Abstract
The circadian system regulates behavior and physiology in many ways important for health. Circadian rhythms are expressed by nearly every cell in the body, and this large system is coordinated by a central clock in the suprachiasmatic nucleus (SCN). Sex differences in daily rhythms are evident in humans and understanding how circadian function is modulated by biological sex is an important goal. This review highlights work examining effects of sex and gonadal hormones on daily rhythms, with a focus on behavior and SCN circuitry in animal models commonly used in pre-clinical studies. Many questions remain in this area of the field, which would benefit from further work investigating this topic.
Keywords: circadian rhythm, suprachiasmatic nucleus, sex difference, gonadal hormone, estrogen, androgen, sex chromosome
1. Introduction
Daily rhythms coordinate behavior and physiology to anticipate predictable changes in the environment. In nearly all cells of the body, a set of molecular gears counts down the hours of the day, programming ca. 24 h rhythms in gene expression and cellular physiology dictated according to the specialized functions of each tissue(124). This diverse system of biological clocks is coordinated by a central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN receives direct input from the retina and entrains the circadian system to the external 24 h solar day — a critical function without which the organism is poorly matched to environmental demands.
A wide range of studies indicates that biological sex interacts with circadian mechanisms in important ways relevant for human health. For example, sex differences in sleep amount and timing are well-recognized in humans and animal models (109). In particular, women tend to be “early birds” and have faster internal clocks relative to men (23, 39, 49). Beyond sleep, biological sex modulates daily rhythms in other critical processes, including hormonal, metabolic, and cellular rhythms (11, 29, 34, 63, 151, 160). Daily rhythms are also modulated by reproductive state and gonadal steroids (15, 89), suggesting that hormones influence circadian processes. This area of work has significance from a women’s health perspective given circadian regulation of female reproduction (80, 106). Indeed, circadian disruption can reduce fecundity (84, 95) and cause other negative effects that differ by sex (7, 130, 139, 153). Further work investigating sex differences in clock function is needed and may shed light on gender disparities in diseases involving circadian timekeeping (92, 94, 102).
In general, there are at least two approaches to study sex differences: 1) study mechanisms in both sexes and 2) manipulate gonadal hormones to evaluate resulting changes in behavior, physiology and/or cellular function (99). Classic circadian studies using the second approach have established that daily rhythms are modulated by activational and organizational effects of gonadal steroids (11, 61, 81, 120). Activational effects are tested in adulthood using gonadectomy and hormone replacement, whereas organizational effects are more permanent changes caused by exposure to sex steroids during critical developmental periods. Sex differences can also be driven by non-hormonal mechanisms, such as sex chromosomes, which can be studied in mice using the four core genotypes model (99). Sex differences in behavior often motivate further research, but lack of behavioral dimorphism does not preclude sex differences in cellular function (38). Indeed, there is growing awareness that differences in cellular mechanisms may provide convergence of function under the different hormonal and genetic conditions present in each sex.
There is a long-standing interest in sex differences in circadian rhythms (11, 61, 81, 120), but many questions remain because very few circadian studies include females (83, 87). Recent metaanalyses indicate that females are not more variable than males in neuroscience studies using rats and mice (12, 128), supporting the call for greater inclusion of female subjects. Here we review current understanding of sex differences in circadian timekeeping. We first introduce basic principles by defining key concepts in the field and describe the neurobiological basis of circadian rhythms. Next, we discuss interactions between circadian and reproductive function, followed by a review of work examining effects of sex and gonadal hormones on circadian rhythms. We focus here on sex differences in behavioral rhythms and SCN circuits in animal models commonly used in pre-clinical studies. We conclude with general inferences that can be drawn from this work and considerations for future studies in this area.
2. Formal and cellular basis of circadian rhythms
Circadian rhythms can be defined by several parameters, including period, precision, phase, amplitude, and waveform (Figure 1A–B). Daily time cues can entrain a circadian rhythm by resetting its phase and/or modulating its period (Figure1C). Period is the duration of one full cycle, typically quantified under free-running conditions devoid of exogenous time cues. Rhythm precision can be measured by the standard deviation of period length over multiple cycles. Phase is one specific timepoint in the rhythm (e.g., onset of locomotor activity), amplitude is the magnitude of change across one cycle, and waveform describes the shape of the rhythm. A phase shift describes the size and direction by which an external stimulus resets a rhythm, either shifting it later (phase delay) or earlier (phase advance) on subsequent cycles. Although changes in phase are most well studied, the amplitude, waveform, and period of a rhythm can also be altered by external stimuli in a phase-dependent manner. Phase angle of entrainment describes the difference between the phase of an endogenous rhythm and that of the entraining stimulus. Lastly, limits of entrainment are the range of external periods to which the rhythm can be entrained (e.g., 22-26 h). Due to its critical role in dictating overt rhythms, many rhythmic parameters are thought to reflect SCN function, but may also be influenced by the properties of other clock tissues.
Figure 1.
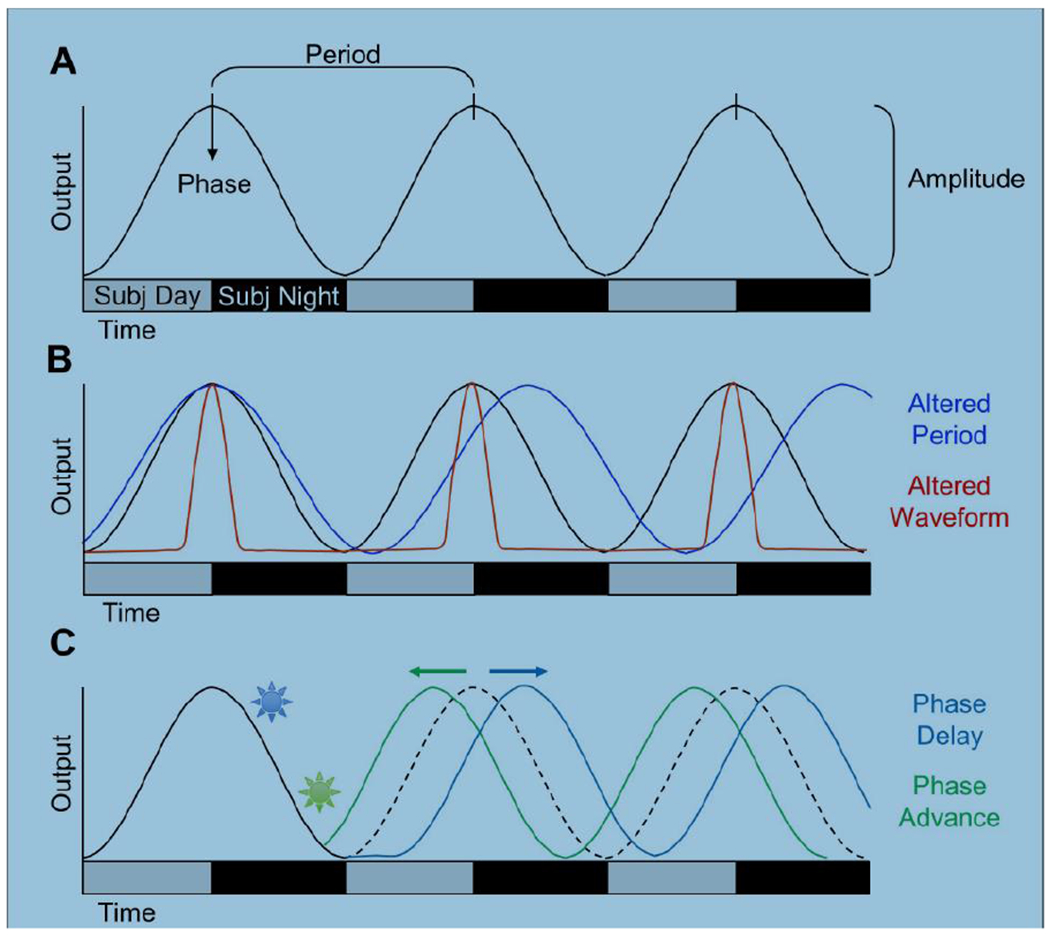
Formal properties of circadian rhythms. A. Schematic illustrating parameters commonly used to quantify circadian rhythms. A sine wave is used to represent the output of the rhythm (e.g., PER2 expression in the SCN). Gray and black bars on the abscissa represent subjective day and subjective night, respectively. B. Schematic illustrating changes in circadian period and waveform. C. Schematic illustrating photic phase resetting. A light pulse during early subjective night causes a phase delay (blue) and a light pulse during late subjective night causes a phase advance (green).
The circadian system is often depicted using a 3-part model (Figure2A). Central to this model is a “clock” that displays self-sustained circadian rhythms, receives inputs, and sends outputs to effector systems. Over the last few decades, this model has expanded to include a multitude of cellular clocks regulated by the central clock in the SCN (108). Cellular circadian rhythms are coordinated by transcriptional-translational feedback loops (TTFLs) that modulate gene and protein expression across the day (124). In mammals, the core TTFL is a negative feedback loop involving activation of repressive elements PERIOD (PER) and CRYPTOCHROME (CRY) by the transcription factors CLOCK and BMAL1 (Figure2B). During the repressive phase, PER and CRY feedback to inhibit their own transcription by interfering with CLOCK: BMAL1-mediated transcription. As PER and CRY are degraded, the repressive phase ends and active CLOCK-BMAL transcription resumes the next day. Additional molecular loops regulate the precision, robustness, and amplitude of core clock gene expression (Figure2B). In addition, clock gene expression can be regulated by other elements in response to extrinsic signals (e.g., cAMP response element). Lastly, there are numerous “clock-controlled genes” regulated by the molecular clock, which is estimated to be 40-60% of protein-coding genes expressed across all tissues in the body (121, 169).
Figure 2.
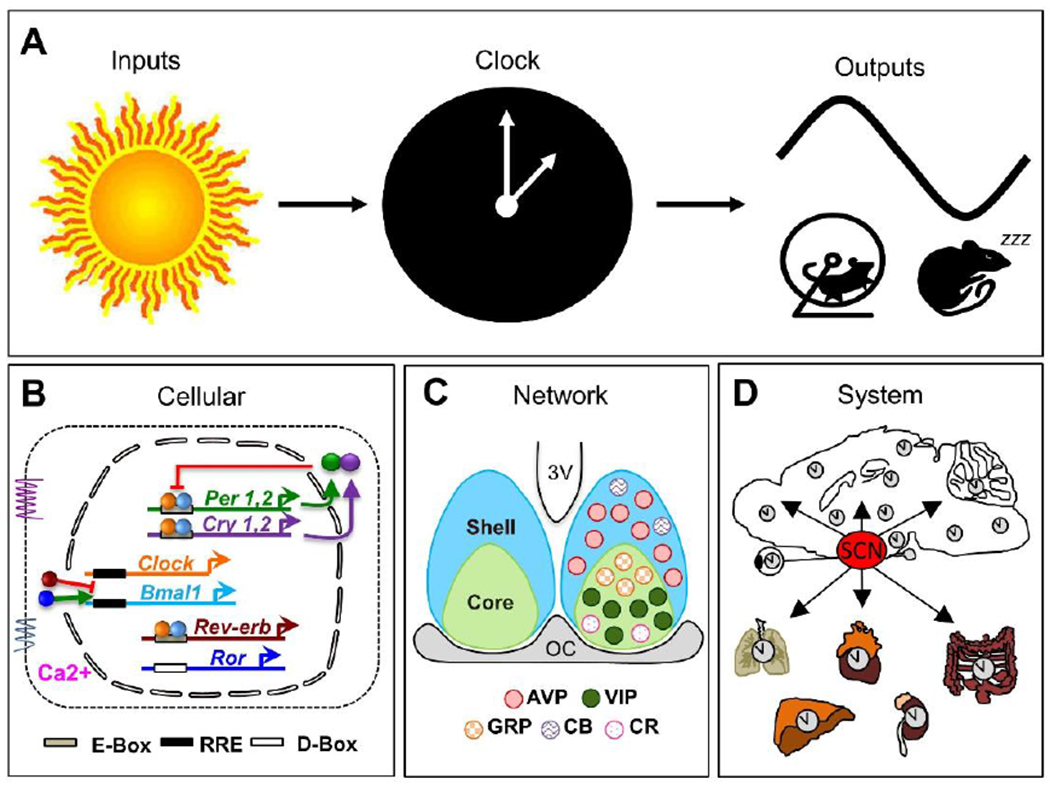
Organization of the circadian system at the conceptual, cellular, network, and systems levels. A. Conceptual three-part model of the circadian system. B. Cellular timekeeping involves transcriptional-translational feedback loops that can be modified by inter- and intracellular signaling. Arrows indicate activation and bars indicate repression. C. The SCN network comprises multiple neuronal subtypes organized into shell and core compartments. Schematic illustrates the location of neuronal subtypes mentioned in text. AVP: arginine vasopressin, VIP: vasoactive intestinal polypeptide, GRP: gastrin releasing peptide, CB: calbindin, CR: calretinin. D. The SCN sends time of day cues to other clock tissues in the brain and body.
For optimal circadian timekeeping, SCN neurons interact as a network and provide time of day cues to cellular and tissue clocks (9, 43). In addition to clock gene expression, many cellular processes fluctuate over the day in SCN neurons (60), including daily rhythms in membrane potential, with highest firing during the day (21, 31). Daily rhythms in electrical activity reflect not only the influence of the molecular clock, but also cytosolic rhythms that interact with the TTFL to regulate cellular physiology (122, 158). Nearly all SCN neurons produce γ-aminobutyric acid (GABA), but different neuronal classes can be distinguished based on peptide expression (Figure 2C). The SCN is often divided into two compartments: the shell and the core (9, 43). Among other cell types, the SCN shell contains a dense population of arginine vasopressin (AVP) neurons, and the SCN core contains vasoactive intestinal polypeptide (VIP) and gastrin releasing peptide (GRP) neurons (Figure 2C). Intercellular communication among SCN neurons is critical for maintaining network function and ensuring coherent output signals can coordinate rhythms at the overt level. For instance, isolated SCN neurons display different periods but coupled SCN cells coordinate to adopt a common speed. Further, cellular rhythms in the SCN network are more precise and robust than in isolated SCN neurons, which ensures daily rhythms are stable from cycle to cycle. Finally, SCN neurons adopt specific phase relationships with one another that encode salient features of the environment, such as seasonal changes in day length (33, 46). The SCN network transmits time of day cues to downstream tissues through synaptic projections and paracrine release of humoral signals (Figure2D). In addition, the SCN controls behavioral and physiological rhythms (e.g., body temperature, glucocorticoids, and melatonin) that can serve as entraining signals for downstream tissues. Finally, the SCN expresses melatonin and sex steroid receptors (11), suggesting that these signals can modulate its function.
In addition, the SCN processes extrinsic time cues to synchronize the circadian system to the 24 h day. Light is the principle entraining cue for the SCN, and photic stimuli are conveyed directly from melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) that release glutamate, GABA, and PACAP (10) via the retinohypothalamic tract (RHT). Photic stimuli induce phase shifts during subjective night, causing phase delays during early night and phase advances during late night (Figure 1C). SCN neurons respond to photic inputs with tonic/phasic excitation or inhibition as well as increased expression of immediate early genes (e.g., cFos) and Period through calcium-dependent signaling pathways (31). Tract tracing studies indicate there is a denser retinal projection to the SCN core than the SCN shell (3, 91), which maps onto findings that light-activated changes in gene expression in the SCN core precede those in the SCN shell (36, 116, 166, 167). Like regional patterning of photic inputs, the SCN core also receives dense inputs from the raphe nuclei and the intergeniculate leaflet (via the geniculohypothalamic tract, GHT). Thus, it is hypothesized that SCN core neurons process inputs, which are relayed to neurons in the SCN shell.
3. Interactions between circadian and reproductive function in females
The circadian system plays a critical role in female reproduction by timing ovulation to coincide with behavioral receptivity (14, 80, 106). In spontaneously ovulating mammals, reproductive rhythms occur with a circadian harmonic and require an intact SCN (50, 146). In most laboratory rodent species, the estrous cycle is a 4-5 day rhythm with four stages: metestrus, diestrus, proestrus, and estrus (Figure3A), with cyclical changes in reproductive hormones and vaginal cytology (4, 101). During metestrus, estradiol levels are low but slowly increasing due to growth of the ovarian follicle. Follicular growth and estradiol release increases through diestrus. On the afternoon of proestrus, high estradiol levels positively feedback to increase hypothalamic release of gonadotropin-releasing hormone (GnRH), which induces a surge in luteinizing hormone (LH) from the anterior pituitary to stimulate ovulation. The LH surge is eliminated by ovariectomy in adulthood demonstrating that it is dependent on activational effects of ovarian hormones. The LH surge is also sexually dimorphic (i.e., absent in male rodents of many laboratory species) due to organizational effects of gonadal hormones during development that are eliminated by postnatal castration of males or perinatal testosterone administration in females. In addition, the SCN provides a neural signal that causes the LH surge to specifically occur before the active phase of the circadian cycle. The SCN provides this signal every day, but it is only effective at stimulating GnRH release when it coincides with high estradiol levels. As such, the LH surge occurs daily in females given estradiol implants that mimic proestrus levels (106). The SCN signal is transmitted through multiple pathways that modulate GnRH release either directly or indirectly (14, 80, 106). SCN AVP- and VIP-ergic inputs to regions controlling reproduction display sexually dimorphic patterns of innervation (68, 134). Reflecting this regulation, changes in the state of the circadian system can impact reproduction (84, 95, 133, 165).
Figure 3.
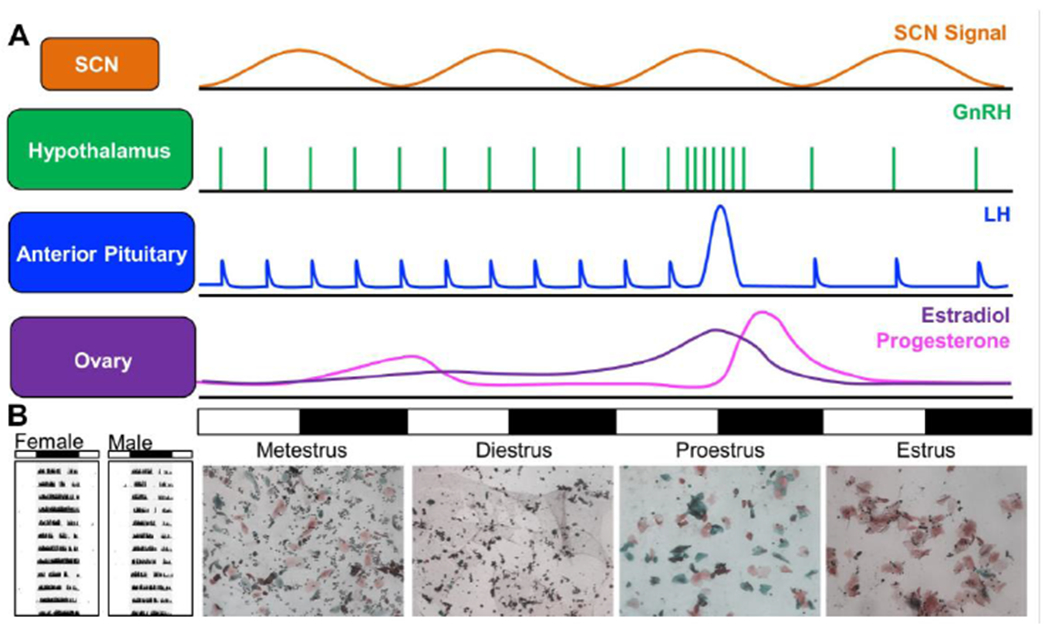
Reciprocal interactions between reproduction and circadian timekeeping. A. The SCN provides a time-of-day signal to regulate timing of the LH surge. Changes in hormone release and vaginal cytology are represented for the mouse. B. Representative single-plotted actograms of wheel-running rhythms in a female and male mouse illustrating cyclical changes in activity levels across the 4–5-day estrus cycle. In the actogram, each line depicts 24 h, with the time of darkness indicated by the black bar above the actogram and the internal gray shading.
In turn, female reproductive cycles modulate daily rhythms, with changes in both the levels and phase of locomotor activity observed in multiple mammalian species (81). In hamsters, activity onsets exhibit a well-defined “scalloping” pattern, with an earlier phase angle of entrainment, higher levels, and/or more consolidated activity on proestrus and estrus (113, 150, 172). Rats also exhibit higher levels of activity levels on estrus along with advanced phase angle of entrainment, increased activity duration, and a more consolidated active phase (6, 164). Likewise, female degus display increased activity levels and a 5 h advance of activity onset on the day of estrus(85). Estrus can modulate daily rhythms in mice (Figure3B), but reports of estrus effects are more varied in this species, with increased activity on proestrus (78, 127), overall more activity in females than males (17), or no difference by sex or estrous phase (83, 123). Discrepancies across studies may reflect differences in mouse strain, diet composition, and/or housing conditions, such as access to a running wheel (78, 104). Further, estrous modulation of activity rhythms can interact with photoperiod and entrainment history (129, 162). Additional studies are needed to evaluate whether estrous modulation of activity rhythms reflects changes in the period, organization, and/or photic responses of the central SCN clock versus properties of other tissues regulating locomotor activity.
4. Effects of sex and hormones on behavioral timekeeping
Sex differences have been reported in many circadian parameters, including period, precision, waveform, and levels of activity. Where possible, we discuss activational and organizational effects of gonadal hormones on behavioral rhythms below, though the loci of these effects remain unclear because steroid receptors are present in many tissues of the circadian system (11). In addition, sex chromosomes can influence circadian rhythms in mice (83).
4.1. Period
In general, sex differences in circadian period are modest in size and variable in direction across species. The largest sex difference in circadian period is seen in the diurnal degu, where adult females display free-running rhythms that are 18-36 min longer than adult males (59, 70). In hamsters and rats, the direction of sex difference is reversed, with females displaying 2-30 min shorter free-running rhythms relative to males (37, 58, 140). In contrast, most studies using mice have found that circadian period is similar in females and males (17, 20, 71, 83), although period is 6-30 min shorter in female mice in some studies (47).
Most rodent species display changes in circadian period when gonadal hormones are manipulated in adulthood. Ovariectomy can increase period length in hamsters and rats (152, 172), although this effect may depend on the presence of a running wheel (138). After ovariectomy, estradiol replacement shortens free-running period by 10-20 min in female hamsters, rats, and mice (5, 113, 137, 150, 172). In ovariectomized female mice, the period-shortening effects of estradiol can be mimicked by agonists for both the estrogen receptor subtype 1 (ESR1) and estrogen receptor subtype 2 (ESR2), with ESR1 agonists effective at lower doses (137). In contrast, progesterone alone does not alter circadian period, but it effectively blocks the effects of estradiol in ovariectomized hamsters (113, 150) Progesterone interference may be important under physiological conditions because period shortening effects of estradiol can persist for 20-65 days after implant removal in hamsters (113). In contrast, activational effects of androgens do not influence period in hamsters, rats, degus, or voles (73, 100, 111, 136). In mice, castration lengthens period by 12-34 min, which can be at least partially restored by testosterone replacement (20, 22, 35, 77, 107). However, individual responses are variable and period lengthening after castration is not universally observed across studies (141). Subsequent work suggests that the effect of castration and exogenous androgens on period in mice is dependent on dim red light exposure during free-running conditions (22). Lastly, gonadectomy and exogenous hormone exposure does not alter period in adult male or female degus (70, 85, 88), indicating that the sex difference in period does not require activational effects of gonadal hormones in this species.
Consistent with classic organizational effects, female hamsters and rats treated with perinatal testosterone do not shorten period in response to estradiol administration in adulthood (5, 172). Further, estradiol shortens period in adult male hamsters castrated at birth (172). Interestingly, this study also found that very few male hamsters castrated at birth exhibited sustained, stable free-running rhythms in adulthood regardless of androgen replacement. In addition, blocking organizational effects of estradiol with aromatase knockout lengthens period in both male and female mice (20). Circadian period is lengthened in male mice by non-classical estrogen receptor knock-in (17), which abolishes genomic effects of ESR1 signaling, but preserves ESR2 and non-genomic effects of ERS1. Lastly, castration prior to puberty prevents age-associated shortening of period in adult male degus (70, 88), suggesting that organizational effects at puberty cause sex differences that manifest later in life in this species.
4.2. Precision
Relative to males, females tend to have less precise activity rhythms due to estrous-related changes in phase and activity levels in mice, hamsters, and rats (81). Female rats exhibit less stable, lower amplitude rhythms, largely driven by higher daytime activity relative to males (24). However, variability of locomotor activity and core body temperature across the day is larger in male BALB/c mice (145). Castration decreases precision of activity rhythms in male hamsters, which is restored by testosterone administration (111). In male and female mice, gonadectomy decreases precision, which is increased by androgen exposure in both sexes (22, 71). Taken together, these findings suggest that activational effects of gonadal hormones influence rhythm precision, which may reflect modulation of interactions between circadian and sleep/wake centers in the brain.
4.3. Locomotor Activity Levels
Higher activity levels are reported in female hamsters, rats, mice, and gerbils (17, 20, 40, 71, 135, 140, 147). However, this finding is not observed in mice given running wheels (83), which may reflect important sex differences in motivational and/or motor circuits that influence wheel running. Gonadectomy reduces activity levels in both sexes in most rodent models, which can be restored at least partially with androgen or estradiol replacement (5, 20, 22, 35, 42, 71, 74, 83, 85, 111, 113, 150). Testosterone and the non-aromatizable dihydrotestosterone (DHT) will at least partially restore activity levels in adult mice of both sexes (5, 71), indicating a key role for androgen signaling. Estrogen signaling also modulates activity levels in male and female mice through multiple mechanisms involving both ERS1 and ERS2 (17, 18, 123). Lastly, progesterone administration decreases activity levels in hamsters and degus (85, 150), complementing its antagonistic effects on circadian period. In addition to modulating activity levels over the estrus cycle, this action of progesterone may also serve to suppress activity levels during pregnancy and lactation.
4.4. Waveform
Although not examined as frequently as other circadian parameters, sex differences in the waveform or distribution of activity have been reported in studies using rats, gerbils, degus, and mice. In one early study conducted in rats, sex differences in activity waveform were mentioned, but were not quantified due to variation in activity levels over the estrous cycle (140). In another study, female gerbils were found to be more nocturnal compared to male gerbils due to more daytime activity in males (135). In rats and degus, sex differences in activity distribution emerge after puberty (58, 59), with a switch from crepuscular to a more diurnal pattern of activity in male but not female degus (59). A delay in activity onset also occurs at puberty in both male and female degus, suggesting that distribution of activity is modulated by gonadal steroids, with either both sexes responding to the same steroid or convergent responses to different steroids. In rats, there is also a post-pubertal switch from bimodality to a more unimodal activity rhythm, which manifests in females 4-7 days earlier (58). As in other circadian parameters, studies examining activity waveform in mice vary in whether sex differences are evident. Activity duration has been found to be longer in female mice than in male mice in some studies (83, 147), but not others (17). The reasons for this discrepancy are unclear, but it could reflect difficulty in measuring sex differences in circadian waveform using average activity profiles given estrus-modulation and individual differences in the timing of the nocturnal “siesta” (Figure3B). Although activity waveform can be difficult to quantify, it remains an important parameter because it may reflect differences in neural circuits regulating circadian timekeeping, wakefulness/sleep, and/or the interaction of these systems.
Activational effects of gonadal hormones modulate activity waveform in a variety of species. In female ovariectomized hamsters held under constant darkness, estradiol increases activity levels specifically during early subjective night (113), suggesting an interaction with timekeeping mechanisms in circadian, motivational and/or motor circuits. Castration of male hamsters causes activity rhythms to become more fragmented, with the appearance of more activity bouts < 6 min, which is reversed by testosterone replacement (111). In mice, castration can alter activity waveform, but ovariectomy does not (20, 71). After castration, activity rhythms in male mice often become more bimodal (22, 35, 71, 77, 107), but the effect on activity duration varies across studies — either becoming longer or shorter depending on the relative intensity of each activity bout. Activational effects of androgen signaling are indicated by studies finding that castration-induced changes in waveform are fully reversed by high doses of testosterone, with partial or full restoration with DHT administration (22, 35, 71, 77). In contrast to peripheral administration, bimodal rhythms may persist when testosterone capsules are implanted directly into the brain (107), although activity waveform was not directly examined in this study. Thus, it remains unknown whether the effects of androgen signaling on activity waveform reflect changes in the SCN, other central circuits, or peripheral tissues.
Sex differences in activity waveform may also reflect organizational and non-hormonal mechanisms. For instance, gonadectomy in adulthood does not fully eliminate the sex difference in activity distribution that emerges after puberty in rats and degus (58, 59). Gonadectomy prior to puberty will eliminate the sex difference in activity offset in male degus; however, differences in circulating hormones cannot fully account for this effect given that it emerges many months after puberty (70). Interestingly, activity duration is longer in XX mice regardless of gonadal sex (83), which was the circadian parameter most strongly modulated by sex chromosome in this study. This finding is consistent with work demonstrating that sex differences in activity duration persist after reproductive senescence in female mice (147). Although many studies suggest potent effects of androgen signaling on activity waveform in mice (22, 35, 71, 77), effects of estrogen signaling cannot be discounted given that ESR1 deletion causes activity rhythms to become more bimodal in both male and female mice (17).
5. Effects of sex and hormones on circadian responses to inputs
Entrainment is the process by which the internal rhythm is adjusted to match the environmental cycle by an external stimulus (e.g., zeitgeber). Daily rhythms can be adjusted by many types of stimuli, although light is the principal stimulus for the SCN and the one for which the most is known. However, a recent metanalysis found that fewer than 7% of behavioral studies on circadian responses to light included females (87). Below we review sex differences in three main types of responses to external stimuli: changes in phase, period, and waveform. Our discussion largely focuses on responses to photic stimuli, though sex differences in non-photic responses are also discussed.
5.1. Light-induced changes in phase
Changes in phase occur in response to a stimulus pulse delivered under otherwise constant conditions (e.g., a bright light pulse in constant darkness), which is often referred to as non-parametric response. The magnitude and direction by which the rhythm is reset is time-dependent and typically illustrated by a phase response curve (PRC, Figure4A). Daily resetting can correct the discrepancy between the internal and external period to promote entrainment. In fact, the period and PRC of a species can be used to predict the phase angle of entrainment, limits of entrainment, and latency to jetlag recovery. Further, daily application of two short pulses of light at dusk and dawn is sufficient for entrainment of nocturnal mammals under a wide variety of circumstances (Figure4B). However, this type of skeleton photoperiod is insufficient to maintain stable entrainment under simulated long day lengths (Figure4B), suggesting that full photophases are necessary in some circumstances.
Figure 4.
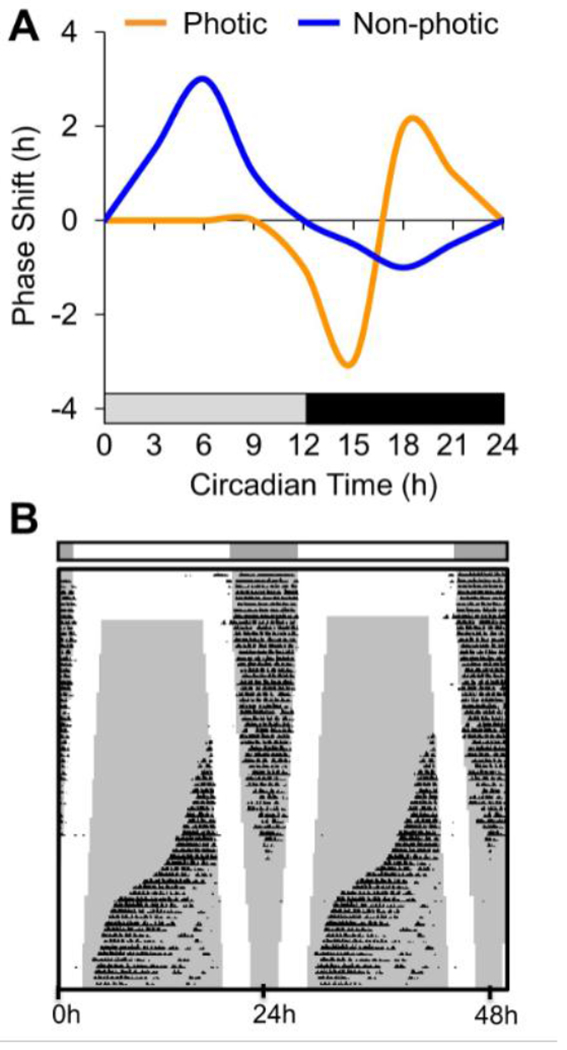
Circadian responses to photic and non-photic cues. A. Schematic phase response curves illustrating that resetting responses to photic and non-photic stimuli dependent on time of day. B. Representative double-plotted actogram illustrating unstable entrainment of running wheel activity under skeleton photoperiods simulating long day lengths. Each line of the actogram depicts 48 h, with the second day of each line also plotted as the first day in the following line. , Darkness is indicated by the black bar above the actogram and the internal gray shading.
The general shape of the photic PRC is similar in males and females, but sex differences in the magnitude of photic resetting have been reported in mice and hamsters. Relative to males, female hamsters exhibit 30 min smaller phase delays to light pulses delivered at CT12-14 (37). In mice, the sex difference is reversed, with females displaying larger phase delays (19, 20). Ovariectomy reduces phase delays and potentiates phase advances in female wild-type mice, but not mice lacking aromatase (20). Similarly, ESR1 knockout also reduces phase delays and potentiates phase advances in female mice (19). Lastly, a primary role of circulating ovarian hormones in modulating photic resetting is supported by results using the four core genotype model (83). Gonadal females displayed larger phase delays than gonadal males regardless of chromosomal complement, although phase delays were greatest in XX mice with ovaries. Activational effects of circulating androgens also modulate photic resetting, although the results vary by study. In one study, castration reduced both phase advances and delays in wild-type mice, with reduced phase advances in aromatase knockout mice (20). In another study the opposite effects was observed; castration increased phase delays in male wild-type mice, which was restored by DHT administration (76).
Sex differences in photic resetting correspond with differences in entrainment between males and females. In hamsters, the sex difference in photic resetting is consistent with the advanced phase angle of entrainment in females and their reduced capacity to entrain to cycles longer than 24 h (37). However, the phase of activity onset after release into constant conditions does not differ by sex or estrous phase (37), suggesting that the sex difference in phase angle of entrainment is caused by a sex difference in the acute response to light (i..e, masking response). In addition, adult ovariectomy of female hamsters eliminates the sex difference in the phase angle of entrainment, which was restored by estradiol administration (113). Further, perinatal testosterone administration to female hamsters abolishes the sex difference in phase angle of entrainment (113), indicating that both activational and organizational effects of gonadal hormones modulate photic processing in hamsters. In mice, phase angle of entrainment does not differ by sex (19, 20, 83), at least not until after 24 weeks of age (147). Nevertheless, jetlag recovery is faster in female mice than males when eastward travel is simulated (47, 132). Jetlag recovery is faster in female mice shifted on the day of proestrus compared to males and females in metestrus, and accelerated re-entrainment is mimicked by estradiol treatment (126). Although the cellular mechanisms by which estrogen signaling modulates photic resetting and jetlag recovery remain unclear, it has been suggested that an ESR1-independent mechanism modulates phase angle and photic resetting in mice (16, 18, 137). Collectively these data suggest that activational and organizational effects of gonadal hormones modulate photic processing, although these results may also reflect a sex difference in pacemaker interactions with sleep/wake circuits and/or peripheral tissues (34, 47, 126, 150).
5.2. Light-induced changes in period and waveform
Changes in period can occur in response to longer stimulus exposure (e.g., constant light), which is often referred to as a parametric response. In nocturnal rodents, exposure to constant bright light lengthens period, suppresses activity levels (i.e., negative masking), and can disrupt consolidation of activity rhythms such that they become bimodal or ultradian (46). Although the neurobiological bases of parametric responses remain unclear, these are relevant for stable entrainment to long day photoperiods (Figure4B) and entrainment in diurnal species such as humans.
Sex differences in parametric responses to light have been reported in hamsters, rats, and mice. Gonadectomized hamsters displayed sex differences in the emergence of split rhythms under constant light, with ovariectomized females displaying more rapid rhythm disturbance that does not resolve into two stable components like in castrated males (112). Estradiol administration after ovariectomy prevents constant-light-induced rhythm disturbances in female hamsters (110), suggesting that estradiol increases the robustness of circadian rhythms in female hamsters. Progesterone does not produce a similar effect (110), nor does manipulation of testicular hormones in male hamsters (112). Similarly, male rats exhibited a higher coherence of rhythms under constant light, and females exhibited longer latency to reconsolidate rhythms after release into constant darkness (24). Further, the strength of circadian rhythms under constant light was decreased after ovariectomy in adult female rats, which was prevented by estradiol treatment (152). In addition, exposing rat pups to constant light during lactation prevents constant-light-induced arrythmia in adulthood (26). The protective effects of constant light during lactation is greater in female rats, suggesting that perinatal light conditions can re-wire circadian circuits in a sexually dimorphic manner. Relative to males, constant light lengthens period more in female mice, and ESR1 knockout attenuates the period lengthening effect in both sexes (17). Lastly, castration lengthens period of male mice exposed to constant dim light, which is blocked by testosterone administration (22). Collectively, these results suggest that estrogens and androgens modulate parametric responses to constant light in a species-specific manner, but the exact cellular mechanisms remain unclear.
Dim nighttime illumination can increase the plasticity of circadian entrainment under exotic lighting conditions in a manner influenced by sex. Under 24 h light:dark:light:dark cycles, hamsters and mice will adopt bimodal activity rhythms when the two daily scotophases are dimly lit (53). Bifurcated activity rhythms under these conditions correspond to bimodal markers of subjective day and night (52, 53) and transients after release into constant conditions (44), indicating that this is bona fide entrainment rather than masking. Under these conditions, female mice exhibit more symmetric bifurcated entrainment that can be induced at a lower critical photophase duration (157). Further, symmetry of bifurcated entrainment decreases in male mice with age, but older females retain high levels of symmetry (157). Females also display stronger entrainment under non-24 h photoperiods (156). Lastly, there is evidence to suggest that there are sex differences in behavioral and physiological responsiveness to seasonal changes in day length (13, 45, 161), which may have relevance for understanding gender disparities in seasonal disease in humans (92, 94, 102). Given the importance of photo-entrainment for circadian function, the cellular basis of sex differences in photic responses warrant further investigation.
5.3. Non-photic responses
Non-photic stimuli include food, melatonin, locomotor activity, and social cues (28). Nonphotic cues typically induce phase advances during subjective daytime and phase delays during subjective night (Figure4A), which is associated with downregulation of SCN gene expression (32, 98, 103). The response to non-photic cues interacts with estrous in hamsters; novelty-induced wheel-running delays the estrous cycle by 1 day when the stimulus is presented on the afternoon of proestrus (168). Further, the resetting response to a novel wheel is smaller on the day of estrus (168). In addition, the response to time restricted food access differs by sex in mice (69, 105), with lower levels of food-anticipatory activity displayed by females. This suggests that there are sex differences in circadian responses to food cues, which may be important to consider in studies evaluating the positive effects of time-restricted eating (27). Lastly, social cues can accelerate recovery to simulated jetlag in degus, with sex and gonadal hormones modulating this effect (51, 72, 75).
6. Effects of sex and hormones on SCN structure and cellular function
In most studies, the gross anatomy of the SCN is similar in males and females, but sex differences in general SCN morphology have been reported. In humans, SCN volume does not differ by sex (64, 65, 148), although it is 14% smaller in men when measurements are adjusted for brain size (64). Likewise, overall cell number, cell density and cellular diameter does not differ by sex in humans (64). However, a sex difference has been found in the shape of the human SCN, with the anteroposterior axis 40% longer in women and the mediolateral axis 35% more spherical in men (64, 66, 148). The functional significance of a sex difference in SCN shape is unclear, although it has been speculated that this could reflect sex differences in subregional SCN organization. As in humans, SCN volume and cell number does not differ by sex in most studies using rat models (25, 65, 93, 154). However, SCN volume was found to be 26% larger in males in one study using rats (131) and up to 45% larger in two studies using gerbils (30, 67). The sex difference in SCN volume in gerbils was eliminated by perinatal castration due to a 62% reduction in males (67). Although there do not appear to be overall sex differences in the number of SCN neurons, the developmental patterns of neurogenesis in the middle and caudal SCN differs by sex in rats, with lower numbers in males due to organizational effects of gonadal hormones (2). In addition, the number of SCN neurons in the ventrolateral core was reported to be lower in males rats (154), although age was not strictly controlled in this study. Sex differences in cellular morphology have also been reported, with larger mean area and nucleoli of SCN neurons in female rats (8, 55). Further, increased neuronal area in female rats is blocked by prepubertal ovariectomy (114, 115), suggesting that gonadal hormones at puberty can alter SCN organization and function.
Other morphological measures indicate SCN circuits differ by sex and gonadal status in rodents. Male rats have a greater number of SCN synapses due to organizational effects of gonadal hormones (86), although perinatal androgen administration markedly disrupted female SCN development in this study. Similarly, it has been reported that male rats display a larger number of axo-somatic, axo-spinal, and asymmetric synapses with more post-synaptic density material relative to females (56, 57). These studies suggest that there are sex differences in SCN signaling, but the exact type and origin of synapses evaluated in these studies is not clear. Further, samples did not always derive from the same region of the SCN, making it difficult to draw rigorous conclusions. Sex differences in SCN organization have also been inferred from GFAP staining, with lower levels in male gerbils and rats (25, 30, 55, 154). In mice, SCN GFAP expression is increased by castration and reduced by DHT administration, with opposite effects on synaptophysin and PSD95 levels (76). Activational effects of ovarian hormones may also modulate SCN circuits, with estradiol injections increasing connexin-36 and connexin-32 levels in the SCN of female rats (142, 143).
Activational and organizational effects of gonadal hormones on SCN organization may reflect local signaling of sex steroids or actions in tissues afferent to the SCN (11). Androgen, estrogen, and progesterone receptors are expressed in the human SCN, with higher levels of androgen receptors in men and higher levels of ESR1 in women (48, 82). Both androgen and estrogen receptors are expressed in the SCN of mice, rats, and degus, often with sex differences in levels (41, 71, 83, 88, 144, 155, 163). It is noteworthy that SCN sex steroid receptor expression tends to be sex-specific in a manner aligned with gonadal output, likely magnifying effects of sex steroid signaling under physiological conditions. In male mice, androgen receptors are expressed constitutively across the day, with the highest levels in SCN GRP neurons, modest expression in VIP neurons, and very little expression in AVP neurons (77). Similarly, AVP neurons do not express androgen receptors in the rat SCN (171). In contrast, ESR1 and ESR2 are highest in the SCN shell of mice, with highest co-expression in neurons positive for calretinin or calbindin (155). SCN androgen and estrogen receptors are modulated by activational effects of gonadal hormones. Specifically, SCN androgen receptor levels are reduced by castration and elevated by androgen administration in both males and females (22, 71, 77, 83, 107). Androgen receptor expression in the SCN is most influenced by gonadal sex, but XX mice with ovaries display the lowest levels (83). In gonadectomized mice, estradiol decreases ESR2 expression in the SCN of both female and male mice but does not influence ESR1 expression (155). In addition, there is a diurnal rhythm of ESR2 expression in the SCN of female rats that peaks at dawn and is reduced in magnitude at 19-24 month of age (163). Thus, androgen and estrogen receptors in the SCN differ in both the spatial and temporal patterns of expression, but modulation by gonadal hormones appears to be retained in both sexes in adulthood despite sex-specific behavioral responses to hormone administration.
In addition, sex differences in SCN neurochemistry are reported in humans and rodent models. Men have twice the number of VIP neurons over 10-40 years of age; however, this sex difference reverses at older ages (149, 170). In contrast, the number of AVP neurons in the SCN does not differ by sex in humans (66, 148). In nocturnal and diurnal rats, sex also modulates SCN expression of VIP, but not AVP (54, 79, 96). In whole hypothalamic punches, higher Vip levels were detected in female rats compared to males (54). This may reflect a sex difference in the rhythm of SCN Vip expression, with an 8-9 h difference in peak time and higher daytime levels in the female SCN of both nocturnal and diurnal rats (79, 96). There also appears to be a sex difference in the phase of expression for the VIP receptor Vipr2 in the SCN of diurnal grass rats (96). Activational and organizational effects of gonadal hormones modulate VIP expression in the SCN (54, 79, 96, 159), although differences across studies limit understanding of the precise nature of this relationship. First, gonadectomy was found to decrease VIP expression in the hypothalamus of female rats, but not male rats (54). Further, VIP levels were increased by estradiol in females and testosterone in males (54). However, subsequent work found that ovariectomy in adulthood produced the opposite effect to increase VIP levels in the rat SCN (79).
In diurnal grass rats, ovariectomy in adulthood delays the phase of Vip rhythms in the female SCN to more resemble that in males, and estradiol replacement will restore the daytime peak of Vip but blunts its expression (96). Lastly, perinatal estradiol treatment increases VIP levels in the SCN when measured in adult female rats (159), although rhythmicity was not considered in this study. Given the phenotypic response to VIP and AVP deficiency can differ between male and female mice (90, 132), the relationship between SCN neurochemistry, biological sex, and gonadal hormones warrants further study.
Although females are rarely included in studies investigating SCN function, some work indicates that sex and gonadal hormones modulate cellular physiology in the central clock. Relative to intact male rats, ovariectomized females display lower glucose utilization in the rostral SCN, which was further decreased by estradiol replacement in a phase-specific manner (62). In addition, sex differences have been reported in ex vivo SCN electrical activity, with higher daytime firing in dorsal SCN neurons from male mice (83). Interestingly, this sex difference was abolished by application of the GABAA receptor antagonist due to reduced spontaneous daytime firing in dorsal SCN neurons from male mice (83), suggesting there are sex differences in SCN GABA circuits. Sex differences in intrinsic membrane properties were also evident in this study, with male SCN neurons displaying lower nighttime action potential thresholds and larger day-night differences in after-hyperpolarization relative to female SCN neurons. In contrast to sex differences in electrical activity, PER2::LUC rhythms do not differ by sex (83). Further, estradiol does not alter SCN period of PER2::LUC rhythms in mice or Per1-luc rhythms in rats (118). Nevertheless, in vivo administration of estradiol to ovariectomized female rats advances the phase of Per2 rhythms and decreases Cry2 in the SCN (117, 119). Although a more indirect measure of SCN activity, daily rhythms in Fos-related antigen differs by sex in degus, with lower peak levels at dusk in the female SCN (97). Daytime expression of cFos measured at a single timepoint does not differ by sex in rats or mice (19, 125); however, ovariectomy reduced daytime cFos expression in the dorsal SCN of female rats in an estradiol dependent manner (125). This same study found that castration increased daytime cFos expression in the ventral SCN of male rats (125), although the meaning of this result is unclear given cFos expression in this compartment is most strongly associated with photic resetting at night.
There is limited work examining sex differences in SCN responses to light. SCN electrical response to NMDA application in vitro does not differ by sex in mice (83), but the spatiotemporal patterning of light-induced molecular responses may differ by sex in degus (97) and mice (19).
However, effects of sex were not compared directly in these studies. Gonadal hormones modulate SCN responses to light in mice, with effects of both androgen and estrogen signaling. In male mice, light-induced cFos expression is reduced by castration and restored by DHT administration (77). Effects of castration and DHT on light-induced clock gene expression have also been described in male mice (76), which are more complex in nature. Castration reduces light-induced Per2 during early subjective night and increases light-induced Per1 during late subjective night. Both responses were normalized by DHT administration (76). These studies indicate that activational effects of androgen signaling modulate SCN molecular responses to light; however, the interpretation of these results is difficult given castration increases phase delays of behavioral rhythms. In contrast, the effects of estrogen signaling are easier to reconcile. Estradiol increases light-induced cFos and pCREB in the SCN of female rats (1), consistent with increases in behavioral resetting (83). It remains unclear whether gonadal hormones modulate photic responses of the SCN directly, via changes in the retina, or other light-responsive structures afferent to the SCN (11). Additional studies investigating how sex and gonadal hormones modulate the spatiotemporal patterning of SCN responses to light may provide further insight into mechanisms of photic signaling in both sexes.
7. Conclusions
Sex hormones and genetic sex regulate nearly all circadian parameters, but many of these effects are species-specific and vary across studies for reasons that remain unclear. Consistent with classic effects, circadian parameters can be modulated by gonadal hormones in a sex-dependent manner (i.e., only one sex responds). For example, ovariectomy lengthens period in female hamsters, but castration does not. However, some work suggests that sex hormones may elicit similar responses in both sexes (e.g., testosterone increases SCN androgen receptors) or induce responses that are distinct in each sex (e.g., phase advances are increased in female mice and decreased in male mice by gonadectomy). It is also suggested that gonadal hormones can act through multiple cellular mechanisms to modulate circadian behavior in a parameter-specific manner. For instance, ESR1 agonists increase wheel running activity, lengthen activity duration, consolidate activity into the dark phase, whereas ESR2 signaling alters distribution of nocturnal activity and activity onset (18, 137). These findings suggest that distinct effects of estradiol on circadian parameters are mediated by specific receptor subtypes. Across studies in mice, there are effects of both gonadal hormones and sex chromosomes, however the precise locus relevant for these effects remains unclear. In particular, it is unknown whether the effects of sex and gonadal hormones on behavioral rhythms reflects changes in the SCN itself because steroid receptors are present in many tissues of the circadian system (11).
More work using both sexes is expected to increase understanding of how sex and gonadal hormones influence cellular mechanisms of circadian timekeeping. Since estrous can influence activity levels and timing in mammals, reductionism has understandably favored the use of male rodents in circadian studies. However, the paucity of female data has impeded our understanding of how circadian clocks function in both sexes. Previous estimates have found that fewer than 20% of circadian studies include females (83), and a recent meta-analysis of published work spanning 1964-2017 found that fewer than 7% of rodent behavioral studies on circadian responses to light included females (87). Federal funding agencies now emphasize the use of both sexes; thus, it is expected that representation in circadian studies will increase in the future. One useful approach to comply with this mandate that has been employed in recent studies is to evaluate average effects across both sexes. However, if adequate numbers of males and females are not included to permit statistical evaluation of sex differences, this will continue to be an obstacle in understanding how circadian circuits may differ by sex. Additional work designed with these considerations in mind can reveal novel mechanisms that have developed under different hormonal and genetic circumstances. Further, better understanding of circadian mechanisms in each sex is an important goal given that sex differences in daily rhythms and consequences of circadian disruption impact human health. Lastly, this work may shed additional light on mechanisms underlying circadian and/or seasonal regulation of reproduction and mental health in women (84, 95, 133, 165).
Acknowledgements
We acknowledge support from the NIH (R01NS091234).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abizaid A, Mezei G, and Horvath TL. Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res 1010: 35–44, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Abizaid A, Mezei G, Sotonyi P, and Horvath TL. Sex differences in adult suprachiasmatic nucleus neurons emerging late prenatally in rats. Eur J Neurosci 19: 2488–2496, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamson EE and Moore RY. Suprachiasmatic nucleus in the mouse: Retinal innervation, intrinsic organization and efferent projections. Brain Res 916: 172–191, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Ajayi AF and Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract 6:5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol 241: R62–66, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Albers HE, Gerall AA, and Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav 26: 21–25, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Alibhai FJ, LaMarre J, Reitz CJ, Tsimakouridze EV, Kroetsch JT, Bolz SS, Shulman A, Steinberg S, Burris TP, Oudit GY, and Martino TA. Disrupting the key circadian regulator CLOCK leads to age-dependent cardiovascular disease. J Mol Cell Cardiol 105: 24–37, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Anderson CH. Nucleolus: changes at puberty in neurons of the suprachiasmatic nucleus and the preoptic area. Exp Neurol 74: 780–786, 1981. [DOI] [PubMed] [Google Scholar]
- 9.Antle MC and Silver R. Orchestrating time: Arrangements of the brain circadian clock. Trends Neurosci 28: 145–151, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Aranda ML and Schmidt TM. Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions. Cell Mol Life Sci 78(3): 889–907, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey M and Silver R. Sex differences in circadian timing systems: Implications for disease. Front Neuroendocrinol 35: 111–139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker JB, Prendergast BJ, and Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ 7: 34, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Hamo M, Tal K, Paz-Cohen R, Kronfeld-Schor N, and Einat H. Differential effects of photoperiod length on depression- and anxiety-like behavior in female and male diurnal spiny mice. Physiol Behav 165: 1–6, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Beymer M, Henningsen J, Bahougne T, and Simonneaux V. The role of kisspeptin and RFRP in the circadian control of female reproduction. Mol Cell Endocrinol 438: 89–99, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Bixler EO, Papaliaga MN, Vgontzas AN, Lin HM, Pejovic S, Karataraki M, Vela-Bueno A, and Chrousos GP. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res 18: 221–228, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blattner MS and Mahoney MM. Changes in estrogen receptor signaling alters the timekeeping system in male mice. Behav Brain Res 294: 43–49, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Blattner MS and Mahoney MM. Circadian parameters are altered in two strains of mice with transgenic modifications of estrogen receptor subtype 1. Genes Brain Behav 11: 828–836, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Blattner MS and Mahoney MM. Estrogen receptor 1 modulates circadian rhythms in adult female mice. Chronobiol Int 31: 637–644, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Blattner MS and Mahoney MM. Photic phase-response curve in 2 strains of mice with impaired responsiveness to estrogens. J Biol Rhythms 28: 291–300, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Brockman R, Bunick D, and Mahoney MM. Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice. Horm Behav 60: 439–447, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Brown TM and Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol 82: 229–255, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Butler MP, Karatsoreos IN, LeSauter J, and Silver R. Dose-dependent effects of androgens on the circadian timing system and its response to light. Endocrinology 153: 2344–2352, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, Schoen MW, Czeisler CA, and Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms 25: 288–296, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cambras T, Castejon L, and Diez-Noguera A. Social interaction and sex differences influence rat temperature circadian rhythm under LD cycles and constant light. Physiol Behav 103: 365–371, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Cambras T, Lopez L, Arias JL, and Diez-Noguera A. Quantitative changes in neuronal and glial cells in the suprachiasmatic nucleus as a function of the lighting conditions during weaning. Brain Res Dev Brain Res 157: 27–33, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Cambras T, Vilaplana J, Torres A, Canal MM, Casamitjana N, Campuzano A, and Diez-Noguera A. Constant bright light (LL) during lactation in rats prevents arrhythmicity due to LL. Physiol Behav 63: 875–882., 1998. [DOI] [PubMed] [Google Scholar]
- 27.Chaix A, Manoogian ENC, Melkani GC, and Panda S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu Rev Nutr 39: 291–315, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Challet E Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 148: 5648–5655, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Chisari A, Carino M, Perone M, Gaillard RC, and Spinedi E. Sex and strain variability in the rat hypothalamo-pituitary-adrenal (HPA) axis function. J Endocrinol Invest 18: 25–33, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Collado P, Beyer C, Hutchison JB, and Holman SD. Hypothalamic distribution of astrocytes is gender-related in Mongolian gerbils. Neurosci Lett 184: 86–89, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci 12: 553–569, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coogan AN and Piggins HD. Dark pulse suppression of P-ERK and c-Fos in the hamster suprachiasmatic nuclei. Eur J Neurosci 22: 158–168, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Coomans CP, Ramkisoensing A, and Meijer JH. The suprachiasmatic nuclei as a seasonal clock. Front Neuroendocrinol 37: 29–42, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, and Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol 205: 807–815, 1963. [DOI] [PubMed] [Google Scholar]
- 35.Daan S, Damassa D, Pittendrigh CS, and Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci 72: 3744–3747, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dardente H, Poirel VJ, Klosen P, Pevet P, and Masson-Pevet M. Per and neuropeptide expression in the rat suprachiasmatic nuclei: compartmentalization and differential cellular induction by light. Brain Res 958: 261–271, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Davis FC, Darrow JM, and Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol 244: R93–105, 1983. [DOI] [PubMed] [Google Scholar]
- 38.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145: 1063–1068, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Duffy JF, Cain SW, Chang AM, Phillips AJ, Munch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP Jr., and Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci 108 Suppl 3: 15602–15608, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duffy JF, Viswanathan N, and Davis FC. Free-running circadian period does not shorten with age in female Syrian hamsters. Neurosci Lett 271: 77–80, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Ehret G and Buckenmaier J. Estrogen-receptor occurrence in the female mouse brain: effects of maternal experience, ovariectomy, estrogen and anosmia. J Physiol Paris 88: 315–329, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Ellis GB and Turek FW. Testosterone and photoperiod interact to regulate locomotor activity in male hamsters. Horm Behav 17: 66–75., 1983. [DOI] [PubMed] [Google Scholar]
- 43.Evans JA. Collective timekeeping among cells of the master circadian clock. J Endocrinol 230: R27–49, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans JA, Elliott JA, and Gorman MR. Dynamic interactions between coupled oscillators within the hamster circadian pacemaker. Behav Neurosci 124: 87–96, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans JA, Elliott JA, and Gorman MR. Individual differences in circadian waveform of Siberian hamsters under multiple lighting conditions. J Biol Rhythms 27: 410–419, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans JA and Gorman MR. In synch but not in step: Circadian clock circuits regulating plasticity in daily rhythms. Neuroscience 320: 259–280, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feillet C, Guerin S, Lonchampt M, Dacquet C, Gustafsson JA, Delaunay F, and Teboul M. Sexual dimorphism in circadian physiology is altered in LXRalpha deficient mice. Plos One 11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Guasti A, Kruijver FP, Fodor M, and Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 425: 422–435, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Fischer D, Lombardi DA, Marucci-Wellman H, and Roenneberg T. Chronotypes in the US - Influence of age and sex. PLoS One 12: e0178782, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzgerald K and Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci 73: 2923–2927, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goel N and Lee TM. Sex differences and effects of social cues on daily rhythms following phase advances in Octodon degus. Physiol Behav 58: 205–213, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Gorman MR and Elliott JA. Entrainment of 2 subjective nights by daily light:dark:light:dark cycles in 3 rodent species. J Biol Rhythms 18: 502–512, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Gorman MR, Elliott JA, and Evans JA. Plasticity of hamster circadian entrainment patterns depends on light intensity. Chronobiol Int 20: 233–248, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Gozes I, Werner H, Fawzi M, Abdelatty A, Shani Y, Fridkin M, and Koch Y. Estrogen regulation of vasoactive intestinal peptide mRNA in rat hypothalamus. J Mol Neurosci 1: 55–61, 1989. [DOI] [PubMed] [Google Scholar]
- 55.Guldner FH. Numbers of neurons and astroglial cells in the suprachiasmatic nucleus of male and female rats. Exp Brain Res 50: 373–376, 1983. [DOI] [PubMed] [Google Scholar]
- 56.Guldner FH. Sexual dimorphisms of axo-spine synapses and postsynaptic density material in the suprachiasmatic nucleus of the rat. Neurosci Lett 28: 145–150, 1982. [DOI] [PubMed] [Google Scholar]
- 57.Guldner FH. Suprachiasmatic nucleus: numbers of synaptic appositions and various types of synapses. A morphometric study on male and female rats. Cell Tissue Res 235: 449–452, 1984. [DOI] [PubMed] [Google Scholar]
- 58.Hagenauer MH, King AF, Possidente B, McGinnis MY, Lumia AR, Peckham EM, and Lee TM. Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Horm Behav 60: 46–57, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagenauer MH, Ku JH, and Lee TM. Chronotype changes during puberty depend on gonadal hormones in the slow-developing rodent, Octodon degus. Horm Behav, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hastings MH, Maywood ES, and Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci 19: 453–469, 2018. [DOI] [PubMed] [Google Scholar]
- 61.Hatcher KM, Royston SE, and Mahoney MM. Modulation of circadian rhythms through estrogen receptor signaling. Eur J Neurosci 51: 217–228, 2020. [DOI] [PubMed] [Google Scholar]
- 62.Hery M, Dusticier G, and Calas A. Application of the 2-deoxy-(1-14C) glucose method to the study of suprachiasmatic nucleus activity and functions: phasic luteinizing hormone secretion and serotonin innervation. Exp Brain Res 47: 465–468, 1982. [DOI] [PubMed] [Google Scholar]
- 63.Hiroshige T, Abe K, Wada S, and Kaneko M. Sex difference in circadian periodicity of CRF activity in the rat hypothalamus. Neuroendocrinology 11: 306–320, 1973. [DOI] [PubMed] [Google Scholar]
- 64.Hofman MA, Fliers E, Goudsmit E, and Swaab DF. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: sex differences and age-dependent changes. J Anat 160: 127–143, 1988. [PMC free article] [PubMed] [Google Scholar]
- 65.Hofman MA and Swaab DF. The sexually dimorphic nucleus of the preoptic area in the human brain: a comparative morphometric study. J Anat 164: 55–72, 1989. [PMC free article] [PubMed] [Google Scholar]
- 66.Hofman MA, Zhou JN, and Swaab DF. Suprachiasmatic nucleus of the human brain: an immunocytochemical and morphometric analysis. Anat Rec 244: 552–562, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Holman SD and Hutchison JB. Differential effects of neonatal castration on the development of sexually dimorphic brain areas in the gerbil. Brain Res Dev Brain Res 61: 147–150, 1991. [DOI] [PubMed] [Google Scholar]
- 68.Horvath TL, Cela V, and van der Beek EM. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res 795: 277–281, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Hsu CT, Patton DF, Mistlberger RE, and Steele AD. Palatable meal anticipation in mice. PLoS One 5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hummer DL, Jechura TJ, Mahoney MM, and Lee TM. Gonadal hormone effects on entrained and free-running circadian activity rhythms in the developing diurnal rodent Octodon degus. Am J Physiol Regul Integr Comp Physiol 292: R586–597, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Iwahana E, Karatsoreos I, Shibata S, and Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav 53: 422–430, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jechura TJ, Stimpson CD, and Lee TM. Odor-facilitated reentrainment in male and female juvenile Octodon degus. Physiol Behav 89: 617–622, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Jechura TJ, Walsh JM, and Lee TM. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav 38: 243–249, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Jechura TJ, Walsh JM, and Lee TM. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav 38: 243–249., 2000. [DOI] [PubMed] [Google Scholar]
- 75.Jechura TJ, Walsh JM, and Lee TM. Testosterone suppresses circadian responsiveness to social cues in the diurnal rodent Octodon degus. J Biol Rhythms 18: 43–50, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Karatsoreos IN, Butler MP, Lesauter J, and Silver R. Androgens Modulate Structure and Function of the Suprachiasmatic Nucleus Brain Clock. Endocrinology, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karatsoreos IN, Wang A, Sasanian J, and Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology 148: 5487–5495, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kopp C, Ressel V, Wigger E, and Tobler I. Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains. Behav Brain Res 167: 165–174, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Krajnak K, Kashon ML, Rosewell KL, and Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology 139: 4189–4196, 1998. [DOI] [PubMed] [Google Scholar]
- 80.Kriegsfeld LJ and Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav 49: 557–574, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krizo JA and Mintz EM. Sex differences in behavioral circadian rhythms in laboratory rodents. Front Endocrinol (Lausanne) 5: 234, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kruijver FP and Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology 75: 296–305, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, and Colwell CS. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology 154: 1501–1512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Labyak S, Lava S, Turek F, and Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int 23: 703–714, 2002. [DOI] [PubMed] [Google Scholar]
- 85.Labyak SE and Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav 58: 573–585., 1995. [DOI] [PubMed] [Google Scholar]
- 86.Le Blond CB, Morris S, Karakiulakis G, Powell R, and Thomas PJ. Development of sexual dimorphism in the suprachiasmatic nucleus of the rat. J Endocrinol 95: 137–145, 1982. [DOI] [PubMed] [Google Scholar]
- 87.Lee R, Tapia A, Kaladchibachi S, Grandner MA, and Fernandez FX. Meta-analysis of light and circadian timekeeping in rodents. Neurosci Biobehav Rev 123: 215–229, 2021. [DOI] [PubMed] [Google Scholar]
- 88.Lee TM, Hummer DL, Jechura TJ, and Mahoney MM. Pubertal development of sex differences in circadian function: an animal model. Ann N Y Acad Sci 1021: 262–275, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Leibenluft E Do gonadal steroids regulate circadian rhythms in humans? J Affect Disord 29: 175–181., 1993. [DOI] [PubMed] [Google Scholar]
- 90.Loh DH, Kuljis DA, Azuma L, Wu Y, Truong D, Wang HB, and Colwell CS. Disrupted reproduction, estrous cycle, and circadian rhythms in female mice deficient in vasoactive intestinal peptide. J Biol Rhythms 29: 355–369, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lokshin M, LeSauter J, and Silver R. Selective distribution of retinal input to mouse SCN revealed in analysis of sagittal sections. J Biol Rhythms 30: 251–257, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lyall LM, Wyse CA, Celis-Morales CA, Lyall DM, Cullen B, Mackay D, Ward J, Graham N, Strawbridge RJ, Gill JMR, Ferguson A, Bailey MES, Pell JP, Curtis AM, and Smith DJ. Seasonality of depressive symptoms in women but not in men: A cross-sectional study in the UK Biobank cohort. J Affect Disord 229: 296–305, 2018. [DOI] [PubMed] [Google Scholar]
- 93.Madeira MD, Sousa N, Santer RM, Paula-Barbosa MM, and Gundersen HJ. Age and sex do not affect the volume, cell numbers, or cell size of the suprachiasmatic nucleus of the rat: An unbiased stereological study. J Comp Neurol 361: 585–601, 1995. [DOI] [PubMed] [Google Scholar]
- 94.Magnusson A An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatr Scand 101: 176–184, 2000. [PubMed] [Google Scholar]
- 95.Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol 2010: 813764, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahoney MM, Ramanathan C, Hagenauer MH, Thompson RC, Smale L, and Lee T. Daily rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent, Arvicanthis niloticus. Eur J Neurosci 30: 1537–1543, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahoney MM, Smale L, and Lee TM. Daily immediate early gene expression in the suprachiasmatic nucleus of male and female Octodon degus. Chronobiol Int 26: 821–837, 2009. [DOI] [PubMed] [Google Scholar]
- 98.Maywood ES, Mrosovsky N, Field MD, and Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci 96: 15211–15216, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, and De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci 32: 2241–2247, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, and Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav 92: 1010–1018, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McQuillan HJ, Han SY, Cheong I, and Herbison AE. GnRH Pulse Generator Activity Across the Estrous Cycle of Female Mice. Endocrinology 160: 1480–1491, 2019. [DOI] [PubMed] [Google Scholar]
- 102.Melrose S Seasonal Affective Disorder: An Overview of Assessment and Treatment Approaches. Depress Res Treat 2015: 178564, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mendoza JY, Dardente H, Escobar C, Pevet P, and Challet E. Dark pulse resetting of the suprachiasmatic clock in Syrian hamsters: behavioral phase-shifts and clock gene expression. Neuroscience 127: 529–537, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, and Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav 6: 192–200, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Michalik M, Steele AD, and Mistlberger RE. A sex difference in circadian food-anticipatory rhythms in mice: Interaction with dopamine D1 receptor knockout. Behav Neurosci 129: 351–360, 2015. [DOI] [PubMed] [Google Scholar]
- 106.Miller BH and Takahashi JS. Central circadian control of female reproductive function. Front Endocrinol (Lausanne) 4: 195, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Model Z, Butler MP, LeSauter J, and Silver R. Suprachiasmatic nucleus as the site of androgen action on circadian rhythms. Horm Behav 73: 1–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mohawk JA and Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci 34: 349–358, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, and Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci 31: 16107–16116, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morin LP. Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol Behav 24: 741–749, 1980. [DOI] [PubMed] [Google Scholar]
- 111.Morin LP and Cummings LA. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol Behav 26: 825–838., 1981. [DOI] [PubMed] [Google Scholar]
- 112.Morin LP and Cummings LA. Splitting of wheelrunning rhythms by castrated or steroid treated male and female hamsters. Physiol Behav 29: 665–675, 1982. [DOI] [PubMed] [Google Scholar]
- 113.Morin LP, Fitzgerald KM, and Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science 196: 305–307, 1977. [DOI] [PubMed] [Google Scholar]
- 114.Morishita H, Kawamoto M, Masuda Y, Higuchi K, and Tomioka M. Quantitative histological changes in the hypothalamic nuclei in the prepuberal, puberal and postpuberal female rat. Brain Res 76: 41–47, 1974. [DOI] [PubMed] [Google Scholar]
- 115.Morishita H, Nagamachi N, Kawamoto M, Tomioka M, Higuchi K, Hashimoto T, Tanaka T, Kuroiwa S, Nakago K, Mitani H, Miyauchi Y, Ozasa T, and Adachi H. The effect of prepuberal castration on the development of the nuclear sizes of the neurons in the hypothalamic nuclei of female rats. Brain Res 146: 388–391, 1978. [DOI] [PubMed] [Google Scholar]
- 116.Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, Meyer-Bemstein E, Sehgal A, and Shigeyoshi Y. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci 23: 6141–6151, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, and Shinohara K. Estrogen differentially regulates expression of Perl and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res 82: 622–630, 2005. [DOI] [PubMed] [Google Scholar]
- 118.Nakamura TJ, Sellix MT, Menaker M, and Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab 295: E1025–1031, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakamura TJ, Shinohara K, Funabashi T, and Kimura F. Effect of estrogen on the expression of Cry1 and Cry2 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Res 41: 251–255, 2001. [DOI] [PubMed] [Google Scholar]
- 120.Nicolaides NC and Chrousos GP. Sex differences in circadian endocrine rhythms: Clinical implications. Eur J Neurosci 52: 2575–2585, 2020. [DOI] [PubMed] [Google Scholar]
- 121.Noya SB, Colameo D, Bruning F, Spinnler A, Mircsof D, Opitz L, Mann M, Tyagarajan SK, Robles MS, and Brown SA. The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 366, 2019. [DOI] [PubMed] [Google Scholar]
- 122.O’Neill JS, Maywood ES, and Hastings MH. Cellular mechanisms of circadian pacemaking: beyond transcriptional loops. Handb Exp Pharmacol: 67-103, 2013. [DOI] [PubMed] [Google Scholar]
- 123.Ogawa S, Chan J, Gustafsson JA, Korach KS, and Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology 144: 230–239, 2003. [DOI] [PubMed] [Google Scholar]
- 124.Partch CL, Green CB, and Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24: 90–99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peterfi Z, Churchill L, Hajdu I, Obal F Jr, Krueger JM, and Parducz A. Fosimmunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience 124: 695–707, 2004. [DOI] [PubMed] [Google Scholar]
- 126.Pilorz V, Kolms B, and Oster H. Rapid Jetlag Resetting of Behavioral, Physiological, and Molecular Rhythms in Proestrous Female Mice. J Biol Rhythms 35: 612–627, 2020. [DOI] [PubMed] [Google Scholar]
- 127.Pilorz V, Steinlechner S, and Oster H. Age and oestrus cycle-related changes in glucocorticoid excretion and wheel-running activity in female mice carrying mutations in the circadian clock genes Per1 and Per2. Physiol Behav 96: 57–63, 2009. [DOI] [PubMed] [Google Scholar]
- 128.Prendergast BJ, Onishi KG, and Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40: 1–5, 2014. [DOI] [PubMed] [Google Scholar]
- 129.Prendergast BJ, Stevenson TJ, and Zucker I. Sex differences in Siberian hamster ultradian locomotor rhythms. Physiol Behav 110-111: 206–212, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qian J, Morris CJ, Caputo R, Wang W, Garaulet M, and Scheer F. Sex differences in the circadian misalignment effects on energy regulation. Proc Natl Acad Sci 116: 23806–23812, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robinson SM, Fox TO, Dikkes P, and Pearlstein RA. Sex differences in the shape of the sexually dimorphic nucleus of the preoptic area and suprachiasmatic nucleus of the rat: 3-D computer reconstructions and morphometrics. Brain Res 371: 380–384, 1986. [DOI] [PubMed] [Google Scholar]
- 132.Rohr KE, Telega A, Savaglio A, and Evans JA. Vasopressin regulates daily rhythms and circadian clock circuits in a manner influenced by sex. Horm Behav 127: 104888, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rojansky N, Benshushan A, Meirsdorf S, Lewin A, Laufer N, and Safran A. Seasonal variability in fertilization and embryo quality rates in women undergoing IVF. Fertil Steril 74: 476–481, 2000. [DOI] [PubMed] [Google Scholar]
- 134.Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, and De Vries GJ. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol 521:2321–2358, 2013. [DOI] [PubMed] [Google Scholar]
- 135.Roper TJ. Sex differences in circadian wheel running rhythms in the Mongolian gerbil. Physiol Behav 17: 549–551, 1976. [DOI] [PubMed] [Google Scholar]
- 136.Rowsemitt CN. Activity of castrated male voles: rhythms of responses to testosterone replacement. Physiol Behav 45: 7–13, 1989. [DOI] [PubMed] [Google Scholar]
- 137.Royston SE, Yasui N, Kondilis AG, Lord SV, Katzenellenbogen JA, and Mahoney MM. ESR1 and ESR2 differentially regulate daily and circadian activity rhythms in female mice. Endocrinology 155: 2613–2623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ruiz de Elvira MC, Persaud R, and Coen CW. Use of running wheels regulates the effects of the ovaries on circadian rhythms. Physiol Behav 52: 277–284, 1992. [DOI] [PubMed] [Google Scholar]
- 139.Santhi N, Horowitz TS, Duffy JF, and Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS One 2: e1233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schull J, Walker J, Fitzgerald K, Hiilivirta L, Ruckdeschel J, Schumacher D, Stanger D, and McEachron DL. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiol Behav 46: 341–346, 1989. [DOI] [PubMed] [Google Scholar]
- 141.Schwartz WJ and Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci 10: 3685–3694, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shinohara K, Funabashi T, Mitushima D, and Kimura F. Effects of estrogen on the expression of connexin32 and connexin43 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Lett 286: 107–110, 2000. [DOI] [PubMed] [Google Scholar]
- 143.Shinohara K, Funabashi T, Nakamura TJ, and Kimura F. Effects of estrogen and progesterone on the expression of connexin-36 mRNA in the suprachiasmatic nucleus of female rats. Neurosci Lett 309: 37–40, 2001. [DOI] [PubMed] [Google Scholar]
- 144.Shughrue PJ, Lane MV, and Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388: 507–525, 1997. [DOI] [PubMed] [Google Scholar]
- 145.Smarr BL, Grant AD, Zucker I, Prendergast BJ, and Kriegsfeld LJ. Sex differences in variability across timescales in BALB/c mice. Biol Sex Differ 8: 7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stetson MH and Watson-Whitmyre M. Nucleus suprachiasmaticus: the biological clock in the hamster? Science 191: 197–199, 1976. [DOI] [PubMed] [Google Scholar]
- 147.Stowie AC and Glass JD. Longitudinal Study of Changes in Daily Activity Rhythms over the Lifespan in Individual Male and Female C57BL/6J Mice. J Biol Rhythms 30: 563–568, 2015. [DOI] [PubMed] [Google Scholar]
- 148.Swaab DF, Fliers E, and Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res 342: 37–44, 1985. [DOI] [PubMed] [Google Scholar]
- 149.Swaab DF, Zhou JN, Ehlhart T, and Hofman MA. Development of vasoactive intestinal polypeptide neurons in the human suprachiasmatic nucleus in relation to birth and sex. Brain Res Dev Brain Res 79: 249–259, 1994. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi JS and Menaker M. Interaction of estradiol and progesterone: effects on circadian locomotor rhythm of female golden hamsters. Am J Physiol 239: R497–504, 1980. [DOI] [PubMed] [Google Scholar]
- 151.ter Haar MB, MacKinnon PC, and Bulmer MG. Sexual differentiation in the phase of the circadian rhythm of (35S) methionine incorporation into cerebral proteins, and of serum gonadotrophin levels. J Endocrinol 62: 257–265, 1974. [DOI] [PubMed] [Google Scholar]
- 152.Thomas EM and Armstrong SM. Effect of ovariectomy and estradiol on unity of female rat circadian rhythms. Am J Physiol 257: R1241–1250, 1989. [DOI] [PubMed] [Google Scholar]
- 153.Toth LA, Trammell RA, Liberati T, Verhulst S, Hart ML, Moskowitz JE, and Franklin C. Influence of Chronic Exposure to Simulated Shift Work on Disease and Longevity in Disease-Prone Inbred Mice. Comp Med 67: 116–126, 2017. [PMC free article] [PubMed] [Google Scholar]
- 154.Tsukahara S, Tanaka S, Ishida K, Hoshi N, and Kitagawa H. Age-related change and its sex differences in histoarchitecture of the hypothalamic suprachiasmatic nucleus of F344/N rats. Exp Gerontol 40: 147–155, 2005. [DOI] [PubMed] [Google Scholar]
- 155.Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, and Kallo I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol 20: 1270–1277, 2008. [DOI] [PubMed] [Google Scholar]
- 156.Walbeek TJ and Gorman MR. Simple Lighting Manipulations Facilitate Behavioral Entrainment of Mice to 18-h Days. J Biol Rhythms 32: 309–322, 2017. [DOI] [PubMed] [Google Scholar]
- 157.Walbeek TJ, Joye DAM, Mishra I, and Gorman MR. Physiological, behavioral and environmental factors influence bifurcated circadian entrainment in mice. Physiol Behav 210:112625, 2019. [DOI] [PubMed] [Google Scholar]
- 158.Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP, Sweedler JV, Cox CL, and Gillette MU. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 337: 839–842, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Watanobe H and Takebe K. A comparative study of the effects of neonatal androgenization and estrogenization on vasoactive intestinal peptide levels in the anterior pituitary and the hypothalamus of adult female rats. Neuroendocrinology 56: 653–659, 1992. [DOI] [PubMed] [Google Scholar]
- 160.Weger BD, Gobet C, Yeung J, Martin E, Jimenez S, Betrisey B, Foata F, Berger B, Balvay A, Foussier A, Charpagne A, Boizet-Bonhoure B, Chou CJ, Naef F, and Gachon F. The Mouse Microbiome Is Required for Sex-Specific Diurnal Rhythms of Gene Expression and Metabolism. Cell Metab 29: 362–382 e368, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Weinert D, Freyberg S, Touitou Y, Djeridane Y, and Waterhouse JM. The phasing of circadian rhythms in mice kept under normal or short photoperiods. Physiol Behav 84: 791–798, 2005. [DOI] [PubMed] [Google Scholar]
- 162.Widmaier EP and Campbell CS. Interaction of estradiol and photoperiod on activity patterns in the female hamster. Physiol Behav 24: 923–930, 1980. [DOI] [PubMed] [Google Scholar]
- 163.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, and Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev 123: 593–601, 2002. [DOI] [PubMed] [Google Scholar]
- 164.Wollnik F and Turek FW. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol Behav 43: 389–396, 1988. [DOI] [PubMed] [Google Scholar]
- 165.Wood S, Quinn A, Troupe S, Kingsland C, and Lewis-Jones I. Seasonal variation in assisted conception cycles and the influence of photoperiodism on outcome in in vitro fertilization cycles. Hum Fertil (Camb) 9: 223–229, 2006. [DOI] [PubMed] [Google Scholar]
- 166.Yan L and Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci 16: 1531–1540, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yan L and Silver R. Resetting the brain clock: Time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci 19: 1105–1109, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Young Janik L and Janik D. Nonphotic phase shifting in female Syrian hamsters: Interactions with the estrous cycle. J Biol Rhythms 18: 307–317, 2003. [DOI] [PubMed] [Google Scholar]
- 169.Zhang R, Lahens NF, Ballance HI, Hughes ME, and Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci 111: 16219–16224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou JN, Hofman MA, and Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging 16: 571–576, 1995. [DOI] [PubMed] [Google Scholar]
- 171.Zhou L, Blaustein JD, and De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology 134: 2622–2627, 1994. [DOI] [PubMed] [Google Scholar]
- 172.Zucker I, Fitzgerald KM, and Morin LP. Sex differentiation of the circadian system in the golden hamster. Am J Physiol 238: R97–101, 1980. [DOI] [PubMed] [Google Scholar]


