Abstract
Although the functions of the peripheral nervous system in whole body homeostasis and sensation have been understood for many years, recent investigation has uncovered new roles for innervation in the musculoskeletal system. This review centers on advances regarding the function of nerves in the development and repair of two connected tissues: tendon and bone. Innervation in healthy tendons is generally confined to the tendon sheaths, and tendon-bone attachment units are typically aneural. In contrast to tendon, bone is an innervated and vascularized structure. Historically, the function of abundant peripheral nerves in bone has been limited to pain and some non-painful sensory perception in disease and injury. Indeed, much of our understanding of peripheral nerves in tendons, bones, and entheses is limited to the source and type of innervation in healthy and injured tissues. However, more recent studies have made important observations regarding the appearance, type, and innervation patterns of nerves during embryonic and postnatal development and in response to injury, which suggest a more expansive role for peripheral nerves in the formation of musculoskeletal tissues. Indeed, tendons and bones develop in a close spatiotemporal relationship in the embryonic mesoderm. Models of limb denervation have shed light on the importance of sensory innervation in bone and to a lesser extent, tendon development, and more recent work has unraveled key nerve signaling pathways. Furthermore, loss of sensory innervation also impairs healing of bone fractures and may contribute to chronic tendinopathy. However, more study is required to translate our knowledge of peripheral nerves to therapeutic strategies to combat bone and tendon diseases.
Keywords: Tendon, bone, nerve, neurotransmitter, innervation
1.0. INTRODUCTION
The association of peripheral nerves with skeletal pain has been understood for some time, with the early identification of nociceptive fibers expressing Substance P (SP) in the human periosteum in the 1980s1. Indeed, initial work to characterize peripheral nerves in bone was motivated by a limited understanding of the mechanisms associated with pain in osteoarthritis, Paget’s disease, and bone cancers2. While innervation of healthy musculoskeletal tissues by peripheral nerves is low to moderate, rapid nerve ingrowth, increases in nerve density, and arborization are remarkable hallmarks of tissue formation and the response to injury and mechanical loading3; 4. Furthermore, recent evidence suggests a more expansive role for peripheral nerves in the spatiotemporal coordination of molecular processes involved in tissue formation and homeostasis. This review presents a brief historical perspective of peripheral nerves in tendons, bones, and tendon-bone attachments as well as the current understanding of the role of nerves and nerve-dependent signaling pathways in the development and healing of these distinct musculoskeletal tissues.
2.0. ANATOMY
Tendons are fibrous connective tissues that transfer force from muscles to bones to enable ambulation. These tissues are primarily composed of a dense, collagenous matrix that consists mainly of collagen type I (65–80%) and elastin (1–2%)5; 6. Bundles of collagen type I microfibrils aggregate to form fibrils, which progressively assemble to form collagen fibers and fascicles, forming the basis of the hierarchical structure of tendon. A connective tissue sheath termed the endotenon surrounds collagen fascicles and is contiguous with the outer epitenon that envelopes the tendon unit. Finally, some tendons are coated by either an elastic, fibrous sheath termed the paratenon or a synovial sheath to augment collagen fiber gliding7; 8.
A complex fibrocartilaginous connective tissue termed the enthesis attaches tendon to bone. While some entheses are primarily fibrous and non-cartilaginous9, the majority of tendon-bone attachment sites employ fibrocartilaginous entheses. Generally, the latter consists of four zones of connective tissue - fibrous tissue originating from tendon, uncalcified fibrocartilage, calcified fibrocartilage, and subchondral bone10. Entheses enable tendons and bones to function as a single unit to facilitate force transfer and joint flexion. While anatomically distinct from tendons, they are derived from tendon precursor cells, can be identified by tendon biomarkers, and are often involved in tendon injuries.
Finally, bone is a complex, hierarchically organized structural composite, comprising both organic (mainly collagen) and inorganic (bone apatite) components. Long bones can be divided into three parts: the diaphysis, which is the shaft of the bone and is comprised of densely mineralized cortical bone, the metaphysis which includes both cortical and trabecular bone, and the extremity of long bones known as the epiphysis which are comprised of trabecular bone covered by a layer of cortical bone. Like tendon, bone is also covered by a thin connective tissue sheath, known as the periosteum, which houses the majority of blood vessels and nerve fibers. However, nerve axons are also found in the bone marrow and traverse nutrient canals in mineralized bone matrix11.
Unlike bone, typical tendon tissue is hypoxic, aneural, and devoid of blood vessels, which may explain the tendon’s general low metabolic turnover and poor healing potential. Most of the nerves and blood vessels found in healthy tendons are localized to the tendon sheaths, namely the endotenon, epitenon, paratenon, and the mesotendons of the synovial sheaths8; 12; 13. As expected, the underlying cartilage in the peri-tendinous enthesis is aneural and avascular, but this can be attributed to the increased expression of the axonal growth inhibitor aggrecan in this region, typical of articular cartilage and other cartilaginous tissues14.
Nonetheless, both sensory and autonomic nerves have been observed in tendons, as reviewed previously15–17. Sensory nociceptors are responsible for painful sensation in tendons and entheses. These nerve fibers are often identified by the expression of SP and calcitonin gene-related peptide (CGRP), neuropeptides synthesized in the cell bodies of sensory nerves that are released from axons in extracellular vesicles. Both SP and CGRP as well as their respective receptors, NK1 and RAMP, have been identified in healthy tendon sheaths, are commonly associated with blood vessels in the sheath, and are primarily involved with the intrinsic tendon healing response post-injury. Encapsulated sensory nerve endings including Pacinian and Ruffini corpuscles have been detected in fatty tissue of the endotenon, and more conspicuously in the epitenon close to the enthesis18. Neurotrophic tyrosine kinase receptors (Trk) are lowly expressed in intact tendons, but the proportion of sensory nerves that express different Trk receptors are unknown19. Sympathetic adrenergic nerve fibers, which appear to regulate blood flow in tendons, can be identified by the presence and synthesis of the pro-inflammatory neurotransmitters epinephrine, norepinephrine, and neuropeptide Y (NPY), and/or expression of tyrosine hydroxylase (TH). Adrenergic receptors, a class of G-protein coupled receptors that bind epinephrine and norepinephrine, have been identified in tendon cells and on closely associated vasculature. Likewise, cholinergic nerves are identified by and modulate release of the anti-inflammatory neurotransmitters acetylcholine and vasoactive intestinal polypeptide (VIP) in response to inflammatory reflexes in tendons16; 20; 21.
Similarly, mature bone is also innervated by both sensory and autonomic peripheral nerve fibers. The sensory afferent peptidergic fibers expressing CGRP and/or SP in bone are either small diameter, unmyelinated axons (0.2–1.5 um) or medium diameter, myelinated axons (1–5 um)22; 23. Surprisingly, up to 80% of the sensory nerves in bone express TrkA, the neurotrophic tyrosine kinase receptor with high affinity for nerve growth factor (NGF)24–26. These sensory nerves can relay signals to the central nervous system in response to external stimuli, such as mechanical loads or noxious heat. Sympathetic and parasympathetic nerve fibers that innervate bone are generally small diameter, unmyelinated axons. These nerves coordinate the involuntary responses in bone required to maintain homeostasis and vascular tone. While the majority of autonomic fibers expressing acetylcholine in bone originate from parasympathetic ganglia, some cholinergic fibers may also be of sympathetic or peptidergic origin, such as found in the periosteum of the rat sternum27.
3.0. DEVELOPMENT
Although the development of tendon and bone are generally discussed separately, these dissimilar musculoskeletal tissues develop in close spatiotemporal coordination. In the embryonic limb bud, chondroprogenitors first populate and establish a primary cartilaginous template for future bone formation28. Bony “eminences” or clusters of scleraxis (Scx)+/Sox9+ progenitor cells induced by signals from the overlying ectoderm form on surfaces of bone anlage and serve as sites of attachment for tendons. Eventually, Scx+ progenitor cells segregate from Sox9+ chondrocytes to differentiate into tenocytes and develop into functional tendons attached to bones at their eminences28. Differentiation of fibrocartilage from tendons occurs postnatally, and appearance of distinct entheseal zones is first observed at P21–28 in mice29.
The limb itself has long been utilized to study the patterning and development of musculoskeletal tissues in the embryo30; 31, however the importance of nerves and neuronal pathways in tendon development specifically is unclear. Nerve ingrowth to the early chick limb mesenchyme originates from the plexus and appears around day 5 (stages 24–25) in development32; 33, suggesting that peripheral nerves exert some degree of influence on tendon development and differentiation at this point. Nerve ingrowth to tendons may originate from regions localized to early, undifferentiated myogenic precursors, from cutaneous nerves of the peripheral musculature, directly from the plexus, or an alternate source. In fact, previous studies suggest that the segmental patterning of the limb skeleton may be derived from a single spinal nerve34; 35. Nonetheless, the specific type, contribution and origins of nerve ingrowth to limb tendons over the time course of development remains poorly understood.
Nonetheless, the extracellular matrix glycoproteins tenascin and versican are widely expressed in tendon and provide some insight to peripheral sensory innervation in the embryonic limb36; 37. In early chick limb buds, tenascin expression is concomitant with peripheral nerves originating from the spinal cord and invading the limb, but at embryonic stage 28 and beyond tenascin is primarily associated with the mesenchyme36. Tenascin mRNA expression is also found adjacent to early tendon primordia in normal chick limb buds. This suggests that tenascin may be associated with transient, regulatory signals for nerve ingrowth and patterning in developing chick tendons. In contrast to tenascin, strong expression of versican was previously found in mesenchymal condensations and chondrocyte precursors of the mouse limb bud, but absent in regions of axonal ingrowth and myogenic precursors37. Hence, versican expression in developing tendon primordia appears to inhibit axonal migration to these regions. Thus, the expression of these extracellular matrix proteins in tendon appears to act to promote or inhibit innervation, similar to traditional neurotrophins. However, in denervated wing bud grafts, tendon primordia appears to develop normally, with steadily increasing tenascin expression over the course of development36. As a result, more study is necessary to delineate the source and neurotrophic roles of tenascin and versican in early limb development.
In contrast to the limited effect of denervation on tendon development, there is a strong association between innervation and muscle mass during limb development. Denervation or restricted innervation of the limb is directly correlated to loss of muscle mass, with lesser impacts on tendon and other soft tissues38–40. In one study, a foil barrier system used to restrict nerve growth to embryonic chick limbs resulted in muscular atrophy without effects tendon morphology and differentiation34. Similarly, surgical excision of the peripheral neural tissue in salamander larvae resulted in aneural limbs with significantly lower muscle mass41. Furthermore, early tendon development was unaffected by the loss of muscle mass, and tendons were detected even in aneural limbs even with very little muscle mass. However, by day 65, the same tendons exhibited lower cellularity and collagen expression compared to normally innervated tendons41. In total, the loss of innervation in embryonic limbs may impact developing tendon morphology and other regulatory pathways in tendon in addition to muscle volume.
Scx, a bHLH transcription factor, is fundamental for the formation of tendon and expressed in both early as well as late differentiated tendons42. In the mouse limb bud, Scx is first induced as dorsal and ventral patches; however, by E12.5, an organized alignment of Scx-expressing progenitor cells can be detected between the muscles and cartilage43. Scx-null mutant mouse embryos are viable, but exhibit moderate to severe structural defects in the limb and tail tendons with either reduced or complete loss of function44. Scx also promotes tenascin expression in response to Fgf4 found at early tendon-muscle attachment sites45. Tenascin, as described earlier has been associated with early nerve ingrowth in chick limbs36. This suggests that Scx itself may regulate the early neuronal ingrowth and patterning of limb tendons, by inducing tenascin expression. Loss of Scx in the limb tendons of Scx-null mice may be impairing innervation necessary for differentiation and maturation of tendon in late embryonic development. However, future studies are necessary to assess this directly.
Adjacent to Scx+ progenitor cells in the embryonic mesenchyme, bone development commences with a cluster of osteoprogenitor cells also known as a mesenchymal condensation46. Although some bones, such as the flat bones of the skull, develop through a process of intramembranous ossification, most bones arise from a cartilaginous template formed when progenitor cells in the condensation differentiate to chondrocytes47. Actively proliferating chondrocytes in this region subsequently enlarge and differentiate to osteoblasts48. Alternatively, they may undergo apoptosis, then be resorbed and replaced by peripheral perichondrial cells that eventually become osteoblasts. Simultaneously, differentiating osteoblasts secrete a collagen type I-rich matrix that is invaded by blood vessels, endothelial cells, osteoclasts, and osteoblast precursors to form the primary ossification centers in long bones49; 50. The remaining cells in the perichondrium or outer layer of the mesenchymal cluster also become osteoblasts, forming a characteristic “bone collar” of nascent cortical bone46. The unorganized developing bone is eventually remodeled towards lamellae, which are layers of bone with anisotropically aligned type I collagen fibrils interspersed with osteocytes51.
Research to determine the precise timeline for when nerve axons successfully invade developing bone has been ongoing for some time. Early studies of peripheral innervation successfully identified PGP9.5+ and GAP43+ nerve fibers at E21 in the perichondrium of the long bones in rats52; 53 (Fig. 1B). At P3, abundant CGRP+ sensory afferent fibers are visible in the periosteum and bone marrow of the diaphysis and closely associated with blood vessels53 (Fig. 1C). Autonomic fibers first appear in long bone postnatally. Sympathetic NPY+ nerve fibers are first visible in the bone marrow of the mouse femur and tibia at P6 (Fig. 1D). Both NPY+ and TH+ fibers are evident in perichondrium of the epiphysis and the cartilage canals at P1054.
Figure 1: Nerves in development.

At E14.5 in the mouse embryo, TrkA+ sensory fibers are first visible in the perichondrium of long bones and may be found in close vicinity of early tendon primordia (A). At E21, abundant GAP43+ and PGP9.5+ nerves are closely associated with peripheral arteries near the POC (B). P3 marks the early ingrowth of CGRP+ sensory nerves from the periosteum into the POC (C). At P6, the first NPY+ sympathetic nerves can be visualized in the bone marrow (D).
To understand the potential role of these peripheral nerves in bone development, researchers have utilized partial denervation and complete nerve resection of developing limbs as well as systemic administration of neurotoxic agents. In one study, complete neurectomy of the feet in one week-old rats resulted in significant reductions in metatarsal bone length thirty days after surgery55. In contrast, treatment of rats with capsaicin or guanethidine in the immediate postnatal period did not affect tibial bone growth or structure when compared to vehicle treated rats56. More recently, capsaicin treatment of neonatal mice resulted in only minor reductions in trabecular bone thickness at the femoral metaphysis at 4, 8, and 12 weeks of age compared to vehicle treated mice, without affecting the mechanical properties of bone or bone formation rate at these time points57. In a subsequent study, the same group showed that neonatal capsaicin treatment resulted in slightly smaller bones, but did not alter parameters of skeletal adaptation to compressive loading58. However, it is important to note that capsaicin is a TRPV1 receptor agonist, and treatment results in sensory denervation of these specific nerves in a dose-dependent manner59. The loss of SP in response to capsaicin treatment during early embryonic development may be overcome by compensatory mechanisms of NGF synthesis and transport to sensory ganglia in postnatal development, thereby restoring SP expression in peripheral axon endings invading bone60. Furthermore, a significant number of sensory nerve fibers innervating bone are myelinated axons that may be resilient to damage below a certain dose threshold of capsaicin61. In total, chemical denervation models appear limited in their ability to recapitulate the effects of denervation on bone development.
Recent evidence suggests that NGF is the major skeletal neurotrophic factor expressed during development to drive innervation of long bones. Nerves expressing TrkA, the high affinity receptor for NGF, have been observed in embryonic mouse femurs as early as E14.5, coincident with the first appearance of primary ossification centers in the diaphysis3 (Fig. 1A). These nerves appear to be established in bone by NGF expressed by the perichondrial cells closely associated with CD31+ vessels adjacent to the sites of incipient ossification3, a paradigm consistent with in vitro studies illustrating NGF expression by mesenchymal progenitor cells62; 63. In postnatal development, the requirement of NGF by nerves for survival appears to diminish64. To determine the functional significance of NGF-TrkA neurotrophic signaling in development, TrkA signaling was inhibited using a chemical-genetic approach. In this case, bones were observed to have significantly reduced nerve density and vascularization as well as significantly decreased bone mass in early postnatal life3. These results are consistent with previous work illustrating that sensory innervation coordinates vascularization in developing embryonic skin65. In addition, NGF-TrkA signaling may be required to induce the release of osteoinductive cues, such as Wnt ligands, which are necessary for normal bone development66; 67. Furthermore, recently work has uncovered that both NGF and TrkA are expressed in differentiating chondrocytes of the early limb bud and late epiphyseal growth plates of long bones68, suggesting a more expansive role for NGF-TrkA signaling in developing bone.
4.0. HEALING
Musculoskeletal injuries involving tendons, bones, and tendon-bone attachments are the leading cause of morbidity and mortality worldwide, and up to 28 million people each year are affected in the United States alone69. In the previous section, we discussed the role of innervation in the development of tendon and bone. In fact, there are many parallels that can be drawn between development and tissue repair70. Traditionally, fracture healing has been considered through this lens, in which much of the signaling and stages recapitulates endochondral bone formation71. For example, aggregation of mesenchymal cells to form condensations in the embryo are mediated by TGFβ and BMP – the same growth factors that activate cell migration and contraction to facilitate fracture repair in adult bone70. In contrast to bone, only fetal tendons can regenerate completely72. Adult tendon repair is less robust and largely results in the formation of scar, leaving up to 40% of repaired tendons non-functional. We refer the reader to recent reviews that have examined bone and tendon repair in great detail73; 74. Here, we will examine the specific contribution of nerves and neurotrophic signaling to the healing responses of tendon and bone.
Acute tendon and enthesis injuries are sustained in a single traumatic event, such as sports-related injuries involving the Achilles tendon, patellar tendon, and/or tendons attached to the lateral epicondyle in the elbow. In contrast, a chronic injury is characterized by degenerative changes in response to the progressive accumulation of microdamage over time by overuse or aging. In either case, the tendon and enthesis healing response can generally be examined in three sequential phases – the inflammatory phase, the proliferative phase, and the remodeling and/or bone formation phase74; 75. Perhaps related to our poor understanding regarding the action of peripheral nerves on tendon development, the contribution of innervation to the different phases of healing is not well understood.
In an acute tendon injury, damaged blood vessels can result in hemorrhaging and edema that leads to the characteristic discoloration or “reddening” of the tendon (Fig. 2A). An initial inflammatory response involves the influx of white blood cells (mainly platelets and neutrophils) to the injury site from peripheral vessels, presumably in response to the release of chemotactic factors by local tenocytes. Within this inflammatory milieu, early extension and ingrowth of GAP43+ and PGP9.5+ nerve fibers has been observed from the paratenon and proximal musculotendinous junctions to the tendon proper76 (Fig. 2B). Abundant sensory nerve axons expressing SP and CGRP appear to co-localize with blood vessels in the paratenon, but whether they infiltrate the injury site at this stage is uncertain77. Next, small blood vessels permeate the injury site, providing a conduit for the transport of inflammatory and mesenchymal progenitor cells. Depending on the severity of the injury and response, inflammation subsides one to six weeks after injury, leading into the proliferative phase of repair.
Figure 2: Nerves in tendon healing.
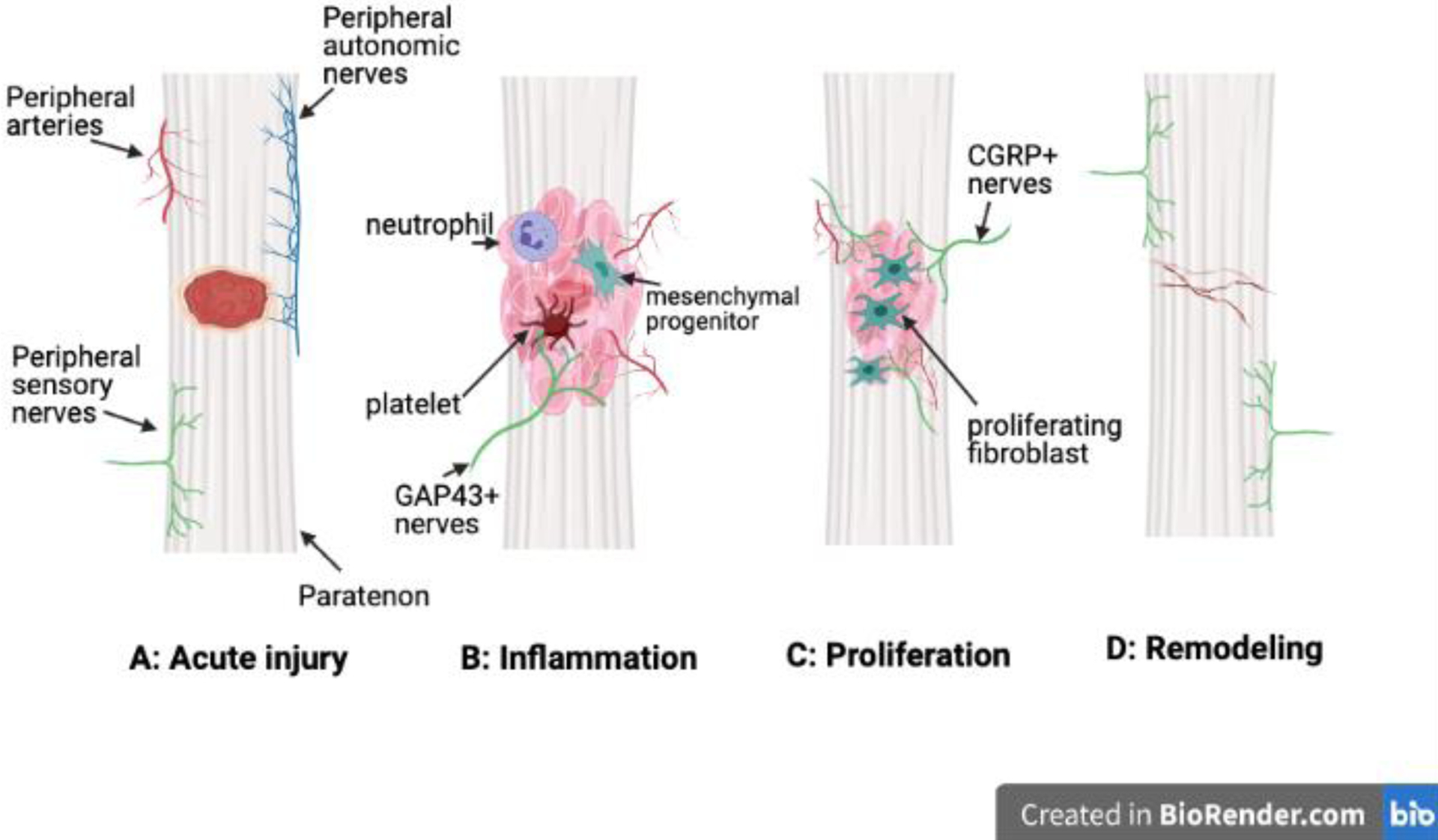
Acute injury results in hemorrhaging and edema (A). Inflammation is marked by the extension of GAP43+ axons from the paratenon to the tendon proper (B). In the proliferative phase, nerve fiber density in the tendon increases and CGRP+ nerve axons are closely associated with vessels (C). The final phase of remodeling is associated with decreases in nerve density in the proper tendon to restore native neuronal networks (D). Tendons heal with scar.
In the proliferative phase, inflammatory cells and tenocytes at the injury site express the neurotrophic factors NGF and BDNF as well as the angiogenic factor VEGF to promote the rapid expansion of nerve and vascular networks towards the tendon proper from the paratenon and peri-tendinous tissues78; 79 (Fig. 2C). Abundant CGRP+ nerve infiltration of the tendon proper is coupled with a pronounced decrease in nerve fiber density in the tendon sheath. In the Achilles tendon, abundant nerve endings expressing GAP and PGP9.5 have been identified within isolated pockets or “zones” of connective tissue at this stage80. In transected rat Achilles tendons at two weeks-post injury, expression of NGF and BDNF, as well as their respective receptors TrkA and TrkB, either decrease or remain unchanged compared to uninjured tendons, perhaps marking the end of the proliferative phase and the beginning of the remodelling phase19. Indeed, tendon remodelling involves the withdrawal of SP+ and CGRP+ nerve fibers from the tendon proper, which may be caused by the elevated expression of NPY by sympathetic nerves in the vicinity of the healing site76 (Fig. 2D). Neo-collagen matrix and tendon fibroblasts are then reorganized and remodeled towards the native directionality of tendon tissue81. However, in the end, adult tendon repair results in a fibrovascular scar that is mechanically inferior to native tissue and prone to re-injury82 (Fig. 2D).
While the enthesis fibrocartilage that connects tendon to bone is typically aneural and avascular, recent studies indicate that the sensory neuropeptides SP and CGRP can be expressed in healthy cartilaginous tissues, including articular cartilage83; 84. Furthermore, expression of SP and CGRP has been observed in the Achilles tendon-enthesis of arthritic hindlimbs as well as the articular cartilage of painful joints, lending direct support for cartilage as an agent of nociception85. In total, these studies suggest that sensory nerve ingrowth to the injured enthesis may be essential to coordinate and promote healing. In fact, previous studies have identified small capillaries or “transcortical microvessels” in the enthesis fibrocartilage10; 75. These microvessels may originate from the adjacent subchondral bone and traverse the enthesis fibrocartilage after injury to facilitate inflammatory and progenitor cell transport in response to the expression of SP and CGRP. However, the source of sensory neuropeptides in a typically aneural enthesis is not well understood. In contrast to the tendon proper and enthesis, the adjoining muscle bellies of tendons, musculotendinous junctions, and adipose tissue contained within the proximal fat pad are densely innervated and vascularized. In fact, a significant fraction of PGP9.5+ nerve fibers within the Kager’s fat pad proximal to the Achilles tendon typically express SP and/or CGRP, confirming their origins in sensory ganglia86. Pacinian and Ruffini corpuscles have also been identified in the musculotendinous junctions of the palmaris longus and plantaris muscles87. Furthermore, damage to adipose tissue in the vicinity of joints is often associated with joint pain18. In total, these studies suggest that innervation from peripheral connective tissues may be a significant source of pain and SP+/CGRP+ sensory nerve ingrowth in inflammatory enthesitis. However, additional study is needed to determine the contributions of SP and CGRP to chondrocyte differentiation, fibrocartilage repair, and re-establishment of the entheseal zones following injury.
Similar to acute and chronic tendon injury, acute bone fractures can be sustained in a single traumatic event, whereas stress fractures result from an accumulation of damage in bone over weeks or months88. However, unlike either tendon or enthesis, healthy bone is highly innervated and vascularized. As a result, fracture results in the abrupt disruption of the vascular and nerve supply followed by a temporal sequence of healing phases. These phases include the inflammatory phase, the fibrovascular phase, the bone formation phase, and the late remodelling phase. Here, we focus on the peripheral nerves, which provide feedback to the central nervous system (pain), as well as the neuropeptides that coordinate a systematic healing response.
In the early inflammatory phase, both macrophages and periosteal mesenchymal cells at the fracture site express the neurotrophins NGF and BDNF89–91. In addition to promoting cell recruitment and proliferation, NGF stimulates the early ingrowth of CGRP+ sensory fibers and TH+ sympathetic fibers to the fracture callus from the periosteum and bone marrow90; 92; 93 (Fig. 3B). Furthermore, increased NGF expression in the callus sensitizes TrkA expressing nociceptors, resulting in inflammatory bone pain, but does not appear to affect expression of TrkA, TRPV1, and Nav1.826. Perhaps surprisingly, innervation of the fracture callus has been observed to precede vascularization in displaced tibial fractures in rats as well as non-displaced stress fractures in mice90; 94.
Fig. 3: Nerves in fracture healing.
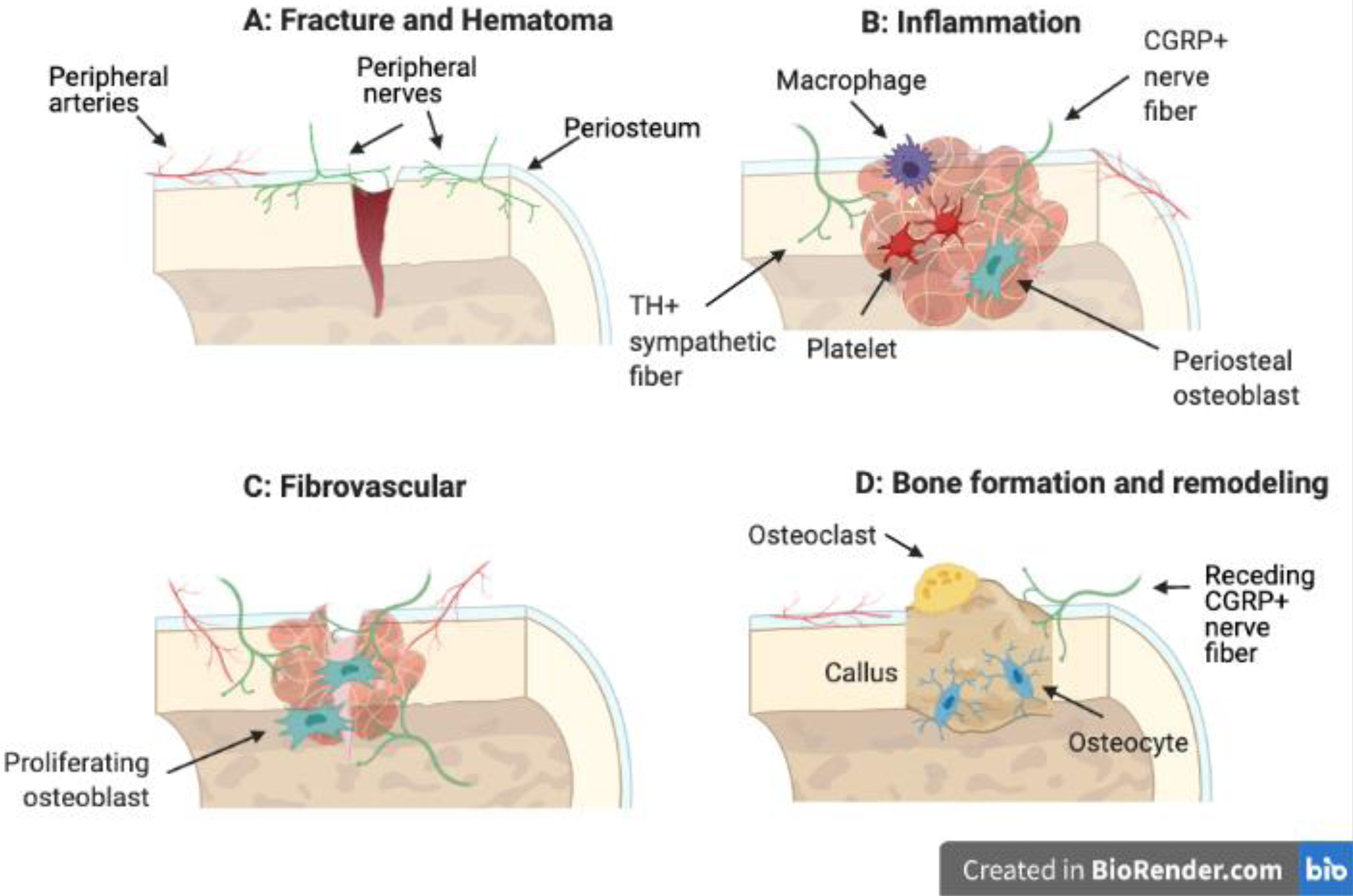
Fracture results in the disruption and bleeding of local vessels which is marked by a hematoma (A). In the inflammatory phase, CGRP+ sensory and TH+ sympathetic nerves first innervate the callus from the periosteum (B). In the fibrovascular phase, nerve density increases in the callus, and nerves are closely associated with ingrowing vessels (C). Bone formation is characterized by a soft cartilage callus at the injury site and marked reductions in nerve density (D). Bone is remodeled to its native structure and strength.
As fracture healing proceeds, the hypoxic fracture callus is vascularized to restore the supply of oxygen, nutrients, and progenitor cells to the callus, accompanied by robust expression of the neuropeptides NGF, BDNF, and NT-368; 89. Certainly, expression of these neurotrophins in the fracture callus results in axon sprouting and increased nerve density90 (Fig. 3C). Nonetheless, the role of innervation to direct angiogenesis during fracture repair is not completely understood. In previous work, topical application of NGF to engineered bone defects increased expression of VEGF and its receptor but did not enhance vascular density in the callus95, contrary to other findings96–98. However, NGF-TrkA signaling may stimulate endothelial cell migration and proliferation through the action of SP99 and/or regulate arterial vessel patterning in the cartilage callus through the CXCL12-CXCR4 chemokine axis100. Additional studies are necessary to determine the precise factors derived from skeletal nerves that influence angiogenesis during bone repair.
Following the fibrovascular phase of endochondral repair, mesenchymal progenitor cells undergo differentiation to chondrocytes while simultaneously depositing woven bone matrix to form a soft cartilage callus (Fig. 3D). In a recent study, expression of SP and NPY in the fracture callus peaked between two and six weeks after injury in rat, suggesting that abundant neuropeptide expression coincides with rapid mesenchymal expansion and cartilage differentiation101. In fact, investigation of femoral fractures in mice following administration of the neurotoxic agent capsaicin or in mice lacking CGRP observed impaired chondrogenesis and callus formation, underscoring the importance of sensory neuropeptides in the fibrovascular phase of healing92; 102. The specific local and systemic cellular sources of SP and CGRP in fracture repair remains to be determined.
In the final phase of repair, remodeling of the fracture callus is associated with a decline in neuropeptide expression in the callus and withdrawal of CGRP+ and GAP43+ nerve fibers from the callus to the periosteum90; 93; 94 (Fig. 3D). In contrast to tendons and tendon-bone attachments that heal with a fibrovascular scar, adult bone is capable of returning to native function and form without scarring following injury. Nonetheless, overall bone regeneration is adversely affected by denervation or loss of critical neuropeptide expression. Either loss of tachykinin-1 or chemical sympathectomy in fractured limbs result in altered callus formation with reduced cartilage differentiation and mechanically inferior bone tissue post-repair compared to native, uninjured bone103; 104. In fact, a recent study demonstrated that blockage of NK1 in fractured femurs was sufficient to reduce the ultimate strength of the repaired bone 6 weeks and 3 months post-fracture105. Interestingly, no differences were noted in the structural parameters of the fracture calluses in response to NK1 blockage in this study, suggesting a novel role for SP and/or NK1 perhaps in secondary bone formation and late-stage bone remodeling post-fracture. Consistent with this finding, previous work observed that loss of SP is associated with decreased bone turnover103, which may prolong the remodelling phase of fracture repair.
5.0. DISCUSSION
In this review, research to uncover the role of nerves and neurotrophic factors in tendon and bone development and repair to date has been summarized. However, the specific contributions of sensory and autonomic nerves to the different stages of development and repair continue to expand and be re-defined as novel methods enable increasingly precise observations. Nonetheless, many contradicting reports remain to be examined more closely. Early models of limb denervation report stunted bone growth, whereas others report minimal if any effects on bone formation and development. More recent work using chemical denervation models as well as specific abrogation of neurotrophic signaling suggests that skeletal nerves are diverse with non-overlapping functions. In soft tissue development, the role and requirement of nerves also remains murky. Direct evidence regarding the role of innervation in embryonic tendons is surprisingly scarce. However, in the embryonic limb, differentiation of individual limb tendons from early tendon primordia and their maturation is dependent on proximal innervated muscles, lending indirect support to a role of peripheral nerves in tendon development106. Furthermore, not all studies on skeletal denervation have analyzed soft tissues in addition to bone, which would provide an orthogonal measure of the extent of denervation in the developing limb. In total, more research regarding the role of nerves in musculoskeletal tissue development is required.
One of the major challenges of harnessing this research for developing therapeutic strategies is that neuropeptides appear to play multiple roles throughout development and repair. For example, expression of SP and CGRP during the inflammatory stage of healing results in pain propagation, vasodilation, increased blood flow, and white blood cell infiltration77; 107. However, expression of these sensory neuropeptides continues to trend upward in the proliferative phase, suggesting a critical anabolic function, specifically regarding the proliferation and differentiation of mesenchymal progenitor cells12; 101; 108. Nonetheless, exogenous SP injection does not appear to strongly promote tenocyte proliferation, suggesting either a complex interplay of molecules involved in cell proliferation or an undetermined spatiotemporal response109; 110. Furthermore, SP and CGRP crosstalk has been implicated in the heterotopic ossification of soft tissues111; a common complication following tendon injuries and in BMP-2-mediated spinal fusion112. Encouragingly, a recent study revealed that SP-mediated heterotopic ossification in the healthy tendon could be inhibited by an injection of exogenous CGRP111. Similarly, CGRP treatment of osteoblast cultures in combination with SP was found to inhibit BMP-2-mediated mineralization and osteogenic gene expression in vitro112. Nonetheless, additional work is required to strengthen our understanding of sensory innervation and determine whether the roles of SP and CGRP in tendon and bone healing are analogous, complimentary, or antagonistic.
Nonetheless, translational efforts to improve bone and tendon healing through the use of neurotrophins and neuropeptides, such as NGF and SP, are currently underway113; 114. A number of stress-related signaling pathways, including Wnt, SMAD, MAPK and NFκB115–122 have been implicated in tendon and bone healing, and likely play a role in neuropeptide expression and axonal ingrowth to the injury site. Furthermore, the anabolic functions of neuropeptides are dictated by their spatiotemporal expression in the inflammatory milieu of bones and tendons post-injury, complicating the delivery strategy for therapeutics that involve these signalling axes90; 123. For example, injection of exogenous NGF into fracture calluses during the endochondral ossification phase of fracture repair promoted osteogenic marker expression, whereas injections during the inflammatory stage of repair reduced expression of osteogenic and angiogenic markers123. Finally, given the dense innervation of bones in contrast to tendons and fibrocartilage, it is reasonable to assume that damage to peripheral nerves may disproportionally affect bone metabolism and physiology in scenarios of polytrauma124–126. We also note that the peripheral axons residing in distinct sheath-like structures enveloping tendons may respond differently to injury. Nonetheless, more research is necessary to compare and contrast the roles of nerves and neurotransmitters in distinct connective tissues in these scenarios.
ACKNOWLEDGEMENTS
Our research is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award numbers AR074953 (RET) and DE028397 (RET). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies. Figures were created with BioRender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
DECLARATION OF INTEREST
None
CITED REFERENCES
- 1.Haegerstam GA. 2001. Pathophysiology of bone pain: a review. Acta Orthop Scand 72:308–317. [DOI] [PubMed] [Google Scholar]
- 2.Mach DB, Rogers SD, Sabino MC, et al. 2002. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113:155–166. [DOI] [PubMed] [Google Scholar]
- 3.Tomlinson RE, Li Z, Zhang Q, et al. 2016. NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone. Cell Rep 16:2723–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomlinson RE, Li Z, Li Z, et al. 2017. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A 114:E3632–E3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien M 1997. Structure and metabolism of tendons. Scand J Med Sci Sports 7:55–61. [DOI] [PubMed] [Google Scholar]
- 6.Kannus P 2000. Structure of the tendon connective tissue. Scand J Med Sci Sports 10:312–320. [DOI] [PubMed] [Google Scholar]
- 7.Muller SA, Evans CH, Heisterbach PE, et al. 2018. The Role of the Paratenon in Achilles Tendon Healing: A Study in Rats. Am J Sports Med 46:1214–1219. [DOI] [PubMed] [Google Scholar]
- 8.Martinoli C, Bianchi S, Derchi LE. 1999. Tendon and nerve sonography. Radiol Clin North Am 37:691–711, viii. [DOI] [PubMed] [Google Scholar]
- 9.Apostolakos J, Durant TJ, Dwyer CR, et al. 2014. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J 4:333–342. [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin M, Toumi H, Suzuki D, et al. 2007. Microdamage and altered vascularity at the enthesis-bone interface provides an anatomic explanation for bone involvement in the HLA-B27-associated spondylarthritides and allied disorders. Arthritis Rheum 56:224–233. [DOI] [PubMed] [Google Scholar]
- 11.Marrella A, Lee TY, Lee DH, et al. 2018. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today (Kidlington: ) 21:362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann PW. 2013. Neuronal regulation of tendon homoeostasis. Int J Exp Pathol 94:271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann PW, Li J, Finn A, et al. 2001. Autonomic innervation of tendons, ligaments and joint capsules. A morphologic and quantitative study in the rat. J Orthop Res 19:372–378. [DOI] [PubMed] [Google Scholar]
- 14.Hering TM, Beller JA, Calulot CM, et al. 2020. Contributions of Chondroitin Sulfate, Keratan Sulfate and N-linked Oligosaccharides to Inhibition of Neurite Outgrowth by Aggrecan. Biology (Basel: ) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann PW, Franklin SL, Dean BJ, et al. 2014. Neuronal pathways in tendon healing and tendinopathy--update. Front Biosci (Landmark: Ed) 19:1251–1278. [DOI] [PubMed] [Google Scholar]
- 16.Ackermann PW, Salo PT, Hart DA. 2009. Neuronal pathways in tendon healing. Front Biosci (Landmark: Ed) 14:5165–5187. [DOI] [PubMed] [Google Scholar]
- 17.Alpantaki K, McLaughlin D, Karagogeos D, et al. 2005. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am 87:1580–1583. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin M, Redman S, Milz S, et al. 2004. Adipose tissue at entheses: the rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann Rheum Dis 63:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed AS, Li J, Abdul AM, et al. 2017. Compromised Neurotrophic and Angiogenic Regenerative Capability during Tendon Healing in a Rat Model of Type-II Diabetes. PLoS One 12:e0170748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danielson P, Andersson G, Alfredson H, et al. 2008. Marked sympathetic component in the perivascular innervation of the dorsal paratendinous tissue of the patellar tendon in arthroscopically treated tendinosis patients. Knee Surg Sports Traumatol Arthrosc 16:621–626. [DOI] [PubMed] [Google Scholar]
- 21.Danielson P, Alfredson H, Forsgren S. 2007. Studies on the importance of sympathetic innervation, adrenergic receptors, and a possible local catecholamine production in the development of patellar tendinopathy (tendinosis) in man. Microsc Res Tech 70:310–324. [DOI] [PubMed] [Google Scholar]
- 22.Nencini S, Ivanusic JJ. 2016. The Physiology of Bone Pain. How Much Do We Really Know? Front Physiol 7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brazill JM, Beeve AT, Craft CS, et al. 2019. Nerves in Bone: Evolving Concepts in Pain and Anabolism. J Bone Miner Res 34:1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aso K, Ikeuchi M, Izumi M, et al. 2014. Nociceptive phenotype of dorsal root ganglia neurons innervating the subchondral bone in rat knee joints. Eur J Pain 18:174–181. [DOI] [PubMed] [Google Scholar]
- 25.Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, et al. 2011. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 178:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nencini S, Ringuet M, Kim DH, et al. 2017. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain 13:1744806917697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asmus SE, Parsons S, Landis SC. 2000. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J Neurosci 20:1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelzer E, Blitz E, Killian ML, et al. 2014. Tendon-to-bone attachment: from development to maturity. Birth Defects Res C Embryo Today 102:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen PT, Lambertsen KL, Frich LH. 2018. Assembly, maturation, and degradation of the supraspinatus enthesis. J Shoulder Elbow Surg 27:739–750. [DOI] [PubMed] [Google Scholar]
- 30.Hopyan S, Sharpe J, Yang Y. 2011. Budding behaviors: Growth of the limb as a model of morphogenesis. Dev Dyn 240:1054–1062. [DOI] [PubMed] [Google Scholar]
- 31.Petit F, Sears KE, Ahituv N. 2017. Limb development: a paradigm of gene regulation. Nat Rev Genet 18:245–258. [DOI] [PubMed] [Google Scholar]
- 32.Landmesser L, Morris DG. 1975. The development of functional innervation in the hind limb of the chick embryo. J Physiol 249:301–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honig MG. 1982. The development of sensory projection patterns in embryonic chick hind limb. J Physiol 330:175–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strecker TR, Stephens TD. 1983. Peripheral nerves do not play a trophic role in limb skeletal morphogenesis. Teratology 27:159–167. [DOI] [PubMed] [Google Scholar]
- 35.Brockes JP. 1984. Mitogenic growth factors and nerve dependence of limb regeneration. Science 225:1280–1287. [DOI] [PubMed] [Google Scholar]
- 36.Wehrle-Haller B, Koch M, Baumgartner S, et al. 1991. Nerve-dependent and -independent tenascin expression in the developing chick limb bud. Development 112:627–637. [DOI] [PubMed] [Google Scholar]
- 37.Snow HE, Riccio LM, Mjaatvedt CH, et al. 2005. Versican expression during skeletal/joint morphogenesis and patterning of muscle and nerve in the embryonic mouse limb. Anat Rec A Discov Mol Cell Evol Biol 282:95–105. [DOI] [PubMed] [Google Scholar]
- 38.Dietz FR. 1989. Effect of denervation on limb growth. J Orthop Res 7:292–303. [DOI] [PubMed] [Google Scholar]
- 39.Dietz FR. 1987. Effect of peripheral nerve on limb development. J Orthop Res 5:576–585. [DOI] [PubMed] [Google Scholar]
- 40.Macpherson PC, Wang X, Goldman D. 2011. Myogenin regulates denervation-dependent muscle atrophy in mouse soleus muscle. J Cell Biochem 112:2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popiela H 1976. In vivo limb tissue development in the absence of nerves: a quantitative study. Exp Neurol 53:214–226. [DOI] [PubMed] [Google Scholar]
- 42.Schweitzer R, Chyung JH, Murtaugh LC, et al. 2001. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128:3855–3866. [DOI] [PubMed] [Google Scholar]
- 43.Huang AH, Lu HH, Schweitzer R. 2015. Molecular regulation of tendon cell fate during development. J Orthop Res 33:800–812. [DOI] [PubMed] [Google Scholar]
- 44.Murchison ND, Price BA, Conner DA, et al. 2007. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134:2697–2708. [DOI] [PubMed] [Google Scholar]
- 45.Edom-Vovard F, Schuler B, Bonnin MA, et al. 2002. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol 247:351–366. [DOI] [PubMed] [Google Scholar]
- 46.Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature 423:332–336. [DOI] [PubMed] [Google Scholar]
- 47.Marsell R, Einhorn TA. 2011. The biology of fracture healing. Injury 42:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinton RJ, Jing Y, Jing J, et al. 2017. Roles of Chondrocytes in Endochondral Bone Formation and Fracture Repair. J Dent Res 96:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berendsen AD, Olsen BR. 2015. Bone development. Bone 80:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maes C, Kobayashi T, Selig MK, et al. 2010. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19:329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartold M, Gronthos S, Haynes D, et al. 2019. Mesenchymal stem cells and biologic factors leading to bone formation. J Clin Periodontol 46 Suppl 21:12–32. [DOI] [PubMed] [Google Scholar]
- 52.Sisask G, Bjurholm A, Ahmed M, et al. 1995. Ontogeny of sensory nerves in the developing skeleton. Anat Rec 243:234–240. [DOI] [PubMed] [Google Scholar]
- 53.Gajda M, Litwin JA, Cichocki T, et al. 2005. Development of sensory innervation in rat tibia: co-localization of CGRP and substance P with growth-associated protein 43 (GAP-43). J Anat 207:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sisask G, Silfversward CJ, Bjurholm A, et al. 2013. Ontogeny of sensory and autonomic nerves in the developing mouse skeleton. Auton Neurosci 177:237–243. [DOI] [PubMed] [Google Scholar]
- 55.Edoff K, Hellman J, Persliden J, et al. 1997. The developmental skeletal growth in the rat foot is reduced after denervation. Anat Embryol (Berl: ) 195:531–538. [DOI] [PubMed] [Google Scholar]
- 56.Hill EL, Turner R, Elde R. 1991. Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience 44:747–755. [DOI] [PubMed] [Google Scholar]
- 57.Heffner MA, Anderson MJ, Yeh GC, et al. 2014. Altered bone development in a mouse model of peripheral sensory nerve inactivation. J Musculoskelet Neuronal Interact 14:1–9. [PMC free article] [PubMed] [Google Scholar]
- 58.Heffner MA, Genetos DC, Christiansen BA. 2017. Bone adaptation to mechanical loading in a mouse model of reduced peripheral sensory nerve function. PLoS One 12:e0187354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilie MA, Caruntu C, Tampa M, et al. 2019. Capsaicin: Physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions. Exp Ther Med 18:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burks TF, Buck SH, Miller MS. 1985. Mechanisms of depletion of substance P by capsaicin. Fed Proc 44:2531–2534. [PubMed] [Google Scholar]
- 61.Nagy JI, Iversen LL, Goedert M, et al. 1983. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci 3:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grassel S, Ahmed N, Gottl C, et al. 2009. Gene and protein expression profile of naive and osteochondrogenically differentiated rat bone marrow-derived mesenchymal progenitor cells. Int J Mol Med 23:745–755. [DOI] [PubMed] [Google Scholar]
- 63.Brohlin M, Kingham PJ, Novikova LN, et al. 2012. Aging effect on neurotrophic activity of human mesenchymal stem cells. PLoS One 7:e45052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazarus KJ, Bradshaw RA, West NR, et al. 1976. Adaptive survival or rat sympathetic neurons cultured without supporting cells or exogenous nerve growth factor. Brain Res 113:159–164. [DOI] [PubMed] [Google Scholar]
- 65.Mukouyama YS, Shin D, Britsch S, et al. 2002. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109:693–705. [DOI] [PubMed] [Google Scholar]
- 66.Dao DY, Jonason JH, Zhang Y, et al. 2012. Cartilage-specific beta-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J Bone Miner Res 27:1680–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Regard JB, Zhong Z, Williams BO, et al. 2012. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su YW, Zhou XF, Foster BK, et al. 2018. Roles of neurotrophins in skeletal tissue formation and healing. J Cell Physiol 233:2133–2145. [DOI] [PubMed] [Google Scholar]
- 69.Mock C, Cherian MN. 2008. The global burden of musculoskeletal injuries: challenges and solutions. Clin Orthop Relat Res 466:2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin P, Parkhurst SM. 2004. Parallels between tissue repair and embryo morphogenesis. Development 131:3021–3034. [DOI] [PubMed] [Google Scholar]
- 71.Silkstone D, Hong H, Alman BA. 2008. Beta-catenin in the race to fracture repair: in it to Wnt. Nat Clin Pract Rheumatol 4:413–419. [DOI] [PubMed] [Google Scholar]
- 72.Tang QM, Chen JL, Shen WL, et al. 2014. Fetal and adult fibroblasts display intrinsic differences in tendon tissue engineering and regeneration. Sci Rep 4:5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bahney CS, Zondervan RL, Allison P, et al. 2019. Cellular biology of fracture healing. J Orthop Res 37:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voleti PB, Buckley MR, Soslowsky LJ. 2012. Tendon healing: repair and regeneration. Annu Rev Biomed Eng 14:47–71. [DOI] [PubMed] [Google Scholar]
- 75.Schett G, Lories RJ, D’Agostino MA, et al. 2017. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol 13:731–741. [DOI] [PubMed] [Google Scholar]
- 76.Ackermann PW, Ahmed M, Kreicbergs A. 2002. Early nerve regeneration after achilles tendon rupture--a prerequisite for healing? A study in the rat. J Orthop Res 20:849–856. [DOI] [PubMed] [Google Scholar]
- 77.Brain SD, Williams TJ. 1985. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol 86:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bagge J, Lorentzon R, Alfredson H, et al. 2009. Unexpected presence of the neurotrophins NGF and BDNF and the neurotrophin receptor p75 in the tendon cells of the human Achilles tendon. Histol Histopathol 24:839–848. [DOI] [PubMed] [Google Scholar]
- 79.Rajpar I, Barrett JG. 2020. Multi-differentiation potential is necessary for optimal tenogenesis of tendon stem cells. Stem Cell Res Ther 11:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spang C, Harandi VM, Alfredson H, et al. 2015. Marked innervation but also signs of nerve degeneration in between the Achilles and plantaris tendons and presence of innervation within the plantaris tendon in midportion Achilles tendinopathy. J Musculoskelet Neuronal Interact 15:197–206. [PMC free article] [PubMed] [Google Scholar]
- 81.Yang G, Rothrauff BB, Tuan RS. 2013. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today 99:203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dakin SG, Dudhia J, Smith RK. 2014. Resolving an inflammatory concept: the importance of inflammation and resolution in tendinopathy. Vet Immunol Immunopathol 158:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szadek KM, Hoogland PV, Zuurmond WW, et al. 2010. Possible nociceptive structures in the sacroiliac joint cartilage: An immunohistochemical study. Clin Anat 23:192–198. [DOI] [PubMed] [Google Scholar]
- 84.Millward-Sadler SJ, Mackenzie A, Wright MO, et al. 2003. Tachykinin expression in cartilage and function in human articular chondrocyte mechanotransduction. Arthritis Rheum 48:146–156. [DOI] [PubMed] [Google Scholar]
- 85.Bring DK, Heidgren ML, Kreicbergs A, et al. 2005. Increase in sensory neuropeptides surrounding the Achilles tendon in rats with adjuvant arthritis. J Orthop Res 23:294–301. [DOI] [PubMed] [Google Scholar]
- 86.Shaw HM, Santer RM, Watson AH, et al. 2007. Adipose tissue at entheses: the innervation and cell composition of the retromalleolar fat pad associated with the rat Achilles tendon. J Anat 211:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jozsa L, Balint J, Kannus P, et al. 1993. Mechanoreceptors in human myotendinous junction. Muscle Nerve 16:453–457. [DOI] [PubMed] [Google Scholar]
- 88.Tommasini SM, Nasser P, Schaffler MB, et al. 2005. Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res 20:1372–1380. [DOI] [PubMed] [Google Scholar]
- 89.Kilian O, Hartmann S, Dongowski N, et al. 2014. BDNF and its TrkB receptor in human fracture healing. Ann Anat 196:286–295. [DOI] [PubMed] [Google Scholar]
- 90.Li Z, Meyers CA, Chang L, et al. 2019. Fracture repair requires TrkA signaling by skeletal sensory nerves. J Clin Invest 129:5137–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Asaumi K, Nakanishi T, Asahara H, et al. 2000. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone 26:625–633. [DOI] [PubMed] [Google Scholar]
- 92.Appelt J, Baranowsky A, Jahn D, et al. 2020. The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing. EBioMedicine 59:102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J, Kreicbergs A, Bergstrom J, et al. 2007. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: A study in rat angulated tibia. J Orthop Res 25:1204–1212. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Ahmad T, Spetea M, et al. 2001. Bone reinnervation after fracture: a study in the rat. J Bone Miner Res 16:1505–1510. [DOI] [PubMed] [Google Scholar]
- 95.Chen WH, Mao CQ, Zhuo LL, et al. 2015. Beta-nerve growth factor promotes neurogenesis and angiogenesis during the repair of bone defects. Neural Regen Res 10:1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang R, Liang Y, Wei S. 2018. The expressions of NGF and VEGF in the fracture tissues are closely associated with accelerated clavicle fracture healing in patients with traumatic brain injury. Ther Clin Risk Manag 14:2315–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Julio-Pieper M, Lara HE, Bravo JA, et al. 2006. Effects of nerve growth factor (NGF) on blood vessels area and expression of the angiogenic factors VEGF and TGFbeta1 in the rat ovary. Reprod Biol Endocrinol 4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calza L, Giardino L, Giuliani A, et al. 2001. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A 98:4160–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu L, Dana R, Yin J. 2020. Sensory neurons directly promote angiogenesis in response to inflammation via substance P signaling. FASEB J 34:6229–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li W, Kohara H, Uchida Y, et al. 2013. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell 24:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zou Zhenlv, Mei Gang, Tang Liying, Xu Yafei, Liu Jun, Wang Shangchong, Fu Su, Wu Jianqun, and Liang Guoguang. 2020. Correlation of neuropeptides substance P and neuropeptide Y and their receptors with fracture healing in rats. Materials Express 10. [Google Scholar]
- 102.Apel PJ, Crane D, Northam CN, et al. 2009. Effect of selective sensory denervation on fracture-healing: an experimental study of rats. J Bone Joint Surg Am 91:2886–2895. [DOI] [PubMed] [Google Scholar]
- 103.Niedermair T, Kuhn V, Doranehgard F, et al. 2014. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol 38:22–35. [DOI] [PubMed] [Google Scholar]
- 104.Nordsletten L, Madsen JE, Almaas R, et al. 1994. The neuronal regulation of fracture healing. Effects of sciatic nerve resection in rat tibia. Acta Orthop Scand 65:299–304. [DOI] [PubMed] [Google Scholar]
- 105.Hofman M, Rabenschlag F, Andruszkow H, et al. 2019. Effect of neurokinin-1-receptor blockage on fracture healing in rats. Sci Rep 9:9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kardon G 1998. Muscle and tendon morphogenesis in the avian hind limb. Development 125:4019–4032. [DOI] [PubMed] [Google Scholar]
- 107.Suvas S 2017. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J Immunol 199:1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang W, Zhuo X, Tang Z, et al. 2015. Calcitonin gene-related peptide stimulates proliferation and osteogenic differentiation of osteoporotic rat-derived bone mesenchymal stem cells. Mol Cell Biochem 402:101–110. [DOI] [PubMed] [Google Scholar]
- 109.Carlsson O, Schizas N, Li J, et al. 2011. Substance P injections enhance tissue proliferation and regulate sensory nerve ingrowth in rat tendon repair. Scand J Med Sci Sports 21:562–569. [DOI] [PubMed] [Google Scholar]
- 110.Andersson G, Backman LJ, Scott A, et al. 2011. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br J Sports Med 45:1017–1022. [DOI] [PubMed] [Google Scholar]
- 111.Tuzmen C, Verdelis K, Weiss L, et al. 2018. Crosstalk between substance P and calcitonin gene-related peptide during heterotopic ossification in murine Achilles tendon. J Orthop Res 36:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tuzmen C, Campbell PG. 2018. Crosstalk between neuropeptides SP and CGRP in regulation of BMP2-induced bone differentiation. Connect Tissue Res 59:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mammoto T, Seerattan RA, Paulson KD, et al. 2008. Nerve growth factor improves ligament healing. J Orthop Res 26:957–964. [DOI] [PubMed] [Google Scholar]
- 114.Zhang YB, Wang L, Jia S, et al. 2014. Local injection of substance P increases bony formation during mandibular distraction osteogenesis in rats. Br J Oral Maxillofac Surg 52:697–702. [DOI] [PubMed] [Google Scholar]
- 115.Eliasson P, Fahlgren A, Aspenberg P. 2008. Mechanical load and BMP signaling during tendon repair: a role for follistatin? Clin Orthop Relat Res 466:1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J, Liu Z, Li Y, et al. 2020. FGF2: a key regulator augmenting tendon-to-bone healing and cartilage repair. Regen Med 15:2129–2142. [DOI] [PubMed] [Google Scholar]
- 117.Kishimoto Y, Ohkawara B, Sakai T, et al. 2017. Wnt/beta-catenin signaling suppresses expressions of Scx, Mkx, and Tnmd in tendon-derived cells. PLoS One 12:e0182051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abraham AC, Shah SA, Golman M, et al. 2019. Targeting the NF-kappaB signaling pathway in chronic tendon disease. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu H, Duan J, Ning D, et al. 2014. Role of Wnt signaling in fracture healing. BMB Rep 47:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu YY, Lieu S, Lu C, et al. 2010. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone 46:841–851. [DOI] [PubMed] [Google Scholar]
- 121.Sharma R, Wu X, Rhodes SD, et al. 2013. Hyperactive Ras/MAPK signaling is critical for tibial nonunion fracture in neurofibromin-deficient mice. Hum Mol Genet 22:4818–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flick LM, Weaver JM, Ulrich-Vinther M, et al. 2003. Effects of receptor activator of NFkappaB (RANK) signaling blockade on fracture healing. J Orthop Res 21:676–684. [DOI] [PubMed] [Google Scholar]
- 123.Rivera KO, Russo F, Boileau RM, et al. 2020. Local injections of beta-NGF accelerates endochondral fracture repair by promoting cartilage to bone conversion. Sci Rep 10:22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ganji E, Killian ML. 2018. Tendon healing in the context of complex fractures. Clin Rev Bone Miner Metab 16:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Davis KM, Griffin KS, Chu TG, et al. 2015. Muscle-bone interactions during fracture healing. J Musculoskelet Neuronal Interact 15:1–9. [PMC free article] [PubMed] [Google Scholar]
- 126.Morioka K, Marmor Y, Sacramento JA, et al. 2019. Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma. Sci Rep 9:12199. [DOI] [PMC free article] [PubMed] [Google Scholar]


