Abstract
Objective:
Recent evidence delineates an emerging role of Periostin (Postn) in osteoarthritis (OA) as its expression subsequent to knee injury is detrimental to the articular cartilage. We hypothesize that intra-articular knockdown of Postn in a murine model of post-traumatic OA would ameliorate OA.
Methods:
Post-traumatic OA was induced in 10-week-old male C57BL/6J mice (n=24) by destabilization of the medial meniscus (DMM) and analyzed 8-week post-surgery. Intra-articular Postn was inhibited by siRNA using a novel peptide-nucleotide polyplex. Cartilage degeneration (OARSI score) and synovitis were assessed histologically. Bone changes were measured by μCT. The effect and mechanism of Postn silencing were investigated in human chondrocytes treated with IL-1β with or without IKK2 inhibitor, SC-514.
Results:
Peptide-siRNA nanoplatform significantly abolished Postn expression. OARSI score was significantly less in mice receiving Postn siRNA (10.94±0.66) compared to both untreated (22.38±1.30,P=0.002) and scrambled siRNA (22.69±0.87,P=0.002) treatment. No differences were observed in synovitis. Subchondral bone sclerosis, BV/TV, vBMD, and heterotopic ossification were significantly low in Postn siRNA treatment. Immunostaining of cartilage revealed that Postn knockdown reduced the DMM-induced MMP-13 intensity, phosphorylation of p65, and immunoreactivity of aggrecan neoepitope, DIPEN. Postn knockdown also suppressed IL-1β-induced MMP-13 and ADAMTS-4 in chondrocytes. Mechanistically, Postn-induced MMP-13 was abrogated by SC-514 demonstrating a link between Postn and NF-κB.
Conclusion:
Intra-articular delivery of Postn siRNA nanocomplex represents a promising clinical approach to mitigate the severity of joint degeneration and provides an unequivocal scientific rationale for longitudinal studies. Employing a cartilage-specific gene knockout strategy will further illuminate the functional role of Postn in OA.
Keywords: Periostin, MMP-13, NF-κB, chondrocytes, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is a painful degenerative disease of the diarthrodial joints and is one of the leading causes of disability and financial burden around the globe. It affects 50+ millions Americans and its prevalence is projected to increase to 78 million by 2040.(1) Current treatment options for OA are not optimal, and no disease-modifying OA therapy has successfully completed clinical trials.(2) Presently, the focus of OA treatment continues to be on established, late-stage disease, which is often recalcitrant to medical therapy and likely entails costly surgical intervention such as arthroplasty to maintain/restore joint mobility.
We posit that pharmacologic intervention represents a more cost-effective approach but would require the targeting of etiologic pathways of early, pre-radiographic OA, prior to irreversible structural joint damage. Post-traumatic OA–which accounts for about 12% of all OA cases–develops after a joint injury and is particularly prevalent in young active adults.(3) Joint injury initiates molecular changes that lead to post-traumatic OA over a course of 10–15 years.(4, 5) In post-traumatic OA, the nature and time of trauma is generally known(4), thus offering a unique window into the early events that are potentially reversible and amenable to disease-modifying therapy.
Recent studies have shown an emergent role of the matricellular and matrix proteins, which regulate important chondrocyte functions.(6) Periostin (Postn) is a matricellular secretory matrix protein expressed by MSCs and periosteum.(7, 8) Emerging evidence delineates that the expression of Postn is increased in human OA.(9–14) In addition, studies have shown that the expression of Postn is increased in the cartilage matrix following knee injury in mice, suggesting its catabolic role in OA progression.(13, 15, 16) We have recently measured and reported the expression of Postn in patients with anterior cruciate ligament (ACL) tear–a common joint injury that often leads to post-traumatic OA–and found that the expression of Postn was relatively low in the first month after injury, which increases within 3-months of injury, peaks at 3–6 months following injury and then declines significantly.(17) Therefore, we reasoned that early intervention after joint injury will prevent the disease sequalae, prior to irreversible damage.
While the mechanism of how Postn exerts its catabolic effects remains elusive, some in-vitro data display that Postn gain-of-function in chondrocytes increases the expression of MMP-13. In contrast, Postn loss-of-function reduces IL-1β-induced MMP-13 expression (15, 16) raising the possibility that it affects NF-κB signaling. Taken together, while Postn appears to be an attractive therapeutic target in joint degeneration, no study to date has investigated the protective effects of Postn knockdown in-vivo.
Given the catabolic role of Postn in OA, we hypothesize that intra-articular knockdown of Postn would reduce joint degeneration. To test this hypothesis, we examined the protective effects of Postn knockdown on cartilage degeneration in a murine model of post-traumatic OA. Moreover, we gained insights into effects of Postn knockdown on the expression of MMP-13 and NF-κB pathway. To achieve Postn knockdown in cells and in the joint, we employed RNAi technology. RNAi effectively induces post‐transcriptional sequence-specific gene silencing with a high degree of specificity using siRNA.(18, 19) The siRNA was delivered by a nanocarrier platform(20) consisting of a peptide-based self-assembled oligonucleotide nanocomplex that penetrates cartilage to deliver siRNA to chondrocytes.(21, 22) Here, we show that intra-articular knockdown of Postn in mice ameliorates cartilage degradation and mitigates changes in the bone. Mechanistically, we show that suppressing Postn dampens the inflammatory NF-κB-MMP-13 signaling axis.
MATERIALS AND METHODS
Ethics statement
All animal procedures were performed following the ethical and statutory approval of the Washington University Institutional Animal Care and Use Committee (Protocol #20190113). All efforts were exercised to minimize animal suffering during this study. The Institutional Review Board approved the use of human discarded cartilage specimens (Protocol #201104119). All patients provided a written and signed informed consent prior to participation.
Mice
C57BL/6J mice were obtained from the Jackson Laboratories. Mice were housed in individually ventilated cages with each cage containing 2–4 mice. All mice were housed in the animal husbandry facility operating at 21–22°C and maintained in a 12h light/dark cycle with unrestricted food and water intake.
p5RHH-siRNA nanoparticles
We used an engineered cationic amphipathic peptide (VLTTGLPALISWIRRRHRRHC) designated as p5RHH,(23, 24) as a cargo for siRNAs. The p5RHH peptide that forms a polyplex with siRNAs was synthesized by Genscript. These peptide nanoparticles have been used for efficient and safe siRNA transfection in rodent joints and human articular cartilage explants.(21, 22, 25) The Postn (#162562) and scrambled (#4390846) siRNAs were purchased from Thermo-Fisher-Scientific. The p5RHH siRNA polyplexes were prepared described previously.(21) Briefly, 10-mM p5RHH peptide and 100-μM siRNA were mixed at equal volumes at a peptide:siRNA of 100:1 in HBSS. The mixture was then incubated at 37°C for 40-min followed by stabilization with albumin at a final siRNA concentration of 500-nM before intra-articular injection.
Intra-articular delivery of p5RHH-siRNA nanoparticles
p5RHH-siRNA nanocomplex was administered immediately after DMM surgery, and then at 1-, 2-, 4- and 6-weeks after surgery for a total of 5 injections. Following sterile technique was used for intra-articular injections: the knee was kept in a flexed position, and 15-μL of p5RHH-siRNA nanoparticle complex were injected intra-articularly using a 30-gauge needle.(21) Each mouse received one of these treatments (n=8 each): (HBSS, untreated), non-targeted scrambled siRNA, or Postn siRNA (Fig–1A). While we did not examine for any leakage of the injected contents, we have shown in a previous study that injected contents stay within the joint cavity.(21)
Fig. 1: Peptide Postn siRNA treatment suppressed Postn upregulation following DMM surgery.
(A) Experimental design: DMM surgery was performed in 10-week-old male mice (n=8 each group). HBSS (untreated control), scrambled siRNA, or Postn siRNA was injected intra-articularly at the following time points: immediately after DMM surgery, at week 1, 2, 4 and 6 post-surgery (5 injections in total). Knee joints were harvested and analyzed at 8-weeks after surgery. (B) Postn immunostaining revealed that Postn was upregulated (green) in the cartilage of knees subjected to DMM surgery compared to the uninjured control knees (see untreated control vs. left control knee). Intra-articular administration of Postn siRNA nanoparticles suppressed the expression of DMM-induced Postn compared with untreated control or scrambled siRNA treatment groups. The dotted lines indicate Col 2 positive cartilage area based on the images in the upper panel. DAPI (blue) was used to stain nuclei. Scale bar=50 μm. (C) Quantification of immunofluorescence intensity showed significant differences in Postn expression between scrambled siRNA and Postn siRNA treatment groups (Kruskal-Wallis test with Dunn’s multiple comparisons, P=0.033). Normalized mean Postn fluorescence intensity per chondrocyte was measured from Z-stack confocal images. Similar lowercase letters indicate statistically significant difference.
Induction of OA
DMM surgery was performed under general anesthesia (inhalation of 2.5% isoflurane in 4-L/min oxygen) to induce OA pathology in the right hind limb of 10-week-old male mice by transecting the anterior attachment of the knee medial meniscotibial ligament.(26, 27) Sustained-release Buprenorphine SR (1.0-mg/kg; SR-Veterinary-Technologies) was administered subcutaneously prior to surgery to minimize pain. Contralateral left limb remained non-operated and served as an internal control. After recovering from anesthesia, all mice were weight-bearing and resumed prior cage activity with normal water and food consumption.
Cartilage degeneration
After 8-week of surgery mice were euthanized in a carbon dioxide chamber. The knees were harvested, fixed with 10% neutral-buffered-formalin for 48h, and maintained in 70% ethyl-alcohol until use. Joints were decalcified for 48h using Inmmunocal™ Decalcifier (StatLab), and then embedded in paraffin for sectioning. Serial coronal sections (5-μm thick) were cut extending throughout the joint.(26) In brief, sections were cut at eight levels, with each level comprising of 12 sections and 80-μm discarded in between, thus covering >75% depth of the joint. Selected sections were stained with Safranin-O/fast green to evaluate cartilage proteoglycans. All images were visualized using NanoZoomer (Hamamatsu). Cartilage damage was measured using OARSI scoring system.(28) We report summed score from four consecutive levels from each mouse knee and from all four knee compartments, the score shown is from a total score of 96 (6 highest score×4 compartments×4 sections). Scoring was performed by two scorers (XD,LC) blinded to sample identity and disparities were resolved by consensus.
Synovitis
Four safranin-O/fast green-stained sections from consecutive levels of each knee were graded in a blinded fashion for synovial pathology in the medial compartment for two parameters:(29) enlargement of the synovial lining cell layer and synovial stroma. Scores obtained from both parameters were averaged separately and the sum of averages was used for analysis on a scale of 0–6.
μCT analysis of bone
Prior to decalcification, knees underwent μCT scanning using vivaCT-40 scanner (Scanco-Medical) to analyze the 3D bone structure and to determine BV/TV, vBMD, and heterotopic ossification.(30) The medial and lateral subchondral bone parameters of each tibia were contoured separately. The relative outcome was determined by dividing the medial side measurement of the right knee by the medial side measurement of the left knee of the same mouse. This method serves as a better internal control since it minimizes variation within each mouse.
Immunofluorescence/confocal microscopy
Paraffin-embedded sections were deparaffinized with xylene and rehydrated using a graded series of ethanol (70%→50%→30%). Proteinase K (10-μg/mL, Abcam) was applied to the sections for antigen retrieval for 20-min at 37°C. The slides were washed with PBS and blocked with 10% normal goat serum (NGS). Subsequently, slides were incubated overnight at 4°C with the following primary antibodies diluted in 2% NGS: anti-Postn (1:100, Abcam), anti-phospho-p65 (1:100, Abcam), anti-MMP-13 (1:200, Abcam), and in-house anti-type-II collagen (Col-2, 1:200). The slides were washed with PBS and incubated with the corresponding Alexa 488-, or Alexa 594-conjugated secondary antibodies in 2% NGS for 1h at room-temperature, and counterstained with Fluoro-Gel II with 4′,6-diamidino-2-phenylindole (DAPI, Electron-Microscopy-Sciences). All images were visualized using a Confocal Laser-Scanning-Microscope (Leica-Biosystems). Signal intensity was quantified in 20–30 cells/section using LAS-X software (Leica-Biosystems).
Detection of DIPEN
We detected cartilage aggrecan neoepitope, DIPEN, by immunohistochemistry using antibody generated at the aggrecan cleavage sites produced by MMP-13 to C-terminal neoepitope DIPEN.(31) Briefly, sections were deparaffinized, rehydrated and digested with proteinase K as above. Then, sections were incubated with H2O2 Blocking Reagent (Abcam) for 15-min to quench endogenous peroxidases. Sections were subsequently incubated over night at 4°C with anti-DIPEN (1:50 dilution in 2% NGS, a gift from Dr. Amanda Fosang), after blocking with 10% NGS. The next day, sections were incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:200 diluted in 2% NGS, Abcam) for 1h at room-temperature. The signal was developed as brown-reaction products by a peroxidase substrate diaminobenzidine (Betazoid DAB Chromogen Kit; Biocare-Medical) and the sections were then counter-stained with hematoxylin 560MX (Leica-Biosystems). Images were acquired with NanoZoomer.
Human chondrocytes: isolation and culture
Human cartilage was obtained from patients undergoing total knee arthroplasty. Chondrocytes were isolated through enzymatic digestion as described previously (16). Cells were counted by hemocytometer and cell viability was determined using 0.4% trypan blue exclusion dye (Sigma-Aldrich). Chondrocytes were seeded at a density of 0.5×105 cells/well in 12-well plates and maintained at 37°C and 5% CO2 with 95% humidity to reach 80–90% confluence. Once confluence was achieved after 2–3 days of culture, cells were used for the following experiments in passage 0.
Preparation of p5RHH-siRNA nanoparticles
The p5RHH-siRNA nanoparticles were prepared for chondrocyte culture as follows: 20-mM p5RHH peptide was diluted to 1:400 (v/v) with Opti-MEM medium (Thermo-Fisher-Scientific); vortexed for 30-sec; followed by addition of 100-μM Postn siRNAs (#S20889 and #HSS116400, Thermo-Fisher-Scientific) or non-targeting scrambled siRNA (#4390846, Thermo-Fisher-Scientific) to achieve a peptide:siRNA ratio of 100:1. The mixture was incubated at 37°C for 40-min with gentle shaking. The Cy5.5-labeled scrambled siRNAs were commercially procured (Sigma-Aldrich).
Transfection efficiency
First, 1.0×105 chondrocytes were seeded on a Lab-Tek chamber slide (Thermo-Fisher-Scientific) and incubated with p5RHH-Cy5.5-labeled siRNA nanoparticles for 5h. Control cells were incubated with Opti-MEM only, peptide only, or Cy5.5-labeled siRNA only. Then, the chambers were washed with PBS and cultured in complete culture medium for 72h. Then, cells were washed in PBS and fixed with 4% paraformaldehyde for 15-min. Phalloidin-iFluor 488 Reagent (1:1000, Abcam) was applied to the chamber slides. Slides were incubated for 1h at room-temperature, washed, and mounted with Fluoro-Gel with DAPI (Electron-Microscopy-Sciences). Images were captured with the confocal microscope.
Effects of Postn siRNA
Human chondrocytes (N=7) were transfected with Postn siRNA nanoparticles, scrambled siRNA nanoparticles, or Opti-MEM only for 5h. Then, cells were washed with PBS and cultured at 37°C for 72h. Cells were washed with PBS, 1-ng/mL human recombinant IL-1β (R&D Systems) was added to each group, and cells were cultured for 24h. Finally, cells were washed with PBS and collected for RNA and protein extraction.
MMP-13 inhibition by IKK-2 inhibitor
Next, we tested whether Postn-induced MMP-13 can be suppressed by IκB kinase 2 (IKK-2) inhibitor, SC-514 (#354812–17-2, Sigma-Aldrich). Normal human chondrocytes were procured from Millipore-Sigma (#402–05a) and cultured as recommended by the manufacturer. Briefly, cells were cultured in a culture dish containing 10 mL of chondrocyte growth medium (Millipore-Sigma) and were incubated at 37°C in humidified incubator with 5% CO2. Once cells reached 80% confluence, cells were subcultured for the following experiments: In one experiment, 0.5×106 cells (n=4) were either left untreated (control) or treated with human recombinant IL-1β (10-ng/mL), or IL-1β and SC-514 combined (the latter was added 30-min before IL-1β). In other experiment (n=6), cells were treated exactly as above, however, human recombinant Postn (10-μg/mL, #3548-F2, R&D Systems) was instilled instead of IL-1β and only SC-514 (20-μmol/L) treatment group was added for comparison. Cells were cultured at 37°C for 24h and then collected for RNA isolation.
Real-time qPCR
Total RNA was extracted using RNeasy-Mini-Kit (Qiagen). RNA (800-ng) was treated with DNase I (1-U/μL, Invitrogen) and reverse-transcribed with High-Capacity cDNA Reverse Transcription Kit (Thermo-Fisher-Scientific). To quantify mRNA expression of POSTN, MMP-13 and IL-1β, real-time qPCR analysis was performed using custom-designed, gene-specific primers (Supplementary Table–1) and standard methods.(32) Target gene expression was normalized with respect to PPIA using 2-ΔΔCt method.
Western blot
Proteins were extracted from chondrocytes using RIPA buffer containing protease inhibitor cocktail (Sigma-Aldrich). The total protein concentration was determined using the Protein Assay Kit (Bio-Rad). Next, 20-μg of total proteins diluted in sodium dodecyl sulfate sample buffer were resolved on 4–20% Mini-PROTEAN-TGX Precast Protein gels (Bio-Rad). Subsequently, proteins were electrophoretically transferred to Polyvinylidene fluoride membrane (Invitrogen). Membranes were blocked with Odyssey® Blocking Buffer (Li-Cor Biosciences) for 1h. Blots were incubated overnight at 4°C with the following primary antibodies: anti-POSTN (1:1000, Abcam), anti-phospho-p65 (1:1000, Abcam), anti-MMP-13 (1:4000, Abcam), or anti-total-p65 (1:1000, Abcam). The next day, membranes were washed and subsequently incubated with fluorescently labeled secondary antibody IRDye 680RD goat anti-rabbit IgG (1:20,000, Li-Cor). For cell lysates, the housekeeping anti-β-actin (1:4000, Sigma-Aldrich) was used as the primary antibody for cell lysates, and the fluorescently labeled IRDye 800CW goat anti-mouse IgG (1:20,000, Li-Cor) was used as the secondary antibody. Blots were imaged using an Odyssey Infrared Imager and the signal intensity was quantified using ImageJ software.
Statistical analysis
Comparisons between DMM-operated and control limbs with and without Postn siRNA for OARSI score was made by 2-way ANOVA with Tukey’s multiple comparison test as a post-hoc. Non-parametric paired t-test (Wilcoxon matched-pairs singed-rank) was used for analysis of real-time qPCR data. For all other comparisons, we used Kruskal-Wallis test followed by Dunn’s correction for multiple comparisons for three groups and Mann-Whitney test for comparison between two groups. All analyses were performed in GraphPad-PRISM (GraphPad Software Inc.). Data are shown as mean±standard error-of-the-mean unless indicated otherwise. Results were rendered significant at a two-tailed P<0.05.
RESULTS
Postn siRNA treatment reduced DMM-induced Postn expression
We observed only minimal immunofluorescence staining of mouse cartilage with an antibody to Postn in non-injured control knees, whereas Postn expression was strongly increased after DMM surgery consistent with our previous observations (16). Expression of Postn remained elevated in both the untreated control and scrambled siRNA treated groups after DMM while Postn siRNA treatment significantly suppressed the expression of Postn protein in the cartilage (Fig–1B). Quantification of signaling fluorescence intensity further confirmed that Postn expression was significantly attenuated in the Postn siRNA treated group compared with scrambled siRNA group (0.63±0.42 vs. 3.91±1.19,P=0.033). Postn expression was low in Postn siRNA group compared with untreated control but did not reach formal cutoff of statistical significance (0.63±0.42 vs. 1.89±0.16,P=0.187). There was a slight increase in expression of POSTN in the scrambled group compared to the control group, which was not statistically significant (Fig–1C). In addition to cartilage, Postn siRNA treatment also suppressed the expression of Postn protein in synovium compared to both untreated control and scrambled siRNA groups (Supplementary Fig–1A–B).
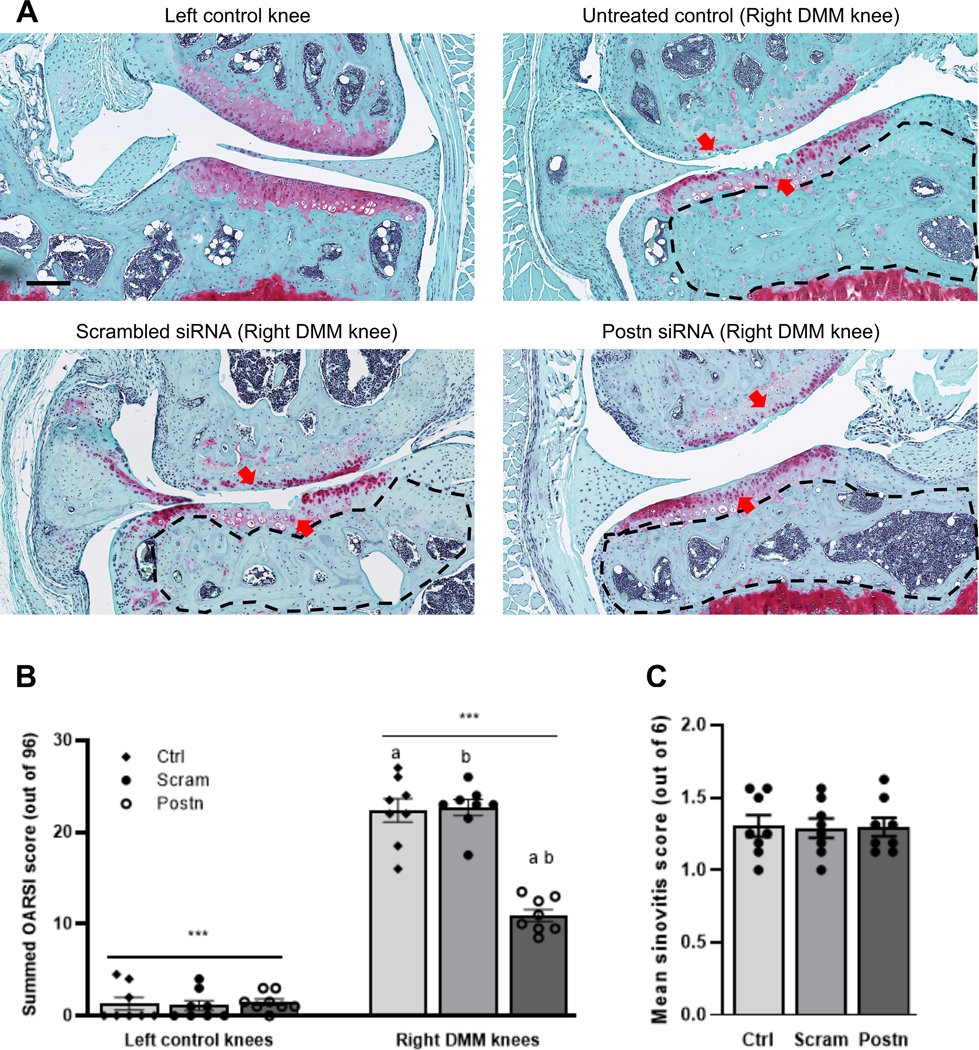
Postn knockdown mitigated DMM-induced cartilage degeneration
We found significantly less cartilage degeneration in the left control knees compared with DMM-operated right knees (1.33±0.69 vs. 22.38±1.30, 2-way ANOVA P<0.001) at 8 weeks after surgery. Next, we examined whether Postn knockdown exhibits protective effects on cartilage. We observed a reduction in the severity of cartilage degeneration in the destabilized knee joint following intra-articular treatment with Postn siRNA (Fig–2A). No differences in cartilage degeneration were observed between untreated control and scrambled siRNA groups. Semi-quantitative analysis of cartilage degradation by the OARSI scoring system (out of 96 possible score) also revealed that untreated control and scrambled siRNA treatment groups did not show any significant differences in OARSI score (22.38±1.30 vs. 22.69±0.87,P=0.999). However, treatment with Postn siRNA significantly reduced OARSI score compared to both untreated control (10.94±0.66 vs. 22.38±1.30,P=0.002) and scrambled siRNA groups (10.94±0.66 vs. 22.69±0.87,P=0.002) (Fig–2B).
Fig. 2: Peptide Postn siRNA treatment attenuated cartilage degeneration in a DMM-induced post-traumatic OA mouse model but did not affect synovitis.
(A) Cartilage sections stained with Safranin-O/Fast green displayed significantly less cartilage degeneration in the left control knees compared with DMM-operated right knees. Treatment with Postn siRNA resulted in reduced cartilage degeneration compared to treatment with untreated control or scrambled siRNA (red arrows). The area within dotted black lines in each image indicates the subchondral bone area. We observed pronounced sclerosis in the untreated control (HBSS) or scrambled siRNA than in the Postn siRNA group. (B) The summed OARSI score (based on the four compartments of each knee; highest possible score = 96) was significantly lower in the left control knee than right DMM knee (***2-way ANOVA with Tukey’s adjusted P<0.001). The summed OARSI score in the Postn siRNA treatment group was significantly lower than in the untreated control (P=0.002) or scrambled siRNA (P=0.002) groups (Kruskal-Wallis test with Dunn’s multiple comparisons). Similar lowercase letters indicate statistically significant differences. Scale bar=200 μm. (C) Histological assessment of synovial pathology (synovitis) displayed no significant differences across treatment groups (Kruskal-Wallis test with Dunn’s multiple comparisons).
Postn knockdown did not improve synovitis score
There were no significant (Kruskal-Wallis P=0.999) differences in synovitis score across groups (Fig–2C). The mean synovitis score was 1.31±0.08 for untreated control, 1.29±0.07 for scrambled siRNA and 1.30±0.06 for Postn siRNA.
Postn knockdown improved DMM-induced bone changes
Subchondral bone plate exhibited reduced sclerosis of the subchondral bone area of the medial tibial compartment in the DMM-operated and Postn siRNA treated knees compared to untreated control or scrambled siRNA groups (Fig–3A). Moreover, histomorphometric analysis revealed significantly lower BV/TV (Fig–3B) values in mice treated with Postn siRNA compared with those untreated control (13.7%,P=0.027) or scrambled siRNA groups (17.1%,P=0.004). Similarly, vBMD was also significantly lower in Postn siRNA treated knees compared to both untreated control (18.5%,P=0.010) and scrambled siRNA groups (18.5%,P=0.022) (Fig–3C). Results for other bone parameters such as structural model index and trabecular thickness did not show any significant differences across groups (not shown). Ectopic calcified nodules (heterotopic ossification) formed in and around the synovium in DMM-operated joints (Fig–3D); however, slightly fewer of these nodules were observed in the Postn siRNA treated group compared to the untreated control (3.00±0.42 vs. 4.00±0.42,P=0.481) and the scrambled siRNA groups (3.00±0.42 vs. 4.75±0.31,P=0.018) (Fig–3E).
Fig. 3: Peptide Postn siRNA treatment blocked DMM-induced subchondral bone changes and heterotopic ossification.
(A) 3D reconstruction of μCT images showed that Postn siRNA treatment resulted in reduced subchondral bone sclerosis (red circles) of the medial tibial compartment in DMM-operated right knees compared to untreated control (HBSS) or scrambled siRNA. L, lateral; M, medial; F, femur; T, tibia. Scale bar=1 mm. (B) BV/TV was significantly lower in the Postn siRNA treatment group than untreated control (P=0.027) or scrambled siRNA (P=0.004) groups. Note: BV/TV of the right DMM-operated limb was normalized to the BV/TV of the left control limb of the same animal to eliminate intraindividual variations. (Kruskal-Wallis test with Dunn’s multiple comparisons). (C) Relative vBMD was significantly lower in the Postn siRNA group compared to the untreated control (P=0.010) or scrambled siRNA (P=0.022) groups. (Kruskal-Wallis test with Dunn’s multiple comparisons). (D) 3D reconstruction of micro-CT images to assess heterotopic ossification (white arrows) in DMM-induced OA knees. (E) Quantification of the number of ossified nodules indicated that Postn siRNA treatment reduced the number of nodules compared the scrambled siRNA group (P=0.018). (Kruskal-Wallis test with Dunn’s multiple comparisons). Similar lowercase letters indicate statistically significant differences.
Postn knockdown suppressed MMP-13 expression and NF-κB signaling in mice
We observed that the expression intensity of MMP-13 protein were induced by DMM, whereas treatment with Postn siRNA significantly reduced MMP-13 intensity (Fig–4A–B). These results suggest that Postn modulates MMP-13 expression in-vivo. We also observed that NF-κB signaling activity was low in control knees, whereas DMM surgery significantly induced the expression of canonical (p65) NF-κB activation as is evident from increased phosphorylation of p65. We further show that Postn siRNA treatment significantly suppressed p65 phosphorylation when compared to those of untreated control and scrambled siRNA groups (Fig–4C–D).
Fig. 4: Peptide Postn siRNA treatment abrogated MMP-13 upregulation and canonical NF-κB signaling following DMM surgery.
(A) Immunofluorescent analysis revealed that MMP-13 levels (green) were higher in DMM-operated knees than in uninjured control knees. Postn siRNA treatment reduced MMP-13 around the injury site. Yellow vertical lines mark the injury site in the cartilage, while white arrows mark MMP-13 in superficial zone chondrocytes. Col 2 (red) staining was used for cartilage. DAPI (blue) = nuclei. Scale bars=100 μm. (B) Quantification of immunofluorescence intensity showed significant differences in MMP-13 expression between scrambled siRNA and Postn siRNA treatment groups (Mann-Whitney test, P=0.029). (C) Upper and lower panels show 2D and 3D views, respectively, of immunofluorescence analysis of phosphorylated p65 subunit (green) in cartilage adjacent to the injury site. Less co-localization of activated/phosphorylated p65 (P-p65, green) was observed in the Postn siRNA treatment group than in the untreated control and scrambled siRNA groups. DAPI (blue) = nuclei. Scale bars=50 μm. Untreated control=DMM knees receiving HBSS. (D) Quantification of immunofluorescence intensity showed significant differences in P-p65 expression between scrambled siRNA and Postn siRNA treatment groups (Mann-Whitney test, P=0.029).
Postn knockdown decreased DIPEN
We noted no staining for aggrecan neoepitope, DIPEN in left control knees, however, there was markedly increased staining in untreated DMM knees or DMM receiving scrambled siRNA. In contrast, we found that Postn siRNA treated knees showed reduced expression of DIPEN (Supplementary Fig–2).
Postn knockdown suppressed MMP-13 and NF-κB in human chondrocytes
We show that the Cy5.5-labeled siRNA nanoparticles were effectively taken up by the human primary chondrocytes as depicted in Fig–5A (red signals) and subsequently stayed in the cells (Fig–5A, white arrows). As expected, naked, non-complexed siRNA was not taken up by chondrocytes. These results further suggest that the peptide-siRNA nanoparticles are required for efficient siRNA transfection of chondrocytes.
Fig. 5: Peptide Postn siRNA nanoparticle treatment attenuated the catabolic effects of IL-1β in human primary chondrocytes.
(A) Human primary chondrocytes treated with p5RHH peptide, Cy5.5-labeled-siRNA, or peptide-Cy5.5-labled siRNA are shown. Our data show that the Cy5.5 labeled siRNAs delivered with nanoparticles were effectively taken up by the human primary chondrocytes (red color, indicated by arrows) and remained in the cells after 72h in culture. Scale bars=100 μm. (B) Real-time qPCR analysis showed that human chondrocytes treated with IL-1β and transfected with peptide Postn siRNA exhibited significantly lower Postn (P=0.016), MMP-13 (P=0.016), IL-1β (P=0.016), ADAMTS-4 (P=0.016), ACAN (P=0.016) and COL2A1 (P=0.016), mRNA levels. There was no effect of treatment on COL1A1 expression (P=0.688). (Wilcoxon matched-pairs signed rank test). (C) Western blot analysis confirmed that IL-1β treatment induced MMP-13 expression and activated p65 (phosphorylated, P-p65). Cells treated with peptide Postn siRNA displayed significantly lower levels of Postn (P=0.025, P=0.025) MMP-13 (P=0.079, P=0.030) and reduced phosphorylation of p65 (P=0.010, P=0.046) than the control and scrambled siRNA groups respectively. (Kruskal-Wallis test with Dunn’s multiple comparisons). Similar lowercase letters indicate statistically significant differences.
After establishing the efficiency of siRNA delivery, we treated chondrocytes with IL-1β to model an in-vitro inflammatory condition and evaluated the therapeutic effects of POSTN knockdown. We observed that IL-1β treatment significantly increased the mRNA expression levels of MMP-13 (~10-fold), and IL-1β (~5500-fold). We confirmed that POSTN siRNA significantly knocked down the expression of POSTN at the mRNA (Fig–5B) and protein level (Fig–5C). We observed that POSTN siRNA treatment significantly suppressed the mRNA expression of IL-1β (P=0.016), MMP-13 (P=0.016), ADAMTS-4 (P=0.016), ACAN (P=0.016) and COL2A1 (P=0.016), compared with the scrambled siRNA. There was no effect of treatment on COL1A1 expression (P=0.688)(Fig–5B). Furthermore, POSTN knockdown also attenuated NF-κB p65 phosphorylation and NF-κB downstream product MMP-13 as determined by Western blot and signal intensity quantified by ImageJ (Fig–5C). Specifically, we noted that signal intensity of Postn (P=0.025) and P-p65 (P=0.010) were significantly lower in Postn treated group than untreated control while that of MMP-13 was at a borderline significance (P=0.079). The signal intensity of Postn (P=0.025), MMP-13 (P=0.030) and P-p65 (P=0.046), was significantly lower in Postn siRNA group compared with scrambled group. Subtle increase in the expression of POSTN and MMP-13 in the scrambled group compared to the control group was noted, which was not statistically significant.
IKK-2 inhibitor suppressed Postn-induced MMP-13 expression
We observed an increased expression of MMP-13 in cells treated with IL-1β (Fig–6A) and Postn (Fig–6B). Treatment with SC-514 significantly suppressed the expression of MMP-13 (Fig–6A–B) indicating that Postn is upstream of NF-κB pathway (Fig–6C).
Fig. 6: Mechanism and Pathway summary:
(A) An increased expression of MMP-13 in cells treated with IL-1β was noted, which was abrogated by SC-514 treatment (Wilcoxon matched-pairs signed rank test). (B) Exogenous recombinant Postn instigated the expression of MMP-13, which was mitigated by SC-514 treatment. (Kruskal-Wallis test with Dunn’s multiple comparisons). (C) Pathway summary: DMM surgery or IL-1β treatment activates NF-κB signaling pathway in chondrocytes, as evidenced by increased p65 phosphorylation, leading to its nuclear translocation, and the expression of catabolic mediators (e.g., MMP-13) that promote extracellular matrix degradation. Administration of p5RHH Postn siRNA reduces the activation of p65, blocks the downstream catabolic effects of NF-κB signaling directly or indirectly, and prevents the progressive cartilage characteristic of post-traumatic OA. i.a.=intra-articular. Similar lowercase letters indicate statistically significant differences.
DISCUSSION
Despite significant advances in our understanding of the affected cellular and molecular pathways in OA, no new therapeutic targets have emerged in the clinic. This study explored the intermittent local joint delivery of Postn siRNA using nanoparticles to mitigate OA pathology in a murine model of knee joint injury. Previous studies reported that Postn is expressed in human and mouse OA cartilage(15, 16) where Postn levels are strongly associated with cartilage degrading enzyme MMP-13.(15, 16) Herein, we show that knockdown of Postn suppresses NF-κB and its downstream catabolic genes including MMP-13 and IL-1β. To the best of our knowledge, this is the first study that reports on the therapeutic effects of Postn knockdown in post-traumatic OA in-vivo, whereas previous studies mainly focused on Postn overexpression or inhibition in-vitro.
We have used the peptide-siRNA nanoplatform previously used for intra-articular administration of NF-κB siRNA and showed that NF-κB knockdown suppressed injury-induced chondrocyte death and the early joint responses to injury.(21, 22). In the present study, we demonstrate, for the first time, the long-term (8-weeks) efficiency of this nanoparticle delivery system. These results further confirm the translational potential of this technology for the treatment of post-traumatic OA.
We noted that intra-articular delivery of Postn siRNA mitigated several aspects of post-traumatic OA, cartilage degeneration, subchondral bone sclerosis, and heterotopic ossification, suggesting that Postn exerts its effects on the whole joint and consistent with previous studies showing differential expression pattern of Postn in various joint tissues.(13, 16, 33, 34). Thus, Postn knockdown effectively suppressed events that lead to structural damage characteristics of OA, in addition to cartilage degeneration. Of note, Postn siRNA treatment reversed subchondral bone changes that were mainly confined to the medial region pointing out that Postn exerts therapeutic effects in the diseased compartment. In the present study, we observed no significant differences in synovitis score across various treatments. While lower Postn intensity in Postn siRNA injected group confirms that the injected particles localized there, a complete knockdown of Postn was not achieved, thus potentially explaining the lack of significant differences in synovitis grade. Moreover, recently we found that Postn-null mice developed significantly less synovitis after DMM compared to wild-type mice, whereas 24-month-old animals revealed no such differences(35). These observations imply that the link between Postn expression and synovitis is context dependent and merits further investigation.
How Postn exerts its effects on these various tissues is still unclear. Postn binding to integrin receptors (αvβ3/αvβ5 or DDR-1) on various cell types(36–38) has been reported to activate intracellular signaling pathways directly(39) or indirectly (via downstream ERK signaling)(40) and lead to NF-κB activation, which in turn regulates the production of inflammatory mediators such as MMPs. In injured joints, NF-κB activation triggers the expression of a myriad of genes that promote destruction of the articular joint and lead to changes that are characteristic of OA.(21, 41).
One NF-κB-dependent gene is MMP-13, which is thought to represent the major collagenase activity in OA, as its expression is upregulated in human OA and experimental OA models.(42, 43). Conditional overexpression of MMP-13 in murine cartilage induces cartilage pathology and OA.(44) By contrast, global knockout of MMP-13 protects against experimental OA in rodents.(45) Our results showed that Postn knockdown suppressed MMP-13 expression in chondrocytes derived from patients with OA and in an experimental post-traumatic OA mouse model. Moreover, we showed that MMP-13 generated C-terminal neoepitope DIPEN was also reduced indicating less proteolytic cleavage of aggrecan.(46) In addition to MMP-13, Postn knockdown also significantly suppressed the expression of ADAMTS-4 and IL-1β and surprisingly reduced the expression of matrix genes (COL2A1, ACAN) suggesting an anti-inflammatory rather than pro-anabolic role of Postn. To test, whether Postn-mediated regulation of MMP-13 expression is NF-κB-dependent, we performed pilot experiments. Preliminary data revealed that Postn-induced MMP-13 expression can be abrogated by IKK2 inhibitor suggesting a link between Postn and NF-κB signaling pathway.
A limitation of current investigation is the lack of multiple time points regarding how Postn expression and treatment effects vary with time. With regard to the expression of Postn, we noted increased Postn signal intensity at 4-weeks post-DMM (not shown). Postn expression remained elevated at 8-weeks (current study) and at 12-weeks(16). Together, these findings, along with findings from ACL tears in humans(16, 17, 34) prompted us to initiate siRNA therapy early, prior to Postn-mediated cartilage degeneration. Our study provides a proof of concept that early intervention to knockdown Postn expression after joint injury leads to significant mitigation of post-traumatic OA progression. However, intervention in the immediate aftermath of joint injury is not always possible. Additional longitudinal studies will assess a therapeutic window during which Postn knockdown will still result in a protective effect. Another limitation is that we did not analyze the role of Postn in meniscus and ligament. However, previous work showed that intra-articular administration of peptide siRNA nanoparticle targets the whole joint.(21) Future work will determine if and how Postn knockdown affects meniscal/ligament biology. It is likely that Postn modulates meniscus and ligament biology through different ways.(16, 34, 47) Yet, another limitation is the lack of measurement of functional tests such as pain, gait, and behavior. Lastly, we used 10-week-old mice, which falls within the standards of the field. Although some authors contemplate age of skeletal maturity to be 12-weeks or more (48, 49), others recommend a minimum age for skeletal maturity of 10-weeks for preclinical modes of OA to gain meaningful insights into human OA.(50) While growth plates do not close completely in mice, OA experiments in mice are recommended after the major growth spurt has occurred and when the animals are sexually mature. Since our treatment of siRNA was delivered from the age of 10-weeks through 16-weeks of age and analysis was performed at 18-weeks of age, it is not clear how skeletal maturation confounds the therapeutic effects of our siRNA treatment. Even if the mice are not skeletally mature, skeletal maturity does not explain the effect of treatment. In all three groups, mice are maturing at the same rate and the differences in OA parameters are exclusively specific to Postn siRNA treatment.
In summary, we demonstrated that intra-articular Postn siRNA nanocomplex represents a promising clinical approach to delay or mitigate the development of post-traumatic OA. Moreover, our data suggest that Postn knockdown suppresses the activity of NF-κB, a classical pathway implicated in OA development. Mechanistically, we show that Postn-induced expression of MMP-13 was abrogated by SC-514 demonstrating a link between Postn and NF-κB. Follow-up mechanistic and cartilage-specific gene knockout studies will further illuminate this link and the functional role of Postn in OA.
Supplementary Material
A. Intra-articular administration of Postn siRNA nanoparticles suppressed the expression of Postn protein compared with untreated control or scrambled siRNA treatment groups. The dotted lines indicate area of synovium based on the image in the left panel (dotted square). DAPI (blue) was used to stain nuclei. Scale bar=100 μm. M = meniscus, S = synovium, F = femur, T = tibia B. Quantification of mean signal intensity per pixel in selected areas of synovium (Kruskal-Wallis test with Dunn’s multiple comparisons, P=0.086).
Immunohistochemistry revealed that intra-articular delivery of Postn siRNA reduced the expression of DIPEN, a neoepitope of aggrecan compared with untreated control or scrambled siRNA treatment groups. The dotted lines indicate area of interest magnified in the inset. DAPI (blue) was used to stain nuclei. Scale bar=200 μm.
Acknowledgements
We thank Crystal Idleburg and Samantha Coleman for technical support (Musculoskeletal Research Center, Washington University School of Medicine). Dr. Ryan Nunley (Orthopaedic Surgery, Washington University School of Medicine) generously provided human cartilage samples. We acknowledge institutional support for the Hamamatsu NanoZoomer (Grant No. NCRR 1S10RR027552) and Leica Confocal Laser Scanning Microscope (NIH P30 AR074992) at Washington University School of Medicine, which were used for the imaging studies.
Financial supporters
This study was supported by research funding by the Department of Orthopaedic Surgery. Dr. Rai is also supported through the Pathway to Independence Award (R00 AR064837) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH). Dr. Abu-Amer is supported by NIH grants AR049192, AR072623, and Shriners grant 85160. The funding support of the following grants is also acknowledged: R01 AR067491, DK102691, P30 AR073752, and P30 AR074992. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIAMS or Shriners.
Footnotes
Conflict of interest statement
All authors have declared that no conflict of interest exists in conjunction with this study.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi:10.1002/ART.41794
REFERENCES
- 1.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai MF, Pham CT. Intra-articular drug delivery systems for joint diseases. Curr Opin Pharmacol. 2018;40:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–44. [DOI] [PubMed] [Google Scholar]
- 4.Rai MF, Brophy RH, Sandell LJ. Osteoarthritis following meniscus and ligament injury: insights from translational studies and animal models. Curr Opin Rheumatol. 2019;31(1):70–9. [DOI] [PubMed] [Google Scholar]
- 5.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–7. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14(5):608–16. [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14(7):1239–49. [DOI] [PubMed] [Google Scholar]
- 8.Coutu DL, Wu JH, Monette A, Rivard GE, Blostein MD, Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem. 2008;283(26):17991–8001. [DOI] [PubMed] [Google Scholar]
- 9.Lourido L, Calamia V, Mateos J, Fernandez-Puente P, Fernandez-Tajes J, Blanco FJ, et al. Quantitative proteomic profiling of human articular cartilage degradation in osteoarthritis. J Proteome Res. 2014;13(12):6096–106. [DOI] [PubMed] [Google Scholar]
- 10.Chijimatsu R, Kunugiza Y, Taniyama Y, Nakamura N, Tomita T, Yoshikawa H. Expression and pathological effects of periostin in human osteoarthritis cartilage. BMC Musculoskelet Disord. 2015;16:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rousseau JC, Sornay-Rendu E, Bertholon C, Garnero P, Chapurlat R. Serum periostin is associated with prevalent knee osteoarthritis and disease incidence/progression in women: the OFELY study. Osteoarthritis Cartilage. 2015;23(10):1736–42. [DOI] [PubMed] [Google Scholar]
- 12.Honsawek S, Wilairatana V, Udomsinprasert W, Sinlapavilawan P, Jirathanathornnukul N. Association of plasma and synovial fluid periostin with radiographic knee osteoarthritis: Cross-sectional study. Joint Bone Spine. 2015;82(5):352–5. [DOI] [PubMed] [Google Scholar]
- 13.Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan MF, Ferguson CM, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64(3):705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou CH, Wu CC, Song IW, Chuang HP, Lu LS, Chang JH, et al. Genome-wide expression profiles of subchondral bone in osteoarthritis. Arthritis Res Ther. 2013;15(6):R190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attur M, Yang Q, Shimada K, Tachida Y, Nagase H, Mignatti P, et al. Elevated expression of periostin in human osteoarthritic cartilage and its potential role in matrix degradation via matrix metalloproteinase-13. FASEB J. 2015;29(10):4107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinzei N, Brophy RH, Duan X, Cai L, Nunley RM, Sandell LJ, et al. Molecular influence of anterior cruciate ligament tear remnants on chondrocytes: a biologic connection between injury and osteoarthritis. Osteoarthritis Cartilage. 2018;26(4):588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brophy RH, Cai L, Duan X, Zhang Q, Townsend RR, Nunley RM, et al. Proteomic analysis of synovial fluid identifies periostin as a biomarker for anterior cruciate ligament injury. Osteoarthritis Cartilage. 2019;27(12):1778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng F, Chen X, Liao Z, Yan Z, Wang Z, Deng Y, et al. A simplified and versatile system for the simultaneous expression of multiple siRNAs in mammalian cells using Gibson DNA Assembly. PLoS One. 2014;9(11):e113064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai MF, Pan H, Yan H, Sandell LJ, Pham CTN, Wickline SA. Applications of RNA interference in the treatment of arthritis. Transl Res. 2019;214:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin Y, Huang M, Guo WW, Huang Q, Zhang LZ, Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol Cancer. 2017;16(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, et al. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci U S A. 2016;113(41):E6199-E208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan H, Duan X, Pan H, Akk A, Sandell LJ, Wickline SA, et al. Development of a peptide-siRNA nanocomplex targeting NF- kappaB for efficient cartilage delivery. Sci Rep. 2019;9(1):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou KK, Pan H, Ratner L, Schlesinger PH, Wickline SA. Mechanisms of nanoparticle-mediated siRNA transfection by melittin-derived peptides. ACS Nano. 2013;7(10):8605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou KK, Pan H, Lanza GM, Wickline SA. Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials. 2013;34(12):3110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou HF, Yan H, Pan H, Hou KK, Akk A, Springer LE, et al. Peptide-siRNA nanocomplexes targeting NF-kappaB subunit p65 suppress nascent experimental arthritis. J Clin Invest. 2014;124(10):4363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto S, Rai MF, Janiszak KL, Cheverud JM, Sandell LJ. Cartilage and bone changes during development of post-traumatic osteoarthritis in selected LGXSM recombinant inbred mice. Osteoarthritis Cartilage. 2012;20(6):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–9. [DOI] [PubMed] [Google Scholar]
- 28.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 Suppl 3:S17–23. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage. 2011;19(7):864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan X, Sandell LJ, Chinzei N, Holguin N, Silva MJ, Schiavinato A, et al. Therapeutic efficacy of intra-articular hyaluronan derivative and platelet-rich plasma in mice following axial tibial loading. PLoS One. 2017;12(4):e0175682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett. 1996;380(1–2):17–20. [DOI] [PubMed] [Google Scholar]
- 32.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am. 2012;94(5):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duchamp de Lageneste O, Julien A, Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018;9(1):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai L, Brophy RH, Tycksen ED, Duan X, Nunley RM, Rai MF. Distinct expression pattern of periostin splice variants in chondrocytes and ligament progenitor cells. FASEB J. 2019;33(7):8386–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attur M, Duan X, Cai L, Han T, Zhang W, Tycksen ED, et al. Periostin loss-of-function protects mice from post-traumatic and age-related osteoarthritis. Arthritis Res Ther. 2021;23(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5(4):329–39. [DOI] [PubMed] [Google Scholar]
- 37.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62(18):5358–64. [PubMed] [Google Scholar]
- 38.Han T, Mignatti P, Abramson SB, Attur M. Periostin interaction with discoidin domain receptor-1 (DDR1) promotes cartilage degeneration. PLoS One. 2020;15(4):e0231501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122(7):2590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert AW, Wong CK, Ozturk S, Papageorgis P, Raghunathan R, Alekseyev Y, et al. Tumor Cell-Derived Periostin Regulates Cytokines That Maintain Breast Cancer Stem Cells. Mol Cancer Res. 2016;14(1):103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11(5):599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol. 2009;182(12):7937–45. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janusz MJ, Little CB, King LE, Hookfin EB, Brown KK, Heitmeyer SA, et al. Detection of aggrecanase- and MMP-generated catabolic neoepitopes in the rat iodoacetate model of cartilage degeneration. Osteoarthritis Cartilage. 2004;12(9):720–8. [DOI] [PubMed] [Google Scholar]
- 47.Brophy RH, Zhang B, Cai L, Wright RW, Sandell LJ, Rai MF. Transcriptome comparison of meniscus from patients with and without osteoarthritis. Osteoarthritis Cartilage. 2018;26(3):422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang H, Huang L, Welch I, Norley C, Holdsworth DW, Beier F, et al. Early Changes of Articular Cartilage and Subchondral Bone in The DMM Mouse Model of Osteoarthritis. Sci Rep. 2018;8(1):2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Kraan PM. Factors that influence outcome in experimental osteoarthritis. Osteoarthritis Cartilage. 2017;25(3):369–75. [DOI] [PubMed] [Google Scholar]
- 50.Poole R, Blake S, Buschmann M, Goldring S, Laverty S, Lockwood S, et al. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18 Suppl 3:S10–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Intra-articular administration of Postn siRNA nanoparticles suppressed the expression of Postn protein compared with untreated control or scrambled siRNA treatment groups. The dotted lines indicate area of synovium based on the image in the left panel (dotted square). DAPI (blue) was used to stain nuclei. Scale bar=100 μm. M = meniscus, S = synovium, F = femur, T = tibia B. Quantification of mean signal intensity per pixel in selected areas of synovium (Kruskal-Wallis test with Dunn’s multiple comparisons, P=0.086).
Immunohistochemistry revealed that intra-articular delivery of Postn siRNA reduced the expression of DIPEN, a neoepitope of aggrecan compared with untreated control or scrambled siRNA treatment groups. The dotted lines indicate area of interest magnified in the inset. DAPI (blue) was used to stain nuclei. Scale bar=200 μm.