Abstract
Intrinsically photosensitive retinal ganglion cells (ipRGCs) respond directly to light by virtue of containing melanopsin which peaks at about 483 nm. However, in primates, ipRGCs also receive color opponent inputs from short-wavelength-sensitive (S) cone circuits that are well-suited to encode circadian changes in the color of the sky that accompany the rising and setting sun. Here, we review the retinal circuits that endow primate ipRGCs with the cone-opponency capable of encoding the color of the sky and contributing to the wide-ranging effects of short-wavelength light on ipRGC-mediated non-image-forming visual function in humans.
1. Retinal Circuits Involved in Circadian Photoentrainment
Retinal circuits adhere to several highly-conserved organizational principles. One striking feature of the visual system is the unusual complexity of the retina. The retina contains five main classes of neuron: photoreceptors, horizontal cells, bipolar cells, amacrine cells and retinal ganglion cells (RGCs). These five classes and their defining features are conserved in all vertebrate retinas1. A second major organizational feature of the retina is the divergence of a single signal from the cones into parallel ON and OFF pathways2–4. Major ganglion cell types have both ON and OFF versions. Though best known for their intrinsic photosensitivity, recent research has established that primate ipRGCs also receive input from S-cone circuits that take full advantage of these retinal motifs.
Both rod and cone photoreceptors provide input to the circadian system; however, our focus here is on the cones inputs which are spectrally opponent such that primate ipRGCs are sensitive to color. Trichromatic primates have three cone types – long (L), medium (M) and short (S)-wavelength sensitive (Figure 1). While the relative numbers of L- and M-cones vary considerably between individuals, the S-cones make up 5–10%5–7. The cone photoreceptor responses are relayed through the retina by the bipolar cells. However, the cone signals transmitted to the bipolar cells have already been the subject of lateral inhibition via the horizontal cells. Each cone terminal gets inhibitory input from surrounding cones. This generates center-surround receptive fields and color opponency in the retina8. In primates, the cone opponent signals carried by ipRGCs are transmitted by S-cone specific ON bipolar cells. The S-cones receive inhibitory feedback exclusively from HII horizontal cells which is gathered from neighboring S, M and L cones, thus S vs. (M+L) cone opponency is predicted to be present in the outputs of S-cones9. As a result, S-ON bipolar cells carry an LM-OFF surround, a prediction supported by recordings from small bistratified ganglion cells that receive their inputs10.
Figure 1.
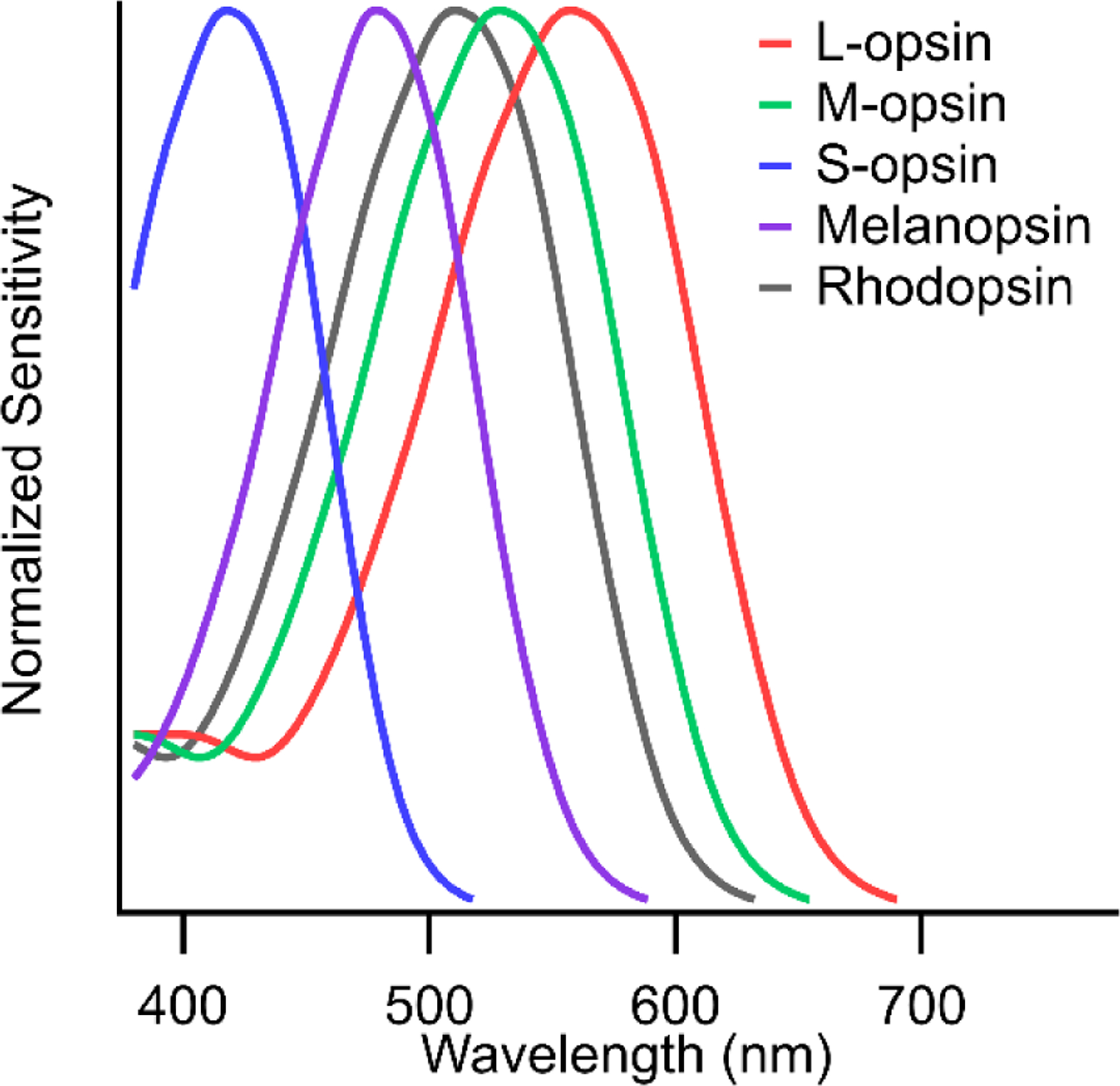
Spectral sensitivity curves of photopigments involved in ipRGC function.
2. Ganglion Cell Photoreceptors (ipRGCs)
Early this century, the anatomy and physiology of an S-ON ganglion cell, the small bistratified ganglion cell, believed to be the basis for blue color sensations had been well studied. However, color vision theory predicted a parallel S-OFF ganglion cell which had not been reported. In 2002, Dacey and colleagues reported the discovery of a large sparse monostratified ganglion cell type with an S-cone OFF/L+M-cone ON color opponent light response and it was speculated that these cells might be the S-OFF counterpart to the small bistratified ganglion cell thought to serve blue-yellow color vision11. Later the group realized that these cells were the primate homolog to the ipRGCs that had been described in rodents12. The early studies in both rodents and primates identified ipRGCs as a single functional type. Later, multiple types were recognized in rodents and, in primates two clear anatomical subtypes of ipRGC have been identified corresponding to the M1 and M2 ipRGCs in mice. A more recent study of human ipRGCs identified three functional subtypes13, however, the relationship to the M1 and M2 subtypes characterized anatomically is unclear. The two anatomical subtypes are distinguished by their stratification at the edges of the inner retina14. M1 “outer-stratifying” ipRGCs have branches in inner plexiform layer (IPL) sublayer 1 (S1) closest to the inner nuclear layer while the branches of M2 “inner-stratifying” ipRGCs are located in sublayer 5 (S5) adjacent to the ganglion cell layer14–16. M1 and M2 ipRGCs are typically monostratified but some are slightly bistratified with sparse branches to the opposite side of the inner retina. These partially bistratified ipRGCs were initially defined in mouse as a separate subtype (M3), but subsequent study reached the consensus that they are not a distinct anatomical type17,18 and have light responses consistent with M2 ipRGCs19. More recently, a third subtype of “giant” ipRGCs has been reported anatomically20,21 and a recent study in human retina reported additional melanopsin-positive RGCs21. However, there is no evidence that the primate retina contains homologs to the subtypes of mouse ipRGCs with low melanopsin expression22–24.
From early recordings of primate ipRGCs it was assumed that M1 and M2 ipRGCs were functionally homogenous12. However, subsequent anatomical work found significant differences in the circuitry and synaptic input of primate ipRGCs14–16. These anatomical differences mirror differences between M1 and M2 ipRGCs reported in the mouse retina: melanopsin expression and intrinsic melanopsin-mediated responses are stronger in M1 ipRGCs and weaker in M2 ipRGCs, which are more strongly driven by rod and cone photoreceptor pathways25.
Rods and cones were long thought to be the only retinal neurons capable of phototransduction. This made the discovery of melanopsin-expressing ipRGCs especially exciting. As such, melanopsin has been the focus of most studies on the ipRGC’s light responses. The spectral sensitivity of melanopsin peaks at 483 nm and is 1000× less sensitive than the cone opsins26. Melanopsin-mediated responses are slow, sustained, require bright light levels and have been proposed to “count photons”, signaling intensity rather than contrast27. However, most of the studies on melanopsin-mediated responses are performed while synaptic inputs from rod and cone retinal circuits are pharmacologically blocked or otherwise isolated. Recent studies of S-cone opponent circuitry associated with primate ipRGCs, reviewed here, demonstrate the diverse spectral and temporal properties of presynaptic circuits carrying cone opponent signals capable of influencing non-image-forming visual functions through ipRGCs.
3. S-cone Circuits of the Primate Retina
3.1. S-cone Input to M1 ipRGCs
As introduced above, from electrophysiology recordings, primate ipRGCs were reported to have a rare yellow-ON, blue-OFF color tuning and respond to increased activity in long (L) and middle (M) wavelength cone pathways and decreased activity in S-cone pathways (i.e., LM-ON/S-OFF cone opponency)3. However, the underlying circuitry for these cone-opponent responses were unclear. The retinal circuits responsible for the primate ipRGC’s color tuning was explored to help clarify this. Paradoxically, the ipRGC’s S-OFF responses are blocked by the drug L-2-Amino-4-phosphonobutyric acid (L-AP4), an ON-pathway agonist that interrupts signaling between S-cones and S-cone ON bipolar cells. This indicated that S-ON bipolar cell are the source of the S-cone signals in ipRGCs and the sign of the S-ON bipolar cells is inverted when transmitted to the ipRGCs.
One possibility was that the S-ON bipolar responses were first communicated to an amacrine cell that, in turn, made inhibitory synapses on to the ipRGCs inverting the S-ON sign to S-OFF33. Testing this idea by identifying the specific amacrine cell type involved was challenging with traditional anatomical methods. The mammalian retina contains 20–40 amacrine cell types28, making selective identification of any one undiscovered type a challenge. Serial electron microscopy (EM) was used to reconstruct the neurons and synapses of the primate S-cone connectome from a volume of macaque retina. The terminals whereby S-cones synapse with bipolar and horizontal cells have a distinctive anatomy compared to L and M cones. Moreover, their identity can be confirmed by their unique post-synaptic contacts. Each S-cone provides input to one to three S-ON bipolar cells and a single OFF midget bipolar cell (Figure 2). The S-ON bipolar cells make contacts specifically with S-cones and they often skip past several L/M-cones to synapse on an S-cone nearby. Thus, it was possible to confidently identify S-cones and S-ON bipolar cells.
Figure 2.
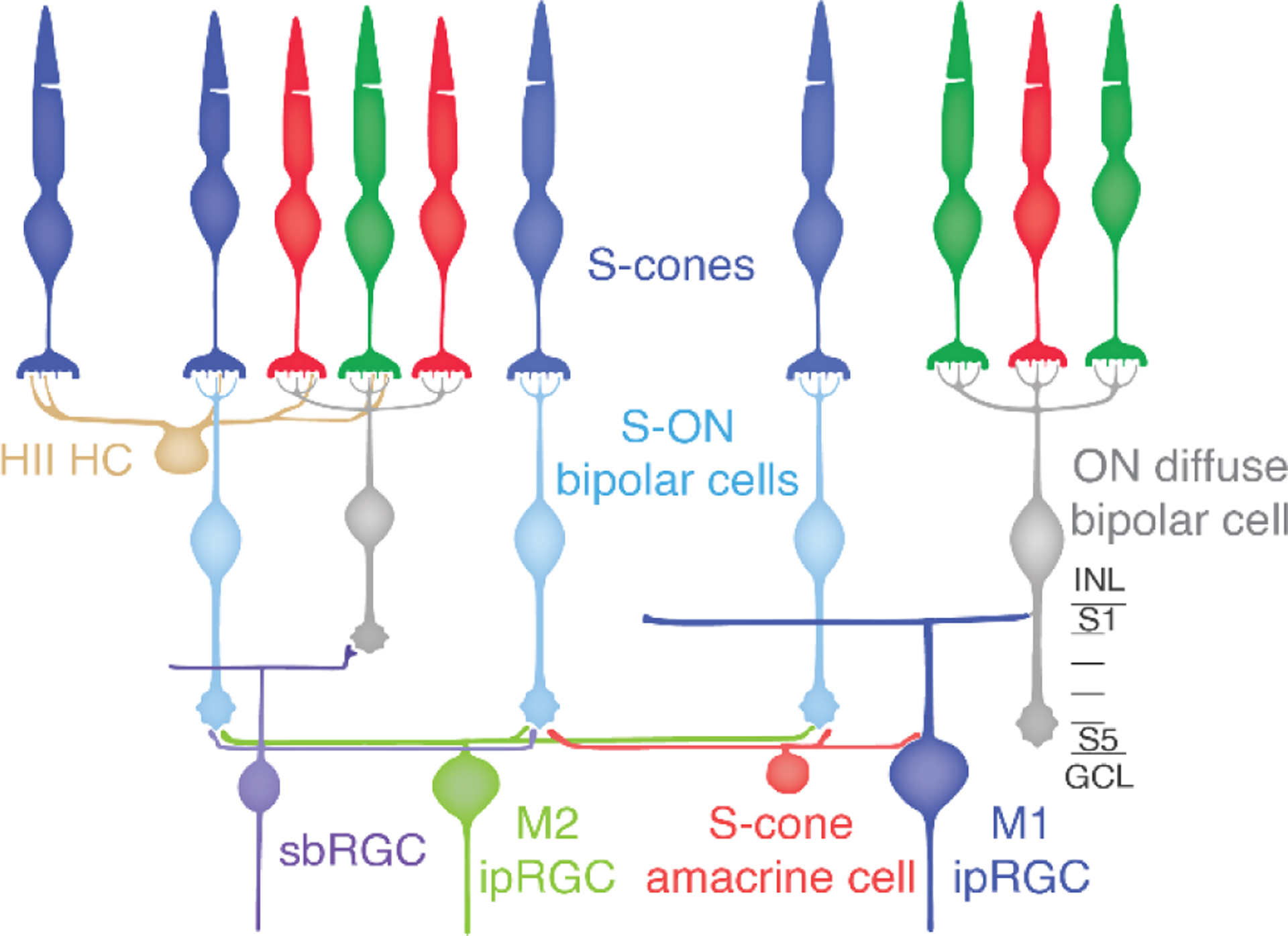
S-cone circuits of the primate retina.
For decades, small bistratified ganglion cells were believed to be the only S-ON RGCs in the primate and the substrate for the perception of blue. These RGCs have a distinctive morphology and receive input in a specific ON sublayer of the IPL from S-ON bipolar cells. Thus, it was possible to further confirm the identity of S-ON bipolar cells by tracing them down to the inner retina and reconstructing the small bistratified RGCs (illustrated in Figure 2). S-ON bipolar cells contact two or three small bistratified ganglion cells, and small bistratified cells collect inputs from several S-ON bipolar cells. The terminals of different bipolar cell types terminate in precise substrata of the IPL and they provide excitatory glutamatergic input to amacrine and RGCs stratifying in the same sublayer. S-ON bipolar cells stratify in S5, the innermost layer of the IPL, closest to the ganglion cell layer (Figure 2).
As introduced above, the two major ipRGC subtypes in the primate retina are distinguished by their stratification in the outermost and innermost edges of the IPL (Figure 2). The anatomical paradox is that M1 ipRGC’s stratify in sublamina 1 (S1) on the opposite side of the IPL from S5 where the S-ON bipolar cells terminate. So, the question was, how could a putative amacrine cell receiving S-ON bipolar cell input relay an OFF response to the dendrites of the M1 ipRGCs?
It was discovered that while many amacrine cells stratifying in S5 receive occasional S-ON bipolar cell input, a population of medium-field displaced amacrine cells contacted S-ON bipolar cells exclusively. Thus, an S-cone amacrine cell was identified in the primate retina as part of an S-cone specific circuit extending from the photoreceptor layer to the innermost layer of the IPL. Based on strong morphological similarities, stratification, and displaced soma, the primate S-cone amacrine cell is likely the homolog to the A12 amacrine cell identified in human retina and the MA-S5 in the mouse retina29,30. However, these had not previously been identified as S-cone specific. The dendritic fields of the newly discovered S-cone amacrine cells covered the S-ON bipolar cell terminal mosaic, each collecting input from over 10 S-ON bipolar cell terminals.
Thus, exactly as predicted, an S-cone specific amacrine cell capable of inverting the sign of S-cone signals from ON to OFF was discovered, but what about the paradox of how the S-cone signals could be communicated to M1 ipRGC whose dendrites stratify on the other side of the IPL in S1? To find out, the two major ipRGC subtypes reported in the primate retina were reconstructed based on their characteristic anatomy and stratification. It was discovered that M1 ipRGCs defy convention in the route they receive S-cone signals from the S-cone amacrine cells. Amacrine cell processes are typically confined to the IPL, however, the S-cone amacrine cells also extend processes into the ganglion cell layer (GCL) to contact M1 ipRGC soma. In addition, thin branches extend from the ipRGC’s cell body to collect input from S-cone amacrine cell processes. Finally, the primary dendrites of the M1 ipRGCs receive input from S-cone amacrine cells as they transit S5 to extend processes that ultimately continue to form their “OFF” strata in S1.
To summarize, the retina contains an amacrine cell capable of transmitting and inverting S-cone color opponent signals to M1 ipRGCs. The cell types and circuits in the retina are highly conserved throughout the vertebrate linage. Some features of different retinas do change as the result of selective pressure. For example, particular cell types and functions may be lost in a species, such as, two of the four cone types found in other vertebrates were lost in the evolution of placental mammals making them dichromats. Also, the relative abundance of different cell types in the retina varies greatly in different lineages depending on environment and lifestyle. For example, in mammals, the relative number of cones to rods varies from 1:200 in the most nocturnal to 20:1 in a few diurnal species31. However, new cell types only arise over very long evolutionary time scales. Thus, we predict that the S-cone amacrine cell is common to vertebrates. This is consistent with the finding that amacrine cells matching the S-cone amacrine cell’s distinctive morphology and stratification have been found in all the other mammals whose retinas have been studied in anatomical detail. If true, color opponency is a fundamental feature of vertebrate ipRGCs serving nonimage forming vision.
3.2. S-cone ON Bipolar Cell Input to M2 ipRGCs
The first report of primate ipRGC physiology did not identify functional differences between the M1 and M2 ipRGCs (their outer- and inner-stratifying types)12. Although the cone opponency of both subtypes was never directly compared, M1 and M2 ipRGCs were generally thought to share the LM-ON/S-OFF opponent responses. In addition, as we have said, small bistratified ganglion cells, were thought to be the only S-ON ganglion cells in primates. Indeed, the majority of RGCs postsynaptic to the ribbon synapses of the S-ON bipolar cells are small bistratified RGCs. However, other RGC types are also encountered at a lower frequency32,33. When the other RGCs receiving S-cone ON bipolar input were reconstructed, a wide-field monostratified RGC type that receives nearly exclusive excitatory input from S-ON bipolar cells was discovered. Based on the morphology and stratification of known primate RGC types, the newly discovered S-ON ganglion cell type corresponded to the M2 ipRGC. The S-cone input to the M2 ipRGC is considerable with each M2 receiving input from more than 20 S-ON bipolar cells. Moreover, S-ON bipolar cells provided 96% of the identified bipolar cell input to M2 ipRGCs34.
S-ON bipolar cell input to M2 ipRGCs is surprising as it confers the opposite spectral tuning as that reported for M1 ipRGCs. Moreover, M2 ipRGCs received very little input from S-cone amacrine cells, even though their processes co-stratified and were often found in close proximity. The major amacrine cell input to the M2 ipRGC appeared to be the axons of a polyaxonal amacrine cell. This could be the polyaxonal amacrine cell previously found presynaptic and gap junction-coupled to rodent M2 ipRGCs35,36.
3.3. S-cone ON and OFF input to non-image forming vision
To summarize, the M1 and M2 ipRGCs receive opposite S-cone input; the S-cone amacrine cell input to M1 ipRGCs is inhibitory while the S-ON bipolar cell input to M2 ipRGCs is excitatory. This indicates the need for a paradigm shift in our understanding of the role of color information being communicated to the primate brain by ipRGCs. First, this research reveals that melanopsin is not the only short-wavelength-sensitive mechanism mediating ipRGC responses. Studies investigating the effects of short-wavelength light on human sleep, mood and health could involve up to three distinct short-wavelength sensitive mechanisms: melanopsin, S-OFF input to M1 ipRGCs and S-ON input to M2 ipRGCs. Fortunately, the distinct properties of each mechanism can inform future experiments. Melanopsin has a distinct spectral tuning and is ~1000× less sensitive than S-opsin26. Sawtooth stimuli37 with opposite ramps preferentially drive S-ON and S-OFF responses. These could be used to probe different non-image forming visual functions that are mediated by different brain centers.
How do downstream visual areas use the opposing S-cone responses of M1 and M2 ipRGCs? Perhaps S-cone ON and OFF responses could work together in a push-pull fashion to modulate downstream visual areas more effectively in response to short-wavelength light vs. long-wavelength light? However, it is worth noting that the underlying retinal circuits suggest far less symmetry between the S-ON and S-OFF responses than seen in traditional ON/OFF pairs mediated by ON and OFF bipolar cells of the same type. In the case of the M1 and M2 ipRGCs, the S-OFF response arises through an amacrine cell-mediated sign inversion of the S-ON bipolar cells output. The influence of this amacrine cell on the S-ON bipolar cell’s output beyond the sign inversion is unknown and could involve changes in gain, kinetics and/or spatial structure. Accordingly, it would make sense that having these two types would be useful if different brain centers that serve different non-image forming vision functions had different needs. The role of ipRGCs in two distinct non-image-forming visual functions is well-established: circadian photoentrainment and the pupillary light reflex. Sunrise and sunset may be important for synchronizing our internal biological clock to the external day-night cycle38–41. S-cone OFF signals could be particularly useful for this since the orange sky at sunrise and sunset would produce strong decrements in short wavelength light. On the other hand, short wavelength light can be particularly damaging to sensitive tissues such as the retina, thus it might be useful if increments in short wavelength light were used to drive pupil constriction.
Distinguishing between these potential roles for the S-ON and S-OFF responses of ipRGCs requires an understanding of which brain areas receive input from each ipRGC subtype. In humans, ipRGCs project to the pregeniculate nucleus, suprachiasmatic nucleus (SCN), olivary pretectal nucleus (OPN), lateral geniculate nucleus and superior colliculus42; however, the exact subtypes projecting to each region remains unclear. More progress in this area has been made in the mouse. There is evidence that both M1 and M2 subtypes project to the SCN for circadian rhythms and to the OPN for control of the pupillary reflex in mice, with the M1 ipRGCs providing the majority input to the SCN and M2 ipRGCs providing the majority input to the OPN43. However, subsequent results revealed distinct groups of M1 ipRGCs project to the SCN and OPN, indicating a greater complexity than initially expected44. Moreover, insights gained from the mouse retina regarding the differential role of M1 and M2 ipRGCs are currently limited by the lack of an M2 ipRGC-specific marker.
4. Insights into Human Visual Function
The discovery of ipRGCs, cells that break the classification between ganglion cells and photoreceptors, was groundbreaking and they continue to challenge long-standing ideas about vision in addition to having direct translational value. For example, color vision research has focused almost exclusively on color perception and discrimination. The S-cone inputs to M1 and M2 ipRGCs are part of a conserved color vision circuit serving the non-image-forming visual pathways and are independent of the well-studied circuits for color perception. This provides a new understanding of the evolutionary origins of color vision and the uses of wavelength information by the primate visual system. Moreover, the S-ON input to M2 ipRGCs confers them with the same spectral tuning as the small bistratified ganglion cell. Classic color vision models are based on the idea that the retina reduces redundancy to efficiently convey wavelength information to the brain and cannot account for the existence of two types of S-ON ganglion cell34. Further study promises to illuminate the neural substrate for the wide-ranging effects of short-wavelength light on ipRGC mediated non-image-forming visual functions and their role in human health.
Acknowledgements:
The authors would like to thank Ethan Buhr for the invitation to contribute to this issue. This work was supported by grant R01 EY027859, T32 EY007125, P30 EY001730 and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The University of Washington has submitted a provisional patent application (628504893) disclosing Systems, Methods, and Devices for Stimulating Circadian Rhythms (authors: S.S.P., M.N., J.N.).
REFERENCES
- 1.Baden T, Euler T & Berens P Understanding the retinal basis of vision across species. Nat. Rev. Neurosci 21, 5–20 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Westheimer G The ON-OFF dichotomy in visual processing: from receptors to perception. Prog. Retin. Eye Res 26, 636–648 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Wässle H Parallel processing in the mammalian retina. Nat. Rev. Neurosci 5, 747–757 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Ellis EM, Frederiksen R, Morshedian A, Fain GL & Sampath AP Separate ON and OFF pathways in vertebrate vision first arose during the Cambrian. Curr. Biol 30, R633–R634 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll J, Neitz J & Neitz M Estimates of L:M cone ratio from ERG flicker photometry and genetics. J. Vis 2, 531–542 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Curcio CA et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J. Comp. Neurol 312, 610–624 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Hofer H, Carroll J, Neitz J, Neitz M & Williams DR Organization of the human trichromatic cone mosaic. J. Neurosci 25, 9669–9679 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson SS, Neitz M & Neitz J Reconciling color vision models with midget ganglion cell receptive fields. Front. Neurosci 13, 865 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer OS, Verweij J, Li PH, Schnapf JL & Dacey DM Blue-yellow opponency in primate S-cone photoreceptors. J. Neurosci 30, 568–572 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field GD et al. Spatial properties and functional organization of small bistratified ganglion cells in primate retina. J. Neurosci 27, 13261–13272 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dacey DM, Peterson BB & Robinson FR Identification of an S-cone opponent OFF pathway in the Macaque monkey retina: Morphology, physiology and possible circuitry. Investig. Ophthalmol. Vis. Sci 43, eAbstract 2983 (2002). [Google Scholar]
- 12.Dacey DM et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Mure LS, Vinberg F, Hanneken A & Panda S Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science (80-.) 366, 1251–1255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao HW et al. Melanopsin-expressing ganglion cells on macaque and human retinas form two morphologically distinct populations. J. Comp. Neurol 524, 2845–2872 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann S, Haverkamp S & Auferkorte ON Intrinsically photosensitive ganglion cells of the primate retina express distinct combinations of inhibitory neurotransmitter receptors. Neuroscience 199, 24–31 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Grünert U, Jusuf PR, Lee SCS & Nguyen DT Bipolar input to melanopsin containing ganglion cells in primate retina. Vis. Neurosci 28, 39–50 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Berson DM, Castrucci AM & Provencio I Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J. Comp. Neurol 518, 2405–2422 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasir-Ahmad S, Lee SCS, Martin PR & Grünert U Melanopsin-expressing ganglion cells in human retina: Morphology, distribution, and synaptic connections. J. Comp. Neurol 527, 312–327 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Schmidt TM & Kofuji P Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J. Comp. Neurol 519, 1492–1504 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra AJ, Lee SCS & Grünert U Melanopsin and calbindin immunoreactivity in the inner retina of humans and marmosets. Vis. Neurosci 36, E009 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Hannibal J, Tolstrup A, Steffen C, Fahrenkrug J & Folke J Melanopsin expressing human retinal ganglion cells: Subtypes, distribution, and intraretinal connectivity. J. Comp. Neurol 525, 1934–1961 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Quattrochi LE et al. The M6 cell: A small-field bistratified photosensitive retinal ganglion cell. J. Comp. Neurol 527, 297–311 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stabio ME et al. The M5 cell: a color-opponent intrinsically photosensitive retinal ganglion cell. Neuron 97, 150–163.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonoda T, Lee SK, Birnbaumer L & Schmidt TM Melanopsin phototransduction is repurposed by ipRGC subtypes to shape the function of distinct visual circuits. Neuron 99, 754–767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt TM & Kofuji P Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J. Neurosci 30, 16262–16271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas RJ et al. Measuring and using light in the melanopsin age. Trends Neurosci. 37, 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong KY A retinal ganglion cell that can signal irradiance continuously for 10 hours. J. Neurosci 32, 11478–11485 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masland RH. The fundamental plan of the retina. Nat. Neurosci 4, 877–886 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Kolb H, Linberg KA & Fisher SK Neurons of the human retina: A Golgi study. J. Comp. Neurol 318, 147–187 (1992). [DOI] [PubMed] [Google Scholar]
- 30.de Sevilla Muller LP, Shelley J & Weiler R Displaced amacrine cells of the mouse retina. J. Comp. Neurol 505, 177–189 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Peichl L Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle. Anat. Rec 287, 1001–1012 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Calkins DJ, Tsukamoto Y & Sterling P Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina. J. Neurosci 18, 3373–3385 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson SS, Kuchenbecker JA, Anderson JR, Neitz M & Neitz J A color vision circuit for non-image-forming vision in the primate retina. Curr. Biol 30, 1269–1274 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson SS et al. Another Blue-ON ganglion cell in the primate retina. Curr. Biol 30, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez De Sevilla Müller L, Do MTH, Yau KW, He S & Baldridge WH Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. J. Comp. Neurol 518, 4813–4824 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reifler AN et al. All spiking, sustained on displaced amacrine cells receive gap-junction input from melanopsin ganglion cells. Curr. Biol 25, 2763–2773 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowen RW, Pokorny J & Smith VC Sawtooth contrast sensitivity: Decrements have the edge. Vision Res. 29, 1501–1509 (1989). [DOI] [PubMed] [Google Scholar]
- 38.Spitschan M, Aguirre GK, Brainard DH & Sweeney AM Variation of outdoor illumination as a function of solar elevation and light pollution. Sci. Rep 6, 1–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woelders T, Wams EJ, Gordijn MCM, Beersma DGM & Hut RA Integration of color and intensity increases time signal stability for the human circadian system when sunlight is obscured by clouds. Sci. Rep 8, 15214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walmsley L et al. Colour as a signal for entraining the mammalian circadian clock. PLoS Biol. 13, e1002127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauers MJ, Kuchenbecker JA, Neitz M & Neitz J Changes in the colour of light cue circadian activity. Anim. Behav 83, 1143–1151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannibal J et al. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J. Comp. Neurol 522, 2231–2248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baver SB, Pickard GE, Sollars PJ & Pickard GE Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur. J. Neurosci 27, 1763–1770 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Chen SK, Badea TC & Hattar S Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 476, 92–96 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


