outside the submitted work.
TE receives research support from AstraZeneca, Bayer, Pfizer; is employed by Roche (from March 2020) and was employed by AstraZeneca (to March 2020) and has stock in AstraZeneca and Roche; is a trustee of Macmillan Cancer Support. AN has current grants and contracts with BRC, DART; Honoraria Aidence BV, AstraZeneca; Support from BLF, and as the clinical lead for NTLHC. No competing interests from all other co-authors.
Acknowledgments
Acknowledgments
The authors wish to thank all the individuals who participated in the UKLS trial. The authors thank all the individuals involved in the UKLS data management, especially the Liverpool Clinical Trials Centre and Nial Hodge, as well as the Research Nurses and recruitment site co-ordinators. The authors wish to thank Tariq Malik and his colleagues at Public Health England (PHE) for their assistance in providing an update of the UKLS dataset. This project involves the data derived from patient-level information collected by the NHS, as part of the care and support of cancer patients. The cancer registration data are collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of Public Health England Access to the data was facilitated by the PHE Office for Data Release. Mortality data was collated by the Office of National Statistics and released by NHS Digital.
Data availability
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) may be made available upon request following publication (for up to 5 years) to researchers who provide a methodologically sound proposal. Proposals should be directed to J.K.Field@liverpool.ac.uk; to gain access, data requestors will need to sign a data access agreement. Analyses will be limited to those approved in appropriate ethics and governance arrangements. All study documents which do not identify individuals (e.g. study protocol, statistical analysis plan, informed consent form) will be freely available on request.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.lanepe.2021.100179.
Appendix. Supplementary materials
References
- 1.Marcus P.M., Bergstralh E.J., Fagerstrom R.M., et al. Lung cancer mortality in the mayo lung project: impact of extended follow-up. J Natl Cancer Inst. 2000;92(16):1308–1316. doi: 10.1093/jnci/92.16.1308. [DOI] [PubMed] [Google Scholar]
- 2.Frost J.K., Ball W.C., Levin M.L., et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis. 1984;130(4):549–554. doi: 10.1164/arrd.1984.130.4.549. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko M., Eguchi K., Ohmatsu H., et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology. 1996;201(3):798–802. doi: 10.1148/radiology.201.3.8939234. [DOI] [PubMed] [Google Scholar]
- 4.Henschke C.I., McCauley D.I., Yankelevitz D.F., et al. Early lung cancer action project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 5.National Lung Screening Trial Research T. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning H.J., van der Aalst C.M., de Jong P.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 7.US. Preventive Services Task Force. Final Recommendation Statement Lung Cancer: Screening, March 09, 2021. [cited 2021 12-07-2021]. Available from:http://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening.
- 8.Field J.K., Aberle D.R., Altorki N., et al. The international association study lung cancer (IASLC) strategic screening advisory committee (SSAC) response to the USPSTF recommendations. J Thorac Oncol Off Pub Int Assoc Study Lung Cancer. 2014;9(2):141–143. doi: 10.1097/JTO.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 9.USPSTF proposes expanded lung cancer screening. Cancer Discov. 2020;10(9):OF1. doi: 10.1158/2159-8290.CD-NB2020-072. [DOI] [PubMed] [Google Scholar]
- 10.Infante M., Cavuto S., Lutman F.R., et al. Long-term follow-up results of the dante trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191(10):1166–1175. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 11.Wille M.M., Dirksen A., Ashraf H., et al. Results of the randomized danish lung cancer screening trial with focus on high-risk profiling. Am J Respir Crit Care Med. 2016;193(5):542–551. doi: 10.1164/rccm.201505-1040OC. [DOI] [PubMed] [Google Scholar]
- 12.Paci E., Puliti D., Lopes Pegna A., et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax. 2017;72(9):825–831. doi: 10.1136/thoraxjnl-2016-209825. [DOI] [PubMed] [Google Scholar]
- 13.Pastorino U., Silva M., Sestini S., et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol. 2019;30(10):1162–1169. doi: 10.1093/annonc/mdz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker N., Motsch E., Trotter A., et al. Lung cancer mortality reduction by LDCT screening-results from the randomized German LUSI trial. Int J Cancer. 2020;146(6):1503–1513. doi: 10.1002/ijc.32486. [DOI] [PubMed] [Google Scholar]
- 15.Tammemagi M.C., Schmidt H., Martel S., et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol. 2017;18(11):1523–1531. doi: 10.1016/S1470-2045(17)30597-1. [DOI] [PubMed] [Google Scholar]
- 16.Nawa T. Low-dose CT screening for lung cancer reduced lung cancer mortality in Hitachi city. Int J Radiat Biol. 2019;95(10):1441–1446. doi: 10.1080/09553002.2018.1511930. [DOI] [PubMed] [Google Scholar]
- 17.Lee J., Lim J., Kim Y., et al. Development of protocol for korean lung cancer screening project (K-LUCAS) to evaluate effectiveness and feasibility to implement national cancer screening program. Cancer Res Treat. 2019;51(4):1285–1294. doi: 10.4143/crt.2018.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oudkerk M., Devaraj A., Vliegenthart R., et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754–ee66. doi: 10.1016/S1470-2045(17)30861-6. [DOI] [PubMed] [Google Scholar]
- 19.Field J.K., deKoning H., Oudkerk M., et al. Implementation of lung cancer screening in Europe: challenges and potential solutions: summary of a multidisciplinary roundtable discussion. ESMO Open. 2019;4(5) doi: 10.1136/esmoopen-2019-000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oudkerk M., Liu S., Heuvelmans M.A., Walter J.E., Field JK. Lung cancer LDCT screening and mortality reduction — evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18:135–151. doi: 10.1038/s41571-020-00432-6. [DOI] [PubMed] [Google Scholar]
- 21.Field J.K., Duffy S.W., Baldwin D.R., et al. The UK lung cancer screening trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess. 2016;20(40):1–146. doi: 10.3310/hta20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field J.K., Duffy S.W., Baldwin D.R., et al. UK lung cancer RCT pilot screening trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax. 2016;71(2):161–170. doi: 10.1136/thoraxjnl-2015-207140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field J.K., Vulkan D., Davies M.P.A., Duffy S.W., Gabe R. Liverpool lung project lung cancer risk stratification model: calibration and prospective validation. Thorax. 2021;76:161–168. doi: 10.1136/thoraxjnl-2020-215158. [DOI] [PubMed] [Google Scholar]
- 24.Raji O.Y., Duffy S.W., Agbaje O.F., et al. Predictive accuracy of the liverpool lung project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med. 2012;157(4):242–250. doi: 10.7326/0003-4819-157-4-201208210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldwin D.R., Duffy S.W., Wald N.J., Page R., Hansell D.M., Field JK. UK lung screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax. 2011;66(4):308–313. doi: 10.1136/thx.2010.152066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ru Zhao Y., Xie X., de Koning H.J., Mali W.P., Vliegenthart R., Oudkerk M. NELSON lung cancer screening study. Cancer Imaging. 2011;11:S79–S84. doi: 10.1102/1470-7330.2011.9020. Spec No A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chien C.R., Chen TH. Mean sojourn time and effectiveness of mortality reduction for lung cancer screening with computed tomography. Int J Cancer. 2008;122(11):2594–2599. doi: 10.1002/ijc.23413. [DOI] [PubMed] [Google Scholar]
- 28.Patz E.F., Pinsky P., Gatsonis C., et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174(2):269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Lung Screening Trial Research T Lung cancer incidence and mortality with extended follow-up in the national lung screening trial. J Thorac Oncol off Publ Int Assoc Study Lung Cancer. 2019;14(10):1732–1742. doi: 10.1016/j.jtho.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heleno B., Siersma V., Brodersen J. Estimation of overdiagnosis of lung cancer in low-dose computed tomography screening: a secondary analysis of the danish lung cancer screening trial. JAMA Intern Med. 2018;178(10):1420–1422. doi: 10.1001/jamainternmed.2018.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blom E.F., Ten Haaf K., de Koning H.J. Trends in lung cancer risk and screening eligibility affect overdiagnosis estimates. Lung Cancer. 2020;139:200–206. doi: 10.1016/j.lungcan.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman R.M., Atallah R.P., Struble R.D., Badgett R.G. Lung cancer screening with low-dose CT: A meta-analysis. J Gen Intern Med. 2020;35(10):3015–3025. doi: 10.1007/s11606-020-05951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebell M.H., Bentivegna M., Hulme C. Cancer-specific mortality, all cause mortality, and overdiagnosis,in lung cancerscreening trials: A meta-panalysis. Ann Fam Med. 2020;18(6):545–552. doi: 10.1370/afm.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y.I., Davies M.P.A., Liu D., Li W., Field JK. Implementation planning for lung cancer screening in China. Precis Clin Med. 2019;2(1):13–44. doi: 10.1093/pcmedi/pbz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W., Qian F., Teng J., et al. Community-based lung cancer screening with low-dose CT in China: results of the baseline screening. Lung Cancer. 2018;117:20–26. doi: 10.1016/j.lungcan.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Duffy S.W., Smith RA. The evaluation of cancer screening: concepts and outcome measures. Med Clin North Am. 2020;104(6):939–953. doi: 10.1016/j.mcna.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Miller A.B., Wall C., Baines C.J., Sun P., To T., Narod S.A. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callister M.E., Baldwin D.R., Akram A.R., et al. British thoracic society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70(Suppl 2):ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 39.Tailor T.D., Tong B.C., Gao J., Henderson L.M., Choudhury K.R., Rubin G.D. Utilization of lung cancer screening in the medicare fee-for-service population. Chest. 2020;158(5):2200–2210. doi: 10.1016/j.chest.2020.05.592. [DOI] [PubMed] [Google Scholar]
- 40.Ghimire B., Maroni R., Vulkan D., et al. Evaluation of a health service adopting proactive approach to reduce high risk of lung cancer: the liverpool healthy lung programme. Lung Cancer. 2019;134:66–71. doi: 10.1016/j.lungcan.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Crosbie P.A., Balata H., Evison M., et al. Implementing lung cancer screening: baseline results from a community-based 'Lung Health Check' pilot in deprived areas of Manchester. Thorax. 2019;74(4):405–409. doi: 10.1136/thoraxjnl-2017-211377. [DOI] [PubMed] [Google Scholar]
- 42.Crosbie P.A., Gabe R., Simmonds I., et al. Yorkshire Lung Screening Trial (YLST): protocol for a randomised controlled trial to evaluate invitation to community-based low-dose CT screening for lung cancer versus usual care in a targeted population at risk. BMJ Open. 2020;10(9) doi: 10.1136/bmjopen-2020-037075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartlett E.C., Kemp S.V., Ridge C.A., et al. Baseline Results of the West London lung cancer screening pilot study - impact of mobile scanners and dual risk model utilisation. Lung Cancer. 2020;148:12–19. doi: 10.1016/j.lungcan.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 44.NHS-Eng-National-Cancer-Programme. Targeted Screening for Lung Cancer with Low Radiation Dose Computed Tomography; Standard Protocol prepared for the Targeted Lung Health Checks Programme. 2019. Available from: https://www.england.nhs.uk/wp-content/uploads/2019/02/targeted-lung-health-checks-standard-protocol-v1.pdf. Access date12-07-2021.
- 45.Quaife S.L., Ruparel M., Dickson J.L., et al. Lung screen uptake trial (LSUT): randomized controlled clinical trial testing targeted invitation materials. Am J Respir Crit Care Med. 2020;201(8):965–975. doi: 10.1164/rccm.201905-0946OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) may be made available upon request following publication (for up to 5 years) to researchers who provide a methodologically sound proposal. Proposals should be directed to J.K.Field@liverpool.ac.uk; to gain access, data requestors will need to sign a data access agreement. Analyses will be limited to those approved in appropriate ethics and governance arrangements. All study documents which do not identify individuals (e.g. study protocol, statistical analysis plan, informed consent form) will be freely available on request.