Abstract
Objectives
Different machine learning algorithms (MLAs) for automated segmentation of gliomas have been reported in the literature. Automated segmentation of different tumor characteristics can be of added value for the diagnostic work-up and treatment planning. The purpose of this study was to provide an overview and meta-analysis of different MLA methods.
Methods
A systematic literature review and meta-analysis was performed on the eligible studies describing the segmentation of gliomas. Meta-analysis of the performance was conducted on the reported dice similarity coefficient (DSC) score of both the aggregated results as two subgroups (i.e., high-grade and low-grade gliomas). This study was registered in PROSPERO prior to initiation (CRD42020191033).
Results
After the literature search (n = 734), 42 studies were included in the systematic literature review. Ten studies were eligible for inclusion in the meta-analysis. Overall, the MLAs from the included studies showed an overall DSC score of 0.84 (95% CI: 0.82–0.86). In addition, a DSC score of 0.83 (95% CI: 0.80–0.87) and 0.82 (95% CI: 0.78–0.87) was observed for the automated glioma segmentation of the high-grade and low-grade gliomas, respectively. However, heterogeneity was considerably high between included studies, and publication bias was observed.
Conclusion
MLAs facilitating automated segmentation of gliomas show good accuracy, which is promising for future implementation in neuroradiology. However, before actual implementation, a few hurdles are yet to be overcome. It is crucial that quality guidelines are followed when reporting on MLAs, which includes validation on an external test set.
Key Points
• MLAs from the included studies showed an overall DSC score of 0.84 (95% CI: 0.82–0.86), indicating a good performance.
• MLA performance was comparable when comparing the segmentation results of the high-grade gliomas and the low-grade gliomas.
• For future studies using MLAs, it is crucial that quality guidelines are followed when reporting on MLAs, which includes validation on an external test set.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-021-08035-0.
Keywords: Machine learning, Glioma, Neuroimaging, Meta-analysis
Introduction
Gliomas are the most frequently occurring primary tumor of the brain [1]. Accurate segmentation of gliomas on clinical magnetic resonance imaging (MRI) scans plays an important role in the quantification and objectivation of diagnosis, treatment decision, and prognosis [2–4]. In current clinical practice, T1-weighted, post-contrast T1-weighted, T2-weighted, and T2-fluid attenuated inversion recovery (FLAIR) sequences are required to characterize the different components and to assess the infiltration of the surrounding brain parenchyma [5, 6]. Glioma segmentation requires the distinguishing of tumor tissue from healthy surrounding tissues by the radiologist [7] and the segmented region of interest or volume of interest can be used to compute feature-based radiomics and quantifiable measurements [8, 9]. However, segmentation is a time-consuming task with high inter-observer variability [10, 11]. Therefore, automatic segmentation methods have been searched for as these could facilitate consistent measures and simultaneously could reduce time spent on the task by radiologists in their daily practice. These developments have been powered by the organization of the annual multimodal Brain Tumor Segmentation (BraTS) challenge (http://braintumorsegmentation.org/). Within the BraTS challenges, the organization committee released multimodal scan volumes of a relatively large number of patients suffering from glioma after which different research groups aim to construct machine learning algorithms (MLAs) to automatically segment the gliomas. The BraTS data were accompanied by corresponding segmentations which served as the ground truth [11]. Recent developments in automatic segmentation by the use of MLAs helped to achieve higher precision [12]. Within the BraTS challenges, the MLAs which yielded the most accurate results included different 2D and 3D convolutional neural networks (CNNs) [13–17], including 3D U-Nets [18, 19].
Despite the large body of scientific literature covering this topic, a comprehensive overview and meta-analysis of the accuracy of MLAs in glioma segmentation is still lacking [20, 21]. Therefore, factors which enable the further development of MLAs for glioma segmentation remain partially elusive. The aim of the current study therefore was to provide a systematic review and meta-analysis of the accuracy of MLA-based glioma segmentation tools on multimodal MRI volumes. By providing this overview, the strengths and limitations of this field of research were highlighted and recommendations for future research were made.
Methods
The systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22]. Prior to initiation of the research, the study protocol was registered in the international open-access Prospective Register of Systematic Reviews (PROSPERO) under number CRD42020191033.
Papers that developed or validated MLAs for the segmentation of gliomas were reviewed. Literature was searched for in MEDLINE (accessed through PubMed), Embase, and The Cochrane Library, between April 1, 2020, and June 19, 2020. No language restrictions were applied. The full search strings, including keywords and restrictions, are available in the Appendix. Studies describing MLA-based segmentation methodologies on MR images in glioma patients were included. Additional predefined inclusion criteria were as follows: (1) mean results were defined as dice similarity coefficient (DSC) score; (2) study results needed to be validated either internally and/or externally. Letters, preprints, scientific reports, and narrative reviews were included. Studies based on animals or non-human samples or that presented non-original data were excluded.
Two researchers screened the papers on title, abstract, and full-text independently. Discussions between both researchers were held to resolve all disagreements about non-consensus papers. The investigators independently extracted valuable data of the included papers using a predefined data extraction sheet after which the data was cross-checked. Data extracted from the included studies comprised the following: (a) first author and year of publication; (b) size of training set; (c) mean age of participants in the training set; (d) gender of participants in the training set; (e) size of internal test set; (f) whether there was an external validation; (g) study design, including the used MRI sequences and the segmentations which formed the ground truth; (h) architecture of the AI-algorithm(s); (i) target condition; (j) performance of the algorithm(s) in terms of DSC score, sensitivity, and specificity for both the training and the internal and/or external test sets. When studies performed external validation of the described AI-system(s), externally validated data were included in data extraction tables. Data from the internal validation were used when studies solely carried out the internal validation of the reported MLAs.
The quality of the included studies was not formally assessed, as a formal quality assessment is a well-known challenge in this area of research [23–25]. Nevertheless, Collins and Moons (2019) announced their initiative to develop a version of the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statement tailored to machine learning methods [26]. Pinto dos Santos suggested on the European Society of Radiology website various items to take into consideration when reviewing literature regarding machine learning [27]. These items were included in this review.
Statistical assessment
An independent statistician was consulted to discuss the statistical analyses and approaches with regard to the meta-analysis. To estimate the overall accuracy of the current MLAs, a random effects model meta-analysis was conducted. To be included in the meta-analysis, studies needed to have reported the outcome of interest (i.e., DSC score), in combination with a standard deviation (SD), standard error (SE), and/or the 95% confidence interval (95% CI). For studies reporting the SE and/or the 95% CI, the SD was statistically assessed [28]. Meta-analysis was performed on aggregated data of all studies providing suitable outcomes. Then, subgroup analyses were conducted on two separate target conditions, for studies describing the segmentation of either HGGs or LGGs.
Statistical analyses were carried out by use of IBM SPSS Statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. IBM Corp.). Variables and outcomes of the statistical assessment were presented as mean with ± SD when normally distributed. When data were not normally distributed, they were presented as the median with range (minimum–maximum). Statistical tests were two-sided and significance was assumed when p < 0.05.
The DSC score represents an overlap index and is the most used metric in validating segmentation images. In addition to the direct comparison between automated and ground truth segmentations, the DSC score is a common measure of reproducibility [29, 30]. The DSC score ranges from 0.0 (no overlap) to 1.0 (complete overlap). In this meta-analysis, a DSC score of ≥ 0.8 was considered good overlap. A DSC score of ≤ 0.5 was considered poor.
The quantitative meta-analysis was partially carried out using OpenMeta[Analyst] software, which is the visual front-end for the R package (www.r-project.org; Metafor) [31]. Forest plots were created to depict the estimated DSC scores from the included studies, along with the overall DSC score performance. When the 95% CI of the different subgroup analyses overlapped, no further statistical analysis was carried out.
The heterogeneity of the included studies was tested with the Higgins I2-test. The Higgins I2-test quantifies inconsistency between included studies, where a value > 75% indicates considerable heterogeneity between groups. A low heterogeneity corresponds with a Higgins I2 between 0 and 40% [28]. Both the meta-analyses of the aggregated groups as the meta-analyses of the subgroups were performed using a random effects model, due to an observed high heterogeneity (Higgins I2 > 75%) between included studies [32].
To showcase possible publication bias, a funnel plot was created by means of Stata (StataCorp. 2019. Stata Statistical Software: Release 16.: StataCorp LLC.).
Results
Initially, 1094 publications were retrieved through database searching. An additional ten publications were identified through cross-referencing. After removing duplicates, the remaining 734 publications were screened. Based on the title and abstract, 509 papers were excluded. A total of 225 full-text articles were assessed for eligibility and 42 studies were included in the systematic review. Ten studies were eligible for inclusion for the meta-analysis as they provided sufficient quantitative data (e.g., only these studies provided the DSC score along with SD for the performance of the MLA) (Fig. 1). Publications describing the use of (automated) segmentations to apply MLAs to classify molecular characteristics of gliomas (n = 135) were excluded. Fourteen papers were excluded as they described the use of MLAs on gliomas to perform texture analyses. Eleven papers did not report the DSC score and another 11 studies showed unclarities in data reporting. Contacting the authors of these papers did not result in the acquisition of the needed data. Five studies did not report results of internal or external validation steps, whereas an additional three studies did not report data from the training-group. Three studies described separate combined features, instead of a coherent MLA methodology. One study was excluded due to the inclusion of other brain tumors next to gliomas (e.g., metastases) (Fig. 1).
Fig. 1.
PRISMA flowchart of systematic literature search
Review of the included studies
Based on the full-text analysis, 42 segmentation studies [13, 17, 33–72] were included for the systematic review, from which the participant demographics and study characteristics are depicted in Table 1. The used MLAs are presented in Table 1 and comprised different types of CNNs [13, 17, 34, 35, 37–43, 45–47, 49–53, 55–57, 60, 61, 63–65, 67] and random forest model [68–70], multiple classifier system [33, 44], and an adaptive superpixel generation algorithm [60]. In addition, one study used semi-automatic constrained Markov random field pixel labeling [64], one study used an end-to-end adversarial neural network [71], and one study used a 3D supervoxel-based learning method [56].
Table 1.
Participant demographics, study characteristics, and outcomes of the included studies and performance evaluation of MLAs of the included studies
| Training set | Test set | Reference segmentations | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (year of publication) (reference) | N | Mean age (years) | M-F | N | External|validation | Target condition | Dataset | MR sequences | Summary of DLA methods | 2D vs. 3D | Subgroups | SN | SP | DSC score (± SD) | Data/code openly available? | |
| Kamnitsas et al (2017) [17] | 274 | NR | NR | 110 | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | 3D CNN with two-scale extracted Features and 3D dense Conditional Random Field as postprocessing | 3D | Whole tumor | 88 | NR | 0.85 | Y/Y |
| Contrast enhancing tumor | 67 | NR | 0.63 | |||||||||||||
| Tumor core | 60 | NR | 0.67 | |||||||||||||
| Amirmoezzi et al (2019) [33] | 80 | NR | NR | 80 | No | HGG and LGG | BraTS 2012 | FLAIR images | BraTS segmentations | A specific region of interest (ROI) that contains tumor was identified and then the intensity non-uniformity in ROI was corrected via the histogram normalization and intensity scaling. Each voxel in ROI was presented using 22 features and then was categorized as tumor or non-tumor by a multiple classifier system | 3D | Simulated data | 84.0 | 98.0 | 0.81 ± 0.10 | Y/N |
| Real data | 89.0 | 98.0 | 0.80 ± 0.10 | |||||||||||||
| Banerjee et al (2020) [34] | 285 | NR | NR | 66 | No | HGG and LGG | BraTS 2018 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Encoder-decoder type CNN model combined with a consensus fusion strategy with a fully connected Conditional random field-based post-refinement | 3D | Whole tumor | 91.4 | 99.3 | 0.902 | Y/Y |
| Contrast enhancing tumor | 86.9 | 99.7 | 0.824 | |||||||||||||
| Central tumor necrosis | 87.4 | 99.7 | 0.872 | |||||||||||||
| Bonte et al (2018) [35] | 287 | NR | NR | 285 | Yes | HGG, LGG, and other tumor types (e.g., meningioma, ependymoma ) | BraTS 2013, BraTS 2017, and original data | T1w c+, and FLAIR images | BraTS segmentations | Random Forests model combining voxel-wise texture and abnormality features on 275 feature maps | 3D | LGG – whole tumor | NR | NR | 0.684 | Y/N |
| LGG – tumor core | NR | NR | 0.409 | |||||||||||||
| HGG – whole tumor | NR | NR | 0.801 | |||||||||||||
| HGG- tumor core | NR | NR | 0.750 | |||||||||||||
| Choi et al (2020) [36] | 45 | 58.7 | 24–21 | 46 | Yes | HGG | Original data, TCIA data, and TCGA data | T2w images | Manual segmentations made by two experienced radiologists | V-Net model using 3D input and output which uses convolution with a stride of factor 2 instead of max-pooling | 3D | Tumor + peritumoral edema | NR | NR | 0.78 ± 0.14 | Y/Y |
| Cui et al (2018) [37] | 240 | NR | NR | 34 | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Fully convolutional network in conjunction with the transfer learning technology combined with a CNN with deeper architecture and smaller kernel to label a defined tumor region into multiple subregions | 2D | Whole tumor | NR | NR | 0.89 | Y/N |
| Hasan et al (2018) [38] | 285 | NR | NR | 146 | No | HGG and LGG | BraTS 2017 and original data | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Nearest neighbor re-sampling based elastic-transformed U-net deep CNN framework | 2D | HGG | NR | NR | 0.899 | Y/N |
| LGG | NR | NR | 0.846 | |||||||||||||
| Combined | NR | NR | 0.872 | |||||||||||||
| Havaei et al (2017) [39] | 30 | NR | NR | 10 | No | HGG and LGG | BraTS 2013 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | A CNN with two pathways of both local and global information | 3D | Whole tumor | 84 | 88 | 0.840 | Y/N |
| Contrast enhancing tumor | 68 | 54 | 0.570 | |||||||||||||
| Tumor core | 72 | 79 | 0.710 | Y/N | ||||||||||||
| Havaei et al (2016) [40] | 30 | NR | NR | 10 | No | HGG and LGG | BraTS 2013 | T2w, T1w c+, and FLAIR images | BraTS segmentations | A cascade neural network architecture in which the output of a basic CNN is treated as an additional source of information for a subsequent CNN | 3D | PKSVM-CRF | 78 | 88 | 0.86 | |
| KSVM-CRF | 82 | 87 | 0.84 | |||||||||||||
| kNN-CRF | 78 | 91 | 0.85 | |||||||||||||
| Hussain et al (2017) [41] | 30 | NR | NR | NR | No | HGG and LGG | BraTS 2013 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | deep cascaded convolutional neural networks | 2D | Whole tumor | 82 | 85 | 0.80 | Y/N |
| Contrast enhancing tumor | 57 | 60 | 0.57 | |||||||||||||
| Tumor core | 63 | 82 | 0.67 | |||||||||||||
| Iqbal et al (2019) [42] | 274 | NR | NR | 110 | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Combination of CNN- and long short-term memory models | 2D | Whole tumor | NR | NR | 0.823 | Y/N |
| Iqbal et al (2018) [43] | 274 | NR | NR | 110 | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | CNN Model | 2D | SkipNet** | 83 | 73 | 0.87 | Y/N |
| SENet** | 86 | 83 | 0.88 | |||||||||||||
| IntNet** | 86 | 73 | 0.90 | |||||||||||||
| Jiang et al (2013) [44] | 80 | NR | NR | 23 | No | HGG and LGG | BraTS 2012 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Method exploiting the global classifier (trained by using samples from the population feature set) and a custom classifier (trained by using samples from seed points in the testing image). The outputs of these two classifiers are weighted and then constructed | 3D | Whole tumor | 87.2 | 83.1 | 0.845 ± 0.09 | Y/N |
| Kao et al (2019) [45] | 285 | NR | NR | 66 | No | HGG and LGG | BraTS 2017 and BraTS 2018 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | 3D CNN with two-scale extracted features and 3D dense conditional random field as postprocessing combined with a separate 3D U-Net | 3D | Whole tumor | NR | NR | 0.908 | Y/N |
| Contrast enhancing tumor | NR | NR | 0.782 | |||||||||||||
| Tumor core | NR | NR | 0.823 | |||||||||||||
| Li et al (2017) [46] | 59 | NR | NR | 101 | No | LGG | Original data | FLAIR images | Manual segmentations made by two experienced neurosurgeons | 3D CNN with two-scale extracted features and 3d dense conditional random field as postprocessing | 3D | Whole tumor | 88.9 | NR | 0.802 | N/N |
| Liu et al (2018) [47] | 200 | NR | NR | 74 | No | HGG and LGG | BraTS 2015 | T1w, T2w, and FLAIR images | BraTS segmentations | 3D patch-based fully convolution network adopting the architecture of V-Net | 3D | Whole tumor | NR | NR | 0.87 ± 0.06 | Y/N |
| Meng et al (2018) [48] | 154 | NR | NR | 22 | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Light noise suppression U-network to achieve end-to-end learning without elaborate pre-processing and postprocessing | 2D | Whole tumor | 82 | 74 | 0.89 | Y/N |
| Naceur et al (2018) [49] | 285 | NR | NR | NR | No | HGG and LGG | BraTS 2017 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Three end-to-end incremental deep convolutional neural network models | 2D | Whole tumor | 82 | 74 | 0.89 | Y/N |
| Naser et al (2020) [50] | 110 | 46 | 54-56 | 110 | No | LGG | TCIA | T1w, T1w c+, and FLAIR images | Manual segmentations made by the investigators | A deep learning approach which combines CNNs based on the U-net for tumor segmentation and transfer learning based on a pre-trained convolution-base of Vgg16 and a fully connected classifier for tumor grading was developed. | 3D | Whole tumor | NR | NR | 0.84 | Y/N |
| Perkuhn et al (2018) [51] | * | NR | NR | 64 | Yes | HGG | Original data | T1w, T2w, T1w c+, and FLAIR images | Manual segmentations made by the investigators following the BraTS challenge workflow | 3D CNN with two-scale extracted features and 3D dense Conditional random Field as postprocessing | 3D | Whole tumor | 84 | NR | 0.86 ± 0.09 | Y/N |
| Contrast enhancing tumor | 78 | NR | 0.78 ± 0.15 | |||||||||||||
| Central tumor necrosis | 57 | NR | 0.62 ± 0.30 | |||||||||||||
| Razzak et al (2019) [52] | 285 | NR | NR | 110 | No | HGG and LGG | BraTS 2013 and BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Two-pathway CNN which simultaneously accommodates the global and local features as well as embedding additional transformations like rotations and reflections in itself by applying not only translation but also rotational and reflection to the filters which result in an increase in the degree of weight sharing | 2D | Whole tumor | 88.3 | NR | 0.892 | Y/N |
| Savareh et al (2019) [53] | 274 | NR | NR | NR | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Fully convolutional network was selected to implement the wavelet-enhanced fully convolutional network model | 3D | Whole tumor | 93 | 99 | 0.918 | Y/N |
| Soltaninejad et al (2018) [54] | 11 | 53 | NR | 11 | No | HGG and LGG | BraTS 2013 and original data | T1w, T2w, T1w c+, FLAIR, and DTI images | Segmentations derived from the BraTS dataset combined with manual segmentations made by the investigators following the BraTS challenge workflow | 3D supervoxel-based learning method. Supervoxels are generated using the information across the multimodal MRI dataset. For each supervoxel, a variety of features including histograms of tex-ton descriptor, calculated using a set of Gabor filters with different sizes and orientations, and first-order intensity statistical features are extracted. Those features are fed into a random forests classifier to classify each supervoxel into tumor core, edema, or healthy brain tissue. | 3D | Whole tumor | NR | NR | 0.84 ± 0.06 | Y/N |
| Sun et al (2019) [55] | 274 | NR | NR | 110 | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | 3D CNN-based method | 3D | Whole tumor | 89 | NR | 0.84 | Y/N |
| Contrast enhancing tumor | 69 | NR | 0.62 | |||||||||||||
| Wang et al (2018) [56] | 100 | NR | NR | NR | No | HGG and LGG | NR | NR | NR | 3D-CNN Model | 3D | Whole tumor | NR | NR | 0.916 | N/N |
| Wu et al (2020) [57] | 285 | NR | NR | 66 | No | HGG and LGG | BraTS 2017 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | 2D U-Nets | 2D | Whole tumor | NR | NR | 0.91 | Y/Y |
| Contrast enhancing tumor | NR | NR | 0.80 | |||||||||||||
| Tumor core | NR | NR | 0.83 | |||||||||||||
| Wu et al (2019) [58] | 228 | NR | NR | 57 | No | HGG and LGG | BraTS 2017 | T2w images | BraTS segmentations | An adaptive superpixel generation algorithm based on simple linear iterative clustering version with 0 parameter (ASLIC0) was used to acquire a superpixel image with fewer superpixels and better fit the boundary of ROI by automatically selecting the optimal number of superpixels. | 2D | Whole tumor | 81.5 | 99.6 | 0.849 ± 0.07 | Y/N |
| Yang et al (2019) [59] | 255 | NR | NR | 30 | No | HGG and LGG | BraTS 2017 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | U-net | 2D | Whole tumor | 90.6 | NR | 0.883 ± 0.06 | Y/N |
| Contrast enhancing tumor | 79.2 | NR | 0.784 ± 0.10 | |||||||||||||
| Tumor core | 88.3 | NR | 0.781 ± 0.10 | |||||||||||||
| Yang et al (2019) [60] | 274 | NR | NR | 274 | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Two-pathway convolutional neural network combined with random forests | 2D | SK-TPCNN – Whole tumor | 95 | NR | 0.86 | Y/N |
| SK-TPCNN – contrast-enhancing tumor | 76 | NR | 0.81 | |||||||||||||
| SK-TPCNN – tumor core | 91 | NR | 0.74 | |||||||||||||
| SK-TPCNN + RF – whole tumor | 96 | NR | 0.89 | |||||||||||||
| SK-TPCNN + RF – contrast-enhancing tumor | 83 | NR | 0.87 | |||||||||||||
| SK-TPCNN + RF – Tumor core | 92 | NR | 0.80 | |||||||||||||
| Yang et al (2020) [61] | 274 | NR | NR | 274 | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | 2D-CNN Model | 2D | Whole tumor | 88 | NR | 0.90 | Y/N |
| Contrast enhancing tumor | 84 | NR | 0.88 | |||||||||||||
| Tumor core | 82 | NR | 0.82 | |||||||||||||
| Zhao et al (2013) [62] | 30 | NR | NR | 30 | No | HGG | BraTS 2012 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Semi-automatic Constrained Markov random field pixel labeling | 3D | HGG | NR | NR | 0.835 ± 0.089 | Y/N |
| LGG | LGG | NR | NR | 0.848 ± 0.087 | ||||||||||||
| Zhou et al (2020) [63] | 285 | NR | NR | 66 | No | HGG and LGG | BraTS 2013, BraTS 2015, and BraTS 2018 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | 3D dense connectivity model | 3D | Whole tumor | NR | NR | 0.864 | Y/N |
| Contrast-enhancing tumor | NR | NR | 0.753 | |||||||||||||
| Tumor core | NR | NR | 0.774 | |||||||||||||
| Zhuge et al (2017) [64] | 20 | NR | NR | 10 | Yes | HGG | BraTS 2013 dataset and original data | T1w, T2w, T1w c+, and FLAIR images | Segmentations derived from the BraTS dataset [11]; original data was manually annotated following the BraTS-protocol [11] | Holistically nested CNN model | 2D | Whole tumor | 85.0 | NR | 0.83 | Y/Y |
| Dong et al (2017) [65] | 274 | NR | NR | NR | No | HGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | U-Net based deep convolutional networks | 3D | LGG - whole tumor | NR | NR | 0.84 | Y/N |
| LGG – tumor core | NR | NR | 0.85 | |||||||||||||
| HGG – whole tumor | NR | NR | 0.88 | |||||||||||||
| HGG – contrast-enhancing tumor | NR | NR | 0.81 | |||||||||||||
| HGG – tumor core | NR | NR | 0.87 | |||||||||||||
| Dvorak and Menze (2015) [66] | 163 | NR | NR | 25 | No | HGG and LGG | BraTS 2013 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Structured prediction was used together with a CNN | 3D | LGG - whole tumor | NR | NR | 0.85 ± 0.06 | Y/N |
| LGG – tumor core | NR | NR | 0.65 ± 0.15 | |||||||||||||
| HGG – whole tumor | NR | NR | 0.80 ± 0.17 | |||||||||||||
| HGG – contrast-enhancing tumor | NR | NR | 0.81 ± 0.11 | |||||||||||||
| HGG – tumor core | NR | NR | 0.85 ± 0.08 | |||||||||||||
| Lyksborg et al (2015) [67] | 91 | NR | NR | 40 | No | HGG and LGG | BraTS 2014 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | An ensemble of 2D CNNs with a three-step volumetric segmentation | 2D | Whole tumor | 82.5 | NR | 0.810 | Y/N |
| Pereira et al (2016) [13] | 30 | NR | NR | NR | No | HGG and LGG | BraTS 2013 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | A CNN with small 3 × 3 kernels | 2D | Whole tumor | 86 | NR | 0.88 | Y/Y |
| Pinto et al (2015) [68] | 40 | NR | NR | 10 | No | HGG and LGG | BraTS 2013 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Using appearance- and context-based features to feed an extremely randomized forest | 2D | Whole tumor | 82 | NR | 0.83 | Y/N |
| Contrast-enhancing tumor | 79 | NR | 0.73 | |||||||||||||
| Tumor core | 75 | NR | 0.78 | |||||||||||||
| Tustison et al (2015) [69] | 30 | NR | NR | 10 | No | HGG and LGG | BraTS 2013 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Combine a random forest model with a framework of regularized probabilistic segmentation | 2D | Whole tumor | 89 | NR | 0.87 | Y/Y |
| Contrast-enhancing tumor | 83 | NR | 0.74 | |||||||||||||
| Tumor core | 88 | NR | 0.78 | |||||||||||||
| Usman and Rajpoot (2017) [70] | 30 | NR | NR | NR | No | HGG and LGG | BraTS 2013 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Automated wavelet-based features + a random forest classifier | 3D | Whole tumor | NR | NR | 0.88 | Y/N |
| Contrast-enhancing tumor | NR | NR | 0.95 | |||||||||||||
| Tumor core | NR | NR | 0.75 | |||||||||||||
| Xue et al (2017) [71] | 274 | NR | NR | NR | No | HGG and LGG | BraTS 2015 | T1w, T2w, T1w c+, and FLAIR images | An end-to-end adversarial neural network | 2D | Whole tumor | 80 | NR | 0.85 | Y/Y | |
| Contrast-enhancing tumor | 62 | NR | 0.66 | |||||||||||||
| Tumor core | 65 | NR | 0.70 | |||||||||||||
| Zikic et al (2012) [72] | 30 | NR | NR | 10 | No | HGG | BraTS 2012 | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | Apply a CNN in a sliding-window fashion in the 3D space | 3D | Whole tumor | NR | NR | 0.90 ± 0.09 | Y/N |
| Contrast-enhancing tumor | NR | NR | 0.85 ± 0.09 | |||||||||||||
| Necrotic tumor core | NR | NR | 0.75 ± 0.16 | |||||||||||||
| Peritumoral edema | NR | NR | 0.80 ± 0.18 | |||||||||||||
Studies included in the meta-analysis were italicized
BraTS, Brain Tumor Image Segmentation Benchmark; CNN, convolutional neural network; DSC, dice similarity coefficient; kNN-CRF, k-nearest neighbor conditional random fields; KSVM-CRF, kernel support vector machine with rbf kernel conditional random fields; LSTM, long short-term memory; MLA, machine learning algorithms; N, no; NR, not reported; PKSVM-CRF, proposed product kernel support vector machine conditional random fields; SD, standard deviation; SK-TPCNN (+RF), small kernels two-path convolutional (+ random forests) neural network; SN, sensitivity; SP, specificity; TCIA, the Cancer Imaging Archive; TCGA, the Cancer Genome Atlas; Y, yes. *The deep learning model is based on the recently published DeepMedic architecture, which provided top scoring results on the BRATS data set [17]. **Data separated by LGG and HGG for each network available in the original paper
For more information on the multivendor BraTS dataset, see Menze et al [11]. Please note that the ground truth of BraTS 2015 was first produced by algorithms and then verified by annotators; in contrast, the ground truth of BraTS 2013 fused multiple manual annotations
Thirty-eight studies combined different combinations of MRI sequences for brain tumor segmentation (Table 1) [13, 17, 33–42, 44, 45, 47–57, 59–72]. Only 3 studies used one MRI sequence for the algorithm to segment [43, 46, 58]. One conference paper did not report on the used MRI sequences [56]. Four studies reported not to have used (any part of) the BraTS datasets [36, 46, 50, 51]. Two of these papers used original data [46, 51]. The other two papers used either data from the Cancer Imaging Archive (TCIA) [50] or a combination of TCIA data and original data [36].
In 36 studies, the ground truth (i.e., segmentations) was derived from the BraTS dataset [13, 17, 33–36, 38–45, 47–49, 52–55, 57–72]. In two of these studies, the researchers added segmentations of additional original data. Segmentations were manually annotated by two experienced professionals independently following the BraTS segmentation protocol[54, 64]. In one paper, only original data with corresponding segmentations were used. These segmentations were made independently by two experienced professionals following the BraTS segmentation protocol [51]. Three papers used segmentations which were obtained without adhering to the BraTS segmentation protocol [36, 46, 50]. In one conference paper, the segmentation methodology was not described [56]. Please note that the ground truth segmentations of BraTS 2015 were first produced by algorithms and then verified by annotators, whereas the ground truth of BraTS 2013 fused multiple manual annotations.
The performance of the MLAs, in terms of sensitivity, specificity, and DSC score, is displayed in Table 1. All studies used retrospectively collected data. Nine studies focused specifically on the segmentation of HGGs, whereas seven studies focused on the segmentation of LGGs. The remaining studies (n = 31) described the segmentation of gliomas in general without the subdivision of LGG and HGG. Five of the included studies [33, 35, 38, 62, 65] described segmentation of multiple target conditions (i.e., segmentation of both HGG and LGG). For these studies, the results of each different target are displayed in Table 1 as well. All of the included studies conducted some version of cross-validation on the MLAs; however, only four studies [35, 36, 51, 64] performed an external validation of performance.
Nine studies [33, 35, 36, 38, 51, 62, 64, 65, 72] described the segmentation of HGGs in particular, with four studies [35, 36, 51, 64] externally validating the performance of the reported MLAs. Performance evaluation of the included studies in terms of the validated DSC score ranged from 0.78 to 0.90. MLA sensitivity ranged from 84 to 85% (n = 3) [33, 51, 64]. Only one study [33] presented the specificity rate (i.e., 98%).
Seven studies [33, 35, 38, 46, 50, 62, 65] described the segmentation of LGGs. External validation of the MLA was performed by one study [35]. The validated DSC score for the included studies ranged from 0.68 to 0.85. Sensitivity was 89% (n = 2) [33, 46], whereas specificity was 98% (n = 1) [33].
Meta-analysis of the included studies
The aggregated meta-analysis comprised twelve MLAs, described in ten individual studies [33, 36, 44, 47, 51, 54, 58, 62, 66, 72], and showed an overall DSC score of 0.84 (95% CI: 0.82 – 0.86) (Fig. 2). Heterogeneity showed to be 80.4%, indicating that studies differed significantly (p < 0.001).
Fig. 2.
Forest plot of the included studies that assessed the accuracy of segmentation of glioma. Legend: DSC, dice similarity coefficient; CI, confidence interval. Forest plot shows that the performance of the MLAs to segment gliomas are centered around a DSC of 0.837 with a 95% CI ranging from 0.820 to 0.855
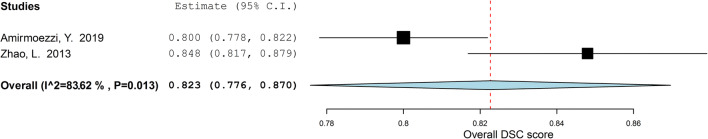
For the subgroup analysis of segmentation studies focusing on HGGs, the results are depicted in Fig. 3. Overall, DSC score for the five included studies [33, 36, 51, 62, 72] was 0.83 (95% CI: 0.80 – 0.87). The estimated I2 heterogeneity between groups showed to be 81.9% (p = 0.001). Two studies [33, 62] focusing on the segmentation of LGGs were included in another subgroup meta-analysis. Overall, the DSC score was found to be 0.82 (95% CI: 0.78–0.87) (Fig. 4). The estimated heterogeneity of included groups was 83.62% (p = 0.013). Hence, the heterogeneity was determined as high for both subgroup meta-analyses.
Fig. 3.
Forest plot of the included studies that assessed the accuracy of segmentation of high-grade glioma. Legend: DSC, dice similarity coefficient; CI, confidence interval. Forest plot shows that the performance of the MLAs to segment HGGs are centered around a DSC of 0.834 with a 95% CI ranging from 0.802 to 0.867
Fig. 4.
Forest plot of the included studies that assessed the accuracy of segmentation of low-grade glioma. Legend: DSC, dice similarity coefficient; CI, confidence interval. Forest plot shows that the performance of the MLAs to segment LGGs are centered around a DSC of 0.823 with a 95% CI ranging from 0.776 to 0.870
Publication bias
Studies included in the funnel plot were the ten studies that were meta-analyzed (Fig. 5). The funnel plot showed an asymmetrical shape, giving an indication for publication bias among included studies. Besides, not all studies were plotted within the area under the curve of the pseudo-95% CI, supporting the indication of possible publication bias [28].
Fig. 5.
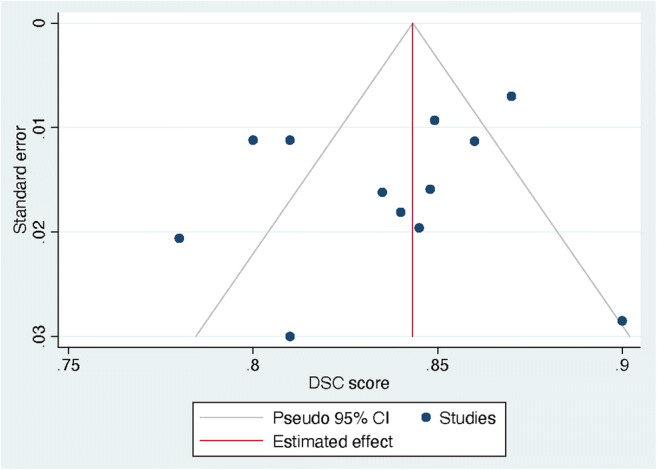
Funnel plot of the included studies. Legend: DSC, dice similarity coefficient; CI, confidence interval. DSC score was displayed on the horizontal axis as the effect size; SE was plotted on the vertical axis of the funnel plot
Discussion
Various MLAs for the automated segmentation of gliomas were reviewed. Although heterogenous, MLAs showed to have a good DSC score with no differences between the segmentation of LGG and HGG. However, there were some indications for publication bias within this field of research.
Currently, segmentation of tumor lesions is a subjective and time-consuming task [58]. By replacing the current manual methods with an automated computer-aided approach, improvement of glioma quantification and subsequently radiomics can be achieved. However, automated segmentation of gliomas is a challenging task, due to the large variety of morphological tumor characteristics among patients [11]. As HGGs usually show more heterogeneous MRI characteristics, their automated segmentation could be expected to be more challenging compared to LGGs. Furthermore, the low proliferative state of LGGs likely results in lower perfusion and higher diffusion values in affected tissue [73, 74]. No performance difference was observed between the segmentation of HGGs and LGGs. Given the differences between HGGs and LGGs, it was expected that significant differences would arise in automatic segmentation tasks. Nevertheless, the ground truth segmentations were based on manual delineation by a (neuro)radiologist, indicating that the performance of automatic segmentation could only be as good as the ground truth segmentations. In addition, the ground truth of BraTS 2015 was first produced by algorithms and then verified by annotators, whereas the ground truth of BraTS 2013 fused multiple manual annotations.
Although MLAs performing automated segmentation show quite promising results (overall DSC score of 0.84; 95% CI: 0.82–0.86), there is still no wide acceptance and implementation of these methodologies in daily clinical practice. One of the explanations for this can be found in the different MLA methodologies; different MLA approaches and their exact details have a significant impact on the outcomes, even when applied to the same dataset. For example, in the BraTS 2019 challenge, the top three with regard to the segmentation task comprised a two-stage cascaded U-Net [75], a deep convolution neural network [76], and an ensemble of 3D-to-2D CNNs [77].
Another reason may be the absence of standardized procedures on how to properly use these segmentation systems. There are substantial differences between advanced systems that offer computer-aided segmentation and the current standards for neuroradiologists, which impedes the integration of MLA methods. CE-certified software is limitedly available in clinical practice, which is one of the reasons for the impediment. Also, the purpose for the use of MLAs varies; where radiologists mainly use these techniques for follow-up, neurosurgeons mostly use MLAs for therapeutic planning. In addition, direct integration into the neuroradiologist’s daily practice without extra time spent on the task will be needed to make automatic glioma segmentation feasible. Moreover, the current automated segmentations still need to be supervised by trained observers. It seems more likely that implementation of MLAs in neuroradiology will lead to an interaction between doctor and computer so that neuroradiologists will utilize more advanced technologies in the establishment of diagnoses [78]. The future implementation of MLAs in the diagnosis of glioma is of great clinical relevance, as these algorithms can support the non-invasive analysis of tumor characteristics without the need of histopathological tissue assessment. More specifically, automatic segmentations form the basis of further sophisticated analyses to clarify meaningful and reliable associations between neuroimaging features and survival rate [79, 80]. In conclusion, as automated segmentation of glioma is considered to be the first step in this process, the implementation of MLAs holds great potential for the future of neuroradiology.
Various publications were found with regard to the automated segmentation of gliomas in the post-operative setting [81–84]. Quantitative metrics are believed to be needed for therapy guidance, risk stratification, and outcome prognostication in the post-operative setting. MLAs could also represent a potential solution for automated quantitative measurements of the burden of disease in the post-operative setting. As shown in Table 2, however, the DSC scores of these studies are lower as compared to the DSC scores of the pre-operative MLA-based segmentations [81–84]. An explanation for these differences in performance could be the post-surgical changes of the brain parenchyma and the presence of air and blood products in the post-operative setting. Together these factors have been reported to affect the performance of MLAs [81].
Table 2.
Overview of the studies on post-operative glioma segmentation
| Training set | Test set | Reference segmentations | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (year of publication) (reference) | N | Mean age (years) | M-F | N | External validation | Target condition | Dataset | MR Sequences | Summary of DLA methods | 2D vs. 3D | Subgroups | SN | SP | DSC score (± SD) | Data/code openly available? | |
| Herrmann et al (2020) [81] | 30 | NR | NR | 30 | No | Brain resection cavity delineation | Original data | T1w, T2w, T1w c+, and FLAIR images | Manual segmentations made by three experienced radiation oncology experts. To improve inter-rater consistency the raters have been instructed by an experienced neuro-radiologist. | A fully convolutional densely connected architecture which builds on the idea of DenseNet was used. | 3D | NA | NR | NR | 0.83 | N/N |
| Meier et al (2016) [82] | 14 | NR | NR | 14 | No | Brain volume delineation during and after therapy with neurosurgery, radiotherapy, chemotherapy, and/or anti-angiogenic therapy | Original data | T1w, T2w, T1w c+, and FLAIR images | Manual segmentations made by two raters (one experienced, one inexperienced); this table only represents the overlap between the MLA and the experienced rater | Machine learning–based framework using voxel-wise tissue classification for automated segmentation | 2D | Non- enhancing T2 hyperintense tissue | NR | NR | 0.673 | N/N |
| Contrast-enhancing T2 hyperintense tissue | NR | NR | 0.183 | |||||||||||||
| Zeng et al (2016) [83] | 218 | NR | NR | 191 | No | Segmenting post-operative scans | BraTS 2016 and original data | T1w, T2w, T1w c+, and FLAIR images | BraTS segmentations | A hybrid generative-discriminative model was used. Firstly, a generative model based on a joint segmentation-registration framework was used to segment the brain scans into cancerous and healthy tissues. Secondly, a gradient boosting classification scheme was used to refine tumor segmentation based on information from multiple patients. | 3D | Post-operative HGG – Whole tumor | NR | NR | 0.72 | N/N |
| Post-operative HGG – contrast-enhancing tumor | NR | NR | 0.49 | |||||||||||||
| Post-operative HGG – tumor core | NR | NR | 0.57 | |||||||||||||
| Tang et al (2020) [84] | 59 | 41.2 ± 12.6 | 32-27 | 15 | No | Post-operative glioma segmentation in CT images | Original data | T1w, T2w, T1w c+, and FLAIR images | Manual segmentations made by one experienced radiation oncology expert | DFFM is a multi-sequence MRI–guided CNN that iteratively learned the deep features from CT images and multi-sequence MR images simultaneously by utilizing a multi-channel CNN architecture, and then combined these two deep features together to produce the segmentation result. The whole network was optimized together via a standard back-propagation. | 3D | NA | NR | NR | 0.818 | N/N |
BraTS, Brain Tumor Image Segmentation Benchmark; CNN, convolutional neural network; DSC, dice similarity coefficient; MLA, machine learning algorithms; N, no; NA, not applicable; NR, not reported; SD, standard deviation; SN, sensitivity; SP, specificity; Y, yes
For more information on the multivendor BraTS dataset, see Menze et al [11]. Please note that the ground truth of BraTS 2015 was first produced by algorithms and then verified by annotators; in contrast, the ground truth of BraTS 2013 fused multiple manual annotations
Several methodological shortcomings of the present meta-analysis should be considered. First, various studies were excluded for the quantitative synthesis, due to missing data. Besides, heterogeneity of all analyses was considerably high, probably caused by technical variances of different MLA methodologies for segmentation. Lastly, only four out of 42 studies performed an out-of-sample external validation, emphasizing the importance of external validation to assess the robustness. It is probable that publication bias was present as there is no interest in the publication of poorly performing MLAs. In addition, differences in MR sequence input, ground truth, and other variables could play a role with regard to the outcomes, although this was considered a minor limitation as the source data across studies was similar in most studies.
Future gains of research on this topic may include an ensemble approach, as this might significantly boost the performance of segmentation. Thus, in addition, to focus current research on training individual segmentation systems, it may be interesting to investigate the fusion of multiple systems as well (i.e., segmentation of different imaging features in order to obtain different imaging biomarkers) [11]. Lastly, all included studies used retrospectively collected data, most of which using data from the BRATS databases. In order to further validate the performance of segmentation systems in clinical practice, larger-scale and external validated studies are preferred. In addition, data availability and providing online tools or downloadable scripts of the used MLAs could enhance future developments within this field of research significantly.
Conclusion
In this study, a systematic review and meta-analysis of different studies using MLA for glioma segmentation shows good performance. However, external validation is often not carried out, which should be regarded as a significant limitation in this field of research. Therefore, further verification of the accuracy of these models is recommended. It is crucial that quality guidelines are followed when reporting on MLAs, which includes validation on an external test set.
Supplementary Information
(DOCX 19 kb)
Acknowledgements
The authors would like to acknowledge Dr. Rogier Donders for his statistical insights.
Abbreviations
- AI
Artificial intelligence
- BraTS
Brain tumor segmentation
- CI
Confidence interval
- DSC
Dice similarity coefficient
- GBM
Glioblastoma multiforme
- HGG
High-grade glioma
- LGG
Low-grade glioma
- MLA
Machine learning algorithm
- MRI
Magnetic resonance imaging
- SD
Standard deviation
- SE
Standard error
Funding
The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is professor Mathias Prokop (MD, PhD) of Radboudumc, Nijmegen, The Netherlands.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Biostatistician Dr. Rogier Donders kindly provided statistical advice for this manuscript. Also, multiple authors have significant statistical expertise.
Informed consent
No informed consent was needed for the conducting of this review.
Ethical approval
Institutional Review Board approval was not required because this review did not include specimens or involve any treatments or interventions.
Study subjects or cohorts overlap
All of the included studies have been previously reported, either as an original research paper or a conference paper.
Methodology
• Systematic review
• meta-analysis
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perry A, Wesseling P (2016) Histologic classification of gliomas handbook of clinical neurology. Elsevier, pp 71–95 [DOI] [PubMed]
- 2.Mazzara GP, Velthuizen RP, Pearlman JL, Greenberg HM, Wagner H. Brain tumor target volume determination for radiation treatment planning through automated MRI segmentation. Int J Radiat Oncol Biol Phys. 2004;59:300–312. doi: 10.1016/j.ijrobp.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Yamahara T, Numa Y, Oishi T, et al. Morphological and flow cytometric analysis of cell infiltration in glioblastoma: a comparison of autopsy brain and neuroimaging. Brain Tumor Pathol. 2010;27:81–87. doi: 10.1007/s10014-010-0275-7. [DOI] [PubMed] [Google Scholar]
- 4.Bauer S, Wiest R, Nolte LP, Reyes M. A survey of MRI-based medical image analysis for brain tumor studies. Phys Med Biol. 2013;58:R97–R129. doi: 10.1088/0031-9155/58/13/R97. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DR, Guerin JB, Giannini C, Morris JM, Eckel LJ, Kaufmann TJ. 2016 Updates to the WHO brain tumor classification system: what the radiologist needs to know. Radiographics. 2017;37:2164–2180. doi: 10.1148/rg.2017170037. [DOI] [PubMed] [Google Scholar]
- 6.Larsen J, Wharton SB, McKevitt F, et al. ‘Low grade glioma’: an update for radiologists. Br J Radiol. 2017;90:20160600. doi: 10.1259/bjr.20160600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordillo N, Montseny E, Sobrevilla P. State of the art survey on MRI brain tumor segmentation. Magn Reson Imaging. 2013;31:1426–1438. doi: 10.1016/j.mri.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Velthuizen RP, Hall LO, Clarke LP. Feature extraction for MRI segmentation. J Neuroimaging. 1999;9:85–90. doi: 10.1111/jon19999285. [DOI] [PubMed] [Google Scholar]
- 9.Ditmer A, Zhang B, Shujaat T, et al. Diagnostic accuracy of MRI texture analysis for grading gliomas. J Neurooncol. 2018;140:583–589. doi: 10.1007/s11060-018-2984-4. [DOI] [PubMed] [Google Scholar]
- 10.Egger J, Kapur T, Fedorov A et al (2013) GBM volumetry using the 3D Slicer medical image computing platform. Sci Rep 3:1364. 10.1038/srep01364 [DOI] [PMC free article] [PubMed]
- 11.Menze BH, Jakab A, Bauer S, et al. The multimodal brain tumor image segmentation benchmark (BRATS) IEEE Trans Med Imaging. 2015;34:1993–2024. doi: 10.1109/TMI.2014.2377694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaddad A, Kucharczyk MJ, Daniel P, et al. Radiomics in glioblastoma: current status and challenges facing clinical implementation. Front Oncol. 2019;9:374. doi: 10.3389/fonc.2019.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira S, Pinto A, Alves V, Silva CA. Brain tumor segmentation using convolutional neural networks in MRI images. IEEE Trans Med Imaging. 2016;35:1240–1251. doi: 10.1109/TMI.2016.2538465. [DOI] [PubMed] [Google Scholar]
- 14.Havaei M, Dutil F, Pal C, Larochelle H, Jodoin P-M (2015) A convolutional neural network approach to brain tumor segmentation BrainLes 2015. Springer, pp 195–208
- 15.Randhawa RS, Modi A, Jain P, Warier P (2016) Improving boundary classification for brain tumor segmentation and longitudinal disease progression. Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries, 2016 10154:65–74
- 16.Long J, Shelhamer E, Darrell T (2015) Fully convolutional networks for semantic segmentation. 2015 Ieee Conference on Computer Vision and Pattern Recognition (Cvpr). 10.1109/cvpr.2015.7298965:3431-3440 [DOI] [PubMed]
- 17.Kamnitsas K, Ledig C, Newcombe VFJ, et al. Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med Image Anal. 2017;36:61–78. doi: 10.1016/j.media.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. In: Navab N, Hornegger J, Wells W, Frangi A (eds) MICCAI 9351. Springer, Cham. 10.1007/978-3-319-24574-4_28
- 19.Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O (2016) 3D U-Net: learning dense volumetric segmentation from sparse annotation International conference on medical image computing and computer-assisted intervention. Springer, pp 424–432
- 20.Sakai K, Yamada K. Machine learning studies on major brain diseases: 5-year trends of 2014-2018. Jpn J Radiol. 2019;37:34–72. doi: 10.1007/s11604-018-0794-4. [DOI] [PubMed] [Google Scholar]
- 21.Lotan E, Jain R, Razavian N, Fatterpekar GM, Lui YW (2018) State of the art: machine learning applications in glioma imaging. AJR Am J Roentgenol 212:26–37 [DOI] [PubMed]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341 [PMC free article] [PubMed]
- 23.Liu XX, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digital Health. 2019;1:E271–E297. doi: 10.1016/S2589-7500(19)30123-2. [DOI] [PubMed] [Google Scholar]
- 24.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 25.Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 26.Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393:1577–1579. doi: 10.1016/S0140-6736(19)30037-6. [DOI] [PubMed] [Google Scholar]
- 27.Pinto dos Santos D (2020) Assessing radiology research on artificial intelligence: a brief guide for authors, reviewers and readers. European Society of Radiology. Available via https://ai.myesr.org/publications/assessing-radiology-research-on-artificial-intelligence-a-brief-guide-for-authors-reviewers-and-readers/. Accessed 5 Oct 2020
- 28.Higgins JPT (2011) GSe Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration
- 29.Yeghiazaryan V, Voiculescu I. Family of boundary overlap metrics for the evaluation of medical image segmentation. J Med Imaging (Bellingham) 2018;5:015006. doi: 10.1117/1.JMI.5.1.015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taha AA, Hanbury A. Metrics for evaluating 3D medical image segmentation: analysis, selection, and tool. BMC Med Imaging. 2015;15:29. doi: 10.1186/s12880-015-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viechtbauer W (2010) Conducting Meta-Analyses in R with the metafor Package. Journal of Statistical Software, 36(3):1–48. 10.18637/jss.v036.i03
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amirmoezzi Y, Salehi S, Parsaei H, Kazemi K, Torabi Jahromi A. A knowledge-based system for brain tumor segmentation using only 3D FLAIR images. Australas Phys Eng Sci Med. 2019;42:529–540. doi: 10.1007/s13246-019-00754-5. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee S, Mitra S. Novel volumetric sub-region segmentation in brain tumors. Front Comput Neurosci. 2020;14:3. doi: 10.3389/fncom.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonte S, Goethals I, Van Holen R. Machine learning based brain tumour segmentation on limited data using local texture and abnormality. Comput Biol Med. 2018;98:39–47. doi: 10.1016/j.compbiomed.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Choi Y, Nam Y, Lee YS, et al. IDH1 mutation prediction using MR-based radiomics in glioblastoma: comparison between manual and fully automated deep learning-based approach of tumor segmentation. Eur J Radiol. 2020;128:109031. doi: 10.1016/j.ejrad.2020.109031. [DOI] [PubMed] [Google Scholar]
- 37.Cui S, Mao L, Jiang J, Liu C, Xiong S. Automatic semantic segmentation of brain gliomas from MRI images using a deep cascaded neural network. J Healthc Eng. 2018;2018:4940593. doi: 10.1155/2018/4940593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasan SMK, Linte CA (2018) A modified U-Net convolutional network featuring a nearest-neighbor re-sampling-based elastic-transformation for brain tissue characterization and segmentation. Proc IEEE West N Y Image Signal Process Workshop 2018 [DOI] [PMC free article] [PubMed]
- 39.Havaei M, Davy A, Warde-Farley D, et al. Brain tumor segmentation with deep neural networks. Med Image Anal. 2017;35:18–31. doi: 10.1016/j.media.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Havaei M, Larochelle H, Poulin P, Jodoin PM. Within-brain classification for brain tumor segmentation. Int J Comput Assist Radiol Surg. 2016;11:777–788. doi: 10.1007/s11548-015-1311-1. [DOI] [PubMed] [Google Scholar]
- 41.Hussain S, Anwar SM, Majid M (2017) Brain tumor segmentation using cascaded deep convolutional neural network. Annu Int Conf IEEE Eng Med Biol Soc 2017:1998–2001 [DOI] [PubMed]
- 42.Iqbal S, Ghani Khan MU, Saba T, et al. Deep learning model integrating features and novel classifiers fusion for brain tumor segmentation. Microsc Res Tech. 2019;82:1302–1315. doi: 10.1002/jemt.23281. [DOI] [PubMed] [Google Scholar]
- 43.Iqbal S, Ghani MU, Saba T, Rehman A. Brain tumor segmentation in multi-spectral MRI using convolutional neural networks (CNN) Microsc Res Tech. 2018;81:419–427. doi: 10.1002/jemt.22994. [DOI] [PubMed] [Google Scholar]
- 44.Jiang J, Wu Y, Huang M, Yang W, Chen W, Feng Q. 3D brain tumor segmentation in multimodal MR images based on learning population- and patient-specific feature sets. Comput Med Imaging Graph. 2013;37:512–521. doi: 10.1016/j.compmedimag.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Kao PY, Shailja S, Jiang J, et al. Improving patch-based convolutional neural networks for MRI brain tumor segmentation by leveraging location information. Front Neurosci. 2019;13:1449. doi: 10.3389/fnins.2019.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, Wang Y, Yu J, et al. Low-grade glioma segmentation based on CNN with fully connected CRF. J Healthc Eng. 2017;2017:9283480. doi: 10.1155/2017/9283480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Shi X, Xia Y, et al. Multi-scale V-Net: a deep learning framework for brain tumor segmentation in multiparametric MRI. Med Phys. 2018;45(6):e568. [Google Scholar]
- 48.Meng Z, Fan Z, Zhao Z, Su F (2018) ENS-Unet: end-to-end noise suppression U-Net for brain tumor segmentation. Annu Int Conf IEEE Eng Med Biol Soc 2018:5886–5889 [DOI] [PubMed]
- 49.Naceur MB, Saouli R, Akil M, Kachouri R. Fully automatic brain tumor segmentation using end-to-end incremental deep neural networks in MRI images. Comput Methods Programs Biomed. 2018;166:39–49. doi: 10.1016/j.cmpb.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Naser MA, Deen MJ. Brain tumor segmentation and grading of lower-grade glioma using deep learning in MRI images. Comput Biol Med. 2020;121:103758. doi: 10.1016/j.compbiomed.2020.103758. [DOI] [PubMed] [Google Scholar]
- 51.Perkuhn M, Stavrinou P, Thiele F, et al. Clinical evaluation of a multiparametric deep learning model for glioblastoma segmentation using heterogeneous magnetic resonance imaging data from clinical routine. Invest Radiol. 2018;53:647–654. doi: 10.1097/RLI.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razzak MI, Imran M, Xu G. Efficient brain tumor segmentation with multiscale two-pathway-group conventional neural networks. IEEE J Biomed Health Inform. 2019;23:1911–1919. doi: 10.1109/JBHI.2018.2874033. [DOI] [PubMed] [Google Scholar]
- 53.Savareh BA, Emami H, Hajiabadi M, Azimi SM, Ghafoori M. Wavelet-enhanced convolutional neural network: a new idea in a deep learning paradigm. Biomed Tech (Berl) 2019;64:195–205. doi: 10.1515/bmt-2017-0178. [DOI] [PubMed] [Google Scholar]
- 54.Soltaninejad M, Yang G, Lambrou T, et al. Supervised learning based multimodal MRI brain tumour segmentation using texture features from supervoxels. Comput Methods Programs Biomed. 2018;157:69–84. doi: 10.1016/j.cmpb.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Sun J, Chen W, Peng S, Liu B. DRRNet: dense residual refine networks for automatic brain tumor segmentation. J Med Syst. 2019;43:221. doi: 10.1007/s10916-019-1358-6. [DOI] [PubMed] [Google Scholar]
- 56.Wang F, Niu J, Fan W, Cao Q. Brain tumor medical image segmentation based on CRF 3D-CNN introduction. Basic Clin Paharmacol Toxicol. 2018;124(Supplement 2):12. [Google Scholar]
- 57.Wu S, Li H, Quang D, Guan Y. Three-plane-assembled deep learning segmentation of gliomas. Radiol Artif Intell. 2020;2:e190011. doi: 10.1148/ryai.2020190011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y, Zhao Z, Wu W, Lin Y, Wang M. Automatic glioma segmentation based on adaptive superpixel. BMC Med Imaging. 2019;19:73. doi: 10.1186/s12880-019-0369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang C, Guo X, Wang T et al (2019) Automatic brain tumor segmentation method based on modified convolutional neural network. Annu Int Conf IEEE Eng Med Biol Soc 2019:998–1001 [DOI] [PubMed]
- 60.Yang T, Song J, Li L (2019) A deep learning model integrating SK-TPCNN and random forests for brain tumor segmentation in MRI. Biocybern Biomed Eng 39(3):613–623. 10.1016/j.bbe.2019.06.003
- 61.Yang T, Song J, Li L, Tang Q. Improving brain tumor segmentation on MRI based on the deep U-net and residual units. J Xray Sci Technol. 2020;28:95–110. doi: 10.3233/XST-190552. [DOI] [PubMed] [Google Scholar]
- 62.Zhao L, Wu W, Corso JJ. Semi-automatic brain tumor segmentation by constrained MRFs using structural trajectories. Med Image Comput Comput Assist Interv. 2013;16:567–575. doi: 10.1007/978-3-642-40760-4_71. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Z, He Z, Shi M, Du J, Chen D. 3D dense connectivity network with atrous convolutional feature pyramid for brain tumor segmentation in magnetic resonance imaging of human heads. Comput Biol Med. 2020;121:103766. doi: 10.1016/j.compbiomed.2020.103766. [DOI] [PubMed] [Google Scholar]
- 64.Zhuge Y, Krauze AV, Ning H, et al. Brain tumor segmentation using holistically nested neural networks in MRI images. Med Phys. 2017;44:5234–5243. doi: 10.1002/mp.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong H, Yang G, Liu F, Mo Y, Guo Y. Automatic brain tumor detection and segmentation using U-Net based fully convolutional networks. In: Valdés Hernández M, González-Castro V, editors. Medical image understanding and analysis. Cham: Springer International Publishing; 2017. pp. 506–517. [Google Scholar]
- 66.Dvorak P, Menze B (2015) Local structure prediction with convolutional neural networks for multimodal brain tumor segmentation. In International MICCAI workshop on medical computer vision. Springer, Cham, pp 59–71
- 67.Lyksborg M, Puonti O, Agn M, Larsen R. An ensemble of 2D convolutional neural networks for tumor segmentation. In: Paulsen RR, Pedersen KS, editors. Image Analysis. Cham: Springer International Publishing; 2015. pp. 201–211. [Google Scholar]
- 68.Pinto A, Pereira S, Correia H, Oliveira J, Rasteiro DMLD, Silva CA (2015) Brain tumour segmentation based on extremely randomized forest with high-level features. 2015 37th Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc):3037–3040 [DOI] [PubMed]
- 69.Tustison NJ, Shrinidhi KL, Wintermark M, et al. Optimal symmetric multimodal templates and concatenated random forests for supervised brain tumor segmentation (simplified) with ANTsR. Neuroinformatics. 2015;13:209–225. doi: 10.1007/s12021-014-9245-2. [DOI] [PubMed] [Google Scholar]
- 70.Usman K, Rajpoot K. Brain tumor classification from multi-modality MRI using wavelets and machine learning. Pattern Anal Applic. 2017;20:871–881. [Google Scholar]
- 71.Xue Y, Xu T, Zhang H, Long LR, Huang XL. SegAN: adversarial network with multi-scale L (1) loss for medical image segmentation. Neuroinformatics. 2018;16:383–392. doi: 10.1007/s12021-018-9377-x. [DOI] [PubMed] [Google Scholar]
- 72.Zikic D, Glocker B, Konukoglu E et al (2012) Decision forests for tissue-specific segmentation of high-grade gliomas in multi-channel MRInternational Conference on Medical Image Computing and Computer-Assisted Intervention. Springer, 369–376 [DOI] [PubMed]
- 73.Durmo F, Lätt J, Rydelius A, et al. Brain tumor characterization using multibiometric evaluation of MRI. Tomography. 2018;4:14–25. doi: 10.18383/j.tom.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Baene W, Rutten GJM, Sitskoorn MM. The temporal pattern of a lesion modulates the functional network topology of remote brain regions. Neural Plast. 2017;2017:3530723. doi: 10.1155/2017/3530723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang Z, Ding C, Liu M, Tao D. Two-stage cascaded U-Net: 1st place solution to BraTS challenge 2019 segmentation task. Cham: Springer International Publishing; 2020. pp. 231–241. [Google Scholar]
- 76.Zhao Y-X, Zhang Y-M, Liu C-L. Bag of tricks for 3D MRI brain tumor segmentation. Cham: Springer International Publishing; 2020. pp. 210–220. [Google Scholar]
- 77.McKinley R, Rebsamen M, Meier R, Wiest R. Triplanar ensemble of 3D-to-2D CNNs with label-uncertainty for brain tumor segmentation. Cham: Springer International Publishing; 2020. pp. 379–387. [Google Scholar]
- 78.Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar V, Gu Y, Basu S, et al. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30:1234–1248. doi: 10.1016/j.mri.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herrmann E, Ermis E, Meier R, et al. Fully automated segmentation of the brain resection cavity for radiation target volume definition in glioblastoma patients. Strahlenther Onkol. 2019;195:586–586. [Google Scholar]
- 82.Meier R, Knecht U, Loosli T et al (2016) Clinical evaluation of a fully-automatic Segmentation method for longitudinal brain tumor volumetry. Sci Rep 6:23376. 10.1038/srep23376 [DOI] [PMC free article] [PubMed]
- 83.Zeng K, Bakas S, Sotiras A, et al. Segmentation of gliomas in pre-operative and post-operative multimodal magnetic resonance imaging volumes based on a hybrid generative-discriminative framework. Brainlesion. 2016;10154:184–194. doi: 10.1007/978-3-319-55524-9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang F, Liang S, Zhong T, et al. Postoperative glioma segmentation in CT image using deep feature fusion model guided by multi-sequence MRIs. Eur Radiol. 2020;30:823–832. doi: 10.1007/s00330-019-06441-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 19 kb)