Abstract
We have previously shown that the Ste20 kinase encoded by misshapen (msn) functions upstream of the c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase module in Drosophila. msn is required to activate the Drosophila JNK, Basket (Bsk), to promote dorsal closure of the embryo. A mammalian homolog of Msn, Nck interacting kinase, interacts with the SH3 domains of the SH2-SH3 adapter protein Nck. We now show that Msn likewise interacts with Dreadlocks (Dock), the Drosophila homolog of Nck. dock is required for the correct targeting of photoreceptor axons. We have performed a structure-function analysis of Msn in vivo in Drosophila in order to elucidate the mechanism whereby Msn regulates JNK and to determine whether msn, like dock, is required for the correct targeting of photoreceptor axons. We show that Msn requires both a functional kinase and a C-terminal regulatory domain to activate JNK in vivo in Drosophila. A mutation in a PXXP motif on Msn that prevents it from binding to the SH3 domains of Dock does not affect its ability to rescue the dorsal closure defect in msn embryos, suggesting that Dock is not an upstream regulator of msn in dorsal closure. Larvae with only this mutated form of Msn show a marked disruption in photoreceptor axon targeting, implicating an SH3 domain protein in this process; however, an activated form of Msn is not sufficient to rescue the dock mutant phenotype. Mosaic analysis reveals that msn expression is required in photoreceptors in order for their axons to project correctly. The data presented here genetically link msn to two distinct biological events, dorsal closure and photoreceptor axon pathfinding, and thus provide the first evidence that Ste20 kinases of the germinal center kinase family play a role in axonal pathfinding. The ability of Msn to interact with distinct classes of adapter molecules in dorsal closure and photoreceptor axon pathfinding may provide the flexibility that allows it to link to distinct upstream signaling systems.
Ste20 kinases play a critical role in mediating activation of the c-Jun N-terminal kinase (JNK) mitogen-activated protein (MAP) kinase pathway (23). Both genetic and biochemical evidence has indicated that Ste20 kinases mediate JNK activation by functioning as MAP kinase kinase kinase kinases (MAP4Ks) (17, 23). Two families of Ste20 kinases have been identified in mammalian cells and lower organisms, the p21-activated protein kinase (Pak) and germinal center kinase (GCK) families (16, 26, 28). In contrast to Paks, GCK family kinases lack binding motifs for Rho family GTPases. In addition, unlike PAKs, which have a C-terminal kinase and an N-terminal regulatory domain, GCK family members contain an N-terminal kinase and a C-terminal regulatory domain (5, 28).
We have recently placed the Ste20 kinase encoded by misshapen (msn), a member of the GCK family of Ste20 kinases, genetically upstream of the JNK MAP kinase pathway in Drosophila (44). The failure to activate JNK in Drosophila leads to embryonic lethality due to defects in dorsal closure; in embryos with mutations in components of the JNK pathway, the lateral epithelial sheets fail to elongate and migrate dorsally (29). This coordinated movement of the lateral epithelia functions to internalize the amnioserosa and connect the two sides of the embryo (6, 48). A number of signaling molecules that are critical for stimulating dorsal closure in Drosophila can now be ordered on a signaling pathway. The most proximal molecule identified on this pathway is the Ste20 kinase encoded by msn (44). Msn likely functions as a MAP4K and activates the JNK pathway by activating a yet-to-be-defined Drosophila MAP3K. A Drosophila MAP3K would likely phosphorylate and activate the JNK kinase Hemipterous (Hep), which in turn phosphorylates and activates Drosophila JNK (DJNK), encoded by basket (bsk) (13, 35, 41). DJNK phosphorylates and activates DJun, which in turn cooperates with DFos to stimulate transcription of dpp, a member of the transforming growth factor-β family (12, 20, 22, 34, 36). Dpp then acts on cells adjacent to the leading-edge cells to promote their elongation, probably through alterations in the cytoskeleton (1, 4, 24, 37a).
The mammalian homolog of Msn, Nck interacting kinase (NIK), was identified in a two-hybrid screen for proteins that interact with the SH3 domains of the adapter protein Nck (43). Nck is a ubiquitously expressed protein composed entirely of a single SH2 and three SH3 domains and thus fits into the adapter class of signaling molecules (32, 38). Nck and related adapter proteins, such as Grb2 and Crk, are thought to regulate signaling pathways downstream of tyrosine kinases by coupling catalytic subunits, bound to their SH3 domains, to phosphotyrosine-containing proteins that interact with their SH2 domains (10, 32, 38).
Recent studies with Drosophila have shed light on the likely function of Nck in mammalian cells. The Drosophila homolog of Nck, encoded by dreadlocks (dock), was identified in a genetic screen for proteins that are critical for the correct targeting of photoreceptor axons (11). The Drosophila compound eye is composed of about 800 repeated units called ommatidia, with each ommatidial unit being composed of eight neurons, R1 to R8 (8). The axons from each ommatidium form a single fascicle and project in a specific topographic pattern into the optic ganglia. R1 to R6 axonal growth cones terminate in the lamina, whereas R7 and R8 growth cones project through the lamina and terminate in the second optic ganglion, the medulla. In dock mutants, photoreceptor axons show abnormal clumping and crossing over, and some axons of R1 to R6 fail to terminate in the lamina and project abnormally into the medulla. In addition, the R7 and R8 axons in dock mutants fail to form an even array in the medulla and lack expanded growth cones (11, 33). It has been proposed that Dock provides a link between a receptor tyrosine kinase located at the axonal growth cone and a downstream signaling pathway by targeting a catalytic molecule bound to its SH3 domain to tyrosine-phosphorylated proteins. The Ste20 kinase Pak has recently been shown to function downstream of Dock in Drosophila (18) and may be such a catalytic molecule. In this study, we have performed a structure-function analysis of Msn in vivo in Drosophila in order to elucidate the mechanism whereby Msn regulates JNK and to determine whether msn, like dock and PAK, is required for the correct targeting of photoreceptor axons.
MATERIALS AND METHODS
Constructs, mutagenesis, and JNK assays.
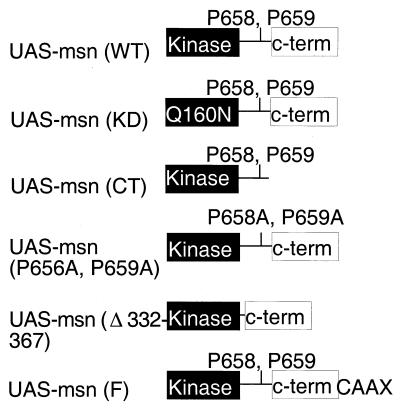
The kinase-defective mutant Msn(KD) contains the substitution of aspartic acid for asparagine at position 160 in the kinase domain and has been described previously (44). The gene for a truncated protein lacking its C-terminal regulatory domain, Msn(−CT), was amplified by PCR using oligonucleotides corresponding to regions that flanked amino acids (aa) 1 to 772 of full-length Msn and that contained appropriate restriction sites. The genes for Msn(P656A, P659A), Msn(Δ332–667), and Msn-F were made using overlapping PCR as previously described (21). To generate the mutation for Msn(P656A, P659A), two complementary primers in opposite orientations spanning the sites of mutation were synthesized. The msn sequence 5′ to the mutation was then amplified using the mutant primer in the reverse orientation together with a primer 5′ to a convenient restriction site in msn, and the msn sequence 3′ to the mutation was amplified using the complementary mutant primer in the forward orientation together with a primer containing the 3′ end of full-length msn and a myc epitope tag. The two fragments were then mixed and used as a template for a second round of PCR using only the nonmutagenic flanking oligonucleotides. The same approach was used to mutate the other PXXP motifs of Msn. A similar strategy was used to generate the mutation for Msn(Δ332–667), with the exception that the complementary mutant primers synthesized for the first PCR contained sequences flanking the regions deleted in the sequence corresponding to Msn(Δ332–667). To add the last 20 aa of Ras containing the Ras farnesylation sequence to the C terminus of Msn, complementary primers containing the 3′ end of msn and the 5′ end of the gene for the Ras farnesylation sequence were synthesized (Msn-Ras). Msn was then amplified using the msn-Ras primer in the reverse orientation together with the same 5′ nonmutant primer mentioned above, and the gene for the Ras farnesylation sequence was amplified using the complementary msn-Ras primer in the forward orientation with a primer containing the 3′ end of the Ras gene. The two fragments were then mixed, and a second PCR was performed as described above to generate Msn-F. All msn constructs were initially cloned into the mammalian expression vector pRK5, and all PCR-generated fragments were subjected to DNA sequencing to eliminate the possibility that errors were introduced during the PCR. In addition, all constructs encoded proteins of the correct molecular size when transfected into 293 cells (data not shown).
GST fusions, in vitro binding studies, and yeast two-hybrid assays.
Glutathione S-transferase (GST) fusion proteins were made using the PCR as previously described (43). Briefly, oligonucleotides flanking the regions of interest and containing appropriate restriction sites were synthesized and used to amplify by PCR the region of interest. The products obtained were subcloned into PGEX3X, and GST fusion proteins were isolated as previously described (43). To assay binding of Msn to the various GST fusions, myc epitope-tagged Msn was overexpressed in 293 cells. Five hundred micrograms of lysates containing myc epitope-tagged Msn was incubated with 5 μg of the various GST fusions coupled to glutathione-agarose beads at 4°C for 4 h. After four washes with lysis buffer, bound proteins were separated by sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis and immunoblotted with the anti-myc antibody 9E10.
The genes for Msn and Msn(P656A, P659A) were amplified by PCR and expressed as a fusion with the LexA DNA binding domain using the vector BTM116 (LexA-Msn). The genes for full-length DTRAF1 and Dock were amplified by PCR and expressed as a fusion with the activation domain of GAL4 by using the vector pGAD (pGAD-DTRAF1 and pGAD-dock) (Clontech) (11, 25). Following cotransfection, interaction was assessed by selecting for growth on selection media lacking histidine and containing 5 mM 3-aminotriazole (43). Yeast transformations and routine growth of yeast were performed as described previously (15).
Ectopic expression in Drosophila.
To express wild-type msn [msn(wt)] and the msn mutants in Drosophila, the GAL4/upstream activation sequence (UAS) system was used (3). All constructs were subcloned from pRK5 into the vector pUAST. Germ line transformations were then performed using standard techniques, and transgenic lines containing the various msn constructs on chromosomes 2 and 3 were obtained (42). To induce expression of msn in the ectoderm, brain, and eye disc, UAS-msn(wt); msn102/SM6.TM6B flies were crossed with 69B-GAL4 msn102/TM6B flies (44). A similar strategy was used for the other UAS constructs. Two or three independent insertions of each UAS construct were evaluated. To rescue the defect in targeting of photoreceptor axons in dockP1 mutants, UAS-msn(wt), dockP1/Gla,Bc,Elp, UAS-msn-F, dockP1/Gla, Bc,Elp, and UAS-msn(Δ332–667), dockP1/Gla,Bc,Elp flies were crossed with ELAV-GAL4 dockP1/Gla,Bc,Elp flies, and photoreceptor axonal targeting of third-instar larvae was analyzed by staining eye-brain complexes with monoclonal antibody (MAb) 24B10 (9).
Immunohistochemistry.
Photoreceptor projection patterns in third-instar larval eye-brain complexes were visualized by MAb 24B10 (1:100) and a horseradish peroxidase-coupled goat anti-mouse secondary antibody.
Genetic mosaic analysis.
To generate msn mutant clones in the eye disc, FRT80, msn/TM6B females were crossed with y, w, eyFLP1; FRT80, M(3)67C/TM6B males, and female larvae were analyzed. To generate dockP1 or bsk1 mutant clones, FRT40, dockP1 (or bsk1) males were crossed with y, w, eyFLP1; FRT40, M(2)24F/CyO females. To produce hep, lic mutant clones, FRT18, H6/FM6 females were crossed with FRT18, ormlacZ; hs FLP38 males and heat shocked at 37°C during the first and second instars, and female third-instar larvae were analyzed.
Cuticle preparations.
Cuticle preparations were performed as previously described (44). Briefly, embryos were collected on yeast agar plates and dechorionated in 100% bleach, rinsed in water, and then fixed for 10 min at 65°C in a solution containing acetic acid and glycerol at a ratio of 3:1. Embryos were then mounted in Hoyer's medium and incubated for 24 h at 65°C.
ATF2 luciferase activity.
A fusion protein consisting of ATF2 (aa 1 to 505) and the GAL4 DNA binding domain was expressed in 293 cells either alone or together with NIK (14). Transcriptional activation of ATF2 was measured by cotransfecting a luciferase reporter plasmid containing five GAL4 DNA binding sites. All transfections were standardized by cotransfecting a control plasmid expressing β-galactosidase (Promega).
RESULTS
The kinase activity and C-terminal domain of Msn are required for JNK activation in the Drosophila embryo.
The requirement for msn to stimulate JNK activation and dorsal closure in the Drosophila embryo afforded us a unique opportunity to identify the domains of Msn that are essential for it to couple to JNK activation in vivo (44). Msn can be divided into three regions, an N-terminal kinase domain, a C-terminal domain that is conserved in several GCK family members, and a region between the kinase and C-terminal domains that probably couples to upstream regulators (Fig. 1C). Previously, we have demonstrated that the mammalian homolog of Msn, NIK, requires both a functional kinase and a C-terminal regulatory domain to activate JNK in 293 cells (43). To determine whether these domains are also critical for Msn to activate JNK in vivo in Drosophila, we determined whether Msn(KD) or Msn(−CT) could rescue the defect in dorsal closure in msn mutant embryos. The 69B-GAL4 driver was used to express wild-type or mutant forms of UAS-msn in the ectoderm of msn mutant embryos. Rescue was determined at the pupal stage by the absence of the TM6B balancer chromosome marked with Tubby. We found that expression of wild-type msn was sufficient to rescue 100% of msn mutant embryos to the pupal stage (Tables 1 and 2). In contrast, none of the msn embryos rescued with Msn(KD) survived to the pupal stage, and only 10% of msn embryos rescued with Msn(−CT) survived to the pupal stage (Tables 1 and 2). The finding that some msn mutants are rescued with Msn(−CT) is consistent with our findings in 293 cells that C-terminally truncated forms of NIK and Msn are able to partially activate JNK (43, 44) (see Fig. 4). Similar results were obtained by crossing the UAS-msn lines with a 69B-GAL4 driver line containing a different inversion allele of msn (msn172, as opposed to msn102) (46), indicating that the results obtained reflect a specific rescue of the loss of msn function.
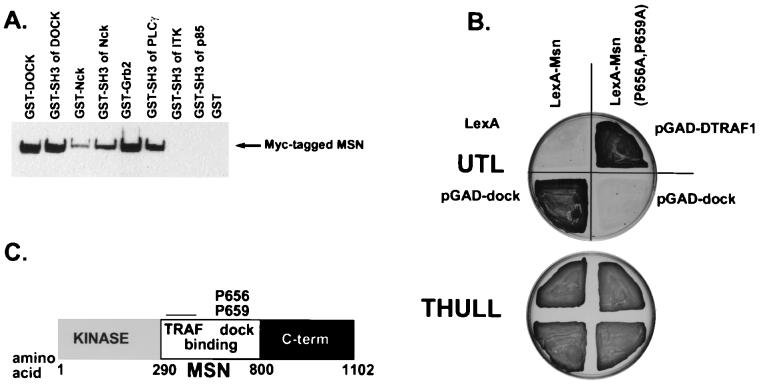
FIG. 1.
Msn binds the SH3 domains of Dock. (A) Various GST fusion proteins (as indicated) were incubated with lysates from 293 cells transfected with myc epitope-tagged Msn. After washing, bound proteins were separated by sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis and visualized by immunoblotting with the anti-myc antibody 9E10. The level of each GST protein was similar as assessed by Coomassie blue staining (data not shown). PLCγ, phospholipase Cγ; ITK, interleukin 2-inducible T-cell kinase. (B) The yeast two-hybrid system was used to identify the PXXP motif in Msn that mediates binding to the SH3 domains of Dock. L40 yeast cells were transfected with LexA-Msn(wt) or LexA-Msn in which two conserved prolines that match potential consensus SH3 binding motifs were mutated to alanine, with Dock expressed as a fusion protein with the activation domain of GAL4. Interaction was determined by selecting for growth on medium lacking histidine in the presence of 5 mM 3-aminotriazole (top). Transfection efficiency was assessed by selecting for growth on media containing histidine (bottom). As a control, LexA-Msn(P656A, P659A) was shown to bind DTRAF1, indicating that LexA-Msn(P656A, P659A) is able to interact with other targets that bind to a different region on Msn. UTL, uracil-tryptophan-leucine; THULL, tryptophan-histidine-uracil-leucine-lysine. (C) Schematic diagram of Msn.
TABLE 1.
Rescue of msn mutantsa
| Protein used for rescue | No. with dorsal closure defect/total (%) | Rescue to pupal stage
|
|
|---|---|---|---|
| No. of long/ short pupae | % Rescued | ||
| None (control) | 28/158 (15.2) | 0/118 | 0 |
| UAS-Msn(wt) | NAb | 47/80 | 118 |
| UAS-Msn(KD) | 9/62 (14.5) | 0/109 | 0 |
| UAS-Msn(−CT) | 26/180 (14.4) | 10/210 | 10 |
| UAS-Msn(P656A, P659A) | 0/120 (0) | 23/125 | 37 |
| UAS-Msn(Δ332–667) | NA | 51/125 | 82 |
To assess defects in dorsal closure, embryos were collected and aged for 24 h. Cuticles were prepared from unhatched embryos, and the total number of embryos with an aberrant dorsal cuticle was determined. The total number of unhatched and hatched embryos was determined by resuspending all the larvae and embryos in Hoyer's solution and counting under a microscope. msn mutant pupae that were rescued because of ectopic expression of Msn were longer due to the absence of the Tubby marker on the balancer. Ectopic expression of Msn using 69B-GAL4 rescued msn mutants only to the pupal stage. The control was an intercross of UAS-msn(wt); msn102/SM6.TM6B flies. The finding that about 15% of embryos had a defect in dorsal closure is similar to previous results that were obtained for the original msn mutant line (46).
NA, not applicable.
TABLE 2.
Rescue of msn−/− embryos
FIG. 4.
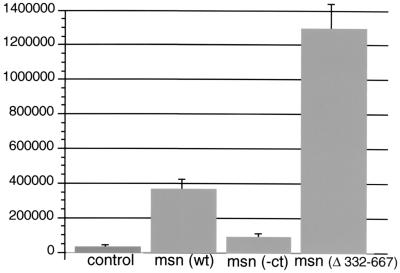
JNK activation by Msn(Δ332–667) in 293 cells. Msn(wt) or various Msn mutant constructs (0.1 μg each) were transfected into 293 cells together with 10 ng of a plasmid expressing a fusion protein of ATF2 and the GAL4 DNA binding domain and 5 μg of a plasmid expressing a GAL4 luciferase reporter. Transfection efficiency was assessed by coexpressing 1 μg of a plasmid expressing β-galactosidase. The means from two experiments performed in triplicate are shown. Luciferase activity is expressed in arbitrary units after being standardized to β-galactosidase activity.
Although these altered forms of Msn were not able to rescue msn mutants as far as the pupal stage, it was possible that they retained some activity in early developmental functions. Thus, we directly determined whether Msn(KD) and Msn(−CT) were able to rescue the defect in dorsal closure. The percentage of msn mutant embryos expressing either of these constructs that were found to have a defect in dorsal closure was about 14, similar to the number in a cross lacking the GAL4 driver line (Tables 1 and 2). However, the finding that a small percentage of msn mutant embryos rescued with UAS Msn(−CT), but not the control, survived to the pupal stage suggests that Msn(−CT) provides some signaling function that is required for survival after hatching. It is not clear whether this signaling function of Msn(−CT) is related to the activation of JNK or to JNK-independent pathways. No defect in dorsal closure was observed when msn embryos were rescued with wild-type Msn. These findings demonstrate that the defect in dorsal closure in msn mutant embryos is due to a loss of msn function and that both the kinase activity and the C-terminal regulatory domain of Msn are essential for it to activate JNK in vivo. In evaluating msn mutant embryos rescued with Msn(KD), we observed that many had ventral cuticular abnormalities, suggesting a defect in gastrulation (data not shown). While embryos zygotically mutant for msn do not show such defects, the function of maternal msn could not be evaluated because msn is required for oogenesis (46). Although the level of expression of maternal msn is sufficient to support gastrulation of embryos lacking zygotic msn, Msn(KD) may function as a dominant negative protein, inhibiting maternal Msn. In the presence of wild-type levels of zygotic Msn we did not observe such a dominant negative effect (data not shown). This role of msn is likely to be independent of JNK activation, because ventral defects are not seen in embryos lacking both maternal and zygotic JNK (bsk) or the JNK kinase encoded by hep (13, 35, 41).
A single PXXP motif in Msn mediates binding to the SH3 domains of the SH2-SH3 adapter molecule Dock.
We isolated NIK in a two-hybrid screen designed to identify proteins that interact with the mammalian homolog of Drosophila Dock, Nck. The region between the kinase and C-terminal regulatory domains of Msn, the Drosophila homolog of NIK, contains several PXXP motifs that match consensus SH3 binding motifs (49). To determine whether Msn may be a physiological target of the SH3 domains of Dock, we determined whether Msn bound the SH3 domains of Dock in vitro. We found that Msn bound full-length Dock as well as a GST fusion protein containing only the SH3 domains of Dock (Fig. 1A). Msn also bound the SH3 domains of mammalian Nck, Grb2, and phospholipase Cγ. The interaction of Msn with these fusion proteins was specific, because Msn did not bind to GST alone or to GST fused to the SH3 domains of p85 or interleukin 2-inducible T-cell kinase.
Using the yeast two-hybrid system, we mapped the binding site in Msn for the SH3 domains of Dock. Msn contains more than 10 PXXP motifs that match consensus SH3 binding motifs in the region between its kinase and C-terminal domains (49). We found that Dock specifically bound prolines 656 and 659 on Msn. Of the 10 mutants we screened, only Msn(P656A, P659A) did not bind Dock (Fig. 1B and data not shown). The inability of Msn(P656A, P659A) to bind Dock was not due to altered expression of this mutant construct, because Msn(P656A, P659A) still interacted with a Drosophila tumor necrosis factor receptor-associated factor (DTRAF) which members of our group have previously shown to bind a different part of the central region of Msn (Fig. 1B) (25). We confirmed that the SH3 domains of Dock mediate binding to Msn, since the SH3 domains of Dock are sufficient to bind Msn in the yeast two-hybrid system, whereas the SH2 domain of Dock does not bind Msn (data not shown). Thus, this finding indicates that we have identified a single PXXP motif that is required for high-affinity binding of Msn to Dock.
Interaction between Msn and Dock is not required for the function of Msn in dorsal closure.
We used this mutant protein to test whether Msn required Dock binding to rescue the defect in dorsal closure in msn mutant embryos. While msn mutants die embryonically due to a defect in dorsal closure, dock mutants survive until the pupal stage (11), suggesting that dock may not function upstream of msn in dorsal closure. Loss of both maternal and zygotic dock causes embryonic lethality, but the embryos survive until after stage 16, when dorsal closure takes place (7), making a role for maternal dock in dorsal closure unlikely. We found that UAS-msn(P656A, P659A) driven by 69B-GAL4 completely rescued the defect in dorsal closure in msn mutant embryos (Tables 1 and 2), allowing 37% of rescued embryos to survive to the pupal stage. This finding is consistent with the idea that Dock does not function upstream of Msn in dorsal closure. Rather, we favor the idea that DTRAF1 acts to couple Msn to upstream signals important in mediating JNK activation and dorsal closure (25).
Formation of normal photoreceptor axonal projections requires the prolines on Msn that interact with SH3 domains.
The finding that msn mutants could be rescued to the pupal stage by expression of either wild-type Msn or Msn(P656A, P659A) led us to test whether larval photoreceptor axonal projections are disrupted in msn mutants rescued with the msn transgenes. Recent evidence has indicated that Pak is one of the downstream signaling molecules activated by Dock and that activation of Pak by Dock is critical for correct targeting of photoreceptor axons (18). However, these studies cannot rule out the possibility that Dock interacts with multiple downstream targets, one of which is Msn.
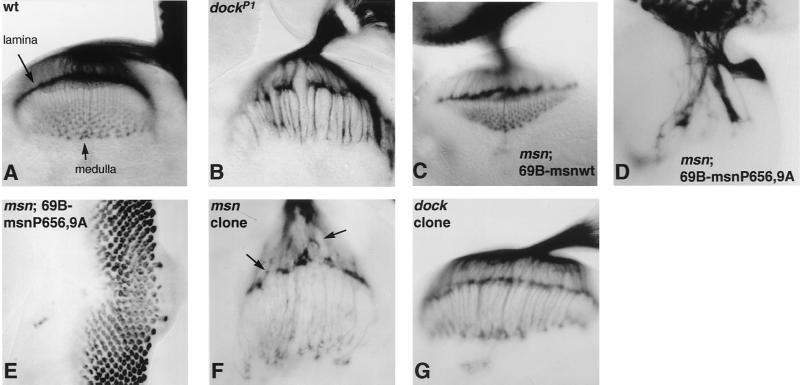
To begin to address whether msn plays a role in photoreceptor axon guidance, we tested whether msn mutant third-instar larvae rescued with UAS-msn(wt) or UAS-msn(P656A, P659A) displayed a defect in pathfinding of photoreceptor axons. We have observed that 69B-GAL4 is expressed in the photoreceptors and the brain as well as in the embryonic ectoderm (data not shown). We reasoned that if binding of Msn to Dock was necessary for Dock to function, photoreceptor axon projections would be rescued by UAS-msn(wt) driven by 69B-GAL4 but would not be rescued by UAS-msn(P656A, P659A). To assess photoreceptor projections, eye-brain complexes were stained with the MAb 24B10, which recognizes the membrane protein chaoptin present on photoreceptors and their axons (9). msn mutants rescued to the third-instar larval stage with UAS-msn(P656A, P659A) exhibited a severe defect in axonal targeting, confirming a requirement for msn in this process. Whereas axons from wild-type photoreceptors or photoreceptors from msn third-instar larvae rescued with UAS-msn(wt) fanned out evenly upon leaving the optic stalk and formed a smooth retinotopic array in both the lamina and the medulla (Fig. 2A and C), photoreceptor axons from msn third-instar larvae rescued with UAS-msn(P656A, P659A) formed very large axonal bundles and failed to defasciculate in the region of either the lamina or the medulla (Fig. 2D). This defect in axonal targeting is more severe than the defect reported for dock mutants (Fig. 2B). It is not due to a nonspecific effect of overexpression of Msn protein (for example, by titrating out an important signaling molecule that functions on this pathway), because these effects were not seen with Msn(wt) (Fig. 2C). In addition, overexpression of msn(P656A, P659A) in wild-type flies did not produce a defect in axonal targeting, indicating that msn(P656A, P659A) does not function as a dominant negative protein in a wild-type background (data not shown).
FIG. 2.
msn is required for correct targeting of photoreceptor axons. (A to D, F, and G) Photoreceptor axonal projection patterns in third-instar larvae were visualized with MAb 24B10. (A) Wild type. (B) dockP1. (C) msn102, 69B-GAL4/msn102; UAS-msn(wt)/+. (D) msn102, 69B-GAL4/msn102; UAS-msn(P656A, P659A)/+. (F) msn102 mutant clone in a Minute background. (G) dockP1 mutant clone in a Minute background. Wild-type photoreceptors or photoreceptors from msn third-instar larvae rescued with UAS-msn(wt) fan out evenly upon exiting the optic stalk and form a smooth retinotopic array in both the lamina and medulla (A and C). In contrast, msn mutants rescued with UAS-msn(P656A, P659A), which is unable to bind the SH3 domains of Dock, exhibit a severe defect in axonal projections (D). The defect in photoreceptor axonal projections in msn mutants rescued with UAS-msn(P656A, P659A) is more severe than in dockP1 mutants (B). (E) Immunostaining with antibodies to Elav. Photoreceptor development appears essentially normal except for a defect near the midline of the eye disc. Photoreceptor cell axons from msn mutant clones (F) fail to terminate at a precise depth in the lamina, resulting in an uneven neuropil. However, growth cones from axons that innervate the medulla appear normal. (G) Removal of dock function from the eye by making clones in a Minute background causes the same phenotype as homozygosity for dockP1 (B).
The defect in photoreceptor axonal targeting in msn mutants rescued with UAS-msn(P656A, P659A) does not appear to be due to an effect on photoreceptor cell fate determination or on the general organization of the eye disc. To demonstrate this, eye discs were stained with antibodies to Elav, a nuclear protein expressed in differentiated photoreceptor cells (Fig. 2E). Although photoreceptor axons were severely disrupted, the photoreceptor clusters appeared mostly normal except for a defect at the midline of the eye disc. Thus, these findings suggest that msn plays a critical role in the correct targeting of photoreceptor axons. However, the finding that photoreceptor axonal targeting was more severely affected in msn mutants rescued with UAS-msn(P656A, P659A) than in null dockP1 mutants indicates that Msn performs some functions that are independent of binding to Dock. Because the defects in axonal targeting were specific to a mutation in a proline motif that matches consensus SH3 binding motifs, these functions of Msn are probably mediated by its interaction with additional SH3 domain-containing proteins.
msn is required in the eye for axonal pathfinding.
To determine whether, like dock, msn acts autonomously in the photoreceptor growth cones, we made use of the FLP-FLP recognition target (FRT) recombinase system, which enables the generation of clones of cells with mutations in msn in a heterozygous animal (46, 47). To generate msn mutant clones in the eye disc, FRT-msn males were crossed with females carrying a Minute mutation, causing dominant slow growth and recessive lethality, on the same chromosome arm as the FRT site. The females also carried a construct expressing FLP recombinase under the control of the eye-specific eyeless promoter (28a). Using this approach, we were able to obtain third-instar larval eye discs in which >90% of the eye disc was mutant for msn. While photoreceptor axons derived from these msn mutant clones extended normally into the brain, the R1 to R6 axons failed to elaborate a smooth retinotopic array in the lamina (Fig. 2F). Whereas R1 to R6 axons from wild-type eye discs terminate at a precise depth in the lamina and therefore form a continuous line of chaoptin immunoreactivity, many R1 to R6 axons in msn mutant clones terminated prematurely, giving rise to an uneven lamina neuropil. Surprisingly, the growth cones of axons that innervate the medulla appeared to expand normally, although their projections were somewhat disorganized. This is in contrast to the R7 and R8 axons in dock mutants, which lack expanded growth cones and fail to form an even array in the medulla. Moreover, the axon bundles projecting between the lamina and medulla appeared to have the same thickness as those in the wild type, whereas in dock mutants these axons form larger bundles. Although a lack of msn in the eye causes defects in the shape of photoreceptor rhabdomeres (46), the relatively normal projection observed for R7 and R8 suggested that the axon guidance defects were not a consequence of these changes. In addition, the differences between the msn and dock phenotypes were not an experimental artifact arising from the generation of msn clones in the eye; the phenotype of dockP1 mutant clones in the eye made in a Minute background was identical to that of dockP1 homozygous mutants (compare Fig. 2G and B). These results confirm that msn is required in photoreceptors for their axons to project normally. However, some of the defects observed are different from those reported for dock mutants, suggesting that Msn performs functions in photoreceptor cells that are independent of those of Dock.
Msn is not the only downstream target of Dock.
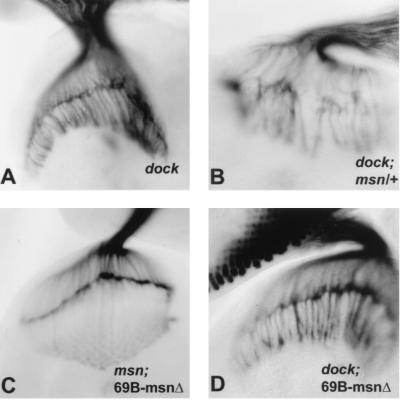
The above-described studies demonstrate that Msn and Dock interact in vitro and that msn plays an essential role in axonal pathfinding. However, these studies do not demonstrate that msn and dock act in the same pathway. In fact, the finding that photoreceptor axonal pathfinding is more severely disrupted in msn mutants rescued with UAS-msn(P656A, P659A) than in dock mutants indicates that Msn must interact with at least one protein other than Dock. To further study the relationship between msn and dock in vivo, we looked for genetic interactions between them. We found that defects in the photoreceptor projection pattern were consistently more severe in dockP1 third-instar larvae that were also heterozygous for msn than in third-instar larvae that were homozygous for the dockP1 mutation alone (compare Fig. 3A and B). Because dockP1 is likely to be a null mutation, its enhancement by msn would be most consistent with msn functioning on a pathway parallel to that of dock.
FIG. 3.
Genetic interaction between msn and dockP1. All panels show third-instar larval eye-brain complexes stained with 24B10. (A) dockP1. (B) dockP1; msn102/+. msn enhances the dock phenotype. Twenty-two eye-brain complexes homozygous for dock and heterozygous for msn were compared to 14 complexes homozygous for dock, and a consistent enhancement was seen in all samples. (C) msn102, 69B-GAL4/msn102, UAS-msn(Δ332–667). (D) dockP1; elav-GAL4/UAS-msn(Δ332–667). UAS-msn(Δ332–667) rescues both the defect in dorsal closure (Table 1) and the defect in photoreceptor axonal targeting in msn mutants (C). However, expression of UAS-msn(Δ332–667) fails to rescue photoreceptor axonal targeting in dockP1 mutant third-instar larvae (D).
If msn mediated some or all the functions of dock, it should be possible to at least partially rescue the dock mutant phenotype with an activated form of msn. We hypothesized that the role of an interaction between Msn and Dock might be to target Msn to a receptor tyrosine kinase localized to the growth cone. We initially tested whether simply overexpressing wild-type Msn at high enough levels would bypass the requirement for Dock to target Msn to a receptor tyrosine kinase. It has previously been shown that either GMR-dock or UAS-dock driven by elav-GAL4 can rescue the dockP1 phenotype (11, 33). However, overexpression of wild-type msn driven by elav-GAL4 failed to rescue the defect in targeting of photoreceptor axons in dockP1 third-instar larvae (data not shown). We next tested whether a farnesylated form of Msn (Msn-F) could rescue the axon defects in dockP1 mutants; if the function of Dock was to target Msn to the plasma membrane, then an alternative plasma membrane targeting signal might make Msn constitutively active. We fused the farnesylation signal from Ras to the C terminus of Msn (see Materials and Methods). This method has been used successfully to create activated versions of several downstream targets of SH3 domain-containing molecules (2, 18). However, expression of msn-F using 69B-GAL4 or elav-GAL4 also failed to rescue the dock mutant phenotype (data not shown).
In the course of our structure-function studies, we inadvertently constructed an activated form of Msn. This protein contained a deletion of the region between the kinase and C-terminal domains (aa 332 to 667) [Msn(Δ332–667)]. The region deleted in this mutant includes both the region that we have shown binds DTRAF1 and prolines 656 and 659, which mediate binding to the SH3 domains of Dock, as well as all the other PXXP motifs. The failure of Msn(Δ332–667) to bind both Dock and DTRAF was confirmed by two-hybrid analysis (data not shown). We initially predicted that if DTRAF1 functioned upstream of Msn and JNK activation in dorsal closure, ectopic expression of UAS-msn(Δ332–667) with 69B-GAL4 would fail to rescue the defect in dorsal closure in msn mutant embryos. However, we found that UAS-msn(Δ332–667) rescued the defect in dorsal closure in msn mutants, allowing them to survive to the pupal stage (Table 1). Surprisingly, and in contrast to the results shown for UAS-msn(P656A, P659A), the photoreceptor projection pattern in larvae rescued with UAS-msn(Δ332–667) was essentially normal (Fig. 3C). Because both Msn(P656A, P659A) and Msn(Δ332–667) are unable to bind Dock, yet Msn(Δ332–667) is able to rescue photoreceptor axonal targeting, Msn(Δ332–667) must be able to function without binding Dock and is probably a constitutively active form of Msn.
To confirm biochemically that Msn(Δ332–667) is an activated version of Msn, we compared the abilities of Msn(wt) and Msn(Δ332–667) to activate JNK in 293 cells. The activation of JNK in 293 cells by NIK and Msn is independent of upstream signals, and previous studies have demonstrated that overexpression of either NIK or Msn is sufficient to activate JNK in these cells (43, 44). To assess JNK activation, Msn(wt) and various Msn mutants were transfected into 293 cells and activation of an ATF2 luciferase reporter was measured; JNK has been shown to phosphorylate and activate ATF2. While overexpression of Msn(wt) led to about a 10-fold increase in luciferase activity compared to that for cells transfected with vector control, overexpression of Msn(Δ332–667) led to a >30-fold increase in luciferase activity (Fig. 4). The increase in JNK activation by Msn(Δ332–667) is not due to an increase in protein expression, because Msn(wt) and Msn(Δ332–667) are expressed at equal levels (data not shown). Thus, these findings are consistent with the idea that Msn(Δ332–667) is an activated form of Msn.
If Msn were a downstream effector of Dock required for correct targeting of photoreceptor axons, like PAK, Msn(Δ332–667) might partially rescue the defect in dock mutant photoreceptors. To test this possibility, we expressed UAS-msn(Δ332–667) in a dockP1 mutant background, using an elav-GAL4 driver. However, the dock mutant phenotype was still observed in these larvae (Fig. 3D). Thus, our studies do not provide conclusive evidence that Msn and Dock act in the same pathway in flies. Since a mutation in Msn sufficient to activate it does not compensate for the lack of dock, either Msn must function on a parallel pathway required for photoreceptor axon guidance or Dock must act through both Msn and additional proteins, such as PAK.
DISCUSSION
Msn couples to distinct pathways to regulate dorsal closure and axon guidance.
While a role for Ste20 kinases in promoting JNK activation has been previously identified, little is known about their regulation or about the specific in vivo function of these kinases. Members of our group have previously shown that the Ste20 kinase Msn functions upstream of the Drosophila JNK, Bsk, to stimulate dorsal closure of the Drosophila embryo (44). We now show that Msn requires both intact kinase activity and a C-terminal regulatory domain conserved in a number of Ste20 kinases of the GCK family in order to activate JNK in vivo in flies. The previous finding that the C-terminal regulatory domain of NIK bound the N-terminal regulatory domain of the Ste11 kinase MEKK1 led us to propose that the interaction of the C-terminal domain of NIK with downstream Ste11 kinases (DMKKK) was critical for NIK and other GCK family members to activate the JNK MAP kinase module (43). However, studies on NIK were performed in assays in which NIK protein was expressed at high levels, and under these circumstances, NIK is able to mediate JNK activation independent of an upstream activating signal. The requirement for both the C-terminal domain and the kinase activity of Msn to promote dorsal closure indicates that these domains are required in order for GCK family members to activate JNK in a physiologically relevant setting and suggests that a Drosophila Ste11 kinase also couples Msn to JNK activation and dorsal closure.
In addition to its role in JNK activation and dorsal closure, Msn is critical for the correct targeting of photoreceptor axons in Drosophila. Thus, our data indicate that msn is important in vivo for regulating at least two distinct biological events: dorsal closure and photoreceptor axon pathfinding. Interestingly, the upstream molecules that regulate msn in these two pathways are distinct, since a mutation eliminating the function of Msn in axon guidance does not affect its activity in dorsal closure. One molecule that may act upstream of Msn in the pathway leading to JNK activation and dorsal closure is a DTRAF; members of our group have recently shown that DTRAF1 can interact with Msn to activate the JNK pathway in cell lines (25). Mutation of a PXXP motif in Msn prevents it from binding to Dock and from rescuing photoreceptor axon pathfinding, indicating that Dock and/or related SH3 domain-containing molecules may act in concert with Msn in this process.
We do not yet know the mechanism by which upstream factors regulate Msn. A common requirement for Msn activation may involve its increased local concentration. This could occur either by the recruitment of Msn to phosphotyrosine-containing proteins or by DTRAF1-induced aggregation of Msn, thereby allowing juxtaposed Msn molecules present in the complex to transphosphorylate and activate each other. Alternatively, the finding that deletion of the region between the kinase and C-terminal domains of Msn leads to its constitutive activation raises the possibility that upstream signals activate Msn by inducing a conformational change and/or displacing a negative regulator bound to this region.
Msn does not behave as a simple downstream effector of Dock.
The ability of axons to make precise connections during development requires the axonal growth cone, localized to the leading edge of projecting axons, to interpret multiple guidance cues that ultimately navigate axons to their destinations (45). Changes in the growth cone's actin cytoskeleton and/or the affinity for binding of the integrins to the matrix are thought to be the key elements whereby guidance cues regulate the path taken by developing axons (45). The finding that dock is required for Drosophila photoreceptor axon guidance and targeting provided a starting point for beginning to dissect the intracellular signaling pathways that are activated at the growth cone to mediate these guidance cues. Dock is a member of a large family of adapter proteins consisting essentially of SH2 and SH3 domains, of which the prototypic member is Grb2. SH2-containing adapter molecules regulate signaling pathways by coupling catalytic molecules bound to their SH3 domains to phosphotyrosine-containing proteins.
While a number of proteins that bind the SH3 domains of Nck and Dock have been identified, which of these serve as targets in vivo has been difficult to resolve. In contrast to the SH2-SH3 adapter molecule Grb2, for which interaction with the downstream SH3 binding partner Sos was demonstrated using genetic evidence, the physiologically relevant binding partners for Nck and Dock and the downstream signaling pathways have only recently begun to be defined (38). In this regard, the Ste20 kinase Pak has been shown to interact with Dock, and expression of a myristylated form of Pak can partially rescue the dock mutant phenotype (18). We show here that Msn also binds to the SH3 domains of Dock and that the amino acids that mediate this binding are required for the correct targeting of photoreceptor axons.
However, our findings do not provide conclusive evidence that msn functions downstream of dock in photoreceptor targeting. Rather, our studies highlight the complex role of msn in photoreceptor targeting and suggest that unraveling the exact functions of msn in this process is unlikely to be simple. For example, it is likely that Msn functions in both photoreceptor cells and the brain. The severe photoreceptor axon guidance defects observed when msn mutants are rescued with UAS-msn(P656A, P659A) are stronger than those caused by either the absence of msn in the eye or the complete loss of function of dock. Interaction between Msn and an SH3 domain-containing protein or proteins other than Dock in nonphotoreceptor cells, such as those in the brain, is a likely explanation. Although we have shown that photoreceptor development in most of the eye disc is normal when rescue is carried out with UAS-msn(P656A, P659A), defects in brain development in these larvae may contribute to the axon guidance phenotype; an enhancer trap insertion in msn shows expression in the optic lobes as well as in the eye (data not shown). This hypothesis is difficult to test directly, as many aspects of optic lobe development are directly dependent on retinal innervation. Because the defects in photoreceptor axonal targeting are specific to a mutation in a proline motif that matches consensus SH3 binding motifs (49), this phenotype is probably due, at least in part, to the loss of interaction of Msn with an SH3 domain-containing protein.
Our finding that the dock phenotype is enhanced by the presence of msn suggests that the signaling pathways regulated by msn, which are critical for the correct targeting of R-cell axons, intersect with the signaling pathways regulated by dock. However, this interaction does not clarify whether msn functions on the same pathway as dock or on a parallel pathway. In addition, expression of a form of Msn that we have shown to be constitutively active is not sufficient to rescue the dock phenotype. One possible explanation for these data is that msn acts downstream of dock but is not the only downstream mediator of its function. As discussed above, the Ste20 kinase Pak has been shown to interact with Dock, and expression of a myristylated form of Pak can partially rescue the dock mutant phenotype. Interestingly, this form of Pak predominantly rescues the expansion of growth cones in the medulla, a process that does not appear to require msn function in the photoreceptor axons. It is possible that msn and Pak mediate separable functions of dock in photoreceptor cells. An alternative possibility is that the function of Msn expressed in photoreceptor cells is mediated by the binding of Msn to an SH3 domain-containing protein other than Dock. The difference in the phenotypes caused by loss of msn and loss of dock in the photoreceptor axons would support this hypothesis. Mutations in the gene encoding such a hypothetical protein, which would function on a pathway parallel to the dock pathway, have yet to be identified.
While this report was under review, Ruan et al. (37) reported a role for msn in photoreceptor axonal targeting and Dock signaling. However, in contrast to our findings that msn mutant R1 to R6 axons terminate prematurely, Ruan et al. reported that the R1 to R6 axons overshoot the lamina and terminate in the medulla. In addition, they found that overexpression of Msn in photoreceptor cells in dock mutants reversed the overshoot of the R1 to R6 axons. These findings and other data led them to conclude that dock and msn act in the same pathway. The reason for the discrepancy between the findings reported here and their results is not clear at present. One possibility is that the expression of Msn was much higher in the studies by Ruan et al., enabling them to see rescue of the dock mutant phenotype; they used an enhancer promoter line containing a UAS element inserted in the 5′ promoter region of msn to overexpress msn. However, Ruan et al. also found that overexpression of msn in a wild-type background led to the premature termination of many R-cell growth cones, essentially the same phenotype as they observed when msn was overexpressed in dock mutants; thus, it is not clear that this in fact constitutes rescue of the dock phenotype. In contrast, expression of a myristylated form of Pak largely rescues the dock mutant phenotype without inducing additional defects (18).
Possible regulators and effectors for Msn in axon guidance.
An attractive hypothesis is that Dock and/or related SH3 domain-containing molecules function as adapters to couple Msn to tyrosine-phosphorylated proteins in response to signaling by a receptor tyrosine kinase localized at the axonal growth cone. Eph receptors, which constitute the largest family of receptor tyrosine kinases, are good candidates for receptors that may function at the axonal growth cone to regulate changes in the actin cytoskeleton and/or adhesion of integrins to the matrix that ultimately facilitate the correct targeting of retinal axons. In this regard, we have recently found that NIK kinase activity is activated in mammalian cells by the EphB1 and EphB2 receptors and that NIK couples EphB1 to both JNK and integrin activation (2a). However, although a Drosophila Eph receptor kinase (DEK) is expressed on retinal axons, misexpression and overexpression of wild-type DEK or a kinase-defective form of DEK do not affect axonal pathfinding in Drosophila (39).
The intracellular signals activated downstream of Msn that mediate the correct pathfinding of photoreceptor axons are not yet known. The finding that regulation of the actin cytoskeleton is critical for growth cones to navigate correctly suggests that Msn may control the targeting of photoreceptor axons by regulating the actin cytoskeleton (45). Our studies have indicated that the downstream pathways regulated by Msn are likely to be diverse and will not be limited to the activation of JNK. This is suggested by the finding that msn is required for oogenesis, while bsk and hep are not, and that ventral defects can be induced by a kinase-defective form of Msn, although maternal and zygotic bsk mutants do not show such a phenotype. We do not think that msn directs axonal guidance via activation of the JNK MAP kinase pathway, because photoreceptor axonal targeting showed only minor defects, including occasional overshooting of R1 to R6 axons, in bsk1 mutant clones made in a Minute background in the eye disc (data not shown). However, because bsk1 is not a complete loss-of-function mutant, these studies cannot definitively rule out a role for JNK. Small clones with mutations in both hep and the other Drosophila p38 MAPK kinase encoded by licorne (44a) also showed an apparent overshoot of R1 to R6 axons, resembling the dock phenotype but not the msn phenotype (data not shown). However, we were unable to rescue the dock phenotype with an activated allele of hep, indicating that activation of the JNK pathway is not sufficient to rescue the dock phenotype (data not shown). While a direct link between Pak family Ste20 kinases and the actin cytoskeleton has been shown, a direct link between GCK family Ste20 kinases and the actin cytoskeleton has not yet been demonstrated (27, 30, 40). Thus, the ability to use genetics to identify and validate potential targets of Msn should provide a valuable tool to uncover not only the relevant biological functions regulated by Ste20 kinases but also their physiological downstream targets.
ACKNOWLEDGMENTS
We thank Barry Dickson, Paul Garrity, Naoto Ito, S. Noselli, M. Mlodzik, and Larry Zipursky for fly stocks and reagents and Zhai Li for technical help.
This work is supported by grants from the NIDDK and American Diabetes Association to E.Y.S. and a grant from the Edward Mallinckrodt, Jr., Foundation to J.E.T.
Y.-C.S. and C.M.-Z. contributed equally to this work.
REFERENCES
- 1.Affolter M, Nellen D, Nussbaumer U, Basler K. Multiple requirements for the receptor serine/threonine kinase thick veins reveal novel functions of TGFβ homologs during Drosophila embryogenesis. Development. 1994;120:3105–3117. doi: 10.1242/dev.120.11.3105. [DOI] [PubMed] [Google Scholar]
- 2.Aronheim A, Engelberg D, Li N, al-Alawi N, Schlessinger J, Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994;78:949–961. doi: 10.1016/0092-8674(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 2a.Becker E, Huynh-Do U, Holland S, Pawson T, Daniel T O, Skolnik E Y. Nck-interacting Ste20 kinase couples EPH receptors to c-Jun N-terminal kinase and integrin activation. Mol Cell Biol. 2000;20:1537–1545. doi: 10.1128/mcb.20.5.1537-1545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand A H, Perrimon N. Targeting gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 4.Brummel T J, Twombly G, Marques G, Wrana J L, Newfeld S J, Attisano L, Massague J. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell. 1994;78:251–261. doi: 10.1016/0092-8674(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 5.Burbelo P D, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29072–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 6.Campos-Ortega J, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin, Germany: Springer-Verlag; 1985. [Google Scholar]
- 7.Desai C J, Garrity P A, Keshishian H, Zipursky S L, Zinn K. The Drosophila SH2-SH3 adapter protein Dock is expressed in embryonic axons and facilitates synapse formation by the RP3 motoneuron. Development. 1999;126:1527–1535. doi: 10.1242/dev.126.7.1527. [DOI] [PubMed] [Google Scholar]
- 8.Freeman M. Cell determination strategies in the Drosophila eye. Development. 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- 9.Fujita S C, Zipursky S L, Benzer S, Ferrus A, Shotwell S L. Monoclonal antibodies against the Drosophila nervous system. Proc Natl Acad Sci USA. 1982;79:7929–7933. doi: 10.1073/pnas.79.24.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galisteo M L, Chernoff J, Su Y C, Skolnik E Y, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 11.Garrity P A, Rao Y, Salecker I, McGlade J, Pawson T, Zipursky S L. Drosophila photoreceptor axon guidance and targeting requires the dreadlocks SH2/SH3 adapter protein. Cell. 1996;85:639–650. doi: 10.1016/s0092-8674(00)81231-3. [DOI] [PubMed] [Google Scholar]
- 12.Glise B, Noselli S. Coupling of Jun amino-terminal kinase and Decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 1997;11:1738–1747. doi: 10.1101/gad.11.13.1738. [DOI] [PubMed] [Google Scholar]
- 13.Glise B, Bourbon H, Noselli S. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. Methods Enzymol. 1992;194:12–18. [PubMed] [Google Scholar]
- 16.Harden N, Lee J, Loh H-Y, Ong Y-M, Tan I, Leung T, Manser E, Lim L. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol. 1996;16:1896–1908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 18.Hing H, Xiao J, Harden N, Lim L, Zipursky S L. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 19.Holland S J, Peles E, Pawson T, Schlessinger J. Cell-contact-dependent signalling in axon growth and guidance: Eph receptor tyrosine kinases and receptor protein tyrosine phosphatase beta. Curr Opin Neurobiol. 1998;8:117–127. doi: 10.1016/s0959-4388(98)80015-9. [DOI] [PubMed] [Google Scholar]
- 20.Hou X S, Goldstein E S, Perrimon N. Drosophila Jun relays the Jun amino-terminal kinase signal transduction pathway to the Decapentaplegic signal transduction pathway in regulating epithelial cell sheet movement. Genes Dev. 1997;11:1728–1737. doi: 10.1101/gad.11.13.1728. [DOI] [PubMed] [Google Scholar]
- 21.Isakoff S J, Cardozo T, Andreev J, Li Z, Ferguson K M, Abagyan R, Lemmon M A, Aronheim A, Skolnik E Y. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 1998;17:5374–5387. doi: 10.1093/emboj/17.18.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kockel L, Zeitlinger J, Staszewski L M, Moldzik M, Bohmann D. Jun in Drosophila development: redundant and nonredundant functions and regulation by two MAPK signal transduction pathways. Genes Dev. 1997;11:1748–1758. doi: 10.1101/gad.11.13.1748. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakis J M. Signaling by the germinal center kinase family of protein kinases. J Biol Chem. 1999;274:5259–5262. doi: 10.1074/jbc.274.9.5259. [DOI] [PubMed] [Google Scholar]
- 24.Letsou A K, Arora K, Wrana J L, Simin K, Twombly V, Jamal J, Staehling-Hampton M B, Gelbert W M, Massague J, O'Connor M B. Drosophila dpp signaling is mediated by the punt gene product: a dual ligand binding type II receptor for the TGFβ receptor family. Cell. 1995;80:899–908. doi: 10.1016/0092-8674(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Su Y C, Becker E, Treisman J, Skolnik E Y. A Drosophila TNF-receptor-associated factor (TRAF) binds the ste20 kinase Misshapen and activates Jun kinase. Curr Biol. 1999;9:101–104. doi: 10.1016/s0960-9822(99)80023-2. [DOI] [PubMed] [Google Scholar]
- 26.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 27.Manser E, Lim L. Roles of PAK family kinases. Prog Mol Subcell Biol. 1999;22:115–133. doi: 10.1007/978-3-642-58591-3_6. [DOI] [PubMed] [Google Scholar]
- 28.Martin G A, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Newsome T P, Asling B, Dickson B J. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 29.Noselli S. JNK signaling and morphogenesis in Drosophila. Trends Genet. 1998;14:33–38. doi: 10.1016/S0168-9525(97)01320-6. [DOI] [PubMed] [Google Scholar]
- 30.Obermeier A, Ahmed S, Manser E, Yen S C, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Leary D D, Wilkinson D G. Eph receptors and ephrins in neural development. Curr Opin Neurobiol. 1999;9:65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 32.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 33.Rao Y, Zipursky S L. Domain requirements for the Dock adapter protein in growth-cone signaling. Proc Natl Acad Sci USA. 1998;95:2077–2082. doi: 10.1073/pnas.95.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riesgo-Escovar J, Hafen E. Drosophila Jun kinase regulates expression of decapentaplegic via the ETS-domain protein Aop and the AP-1 transcription factor DJun during dorsal closure. Genes Dev. 1997;11:1717–1727. doi: 10.1101/gad.11.13.1717. [DOI] [PubMed] [Google Scholar]
- 35.Riesgo-Escovar J R, Jenni M, Fritz A, Hafen E. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- 36.Riesgo-Escovar J R, Hafen E. Common and distinct roles of DFos and DJun during Drosophila development. Science. 1997;278:669–672. doi: 10.1126/science.278.5338.669. [DOI] [PubMed] [Google Scholar]
- 37.Ruan W, Pang P, Rao Y. The SH2/SH3 adaptor protein dock interacts with the Ste20-like kinase misshapen in controlling growth cone motility. Neuron. 1999;24:595–605. doi: 10.1016/s0896-6273(00)81115-0. [DOI] [PubMed] [Google Scholar]
- 37a.Ruperte E T, Nellen M D, Affolter M, Basler K. An absolute requirement for both type II and type I receptors, punt and thick veins, for dpp signaling in vivo. Cell. 1995;80:890–898. doi: 10.1016/0092-8674(95)90292-9. [DOI] [PubMed] [Google Scholar]
- 38.Schlessinger J. SH2/SH3 signaling molecules. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 39.Scully A L, McKeown M, Thomas J B. Isolation and characterization of Dek, a Drosophila eph receptor protein tyrosine kinase. Mol Cell Neurosci. 1999;13:337–347. doi: 10.1006/mcne.1999.0752. [DOI] [PubMed] [Google Scholar]
- 40.Sells M A, Knaus U G, Bagrodia S, Ambrose D M, Bokoch G M, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 41.Sluss K K, Han Z, Barrett T, Davis R J, Ip Y T. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 42.Spradling A C, Rubin G M. Transposition of cloned P-elements into Drosophila germline chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 43.Su Y, Han J, Xu S, Cobb M, Skolnik E Y. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO J. 1997;16:1279–1290. doi: 10.1093/emboj/16.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su Y-C, Treisman J E, Skolnik E Y. The Drosophila Ste20-related kinase misshapen is required for embryonic dorsal closure and acts through a JNK MAPK module on an evolutionarily conserved signaling pathway. Genes Dev. 1998;12:2371–2380. doi: 10.1101/gad.12.15.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Suzanne M, Irie K, Glise B, Agnes F, Mori E, Matsumoto K, Noselli S. The Drosophila p38 MAPK pathway is required during oogenesis for egg asymmetric development. Genes Dev. 1999;13:1464–1474. doi: 10.1101/gad.13.11.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka M, Gupta R, Mayer B J. Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol Cell Biol. 1995;15:6829–6837. doi: 10.1128/mcb.15.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Treisman J E, Ito N, Rubin G M. misshapen encodes a protein kinase involved in cell shape control in Drosophila. Gene. 1997;186:119–125. doi: 10.1016/s0378-1119(96)00694-4. [DOI] [PubMed] [Google Scholar]
- 47.Xu T, Rubin G M. Analysis of genetic mosaics in developing and adult Drosophila tissue. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 48.Young P E, Pesacreta T C, Kiehart D P. Dynamic changes in the distribution of cytoplasmic myosin during Drosophila embryogenesis. Development. 1991;111:1–14. doi: 10.1242/dev.111.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Yu H, Chen J K, Feng S, Dalgarno D C, Brauer A W, Schreiber S L. Structural basis for the binding of proline-rich peptides to the SH3 domains. Cell. 1994;76:933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]