Abstract
Complications following surgery are common and frequently occur the following discharge. Mobile and wearable digital health interventions (DHI) provide an opportunity to monitor and support patients during their postoperative recovery. Lack of high-quality evidence is often cited as a barrier to DHI implementation. This review captures and appraises the current use, evidence base and reporting quality of mobile and wearable DHI following surgery. Keyword searches were performed within Embase, Cochrane Library, Web of Science and WHO Global Index Medicus databases, together with clinical trial registries and Google scholar. Studies involving patients undergoing any surgery requiring skin incision where postoperative outcomes were measured using a DHI following hospital discharge were included, with DHI defined as mobile and wireless technologies for health to improve health system efficiency and health outcomes. Methodological reporting quality was determined using the validated mobile health evidence reporting and assessment (mERA) guidelines. Bias was assessed using the Cochrane Collaboration tool for randomised studies or MINORS depending on study type. Overall, 6969 articles were screened, with 44 articles included. The majority (n = 34) described small prospective study designs, with a high risk of bias demonstrated. Reporting standards were suboptimal across all domains, particularly in relation to data security, prior patient engagement and cost analysis. Despite the potential of DHI to improve postoperative patient care, current progress is severely restricted by limitations in methodological reporting. There is an urgent need to improve reporting for DHI following surgery to identify patient benefit, promote reproducibility and encourage sustainability.
Subject terms: Rehabilitation, Outcomes research
Introduction
The worldwide use of surgical treatments is increasing, with approximately one in ten people undergoing a surgical procedure each year in high-income countries1,2. Following discharge, patients assume primary responsibility for monitoring their own recovery and differences in adhering with both this and related self-care recommendations, can produce variable outcomes. More than 10% of patients over 45 years old experience a major postoperative complication3–5, often following discharge6, which typically prompts readmission7 and is associated with increased postoperative mortality across a range of surgical populations7,8. However, even minor events following surgery, such as nausea and pain, are known to significantly affect patient satisfaction and wellbeing9–13.
Studies have already demonstrated that using digital health interventions (DHI) can help identify postoperative complications earlier, improve recovery, and provide safe follow-up which is acceptable to patients10,14–18. DHI, defined as ‘the use of mobile and wireless technologies for health to improve health system efficiency and health outcomes’19, provide the opportunity to connect patients and healthcare providers in real-time. For example, embedded sensors in mobile phones and wearable technology can capture data remotely, passively and continuously, providing opportunities to track physiological parameters and enable patients to self-report symptoms and signs, which can indicate their postoperative status. In surgery, DHI may include wearable activity trackers20, mobile phone applications21, real-time collection of patient-reported outcomes22 and/or multiple electronic devices forming a digital health kit23.
A growing body of literature evaluating DHI in surgery exists, including studies reporting its value in the assessment of postoperative recovery24–26 and its cost-effectiveness27. Meanwhile, the COVID-19 pandemic has accelerated the adoption of remote monitoring applications and use of digital health in all aspects of surgical workflow22. Medical professionals have increasingly utilised digital health interventions to monitor and review patients remotely28, encouraging resource expansion and potentially representing a paradigm shift in patient management29.
Previous systematic reviews reporting on digital health and surgery have focused on web-based interventions, where the use of mobile devices or real-time measurement of patient data was excluded27,30,31. In addition, the use of narrow inclusion criteria limit comparisons across the research field and hinder the identification of critical evidence gaps19. Despite the emergence of numerous DHI initiatives in surgery, there has been little discussion of the importance of rigorous reporting in this literature30,31.
We aimed to determine the current use, evidence base and reporting quality for mobile DHI in the postoperative period following surgery.
Results
Study characteristics
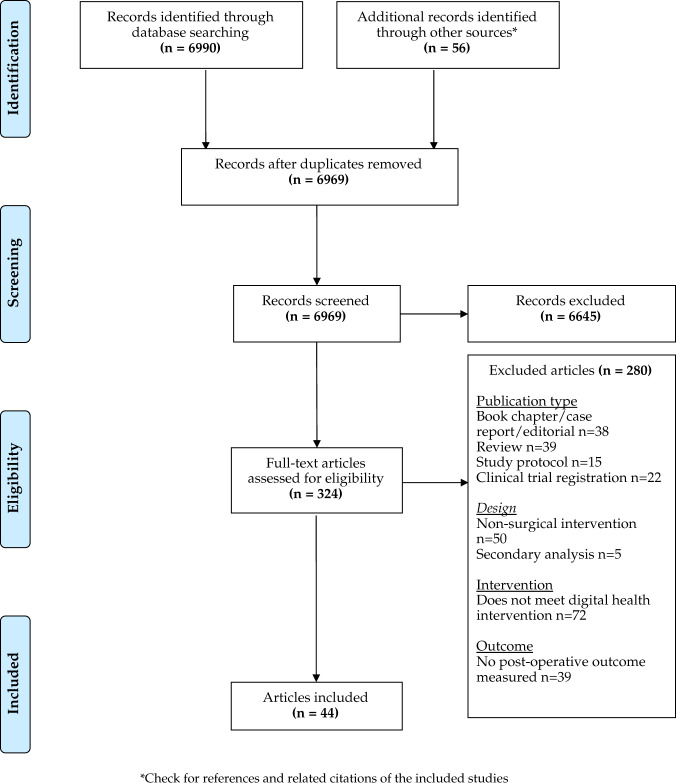
Our review resulted in 324 full-text articles assessed for eligibility after initially screening 6969, with 44 articles (Fig. 1) ultimately included in this review9,23,25,32–72. Tables 1 and 2 provide descriptions of each study, recruiting 3890 patients in total across ten randomised controlled trials9,32–40, 17 prospective studies25,42–54,71 and 17 pilot or feasibility studies23,56–70,72.
Fig. 1. PRISMA diagram.
Articles were published between January 2000 and May 2021, based on a search of Embase, Cochrane library, Web of Science, WHO Global Index Medicus, clinical trial registries and Google scholar databases (for details, see “Methods”).
Table 1.
Summary of included randomised control trials.
| Author | Procedures | Patient number | Digital health intervention (DHI) | Data collected | Control group | Length of intervention | Assessment of patient adherence | Measured patient adherence (%) |
|---|---|---|---|---|---|---|---|---|
| Mangieri et al., 2019 | Laparoscopic sleeve gastrectomy | 56 | iPad mini with MyFitnessPal© application | Calorie counting & exercise tracking | Usual care | 24 months | None | – |
| Campbell et al., 2019 | Hip or knee replacement | 159 | SMS bot (StreaMD) | Pain and patient activity | Usual care | 42 days | None | – |
| Hou et al., 2019 | Lumbar spinal surgery | 168 | Mobile phone- based mHealth programmea | Guide and monitor patient rehabilitation | Usual care | 90 days | Number of training sessions completed per week (arbitrary number) | 65 |
| Mousa et al., 2019 | Infra-inguinal procedures | 30 | Tablet computer with an application (Enform) | Physiological parameters and QoL questionnaire | Usual care | 30 days | None | – |
| Graetz et al., 2018 | Gynaecological cancer surgery | 29 (pilot) | Mobile application (Patient Care Monitor™)a | Postoperative symptoms. Automatic patient alerts using predetermined thresholds | Mobile app (no reminder) | 30 days | Completion of all surveys | 93 |
| van der Meij et al., 2018 | Laparoscopic abdominal procedures | 344 | Mobile application and activity tracker (UP MOVE, Jawbone)a | Postoperative recovery (PROMIS questionnaire) and daily step count | Usual care & placebo website | 6 months | Completion of all questionnaires | 87 |
| Jaensson et al., 2017 | Predominantly orthopaedic and general cases | 997 | Mobile application (RAPP)a | Postoperative recovery using SwQoR questionnaire | Usual care | 14 days | None | – |
| Park et al., 2017 | Total knee replacement | 40 | SMS messaginga | General health, pain, joint symptoms | Telephone consult | 90 days | None | – |
| Armstrong et al, 2017 | Breast reconstruction | 65 | Mobile applicationa | Wound images and pain scores. Red flags prompting in-person review | Usual care | 30 days | None | – |
| Dabbs et al., 2016 | Lung transplant | 201 | Mobile application (PocketPATH®)a | Self-monitored physiological parameters | Usual care | 12 months | None | – |
aStudy inclusion criteria required the patient to own a mobile phone
Table 2.
Summary of included prospective studies.
| Author | Procedures | Patient n | Digital health intervention (DHI) | Data collected | Length of intervention | Assessment of patient adherence | Measured patient adherence (%) |
|---|---|---|---|---|---|---|---|
| Jonker et al., 2021 | Oncological surgery | 47 | Mobile applicationa (Self-Management system) and Fitbit Charge 2 | Physical activity, temperature, blood pressure, weight, pain and symptoms | 90 days | Completion of study follow-up assessment | 79 |
| Gräfitsch et al., 2020 | Abdominal wall hernia repair | 16 | Santiago® tablet, actimeter and pulse oximeter | Continuous activity levels; pain, oxygen saturation and wound images | 30 days | Activity measurements available for the entire postoperative period | 69 |
| Panda et al., 2019 | Soft tissue and abdominal | 62 | Mobile application (Beiwe)a | Continuous passive collection of raw smartphone accelerometer data | 6 weeks | None | – |
| Carmichael et al., 2019 | Inguinal hernia (most common), abdominal and thoracic procedures | 175 | Vivofit 3 (Garmin) | Mean daily step count calculated for each elective procedure type, including preoperative baseline | 30 days | At least 2 weeks postoperative data available | 68 |
| Thijs et al., 2019 | CABG | 22 | Fitbit Charge HR | Weekly average step count data downloaded at end of the study period | 14 days | Accelerometer worn for the entirety of postoperative study period | 77 |
| Cole et al., 2019 | Transsphenoidal surgery | 7 | Wristband device (Wavelet Health) | Multiple physiological parameters tracked (step count, calories, distance, heart rate, RR and sats) | Up to 13 days (average 8 days) | Transfer of data from device to cloud storage system | 84 |
| Argent et al., 2019 | Total knee replacement | 15 | Shimmer3 inertial measurement unit | Accelerometer data used to guide and provide feedback on rehabilitation exercises | 14 days | None | – |
| Scheper et al., 2019 | Joint arthroplasty | 69 | Mobile applicationa | Wound symptoms and images | 30 days | Use of application until day 30 | 59 |
| Khanwalkar et al., 2018 | Sinus surgery | 288 | Mobile applicationa | Pain and PROMIS pain interference | 14 days | Completion of follow-up survey | 89 |
| Felbaum et al., 2018 | Spinal surgery | 56 | Mobile application (TrackMyRecovery®)a | Patient education, pain scores and wound images | 30 days | Downloaded and sent data through app | 96 |
| Anthony et al., 2018 | Hand surgery | 47 | Text messaging via software robota | Patient-reported pain and opiate use through daily automated text messages | 7 days | Completion of all questionnaires | 88 |
| Gunter et al., 2018 | Lower limb vascular surgery | 40 | Mobile application (WoundCheck) | Participant satisfaction and wound status (via app) | 14 days | Daily submission of data | 45 |
| Ghomrawi et al., 2018 | Range of elective paediatric surgical procedures | 60 | Actigraph wearable accelerometer | Time spent in grades of physical activity (light to intense). Data acquired at end of the study period | 14 days | Wear accelerometer for at least 10 h each day of the study period | 42 |
| Pozza et al., 2018 | Cosmetic surgery | 57 | Mobile messaging (SMS and MMS)a | Text message and wound images | 7 days | Completed postoperative survey | 91 |
| Agarwal et al., 2018 | Robotic laparoscopic prostatectomy | 46 | Fitbit Charge HR and mobile applicationa | Pre- and postoperative physical activity (measured by average step count) | Up to 15 days | None | – |
| Scott et al., 2017 | Colorectal surgery | 20 | Mobile application (mHealth app from Seamless Mobile Health)a | Daily postoperative symptom tracker using pre-developed algorithm | 14 days | Completed follow-up | 85 |
| Symer et al., 2017 | Open and laparoscopic abdominal surgery | 31 | FitBit Charge HR and mobile applicationa | Daily symptom questionnaire and wound images. Automated alerts via app | 30 days | Completed at least one app-related task ≥70% of the time | 84 |
| Sosa et al., 2017 | Head and neck cancer resection | 23 | Mobile messaging (SMS and MMS)a | Text messages and wound images (on the SenseHealth app platform) | 7 days | None | – |
| Castillo et al., 2017 | C-section | 105 | Mobile application (how2trak)a | Wound images and surgical site infection symptoms | 30 days | Submission of wound images up to 30 days | 45 |
| Higgins et al., 2017 | ACL reconstruction | 32 | Mobile application (web-based) | Mobile app collecting pain scores, QoL (QoR-9) and wound images | 6 weeks | None | – |
| Chiang et al., 2017 | Total knee replacement | 18 | Accelerometer (brand not stated) | Accelerometer used periodically to measure the range of postoperative activity | 6 weeks | None | – |
| Sun et al., 2017 | Major gastrointestinal resection | 20 | VivoFit2 | Daily steps are continuously collected using a secure group account | 14 days | Wore device for at least 1 week after discharge | 83 |
| Abraham et al., 2017 | Breast reconstruction | 4 | Smartwatch (Microsoft Band 2)a | Step count and physiological parameters streamed continuously via Wi-Fi | 28 days | Daily collection of data | 50 |
| Carrier et al., 2016 | Major colorectal resections | 111 | Mobile messaginga | Pain and postoperative symptoms captured using text messaging | 7 days | Reply to all messages | 90 |
| Toogood et al., 2016 | Total hip arthroplasty | 33 | Fitbit and mobile phone | Daily step count used as marker of patient activity | 30 days | Transmit data for seven consecutive days | 89 |
| Tenhagen et al., 2016 | Gastric sleeve or bypass | 14 | Internet-enabled weighing scales | Patients requested to weigh themselves at least once a week | 1 year | Provided weight for ≥80% weeks | 50 |
| Debono et al., 2016 | Lumbar discectomy | 60 | Mobile applicationa | Predetermined patient responses for pain and postoperative symptoms triggered response alarm | 16 days | None | – |
| Mobbs et al., 2016 | Lumbar spine surgery | 30 | FitBit zip | Average daily activity over each month. Data accessed through shared patient-investigator login | 90 days | Accelerometer worn for an entire study period | 93 |
| McElroy et al., 2016 | Cardiac surgery | 27 | Bluetooth-enabled tablet | Tablet linked to digital health kit (pulse oximeter, heart rate blood pressure cuff and weight scales) | 30 days | None | – |
| Semple et al., 2015 | Breast reconstruction and ACL repair | 65 | Mobile application | Postoperative pain, QoL (QoR-9) and wound photographs | 30 days | Upload of at least one wound photograph each day | 71 |
| Dawes et al., 2015 | Any colorectal procedure | 20 | Pre-programmed tablet computer | Postoperative health status survey completed daily | 14 days | None | – |
| Palombo et al., 2009 | Carotid endarterectomy | 36 | UMTS technology internet-linked video phone | Surgical wound, blood pressure and heart rate monitored every 4 h for 2 days | 2 days | None | – |
| Martinez-Ramos et al., 2009 | Range of ambulatory procedures | 96 | GPRS phone-based system | Wound images | 14 days | None | – |
| Perez et al., 2006 | Predominantly orthopaedic procedures | 49 | Mobile application(symbian OS phone) | Portable saturations probe readings and wound images | Not stated | None | – |
aStudy inclusion criteria required the patient to own a mobile phone
More than half of the studies were conducted in the United States (n = 24; 1556 patients)23,32,33,35,36,39–42,44–50,52,56,59–61,65–67, with only one originating from a low- or middle-income setting34. Orthopaedic procedures were represented in a quarter of studies (n = 10; 611 patients)25,33,34,38,46,52,57,58,63,64, with interventions taking place predominantly within the first 30 postoperative days9,23,34,36,39,42–52,54–62,65–69,72. Real-time data collection and autonomous delivery to clinicians for immediate review occurred in 31 studies9,23,32,34,36,37,39,40,44–48,50–56,58,60–63,65–69,71.
Mobile phone-based interventions
Thirty one of the eligible studies used a mixture of mobile phone-based interventions9,32–34,36–41,44–52,54–56,58,60–63,68–71, with 20 using smartphone applications9,32,36,37,39–41,44,45,47–50,54,55,58,62,63,70,71. Remote assessment of wound images taken by the patient and evaluation of symptoms reported using validated tools were the most frequent aims of the mobile phone-based interventions39,45,47,49,50,55,58,60,62,63,68–70. In total, 19 individual mobile applications were described (Table 3). Only three of these were publicly available to download from either Android or Apple platforms32,41,48, while it was unclear what platform the others used. One application was available as a demonstration version, however, patient data entry was restricted62. Five studies used predetermined thresholds or algorithms to generate clinician alerts from patient responses36,39,48,49,54.
Table 3.
Studies using mobile applications.
| Author | Patient number | Surgical speciality | Study design | Mobile application | Industry or commercial interest | Platform | Purpose | Availability |
|---|---|---|---|---|---|---|---|---|
| Jonker et al., 2021 | 47 | Oncologic surgery | Prospective | Self-management system (SMS) | Yes | Android | Activity monitoring, observations and postoperative symptoms | Not available |
| Panda et al., 2019 | 62 | Soft tissue and abdominal | Prospective | Mobile application (Beiwe) | No | Android, iOS | Continuous passive collection of accelerometer data | Android and iOS |
| Mangieri et al., 2019 | 56 | Bariatric surgery | RCT | MyFitnessPal© | Yes | Android, iOS | Encourage patient activity and weight loss | Android and iOS |
| Scheper et al., 2019 | 69 | Orthopaedics | Prospective | Innovattic | Yes | Not stated | Symptom tracker and uploading of wound images | Not available |
| Graetz et al., 2018 | 29 | Obstetrics and gynaecology | RCT | Adapted version of Patient Care Monitor™ | Yes | Web-based | Records postoperative symptoms | Not available |
| van der Meij et al., 2018 | 344 | Gastrointestinal surgery | RCT | Unnamed | Web-based | Provided information on recovery and tracked recovery | Not available | |
| Khanwalkar et al., 2018 | 288 | ENT surgery | Prospective | Unnamed | Not stated | Measures PROMs | Not available | |
| Felbaum et al., 2018 | 56 | Neurosurgery | Prospective | TrackMyRecovery® | Android, iOS | Sends reminders, measures pain scores and wound images | Not available | |
| Gunter et al, 2018 | 40 | Vascular surgery | Prospective | WoundCheck | iOS | Uploading of wound images and recovery progress | Not available | |
| Jaensson et al., 2017 | 997 | Day surgery | RCT | RAPP | Not stated | Assesses postoperative recovery | Not available | |
| Armstrong et al., 2017 | 65 | Breast surgery | RCT | Unnamed | Not stated | Wound images, pain and QoL | Not available | |
| Scott et al., 2017 | 20 | Colorectal surgery | Prospective | Seamless mobile health | Yes | Android, iOS, Blackberry OS | Symptom tracker | Android and iOS |
| Symer et al., 2017 | 31 | Gastrointestinal surgery | Prospective | Unnamed | Android, iOS | Symptom tracker and uploading of wound images | Not available | |
| Sosa et al., 2017 | 23 | Head and Neck | Prospective | SenseHealth | Android, iOS | Symptom tracker and uploading of wound images | Not available | |
| Castillo et al., 2017 | 105 | Obstetric and gynaecology | Prospective | How2trak | Not stated | Symptom tracker and uploading of wound images | Android only (demonstration only) | |
| Higgins et al., 2017 | 32 | Orthopaedics | Prospective | QoC Health | Not stated | Symptom tracker measures recovery and uploading of wound images | Not available | |
| Dabbs et al., 2016 | 201 | Transplantation | RCT | PocketPATH® | Not stated | Records daily health indicators | Not available | |
| Debono et al., 2016 | 60 | Neurosurgery | Prospective | SOVINTY e-Healthcare services software | Not stated | Records postoperative symptoms | Not available | |
| Semple et al., 2015 | 65 | Multiple specialties | Prospective | Unnamed | Not stated | Measures pain and recovery scores | Not available | |
| Perez et al., 2006 | 49 | Day surgery | Prospective | Unnamed | Symbian OS phones | Uploading of wound images | Not available |
ENT ear, nose and throat surgery, iOS apple mobile device operating system, QoL quality of life, PROMS patient-reported outcome measures.
Twenty-one studies required patients to own a mobile device9,34,36–41,44–46,48–51,54,58,60–62,66,71 excluding up to a third of patients approached as a result47,48. Where participants were provided with a mobile device, participant age was higher (56.1 vs. 53.1 years), with only two studies explicitly recruiting older patients (≥60 years old)52,71.
Mobile phone-based interventions included multimodal patient feedback programmes32,34,37, postoperative recovery tracking39,57 and patient education9. These frequently reduced the requirement for postoperative in-person reviews and reduced inappropriate patient emergency department use39,45,54,70. Some interventions were demonstrated to encourage quicker postoperative recovery and reduce analgesic requirements33,37,41,46 while postoperative complications could be identified earlier through both mobile messaging and wound photographs60,63. However, complication rates were similar to control groups in all studies where reported (range 2.0–7.1%)35,37. In those studies utilising predefined algorithms and thresholds, none had been previously validated within another patient cohort36,39,48,49,54.
Wearable devices
Accelerometer-based devices were the most commonly represented wearable device, measuring postoperative patient physical activity or intensity (n = 14) via FitBit25,43,49,52,61,72 smartwatch42,65,66 or other devices37,56,59,64,71. Eight studies required the synchronisation of wearable devices to a mobile phone, together with manual download by a clinician on study completion, to allow data analysis25,42,43,49,57,59,64,72. Studies using wearables for continuous patient monitoring were less common, with only three studies reporting the use of automated data feeds for real-time clinical analytics and feedback49,54,66.
Studies demonstrated that increases in step count postoperatively correlated with age52,61, body build61 and operative approach (open versus keyhole procedures)43,52. Accelerometer activity data also demonstrated postoperative complications could be identified at an earlier stage42, were associated with other physiological parameters56 and correlated with complication scores such as the Comprehensive Complication Index65. Activity recovery curves were also demonstrated for common abdominal and thoracic procedures42. Only one study utilised in-built smartphone accelerometers, which demonstrated postoperative complications reduced daily exertional activity compared to baseline up to 6 weeks after surgery41.
A single randomised trial37 used a wearable device as part of a multimodal intervention, however, only a proportion of patients received this device, as patients were required to own a compatible smartphone. The study’s authors did not report results based on device data, with a return to normal activity measured through the validated Patient-Reported Outcomes Measurement Information System® (PROMIS) score.
Measured outcomes
The majority of studies reported postoperative recovery as their main outcome (Table 4)9,25,33,34,37,38,41–43,52,54,56,59,61,64–67,72. Additional primary outcomes included the impact of DHI on pain management33,34,44,46, postoperative complications50,51,58,60,68, symptom monitoring36,40, surgical site infection35,47,55,62,69,70 and hospital resource use23,35,39,45,63. Two studies determined the ability of DHI to aid postoperative weight loss following bariatric surgery32,53, while four studies solely focused on determining the feasibility of a DHI in postoperative follow-up48,49,57,71.
Table 4.
Outcomes measured across included studies.
| Primary outcome | Author | Study design | Procedures | Patient number | Length of intervention | Main finding |
|---|---|---|---|---|---|---|
| Postoperative pain management | Campbell et al., 2019 | RCT | Hip or knee replacement | 159 | 42 days | Stopped taking narcotics 10 days sooner (P < 0.001) |
| Postoperative pain management | Hou et al., 2019 | RCT | Lumbar spinal surgery | 168 | 90 days | No difference in pain scores |
| Postoperative pain management | Khanwalkar et al., 2018 | Prospective | Sinus surgery | 288 | 14 days | Similar analgesic requirements across all included procedures |
| Postoperative pain management | Anthony et al., 2018 | Prospective | Hand surgery | 47 | 7 days | Pain trended down sequentially over the first week |
| Postoperative complications | Scheper et al., 2019 | Prospective | Joint arthroplasty | 69 | 30 days | 80% patient-reported complications concorded with physician diagnosis. |
| Postoperative complications | Pozza et al., 2018 | Prospective | Cosmetic surgery | 57 | 7 days | All three complications were detected earlier in the postoperative period |
| Postoperative complications | Sosa et al., 2017 | Prospective | Head and neck cancer resection | 23 | 7 days | Patients with postoperative complications are more likely to use a platform (P < 0.001) |
| Postoperative complications | Carrier et al., 2016 | Prospective | Major colorectal resections | 111 | 7 days | Alerts led to early, timely detection of postoperative complications |
| Postoperative complications | Palombo et al., 2009 | Prospective | Carotid endarterectomy | 36 | 2 days | The intervention allowed safe early discharge in selected patients |
| Postoperative symptom monitoring | Graetz et al., 2018 | RCT | Gynaecological cancer surgery | 29 (pilot) | 30 days | Feasible and acceptable to the patient population. Reminders increased use of a mobile application. |
| Postoperative symptom monitoring | Dabbs et al., 2016 | RCT | Lung transplant | 201 | 12 months | Self-monitoring increased with app use, with patients more likely to report critical indicators (OR 5.11; P < 0.001) |
| Postoperative recovery | Gräfitsch et al., 2020 | Prospective | Abdominal wall hernia repair | 16 | 30 days | 60% of patients regained preoperative activity levels within 3 weeks |
| Postoperative recovery | Panda et al., 2019 | Prospective | Cancer surgery | 62 | 6 weeks | Patients with postoperative complications showed lower activity and ability to achieve 60 min of exertional activity |
| Postoperative recovery | Campbell et al., 2019 | RCT | Hip or knee replacement | 159 | 42 days | Patients in the intervention group exercised for longer (8.6 min per day; P < 0.001) |
| Postoperative recovery | Hou et al., 2019 | RCT | Lumbar spinal surgery | 168 | 90 days | Disability improved in mHealth group |
| Postoperative recovery | Carmichael et al., 2019 | Prospective | Inguinal hernia (most common), abdominal and thoracic procedures | 175 | 30 days | Recovery trajectories have the potential to predict postoperative complications up to 3 days before readmission |
| Postoperative recovery | Thijs et al., 2019 | Prospective | CABG | 22 | 14 days | Higher physical activity has seen following minimally invasive procedures |
| Postoperative recovery | Cole et al., 2019 | Prospective | Transsphenoidal surgery | 7 | Up to 13 days (average 8 days) | Step count fell by 45% following surgery |
| Postoperative recovery | van der Meij et al., 2018 | RCT | Laparoscopic abdominal procedures | 344 | 6 months | Five-day reduction in return to normal activities (21 days vs. 26 days; P = 0.007) |
| Postoperative recovery | Ghomrawi et al., 2018 | Prospective | Range of elective paediatric surgical procedures | 60 | 14 days | Different activity curves demonstrated for patients undergoing in-patient and out-patient procedures |
| Postoperative recovery | Agarwal et al., 2018 | Prospective | Robotic laparoscopic prostatectomy | 46 | Up to 15 days | Greatest reduction in postoperative step count seen in obese and men aged >65 years old |
| Postoperative recovery | Jaensson et al., 2017 | RCT | Predominantly orthopaedic and general cases | 997 | 14 days | Improved recovery in several symptom domains |
| Postoperative recovery | Park et al., 2017 | RCT | Total knee replacement | 40 | 90 days | SMS messages achieved similar postoperative recovery compared to routine care |
| Postoperative recovery | Chiang et al., 2017 | Prospective | Total knee replacement | 18 | 6 weeks | Postoperative range of motion improved if haemostatic agent used intra-operatively |
| Postoperative recovery | Sun et al., 2017 | Prospective | Major gastrointestinal resection | 20 | 14 days | Median step count at day 7 correlated with the Comprehensive Complication Index (CCI) |
| Postoperative recovery | Abraham et al., 2017 | Prospective | Breast reconstruction | 4 | 28 days | Variance in total sleep duration is a potential marker of recovery |
| Postoperative recovery | Toogood et al., 2016 | Prospective | Total hip arthroplasty | 33 | 30 days | Activity increased in a step-wise fashion post-discharge. Age and operative approach were associated with postoperative activity |
| Postoperative recovery | Debono et al., 2016 | Prospective | Lumbar discectomy | 60 | 16 days | Deviations in expected postoperative recovery were identified early, reducing emergency department admissions |
| Postoperative recovery | Mobbs et al., 2016 | Prospective | Lumbar spine surgery | 30 | 90 days | Daily mean step count and distance had improved at follow-up |
| Postoperative recovery | Dawes et al., 2015 | Prospective | Any colorectal procedure | 20 | 14 days | Patients felt more aware of the recovery process and connected with their surgical team |
| Surgical site infection | Mousa et al., 2019 | RCT | Infra-inguinal procedures | 30 | 30 days | No difference in 30-day surgical site infection rates |
| Surgical site infection | Gunter et al., 2018 | Prospective | Lower limb vascular surgery | 40 | 14 days | Surgical site infection correctly identified in 87% of cases |
| Surgical site infection | Castillo et al., 2017 | Prospective | C-section | 105 | 30 days | One surgical site infection identified through intervention |
| Surgical site infection | Semple et al., 2015 | Prospective | Breast reconstruction and ACL repair | 65 | 30 days | All wound complications were correctly identified |
| Surgical site infection | Martinez-Ramos et al., 2009 | Prospective | Range of ambulatory procedures | 96 | 14 days | Two-thirds of patients had their wound concerns successfully resolved without need for hospital review |
| Surgical site infection | Perez et al., 2006 | Prospective | Predominantly orthopaedic procedures | 49 | Not stated | Images modified original treatment plans and avoided emergency department attendance for 88% |
| Follow-up requirements | Mousa et al., 2019 | RCT | Infra-inguinal procedures | 30 | 30 days | No difference in 30-day readmission rates |
| Follow-up requirements | Felbaum et al., 2018 | Prospective | Spinal surgery | 56 | 30 days | Mobile application reduced hospital visits |
| Follow-up requirements | Armstrong et al., 2017 | RCT | Breast reconstruction | 65 | 30 days | Fewer in-person follow-up care visits in mHealth group (0.4; P < 0.001) |
| Follow-up requirements | Higgins et al., 2017 | Prospective | ACL reconstruction | 32 | 6 weeks | Intervention reduced the need for routine follow-up |
| Follow-up requirements | McElroy et al., 2016 | Prospective | Cardiac surgery | 27 | 30 days | Readmissions similar between intervention and control groups |
| Weight loss | Mangieri et al., 2019 | RCT | Laparoscopic sleeve gastrectomy | 56 | 24 months | Application aided longer-term weight loss at 12 months post-surgery |
| Weight loss | Tenhagen et al., 2016 | Prospective | Gastric sleeve or bypass | 14 | 1 year | Excess weight loss >40% in all patients |
| Feasibility | Jonker et al., 2021 | Prospective | Oncological procedures | 47 | 90 days | Older patients (≥65 years old) can successfully perform home monitoring using DHIs, with good usability and acceptability |
| Feasibility | Argent et al., 2019 | Prospective | Total knee replacement | 15 | 14 days | Biofeedback system improved rehabilitation experience for patients |
| Feasibility | Scott et al., 2017 | Prospective | Colorectal surgery | 20 | 14 days | Low use of mobile application associated with inappropriate emergency department presentation in 63% of cases |
| Feasibility | Symer et al., 2017 | Prospective | Open and laparoscopic abdominal surgery | 31 | 30 days | Patients generated an average of 1.1 alerts, but 50% of patients struggled to upload photographs |
Differences in study methodology and outcome definitions limit conclusions on the effectiveness of DHI across each outcome. However, DHI demonstrated a strong ability to track postoperative analgesic requirements33,34,44,46 and patient recovery9,25,33,34,37,38,41–43,52,54,56,59,61,64–67,72 while consistently reducing hospital resource use in the postoperative period39,45,63,70. The capture of longer-term outcomes were also possible beyond 30 days, particularly for orthopaedic procedures25,34,38,63,64 and to monitor weight loss32,53. DHI were also able to identify complications at an early stage51,60 and correctly classify wound infection in the majority of patients47,55,62, demonstrating good agreement with physicians55,58.
Patient adherence
Twenty-five studies reported patient adherence with digital health interventions25,34,36,37,42–49,51–53,55,56,58–60,62,65,66,72 however this assessment varied widely (Tables 1 and 2). Patient adherence ranged between 42 to 96%, however, no included studies used a validated assessment method. Adherence was generally found to be highest within the first 2 weeks postoperatively55,58,72 with adherence falling for longer-term interventions34,55,62. Patients with complications were more likely to use DHI50, while limited use of mobile applications was associated with high rates of inappropriate emergency department presentation following major colorectal resection48. High patient satisfaction was reported in multiple studies23,33,39,45,47,53,54,57,69,71 however patients also found some DHI to be intrusive36,53,58,71 while none reported the carers’ use or experience of the intervention.
Reporting quality and bias
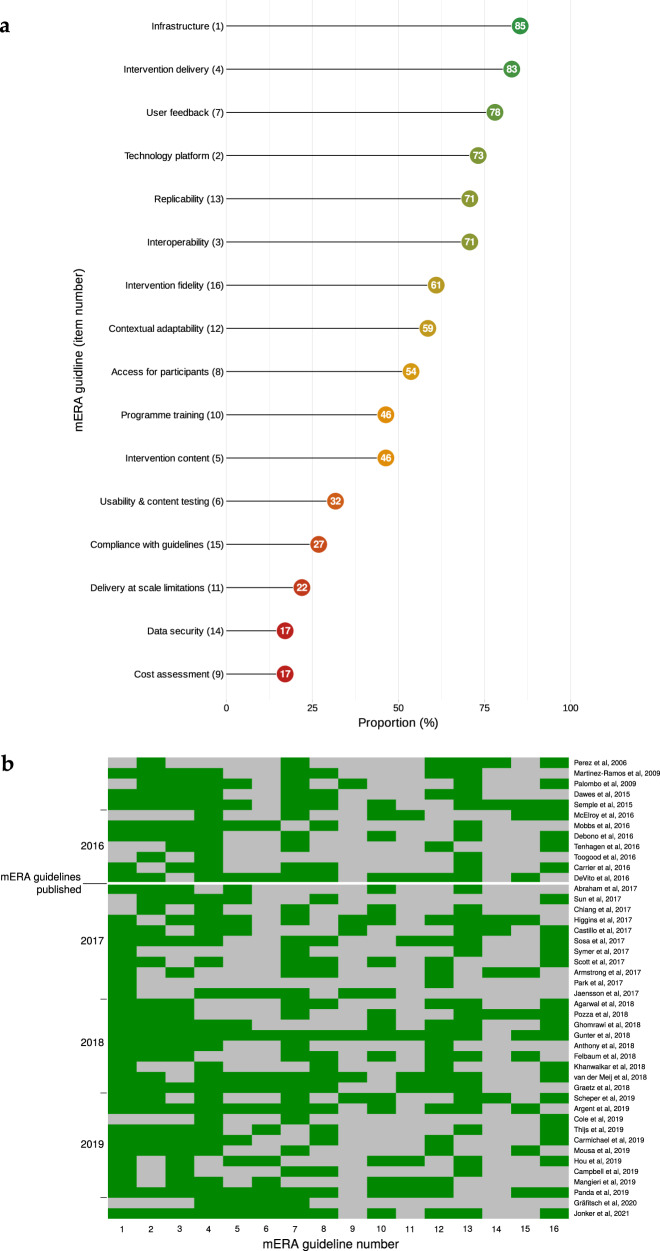
Overall, reporting quality was suboptimal, particularly within the items of data security, cost assessment and patient engagement during intervention development (Fig. 2a). Only one domain, the presentation of infrastructure availability to support technology within the study location (item 1), was consistently reported across all studies. Other domains, including data security, cost assessment and scalability; were frequently under-reported, demonstrating poor standardisation and limiting comparability across studies. The median score was 8 (range 2 to 15), while only nine (19%) studies scored above 1036,37,40,41,47,55,57,63,71 No obvious trends in reporting quality were detected over time, despite the publication of a mobile health evidence reporting and assessment (mERA) and World Health Organisation Monitoring and evaluating digital health interventions in 2016 (Fig. 2b). No association was found between study design, device and quality score.
Fig. 2. Reporting quality across included studies.
Reporting quality for each mERA guideline domain (a) and temporal relationship (b). mERA guideline item number contained within parentheses.
Critical appraisal revealed that all the eligible randomised studies had a high risk of bias in at least one defined outcome, primarily at the allocation and blinding stages (Fig. 3). Prospective studies also showed a high risk of bias, demonstrated during blinding and recruitment of consecutive patients (Supplementary Table 1). Furthermore, only two studies included a control group23,68 and only one performed a sample size calculation a priori56.
Fig. 3. Risk of bias assessment.
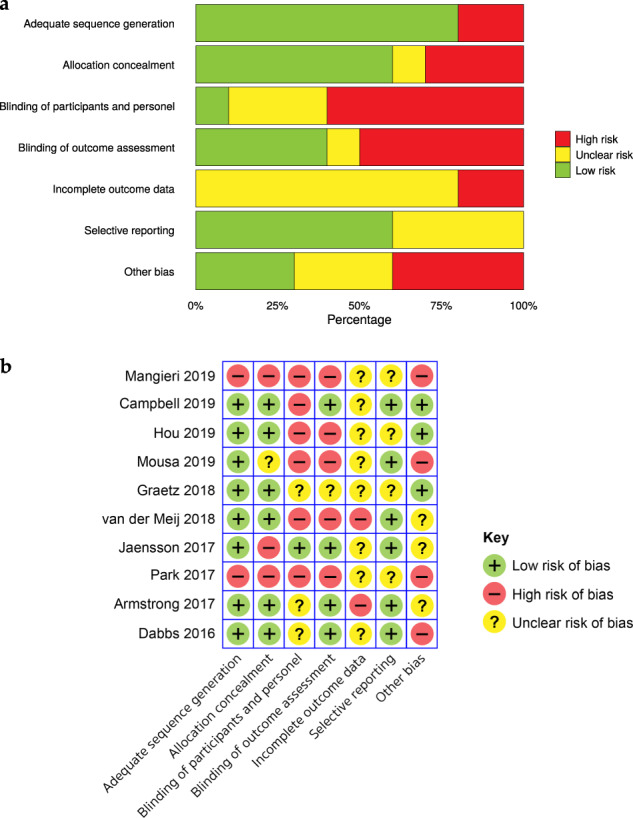
Overall summary (a) and individual bias assessment (b) for included randomised controlled trials assessed using the Cochrane collaboration tool.
Discussion
To our knowledge, this is the first systematic review to have investigated the use and effectiveness of mobile DHI in postsurgical care, including a rigorous assessment of current reporting quality. The increasing affordability and widespread use of mobile technologies presents new opportunities to remotely monitor patient-centred health metrics during the postoperative period. In this review, we evaluated the use of DHI to complement conventional postoperative care across 42 studies. The wide diversity in the types of patient population, intervention and outcome measures were reported, while methodological reporting was found to be suboptimal across multiple domains.
Overall, the results indicate that regular acquisition of objective wound data (from images), patient-reported outcome data (from validated self-report tools) or continuous activity data (from wearables) can improve the assessment of postoperative recovery26. Combining remote assessment with active clinical prompts or patient advice (whether via automated or manual checking) also has the potential to reduce complication rates. Randomised studies included in this review demonstrated that DHI may facilitate patient recovery following major operations9,37, reduce inappropriate service use39,40 and improve longer-term outcomes in bariatric surgery32,33. Despite these opportunities, our review revealed a number of issues with the existing evidence base which require to be addressed if this potential is to be fulfilled.
DHI can provide an opportunity for patient engagement, support and self-care73,74, providing a bridge between clinical services and patients’ homes and helping to mitigate social isolation paving new ways to explore two-way interactions. Despite these opportunities, the research studies reviewed herein captured in this review made little reference to engaging patients in the development of the DHI and only one study was designed to engage patients in their care or in reviewing their own data37. Given the critical role of clinician-patient partnerships in the successful delivery of interventions and in supporting shared care, this seems like a missed opportunity and we would encourage future patient-centred research and interventions73. Many of the studies reported high levels of exclusion amongst patients who did not possess the relevant mobile technology, suggesting that more work on inclusive design is needed to avoid exacerbating the ‘digital health divide’75. The case for better patient engagement, or carers supporting an individual’s recovery, may also mitigate the well-known problem of patient attrition from digital health interventions76.
Published studies on the use of DHI in surgical populations came almost exclusively from high-income countries, particularly the USA. This is likely reflects both the research funding environment in different regions and the lack of financial accessibility of smartphones and wearables in resource-constrained countries. However, the often significant distance patients travel for surgical care in low- and middle-income countries, combined with difficulties in determining early outcomes in these settings77, offers huge potential for postoperative patient outcome reporting and is a legitimate candidate for global health research funding26.
Aggregated day level summaries of patient activity were commonly reported, with few exploring the potential of other accelerometer metrics to predict postoperative complications, such as sleep quality78,79 or activity intensity26,80. Wearable devices were found to generally associate well with operative characteristics and complication severity, however considerable variability within patient cohorts existed, highlighting the need to be developing more personalised models42,56,65,81 Large error values originating from manufacturers’ algorithms82,83, lack of standardised procedures for optimising accuracy82 and small patient cohorts may explain this variance. Data were also frequently only available to clinicians for ‘offline’ analysis upon study completion, demonstrating the current limited ability of accelerometer technology to assist management of a larger population through preloaded signal analysis algorithms and timely clinical review84.
Companies often have a market strategy that relies on proprietary algorithms and closed data sets, making it difficult to evaluate these innovations. This problem is exacerbated when such algorithms are updated, complicating longitudinal comparisons of measures even within the same brand device. We recommend further research investment in Open Software and the sharing of appropriately anonymised datasets for meta-analysis, to encourage sustainable and trustworthy innovations of this type. This is particularly important as we move towards more automated methods involving artificial intelligence, where the ability to scrutinise algorithmic decision making will become increasingly crucial for patient safety and clinical accountability84.
Methodological reporting across the included studies was of variable quality. Current reporting inconsistency is problematic, limiting researchers’ and policy makers’ ability to understand programme details and determine the impact on health systems85. Moreover, continued suboptimal reporting will limit future comparison and study reproducibility. The lack of data security information is particularly concerning and in contrast to the high priority given to security and privacy in electronic health records in general55,86,87. Patients identify security as the single most important barrier to technology use postoperatively15 and future public confidence in DHI may be eroded if patient confidentiality is felt to be at-risk88,89.
Patient adherence reporting is a key component of the mERA guidelines to determine patient engagement, user interaction and DHI fidelity. However, there was wide variation in the definition and assessment of patient adherence within included studies, which restricted more detailed comparison. This suggests the development and validation of a standardised tool, detailing specific metrics on how patient adherence should be defined in DHI studies is needed.
Furthermore, cost assessment was also limited, with basic information on financial costs to design and develop DHI from the perspective of all end-users omitted. Digital health is often assumed to be cost-effective27, however a lack of evidence to substantiate this remains a barrier to implementation and policy investment90. Insufficient detail prevents meaningful comparison with existing care, while the cost of adoption in postoperative surgical settings cannot currently be justified without assessment relative to meaningful clinical outcomes91.
Despite widespread publication and being extensively accessed19,85,92 mERA guidelines were poorly represented within included studies. Designed to address the gaps in comprehensiveness and quality of reporting on the effectiveness of digital health programmes, by an expert committee convened by the World Health Organization (WHO), implementation of all items should be achievable across all income strata. We found no evidence of temporal change in reporting quality, with our findings demonstrating urgent action is required to achieve consistent and comprehensive reporting of digital health interventions. Therefore, we strongly recommend journal editors make mERA checklist completion a mandatory condition for acceptance, similar to other reporting guidelines93–95.
Some limitations should be highlighted. As our search was only limited to the English language, we may have excluded relevant publications if they were not published in English. In addition, the omission of studies originating from low and middle-income countries is possible, with underreporting of DHI known to occur in studies outside the United States or without an industry sponsor96. Due to the heterogeneity of included studies and the quality of methodological reporting, we were unable to answer how DHI can impact specific clinical outcomes. Therefore, reported findings should be cautiously interpreted towards the current assessment of how digital health can improve patient outcomes following surgery until additional, higher-quality studies are available.
DHI to monitor postoperative recovery has been used across a broad range of surgical specialities, particularly within the United States. Devices are generally acceptable to patients and have been shown to identify postoperative complications early. Current studies report findings on small cohorts, infrequently engage patients during the design or delivery of the intervention and utilise patient-generated data in a passive manner. The requirement to own a mobile device considerably limits patient inclusion, while urgent improvements in the reporting of data security and cost-effectiveness is needed.
In order to advocate for the widespread use of digital health in the monitoring of postoperative patient recovery, additional high-quality research is needed prior to integration into the healthcare environment. Particular attention to reporting quality is advised, to ensure these studies can be replicated and provide the opportunity for equitable comparison.
Methods
Design
An electronic systematic search of Embase, Cochrane Library, Web of Science, WHO Global Index Medicus, clinical trial registries and Google scholar databases in accordance with the PRISMA guidelines was performed93. The PROSPERO international systematic review registry97 was searched to ensure a similar review had not previously been performed and the protocol was registered accordingly (CRD42019138736).
A thorough search was undertaken using the following Medical Subject Heading (MeSH) terms: ‘cellular phone’; ‘microcomputers’; ‘smartphone’, ‘iphone’; 'android’; ‘mobile’; ‘ipad’; ‘tablet’; ‘text message’; ‘sms’; ‘e-health’; ‘telemedicine’; ‘digital health’; ‘wearable’; ‘mobile health’; ‘mHealth’; and ‘surgery’; ‘postoperative’. The search was structured to ensure variations such as capitalisation, plurals and alternative phrases were captured (Supplementary Information 1). Search limits applied were English language, full-text, humans and articles published from 2000 (last search 18 May 2021). Case reports and editorials were excluded, with conference abstracts and reviews screened to assist in identifying related full-text articles prior to exclusion.
The title and abstract of all identified articles were screened independently by two authors (S.R.K. and N.N.), with those meeting the inclusion criteria screened further by full-text review. Any disagreements were resolved by discussion to reach a consensus. Reference lists of relevant articles were reviewed, together with a search of grey literature and the National Clinical Trials Register (clinicaltrials.gov) to identify any further studies for inclusion.
Studies involving patients undergoing any surgery requiring skin incision where postoperative outcomes were measured using a DHI following hospital discharge were included. DHI were defined according to the mobile health evidence reporting and assessment (mERA) guidelines; 'the use of mobile and wireless technologies for health to improve health system efficiency and health outcomes'19, with web-based interventions excluded if stationary devices, such as a desktop computer, were only used27. The more generic term ‘digital health’ was selected to ensure all potential approaches, including mhealth, were systematically captured within this review98. Interventions containing only teleconsultation or patient education components were excluded due to the number of previously published reviews in this area27,30,31.
Data extraction
Data were extracted using a standardised proforma (Supplementary Information 2), with partial duplication to ensure consistency. Included studies were evaluated for study design, participant number, participant characteristics, DHI and origin, study duration and main findings. The method used to assess patient adherence was also extracted and reported based on the original study authors’ criteria. A wearable device was defined as a small computing device containing a sensor worn somewhere on the body99.
Quality assessment
Reporting quality was analysed using the validated mERA 16-item core checklist, which systematically assesses transparency and completeness in digital health studies19. All included publications and associated study protocols were reviewed independently for potential risk of bias by two authors (S.R.K. and N.N.), using the Cochrane Collaboration tools for randomised studies100 and the methodological index for non-randomised studies (MINORS)101, with the global ideal score varying between non-comparative (16) and comparative studies (24).
We aimed to determine the current use, evidence base and reporting quality for mobile DHI in the postoperative period following surgery.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Author contributions
S.R.K. and N.N. wrote the review protocol, conducted the literature searches, performed data extraction, and wrote the paper including introduction, methods, results, and discussion. S.R.K. and E.M.H. conceptualised oversaw development of the review. All authors read and critically commented on drafts of the study, including the latest version, and jointly take responsibility for the decision to submit this work for publication.
Data availability
No new or unpublished data is included within the study and all data is freely available.
Code availability
All code relating to summary figure development is available on request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-021-00525-1.
References
- 1.Weiser TG, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(Suppl 2):S11. doi: 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 2.Abbott TEF, et al. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br. J. Anaesth. 2017;119:249–257. doi: 10.1093/bja/aex137. [DOI] [PubMed] [Google Scholar]
- 3.Writing Committee for the VISION Study Investigators. et al. Association of Postoperative High-Sensitivity Troponin Levels With Myocardial Injury and 30-Day Mortality Among Patients Undergoing Noncardiac Surgery. JAMA. 2017;317:1642–1651. doi: 10.1001/jama.2017.4360. [DOI] [PubMed] [Google Scholar]
- 4.Jhanji S, et al. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia. 2008;63:695–700. doi: 10.1111/j.1365-2044.2008.05560.x. [DOI] [PubMed] [Google Scholar]
- 5.Khuri, S. F. et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann. Surg. 242, 326–341; discussion 341–343 (2005). [DOI] [PMC free article] [PubMed]
- 6.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators. et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 7.Tevis SE, Kohlnhofer BM, Weber SM, Kennedy GD. Postdischarge complications are an important predictor of postoperative readmissions. Am. J. Surg. 2014;208:505–510. doi: 10.1016/j.amjsurg.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenblatt DY, et al. Causes and Implications of Readmission after Abdominal Aortic Aneurysm Repair. Ann. Surg. 2012;256:595–605. doi: 10.1097/SLA.0b013e31826b4bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaensson M, Dahlberg K, Eriksson M, Nilsson U. Evaluation of postoperative recovery in day surgery patients using a mobile phone application: A multicentre randomized trial. Br. J. Anaesth. 2017;119:1030–1038. doi: 10.1093/bja/aex331. [DOI] [PubMed] [Google Scholar]
- 10.Sanger PC, et al. Patient perspectives on post-discharge surgical site infections: towards a patient-centered mobile health solution. PLoS ONE. 2014;9:e114016. doi: 10.1371/journal.pone.0114016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehmann M, Monte K, Barach P, Kindler CH. Postoperative patient complaints: a prospective interview study of 12,276 patients. J. Clin. Anesth. 2010;22:13–21. doi: 10.1016/j.jclinane.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999;27:97–132. [PubMed] [Google Scholar]
- 13.Kazaure HS, Roman SA, Sosa JA. Association of postdischarge complications with reoperation and mortality in general surgery. Arch. Surg. 2012;147:1000–1007. doi: 10.1001/2013.jamasurg.114. [DOI] [PubMed] [Google Scholar]
- 14.Stomberg, M. W., Platon, B., Widén, A., Wallner, I. & Karlsson, O. Health Information: What Can Mobile Phone Assessments Add? Perspect Health Inf. Manag. 9, (2012). [PMC free article] [PubMed]
- 15.Abelson JS, Symer M, Peters A, Charlson M, Yeo H. Mobile health apps and recovery after surgery: What are patients willing to do? Am. J. Surg. 2017;214:616–622. doi: 10.1016/j.amjsurg.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Nehra AK, et al. A Survey of Perceptions and Acceptance of Wearable Technology for Health Monitoring in a Urological Patient Population. Urol. Pract. 2017;4:508–514. doi: 10.1016/j.urpr.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Hwa K, Wren SM. Telehealth follow-up in lieu of postoperative clinic visit for ambulatory surgery: results of a pilot program. JAMA Surg. 2013;148:823–827. doi: 10.1001/jamasurg.2013.2672. [DOI] [PubMed] [Google Scholar]
- 18.Mishra A, Kapoor L, Mishra SK. Post-operative care through tele-follow up visits in patients undergoing thyroidectomy and parathyroidectomy in a resource-constrained environment. J. Telemed. Telecare. 2009;15:73–76. doi: 10.1258/jtt.2008.080808. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal S, et al. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. 2016;352:i1174. doi: 10.1136/bmj.i1174. [DOI] [PubMed] [Google Scholar]
- 20.Robinson, A., Oksuz, U., Slight, R., Slight, S. & Husband, A. Digital and Mobile Technologies to Promote Physical Health Behavior Change and Provide Psychological Support for Patients Undergoing Elective Surgery: Meta-Ethnography and Systematic Review. JMIR Mhealth Uhealth8, (2020). [DOI] [PMC free article] [PubMed]
- 21.Patel B, Thind A. Usability of Mobile Health Apps for Postoperative Care: Systematic Review. JMIR Perioperative Med. 2020;3:e19099. doi: 10.2196/19099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aalami O, Ingraham A, Arya S. Applications of Mobile Health Technology in Surgical Innovation. JAMA Surg. 2021;156:414–415. doi: 10.1001/jamasurg.2020.6251. [DOI] [PubMed] [Google Scholar]
- 23.McElroy I, et al. Use of digital health kits to reduce readmission after cardiac surgery. J. Surgical Res. 2016;204:1–7. doi: 10.1016/j.jss.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Appelboom, G. et al. Mobile Phone-Connected Wearable Motion Sensors to Assess Postoperative Mobilization. JMIR Mhealth Uhealth3, (2015). [DOI] [PMC free article] [PubMed]
- 25.Mobbs RJ, Phan K, Maharaj M, Rao PJ. Physical Activity Measured with Accelerometer and Self-Rated Disability in Lumbar Spine Surgery: A Prospective Study. Glob. Spine J. 2016;6:459–464. doi: 10.1055/s-0035-1565259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appelboom G, et al. The promise of wearable activity sensors to define patient recovery. J. Clin. Neurosci. 2014;21:1089–1093. doi: 10.1016/j.jocn.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Iribarren SJ, Cato K, Falzon L, Stone PW. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE. 2017;12:e0170581. doi: 10.1371/journal.pone.0170581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiani, B., Siddiqi, I., Lee, S. C. & Dhillon, L. Telerehabilitation: Development, Application, and Need for Increased Usage in the COVID-19 Era for Patients with Spinal Pathology. Cureus12. [DOI] [PMC free article] [PubMed]
- 29.Radanliev P, et al. COVID-19 what have we learned? The rise of social machines and connected devices in pandemic management following the concepts of predictive, preventive and personalized medicine. EPMA J. 2020;11:311–332. doi: 10.1007/s13167-020-00218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Meij E, Anema JR, Otten RHJ, Huirne JAF, Schaafsma FG. The Effect of Perioperative E-Health Interventions on the Postoperative Course: A Systematic Review of Randomised and Non-Randomised Controlled Trials. PLoS ONE. 2016;11:e0158612. doi: 10.1371/journal.pone.0158612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunter RL, et al. Current Use of Telemedicine for Post-Discharge Surgical Care: A Systematic Review. J. Am. Coll. Surg. 2016;222:915–927. doi: 10.1016/j.jamcollsurg.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangieri CW, Johnson RJ, Sweeney LB, Choi YU, Wood JC. Mobile health applications enhance weight loss efficacy following bariatric surgery. Obes. Res. Clin. Pract. 2019 doi: 10.1016/j.orcp.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Campbell KJ, et al. A Novel, Automated Text-Messaging System Is Effective in Patients Undergoing Total Joint Arthroplasty. J. bone Jt. Surg. Am. 2019;101:145–151. doi: 10.2106/JBJS.17.01505. [DOI] [PubMed] [Google Scholar]
- 34.Hou J, et al. The Effectiveness and Safety of Utilizing Mobile Phone-Based Programs for Rehabilitation After Lumbar Spinal Surgery: Multicenter, Prospective Randomized Controlled Trial. Jmir Mhealth Uhealth. 2019;7:e10201. doi: 10.2196/10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mousa AY, et al. Results of Telehealth Electronic Monitoring for Post Discharge Complications and Surgical Site Infections following Arterial Revascularization with Groin Incision. Ann. Vasc. Surg. 2019 doi: 10.1016/j.avsg.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Graetz I, et al. Use of a web-based app to improve postoperative outcomes for patients receiving gynecological oncology care: A randomized controlled feasibility trial. Gynecologic Oncol. 2018;150:311–317. doi: 10.1016/j.ygyno.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 37.van der Meij E, et al. Personalised perioperative care by e-health after intermediate-grade abdominal surgery: a multicentre, single-blind, randomised, placebo-controlled trial. Lancet. 2018;392:51–59. doi: 10.1016/S0140-6736(18)31113-9. [DOI] [PubMed] [Google Scholar]
- 38.Park KH, Song MR. The Effects of Postdischarge Telephone Counseling and Short Message Service on the Knee Function, Activities of Daily Living, and Life Satisfaction of Patients Undergoing Total Knee Replacement. Orthopedic Nurs. 2017;36:229–236. doi: 10.1097/NOR.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong KA, Coyte PC, Brown M, Beber B, Semple JL. Effect of Home Monitoring via Mobile App on the Number of In-Person Visits Following Ambulatory Surgery: A Randomized Clinical Trial. JAMA Surg. 2017;152:622–627. doi: 10.1001/jamasurg.2017.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dabbs AD, et al. A Randomized Controlled Trial of a Mobile Health Intervention to Promote Self-Management after Lung Transplantation. Am. J. Transpl. 2016;16:2172–2180. doi: 10.1111/ajt.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panda, N. et al. Using Smartphones to Capture Novel Recovery Metrics After Cancer Surgery. JAMA Surg. 1–7 10.1001/jamasurg.2019.4702 (2019). [DOI] [PMC free article] [PubMed]
- 42.Carmichael H, et al. Wearable Technology-A Pilot Study to Define ‘Normal’ Postoperative Recovery Trajectories. J. Surg. Res. 2019;244:368–373. doi: 10.1016/j.jss.2019.06.057. [DOI] [PubMed] [Google Scholar]
- 43.Thijs I, Fresiello L, Oosterlinck W, Sinnaeve P, Rega F. Assessment of Physical Activity by Wearable Technology During Rehabilitation After Cardiac Surgery: Explorative Prospective Monocentric Observational Cohort Study. Jmir Mhealth Uhealth. 2019;7:e9865. doi: 10.2196/mhealth.9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khanwalkar AR, et al. Utilization of a novel interactive mobile health platform to evaluate functional outcomes and pain following septoplasty and functional endoscopic sinus surgery. Int. Forum Allergy Rhinol. 2018 doi: 10.1002/alr.22273. [DOI] [PubMed] [Google Scholar]
- 45.Felbaum DR, et al. Implementation and evaluation of a smartphone application for the perioperative care of neurosurgery patients at an Academic Medical Center: Implications for patient satisfaction, surgery cancelations, and readmissions. Operative Neurosurg. 2018;14:303–311. doi: 10.1093/ons/opx112. [DOI] [PubMed] [Google Scholar]
- 46.Anthony CA, Lawler EA, Ward CM, Lin IC, Shah AS. Use of an Automated Mobile Phone Messaging Robot in Postoperative Patient Monitoring. Telemed. J. e-Health.: Off. J. Am. Telemed. Assoc. 2018;24:61–66. doi: 10.1089/tmj.2017.0055. [DOI] [PubMed] [Google Scholar]
- 47.Gunter RL, et al. Feasibility of an Image-Based Mobile Health Protocol for Postoperative Wound Monitoring. J. Am. Coll. Surg. 2018;226:277–286. doi: 10.1016/j.jamcollsurg.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott AR, Alore EA, Naik AD, Berger DH, Suliburk JW. Mixed-Methods Analysis of Factors Impacting Use of a Postoperative mHealth App. Jmir Mhealth Uhealth. 2017;5:e11. doi: 10.2196/mhealth.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Symer MM, Abelson JS, Milsom J, McClure B, Yeo HL. A Mobile Health Application to Track Patients After Gastrointestinal Surgery: Results from a Pilot Study. J. Gastrointest. Surg.: Off. J. Soc. Surg. Alimentary Trac. 2017;21:1500–1505. doi: 10.1007/s11605-017-3482-2. [DOI] [PubMed] [Google Scholar]
- 50.Sosa A, et al. Improving patient health engagement with mobile texting: A pilot study in the head and neck postoperative setting. Head. Neck. 2017;39:988–995. doi: 10.1002/hed.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrier G, et al. Post-discharge follow-up using text messaging within an enhanced recovery program after colorectal surgery. J. Visc. Surg. 2016;153:249–252. doi: 10.1016/j.jviscsurg.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Toogood PA, et al. The monitoring of activity at home after total hip arthroplasty. Bone Jt. J. 2016;98-B:1450–1454. doi: 10.1302/0301-620X.98B11.BJJ-2016-0194.R1. [DOI] [PubMed] [Google Scholar]
- 53.Tenhagen M, van Ramshorst GH, Demirkiran A, Hunfeld MAJM, Cense HA. Perioperative Online Weight Monitoring in Bariatric Surgery with a Digital Internet-Connected Scale. Obes. Surg. 2016;26:1120–1126. doi: 10.1007/s11695-016-2136-x. [DOI] [PubMed] [Google Scholar]
- 54.Debono B, et al. Postoperative monitoring with a mobile application after ambulatory lumbar discectomy: an effective tool for spine surgeons. Eur. Spine J. 2016;25:3536–3542. doi: 10.1007/s00586-016-4680-4. [DOI] [PubMed] [Google Scholar]
- 55.Semple JL, Sharpe S, Murnaghan ML, Theodoropoulos J, Metcalfe KA. Using a Mobile App for Monitoring Post-Operative Quality of Recovery of Patients at Home: A Feasibility Study. JMIR mHealth uHealth. 2015;3:e18. doi: 10.2196/mhealth.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cole TS, et al. Use of a wrist-mounted device for continuous outpatient physiologic monitoring after transsphenoidal surgery: a pilot study. Pituitary. 2019 doi: 10.1007/s11102-019-00946-y. [DOI] [PubMed] [Google Scholar]
- 57.Argent R, et al. Wearable Sensor-Based Exercise Biofeedback for Orthopaedic Rehabilitation: A Mixed Methods User Evaluation of a Prototype System. Sensors. 2019;19:432. doi: 10.3390/s19020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheper H, et al. A mobile app for postoperative wound care after arthroplasty: Ease of use and perceived usefulness. Int. J. Med. Inform. 2019;129:75–80. doi: 10.1016/j.ijmedinf.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Ghomrawi HM, et al. Using accelerometers to characterize recovery after surgery in children. J. Pediatr. Surg. 2018;53:1600–1605. doi: 10.1016/j.jpedsurg.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Pozza ED, et al. Patient satisfaction with an early smartphone-based cosmetic surgery postoperative follow-up. Aesthetic Surg. J. 2018;38:101–109. doi: 10.1093/asj/sjx079. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal DK, et al. Physical activity monitors can be successfully implemented to assess perioperative activity in urologic surgery. Mhealth. 2018;4:43. doi: 10.21037/mhealth.2018.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castillo E, McIsaac C, MacDougall B, Wilson D, Kohr R. Post-Caesarean Section Surgical Site Infection Surveillance Using an Online Database and Mobile Phone Technology. J. Obstet. Gynaecol. Can. 2017;39:645. doi: 10.1016/j.jogc.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 63.Higgins J., Semple J., Murnaghan L., Sharpe S., & Theodoropoulos J. Mobile Web-Based Follow-up for Postoperative ACL Reconstruction: A Single-Center Experience. Orthop. J. Sports Med.5, (2017). [DOI] [PMC free article] [PubMed]
- 64.Chiang C.-Y., Chen K.-H., Liu K.-C., Hsu S. J.-P., & Chan C.-T. Data Collection and Analysis Using Wearable Sensors for Monitoring Knee Range of Motion after Total Knee Arthroplasty. Sensors (Basel, Switzerland)17, (2017). [DOI] [PMC free article] [PubMed]
- 65.Sun V, et al. Wireless Monitoring Program of Patient-Centered Outcomes and Recovery Before and After Major Abdominal Cancer Surgery. JAMA Surg. 2017;152:852–859. doi: 10.1001/jamasurg.2017.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abraham, B. et al. Using Patient-Generated Health Data to Facilitate Preoperative Decision Making for Breast Cancer Patients. (2017).
- 67.Dawes AJ, et al. Wireless technology to track surgical patients after discharge: A pilot study. Am. Surg. 2015;81:1061–1066. [PubMed] [Google Scholar]
- 68.Palombo D, et al. Role of Interactive Home Telemedicine for Early and Protected Discharge 1 Day after Carotid Endarterectomy. Ann. Vasc. Surg. 2009;23:76–80. doi: 10.1016/j.avsg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 69.Martinez-Ramos C, Cerdan MT, Lopez RS. Mobile phone-based telemedicine system for the home follow-up of patients undergoing ambulatory surgery. Telemed. e-Health. 2009;15:531–537. doi: 10.1089/tmj.2009.0003. [DOI] [PubMed] [Google Scholar]
- 70.Perez F, et al. Evaluation of a mobile health system for supporting postoperative patients following day surgery. J. Telemed. telecare. 2006;12(Suppl 1):41–43. doi: 10.1258/135763306777978506. [DOI] [PubMed] [Google Scholar]
- 71.Jonker LT, et al. Remote Home Monitoring of Older Surgical Cancer Patients: Perspective on Study Implementation and Feasibility. Ann. Surg. Oncol. 2021;28:67–78. doi: 10.1245/s10434-020-08705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gräfitsch A, et al. Perioperative Tablet-Based Telemonitoring After Abdominal Wall Hernia Surgery: Pilot Prospective Observational Cohort Study. JMIR Perioperative Med. 2020;3:e15672. doi: 10.2196/15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang, W. E. et al. The role of a clinician amid the rise of mobile health technology. J. Am. Med. Inform. Assoc.10.1093/jamia/ocz131. [DOI] [PMC free article] [PubMed]
- 74.Zeevi D, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 75.Hall AK, Bernhardt JM, Dodd V, Vollrath MW. The digital health divide: evaluating online health information access and use among older adults. Health Educ. Behav. 2015;42:202–209. doi: 10.1177/1090198114547815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murray E, et al. Evaluating Digital Health Interventions: Key Questions and Approaches. Am. J. Prev. Med. 2016;51:843–851. doi: 10.1016/j.amepre.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.GlobalSurg Collaborative and National Institute for Health Research Global Health Research Unit on Global Surgery. Global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet. 2021;397:387–397. doi: 10.1016/S0140-6736(21)00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su X, Wang D-X. Improve postoperative sleep: what can we do? Curr. Opin. Anaesthesiol. 2018;31:83–88. doi: 10.1097/ACO.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hussain, M. S. Supporting the Delivery of Total Knee Replacements Care for Both Patients and Their Clinicians With a Mobile App and Web-Based Tool: Randomized Controlled Trial Protocol. [DOI] [PMC free article] [PubMed]
- 80.Donaire-Gonzalez D, et al. Benefits of Mobile Phone Technology for Personal Environmental Monitoring. JMIR Mhealth Uhealth. 2016;4:e126. doi: 10.2196/mhealth.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Górriz JM, et al. Artificial intelligence within the interplay between natural and artificial computation: Advances in data science, trends and applications. Neurocomputing. 2020;410:237–270. [Google Scholar]
- 82.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int. J. Behav. Nutr. Phys. Act. 2015;12:159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takacs J, et al. Validation of the Fitbit One activity monitor device during treadmill walking. J. Sci. Med Sport. 2014;17:496–500. doi: 10.1016/j.jsams.2013.10.241. [DOI] [PubMed] [Google Scholar]
- 84.Yetisen AK, Martinez-Hurtado JL, Ünal B, Khademhosseini A, Butt H. Wearables in Medicine. Adv. Mater. 2018;30:1706910. doi: 10.1002/adma.201706910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agarwal S, Lefevre AE, Labrique AB. A Call to Digital Health Practitioners: New Guidelines Can Help Improve the Quality of Digital Health Evidence. MHealth UHealth. 2017;5:e136. doi: 10.2196/mhealth.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whittaker R. Issues in mHealth: Findings From Key Informant Interviews. J. Med. Internet Res. 2012;14:e129. doi: 10.2196/jmir.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu D, Li T, Wang X, Yang X, Yu Z. Visualizing the intellectual structure and evolution of electronic health and telemedicine research. Int J. Med Inf. 2019;130:103947. doi: 10.1016/j.ijmedinf.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Atienza AA, et al. Consumer Attitudes and Perceptions on mHealth Privacy and Security: Findings From a Mixed-Methods Study. J. Health Commun. 2015;20:673–679. doi: 10.1080/10810730.2015.1018560. [DOI] [PubMed] [Google Scholar]
- 89.Ancker JS, Edwards AM, Miller MC, Kaushal R. Consumer perceptions of electronic health information exchange. Am. J. Prev. Med. 2012;43:76–80. doi: 10.1016/j.amepre.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 90.Chib A, van Velthoven MH, Car J. mHealth adoption in low-resource environments: a review of the use of mobile healthcare in developing countries. J. Health Commun. 2015;20:4–34. doi: 10.1080/10810730.2013.864735. [DOI] [PubMed] [Google Scholar]
- 91.Edlin, R., McCabe, C., Hulme, C., Hall, P. & Wright, J. Cost Effectiveness Modelling for Health Technology Assessment: A Practical Course. (ADIS, 2015).
- 92.Altmetric. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist: overview of attention for article published in British Medical Journal. https://www.altmetric.com/details/6208109. [DOI] [PubMed]
- 93.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schulz, K. F., Altman, D. G. & Moher, D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ340, (2010). [DOI] [PMC free article] [PubMed]
- 95.von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 96.Al-Durra M, Nolan RP, Seto E, Cafazzo JA, Eysenbach G. Nonpublication Rates and Characteristics of Registered Randomized Clinical Trials in Digital Health: Cross-Sectional Analysis. J. Med. Internet Res. 2018;20:e11924. doi: 10.2196/11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.PROSPERO: International prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/ (2021). [Accessed 20 May 2021].
- 98.Health, C. for D. and R. Digital Health. FDAhttps://www.fda.gov/medical-devices/digital-health (2020).
- 99.Neff, G. & Nafus, D. Self-Tracking. (MIT Press, 2016).
- 100.Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Slim K, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new or unpublished data is included within the study and all data is freely available.
All code relating to summary figure development is available on request to the corresponding author.