Highlights
-
•
Three halogenated pharmaceuticals of different therapeutic groups were treated in urine.
-
•
Selectivity comparison for sono-treatment, UVC/H2O2 and electrochemical oxidation.
-
•
Kinetics depended on chemical properties of pollutants and degradation routes of processes.
-
•
Sono-treatment showed higher selectivity for pollutants degradation in urine.
-
•
Sono-treatment decreased biological activity associated with the pharmaceuticals.
Keywords: Biological activity changes, Degradation routes, Halogenated pharmaceuticals elimination, Processes selectivity, Sonochemical system, Urine treatment
Abstract
This work considered the sonochemical degradation (using a bath-type reactor, at 375 kHz and 106.3 W L-1, 250 mL of sample) of three representative halogenated pharmaceuticals (cloxacillin, diclofenac, and losartan) in urine matrices. The action route of the process was initially established. Then, the selectivity of the sonochemical system, to degrade the target pharmaceuticals in simulated fresh urine was compared with electrochemical oxidation (using a BDD anode, at 1.88 mA cm−2), and UVC/H2O2 (at 60 W of light and 500 mol L-1 of H2O2). Also, the treatment of cloxacillin in an actual urine sample by ultrasound and UVC/H2O2 was evaluated. More than 90% of the target compounds concentration, in the simulated matrix, was removed after 60 min of sonication. However, the sono-treatment of cloxacillin in the real sample was less efficient than in the synthetic urine. The ultrasonic process achieved 43% of degradation after 90 min of treatment in the actual matrix. In the sonochemical system, hydroxyl radicals in the interfacial zone were the main degrading agents. Meanwhile, in the electrochemical process, electrogenerated HOCl was responsible for the elimination of pharmaceuticals. In turn, in UVC/H2O2 both direct photolysis and hydroxyl radicals degraded the target pollutants. Interestingly, the degradation by ultrasound of the pharmaceuticals in synthetic fresh urine was very close to the observed in distilled water. Indeed, the sonodegradation had a higher selectivity than the other two processes. Despite the sono-treatment of cloxacillin was affected by the actual matrix components, this contrasts with the UVC/H2O2, which was completely inhibited in the real urine. The sonochemical process led to 100% of antimicrobial activity (AA) elimination after 75 min sonication in the synthetic urine, and ∼ 20% of AA was diminished after 90 min of treatment in the real matrix. The AA decreasing was linked to the transformations of the penicillin nucleus on cloxacillin, the region most prone to electrophilic attacks by radicals according to a density theory functional analysis. Finally, predictions of biological activity confirmed that the sono-treatment decreased the activity associated with cloxacillin, diclofenac, and losartan, highlighting the positive environmental impact of degradation of chlorinated pharmaceuticals in urine.
1. Introduction
According to the Water Framework Directive of the European Union, halogenated pharmaceuticals require special attention when addressing groundwater legislation and environmental risks. These kinds of substances are designed to ensure high stability and bioavailability in patients [1]. Thus, due to the high stability of halogenated pharmaceuticals, they may be considered as concern environmental pollutants [2].
The use of chlorine in medicinal chemistry is one of the hottest areas in the synthesis of pharmaceuticals. In fact, among the four halogens, chlorine (Cl) is the most frequently found in drugs than others, even fluorine (F) [3]. Chlorine atom in the pharmaceutical structures induces special properties such as the high electrophilicity at the carbon connected to the chlorine atom, and an increase in lipophilicity of the molecule [3], [4]. Indeed, the increase in lipophilicity of the whole molecule by the chlorine presence enhances the partitioning of the pharmaceutical into the lipophilic phase of cell membranes or lipophilic domains of proteins [3]. The high lipophilicity could potentially promote the bioaccumulation of pharmaceuticals in aquatic organisms, which may have ecotoxicological effects or have human exposure through food (e.g., fishes) [2]. Therefore, the input of this kind of pharmaceuticals into the environment should be limited.
Most human and veterinary pharmaceuticals enter the environment through effluents of municipal wastewater treatment plants, where conventional processes are not able to degrade them [5], [6]. Thereby, a strategy to limit the pharmaceuticals input the environment could be the degradation of these substances in primary such as urine and feces. It is well-known that urine is a main route of pharmaceuticals excretion [7], and this matrix can contain these substances up to mg L-1 levels, which may favor their elimination compared with other matrices where pharmaceuticals are more diluted [8], [9].
Advanced oxidation processes (AOPs) are based on the generation and use of strong oxidizing species such as the hydroxyl radical (HO•). These processes are applicable for the degradation of pharmaceuticals in the urine matrix [10], [11], [12], [13]. Hydroxyl radicals can be efficiently produced using high-frequency ultrasound waves (100–1000 kHz), involving the acoustic cavitation phenomenon [14]. Ultrasonic action cleavages water molecules and dissolved oxygen, generating hydroxyl radicals [15]. Moreover, this system also produces hydrogen peroxide from the combination of hydroxyl radicals. In fact, the formation of H2O2 is an indicator of sonochemical activity [16].
Hydroxyl radicals can also be produced throughout electrochemical systems such as anodic oxidation using a boron-doped diamond (BDD) anode. BDD anodes promote water electrolysis to generate HO•. When chloride ion is present in the aqueous solution, active chlorine species (such as Cl2, HOCl, and OCl-) are also produced. These chlorine species can degrade organic pollutants, and their speciation is a function of the pH of the solution, Cl2 predominates (E° = 1.36 V) at pH < 3, HOCl (E° = 1.49 V) is the main species at pH between 3.0 and 7.5; and OCl− (E° = 0.89 V) prevails at pH greater than 7.5 [17].
The combination of UVC light with hydrogen peroxide (UVC/H2O2) is also employed to form the hydroxyl radical. In this AOP, the UVC irradiation (e.g., photons of 254 nm) leads to the homolytic cleavage of the hydrogen peroxide, generating two hydroxyl radicals, to transform diverse pollutants [18].
Due to the high reactivity of hydroxyl radicals, matrix components can also interact with HO•, which reduces the efficiency of the treatment [19]. Therefore, some works have recently been addressed to evaluate the selectivity of a process to preferentially degrade a target compound in a multicomponent aqueous matrix [13], [20]. The selectivity aspect is very important, especially for eliminating organic pollutants in complex matrices such as urine or wastewater. Interestingly, in a previous work from our research team, it was evidenced that the sonochemical AOP was highly selective for the degradation of a pharmaceutical in simulated urine [13]. Nonetheless, the sono-treatment of other compounds in this matrix is required to confirm that the selectivity of ultrasound is not exclusive for a singular compound.
It should be remarked that photochemical (e.g., UVC/H2O2), electrochemical (e.g., anodic oxidation), and sonochemical AOPs have been successfully applied for the elimination of pharmaceuticals in urine [10], [11], [12], [13], [21], [22]. However, most of the previous works consider the treatment of only one compound. Additionally, the comparison of ultrasound with photochemical and electrochemical AOPs for the degradation of pharmaceuticals in urine is not reported. Hence, in this research, the treatment of three relevant chlorine-containing pharmaceuticals (cloxacillin (CLX), diclofenac (DFC) and losartan (LOS)) in urine by high-frequency ultrasound, UVC/H2O2, and electrochemistry using a BDD anode, is presented. CLX, DFC, and LOS were selected because they belong to different therapeutic groups (antibiotics, analgesics, and antihypertensives, respectively). Also, they are widely consumed, frequently found in wastewaters, and able to induce negative environmental impacts [23], [24], [25], [26], [27], [28], [29].
In the present work, three missed aspects in the current literature were considered: 1) the treatment of pharmaceuticals of different therapeutic groups and chemical structure in the urine matrix, 2) the comparison of diverse AOPs in terms of the selectivity toward degradation of target pharmaceuticals in the urine matrix, and 3) the relationship between the primary transformations and the change in the biological activity of treated samples. To cover these topics, the action routes of the three AOPs (sonochemical, electrochemical, and photochemical processes) involved in the elimination of the three target pharmaceuticals were determined. Then, the selectivity of the processes in the degradation of the pharmaceuticals in simulated fresh urine was assessed. Due to the sonochemical system showed the highest selectivity for the elimination of the three pollutants, the primary transformations and modifications of the activity of pollutants under the ultrasonic action were established. Also, the ability of the sonochemical process to degrade a target pollutant and decrease the biological activity in an actual urine sample was tested. Finally, to evaluate the application feasibility of the sonochemical technology from the electric energy point of view, the specific electrical energy consumption was calculated.
2. Materials and methods
2.1. Reagents and samples
2.1.1. Reagents
Sodium cloxacillin (CLX) was provided by Syntofarma S.A. Sodium diclofenac (DFC) was obtained from Laproff laboratories. Potassium losartan (LOS) was purchased from La Santé S.A. Acetonitrile, ammonium heptamolybdate, methanol, nutrient agar, potassium hydrogen phthalate, potassium iodide, potassium perchlorate, sodium acetate, sodium chloride, sodium dihydrogen phosphate, sodium hydroxide, sodium sulfate, sulfuric acid, and urea were provided by Merck. Ammonium chloride, calcium chloride, ferrous sulfate heptahydrate, formic acid, hydrogen peroxide, and magnesium chloride were provided by PanReac.
2.1.2. Samples
The solutions of the pharmaceuticals and synthetic fresh urine were prepared using distilled water. In all cases, the initial concentrations of the target pharmaceuticals were 43.38 µmol L-1 (in the order of mg L-1, which is plausible to be found in human urine [8], [9], [30]). The pharmaceuticals were treated individually. The composition of the synthetic fresh urine is presented in Table 1, which was taken from Amstutz et al. [31].
Table 1.
Composition of the synthetic fresh urine.
| Compound | Concentration [in mg L-1] |
Concentration [ in mol L-1] |
|---|---|---|
| Urea | 16,000 | 0.2664 |
| NaCH3COO | 10,250 | 0.1250 |
| Na2SO4 | 2300 | 0.01619 |
| NH4Cl | 1800 | 0.03365 |
| NaH2PO4 | 2900 | 0.02417 |
| KCl | 4200 | 0.05634 |
| MgCl2 | 370 | 0.003886 |
| CaCl2 | 510 | 0.004595 |
| NaOH | 120 | 0.00300 |
| Conductivity = 19.5 mS cm−1 | ||
| Absorbance at 254 nm = 0.208 | ||
| Total organic carbon = 6197 mg L-1 | ||
| pH = 6.1 | ||
The actual fresh urine sample was provided by a healthy volunteer. The sample (a composite of 1.0 L) was collected during a 12 h period in a sterile amber flask, and this was stored under refrigeration at 4 °C. The experiments were performed within 48 h after the sample collection. For the degradation experiments, the real urine sample was spiked with the target pollutant to obtain an initial concentration of 43.38 µmol L-1 (the same concentration as used for the experiment in distilled water and simulated fresh urine).
2.2. Analyses
2.2.1. Evolution of pharmaceuticals
The evolution of the pharmaceuticals during treatments was followed by using a UHPLC Thermo Scientific Dionex UltiMate 3000 instrument equipped with an Acclaim™ 120 RP C18 column (5 µm, 4.6 x150 mm) and a diode array detector. The injection volume was 20 µL. The chemical structure, composition of mobile phase, flow, and detection wavelength for each pharmaceutical are detailed in Table 2. The evolution of the target pharmaceuticals followed pseudo-first-order kinetics, and the degradation rate constant (k) was determined as the slope of the plot of Ln (Ct/Co) vs. time [13]. All experiments were performed at least by duplicate.
Table 2.
Chromatographic conditions for analysis of the pharmaceuticals.
| Pharmaceuticals | Mobile phase: Formic acid /Acetonitrile /methanol (% v/v/v) |
Flow: Isocratic mode (mL min−1) |
Wavelength of detection (nm) |
|---|---|---|---|
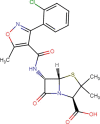 Cloxacillin (CLX) |
50/50/0 | 0.7 | 225 |
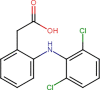 Diclofenac (DFC) |
30/70/0 | 0.5 | 260 |
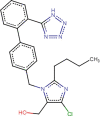 Losartan (LOS) |
46/44/10 | 0.6 | 230 |
2.2.2. Primary transformation products
The primary transformation products were elucidated by chromatographic separation and mass spectrometry detection, using a UHPLC-QTOF MS instrument. A Waters Acquity UHPLC system (Waters, Milford, MA, USA) coupled to a hybrid quadrupole-orthogonal acceleration-TOF mass spectrometer (XEVO G2 QTOF, Waters Micromass, Manchester, UK), using an orthogonal Z-spray-ESI interface. The chromatograph was equipped with a Cortecs BEH C18 analytical column (2.1 mm × 100 mm, 2.1 μm) from Waters. The mobile phase consisted of a mixture of methanol acidified with 0.01% formic acid (A) and water acidified with 0.01% formic acid (B), at a flow rate of 300 µL min−1. The initial percentage of A was 10%, which was linearly increased to 90% in 14 min, followed by 2 min in isocratic mode, and then returned to initial conditions over 2 min. The QTOF MS was operated in both ionization modes under the following conditions: capillary voltages of 700 (ESI + ) and 2000 V (ESI-), cone voltage of 20 V, at 600 °C of desolvation temperature, source temperature set to 130 °C, and column temperature set to 40 °C [16].
2.2.3. Oxidizing species
Accumulation of sonogenerated hydrogen peroxide and electrogenerated active chlorine species was estimated by iodometry [32]. An aliquot of 600 µL from the reactors was added to a quartz cell containing 1350 µL of potassium iodide (0.1 mol L-1) and 50 µL of ammonium heptamolybdate (0.01 mol L-1). After 5 min, the absorbance at 350 nm (for the formed triiodide ion) was measured using a Mettler Toledo UV5 spectrophotometer. Then, the concentration of sonogenerated hydrogen peroxide or electrogenerated active chlorine species was determined using the Beer-Lambert law and the molar absorptivity at 350 nm for the triiodide ion (which is 26,400 L mol−1 cm−1). The rate of H2O2 accumulation (Ra), in the sonochemical process, was calculated as the slope of the plot of oxidants concentration vs. time [33].
2.2.4. Antimicrobial activity
Antimicrobial activity was determined by the Kirby-Bauer method, using Staphylococcus aureus (ATCC 25923) as the indicator bacteria. The samples to be analyzed (30 μL) were seeded on Petri dishes containing 12 mL of nutrient agar (which has been inoculated with 10 μL of S. aureus (with an optical density of 0.600 at 580 nm). After 24 h at 37 °C in a Memmert-Schwabach incubator, the diameter of the inhibitory halo was measured with a vernier [34]. The antimicrobial activity tests were performed by triplicate, and in the plots, the results were reported as the average value, and the error bar represents the corresponding standard deviation.
2.2.5. Urine characterization
The pH was directly measured using a pH93 pH-meter. Conductivity was determined by direct measurement using a Lab945 SI Analytics conductimeter. The absorbance at 254 nm was measured using a Mettler Toledo UV5 spectrophotometer. Total organic carbon was established through a Shimadzu TOC-L analyzer. This apparatus comprised catalytic oxidation at 680 °C using a Pt-catalyst and high-purity compressed air as a carrying gas, and a non-dispersive infrared detector. The injection volume was 50 μL. The calibration curve for the analysis was prepared with standard solutions of potassium hydrogen phthalate.
2.3. Reaction systems
2.3.1. Sonochemical system
Sonochemical experiments were carried out in a Meinhardt Ultrasonics bath-type reactor equipped with a cylindrical glass vessel (500 mL maximum capacity), which contained 250 mL of sample to be treated. The reactor temperature was controlled at 20 °C using a Huber Minichiller. The ultrasound waves were emitted from a transducer placed at the bottom of the reactor. The reactor was operated at 375 kHz of frequency, and 26.6 W (power density: 106.3 W L-1) of actual ultrasound power, which was determined by the calorimetric method [35]. The experimental conditions were selected based on previous works [13], [36]. For the experiments in distilled water and simulated fresh urine, the total sono-treatment time was 60 min (aliquots of 1.1 mL were taken from the reaction system at 0, 5,10, 20, 30, 45, and 60 min of treatment for the analyses). Meanwhile, in the actual urine experiments, the sample was sonicated for 90 min, taking aliquots of 1.1 mL from the reaction system at 0, 10, 20, 30, 45, 60, 75, and 90 min of treatment for the analyses. As the real sample was matrix more complex than the simulated fresh urine, the treatment time was higher regarding the synthetic one.
2.3.2. Electrochemical system
Electrochemical experiments were conducted in an undivided electrolytic cell (a beaker), containing 50 mL of the solution to be treated, under constant magnetic stirring (750 rpm). Degradation experiments were performed applying a constant current density (J: 1.88 mA cm−2, galvanostatic conditions) and using a BBD anode (8 cm2). The cathode was a zirconium spiral electrode (10 cm2). The separation between the electrodes was ∼ 1 cm. The experimental conditions were selected based on literature [37], [38]. For the experiments in distilled water and simulated urine, the total electrolysis time varied between 90 (1.5) and 300 s (5 min), depending on the target pollutant. During the treatment of DFC, aliquots of 0.7 mL were taken from the reaction system at 0, 15, 30, 45, 60, 75, and 90 s. For LOS, the aliquots of 0.7 mL were taken at 0, 15, 30, 60, 90, 120, and 180 s. in the case of CLX the aliquots of 0.7 mL from the reaction system were taken at 0, 30, 60, 120, 180, and 300 s for the corresponding analyses.
2.3.3. Photochemical system
For the UVC/H2O2 process, a homemade aluminum reflective box (L: 55 cm, H: 40 cm, and W: 30 cm) containing three UVC lamps (OSRAM HNS®, with the main emission peak at 254 nm, 60 W) was used. The solutions of pharmaceuticals (50 mL) were placed in beakers below the lamps (at 25 cm) under constant stirring (750 rpm). The hydrogen peroxide was added to the reaction systems at an initial concentration of 500 µmol L-1. Immediately added the H2O2, the UVC light was switched on. The experimental conditions were selected based on the literature [13]. For the experiments in distilled water and simulated urine, the total treatment time was 20 min (aliquots of 0.5 mL were taken from the reaction system at 0, 1, 3, 5, 10, 15, and 20 min of treatment for the analyses). Meanwhile, for the experiments in actual urine, the samples were treated for 30 min (aliquots of 0.5 mL were taken from the reaction system at 0, 1, 3, 5, 10, 15, 20, and 30 min of treatment to carry out the analyses).
2.4. Theoretical calculations
2.4.1. Density functional theory (DFT) calculations
For the determination of electron-rich regions susceptible to electrophilic attacks by the hydroxyl radical, computational analyses were performed by applying the framework of density functional theory (DFT). The structure was optimized with the B3LYP hybrid functional density [39], 6–311++G(2d,2p) method [40] using the dielectric constant for water.
2.4.2. Predictions of biological activity
Predictions of biological activity for the target pollutants and their primary transformation products were carried out on the PASS software (free online version) [41]. For these calculations, the chemical structures of the parent pharmaceuticals and their corresponding products were uploaded individually to the PASS software in the SMILE format. After running the theoretical calculation, the software outputs tables containing a set of biological activities with their probabilities (Pa).
3. Results and discussion
3.1. Degradation routes assessment of the pharmaceuticals upon the evaluated processes
3.1.1. Sonochemical process
Initially, the sonochemical process was applied to degrade DFC, CLX, and LOS individually, in distilled water. Fig. 1 shows the evolution of the pharmaceuticals, indicating that the process was able to degrade ∼ 90% of the three pollutants after 60 min of treatment. It can be noted that the degradation profiles for the target compounds were very close and similar. Thus, it can be expected that DFC, CLX, and LOS present the same degradation route under the action of the sonochemical process.
Fig. 1.
Degradation of the target pharmaceuticals by ultrasound. Inset: accumulation of H2O2 in absence of pharmaceutical (BK), and in presence of the pharmaceuticals (DFC, CLX or LOS). Experimental conditions: [Pharmaceutical] = 43.38 μmol L-1, acoustic power = 26.6 W, frequency = 375 kHz, pH = 6.1.
As the considered pollutants are non-volatile compounds, they are not degraded inside the cavitation bubbles [42], [43], [44]. Then, DFC, CLX, and LOS can be degraded at the interfacial region or in the solution bulk by the action of sonogenerated hydroxyl radical [45], [46]. To corroborate the participation of hydroxyl radical in the sonodegradation of the pollutants, a control test in the presence of 2-propanol (2-prop), which is a well-known scavenger of hydroxyl radicals [47], was performed. As the three pharmaceuticals had similar behavior (Fig. 1), one compound, specifically LOS, was selected for the test of sonodegradation in presence of 2-prop.
Fig. S1 (in Supplementary material) compares the k values for degradation of LOS alone and LOS in presence of 2-prop. We can note that the rate constant in the presence of 2-propanol was significantly decreased (∼60% lower). Therefore, the decrease in k value in the 2-prop presence indicates that HO• is the main degradation route for these pharmaceuticals. Besides, the initial rate of accumulation of H2O2 (Ra) during the degradation of the pollutant was determined, and compared with a control experiment (BK), in which distilled water was sonicated in absence of pharmaceuticals. Inset in Fig. 1 shows that the Ra values in the absence (BK) and presence of the pharmaceuticals were ∼ 3.6, and ∼ 2.5 µmol L-1 min−1, respectively. The lower rate of H2O2 accumulation also confirms the reaction between the sonogenerated HO• and DFC, CLX, and LOS.
To determine the region of degradation of the target pharmaceuticals. An experiment in the presence of ferrous ions was performed. Fe (II) was added to the sonochemical reactor at 90 µmol L-1 (pH 6.1), and then the sonication started. This low concentration of iron was used to limit the precipitation of iron at the experimental pH, which is higher than 3.0 [48]. The Fe (II) reacts with the sonogenerated hydrogen peroxide producing HO• (Eq. (1)) in the solution bulk, thus improving the degradation of compounds placed in this zone [49]. As the three pharmaceuticals had similar behavior (Fig. 1), one of the compounds, specifically LOS, was selected for the tests of sonodegradation in presence of Fe (II).
| (1) |
Fig. S2 compares the k values for the sonochemical degradation of LOS alone and LOS in presence of Fe (II). We can note that the rate constants in the absence and presence of ferrous ions were the same. Hence, the non-enhancement of the LOS degradation by the Fe (II) addition indicates that this pharmaceutical (also DFC and CLX) is mainly degraded in the bubble-solution interface. It is important to mention that the values of Log KOW (which is an indicator of hydrophobicity [36]) for DFC, CLX, and LOS are 4.51, 2.48, 4.01, respectively [50]. This indicates that these target compounds have hydrophobic characteristics, and they tend to accumulate at the interfacial zone of the system, thus having a high opportunity to be degraded by the sonogenerated HO• [51]. Moreover, it can be suggested that the no differentiation in the degradation of these pharmaceuticals is due to the high similarity in their hydrophobicity.
3.1.2. Electrochemical process
The electrochemical treatment of the pharmaceuticals was carried out in the presence of chloride ions (which is the main anion in urine). The evolution of the pollutants is presented in Fig. 2, showing that under this process, the degradation order was DCF > LOS > CLX. Interestingly, the elimination of pharmaceuticals was so fast, after 3 min of electrolysis, more than 90% of degradation was achieved.
Fig. 2.
Degradation of the target pharmaceuticals by the electrochemical oxidation. Inset: Accumulation of HOCl in absence of pharmaceutical (BK), and in presence of diclofenac (DFC). Experimental conditions: [Pharmaceutical] = 43.38 μmol L-1, [NaCl] = 0.1 mol L-1, BDD anode, Zr cathode, J = 1.88 mA cm−2, pH = 6.1.
In this electrochemical system, two degradation routes are possible: 1) direct oxidation by interaction with anode surface [52], or with hydroxyl radicals superficially adsorbed [53], and 2) indirect oxidation by species generated from the supporting electrolyte [54], [55]. As mentioned above, reactive active chlorine species can be formed from the oxidation of chloride ions from the supporting electrolyte. Then, to verify the participation of the indirect route, the accumulated concentration of active chlorine species in both absence (BK) and presence of the pharmaceuticals was measured for the experiment when NaCl was used as the supporting electrolyte (inset in Fig. 2, and Fig. S3). It can be noted that the accumulation of active chlorine species in the presence of pollutants was ∼ 50% or less than the found in their absence (BK). These results indicate that active chlorine species are strongly involved in the degradation of the target compounds, and according to the experimental pH (6.1), the predominant chlorine species is the hypochlorous acid (HOCl, [17]).
To determine the participation of the direct oxidation, an experiment using KClO4 (0.1 mol L-1) as the supporting electrolyte was carried out for the three pollutants. In contrast to NaCl, KClO4 limits active chlorine species formation, Fig. S4, favoring the interaction with the BDD surface). With the use of KClO4 as the supporting electrolyte, less than 10% of pharmaceuticals were degraded after 5 min of treatment (Fig. S5), indicating that under the tested conditions, the direct route has a minor contribution to the degradation of the pollutants [56].
As upon the considered electrochemical system, the main route of degradation involves the electrogenerated HOCl, the differences observed in Fig. 2 for the elimination of pharmaceuticals can be associated with the reactivity of the pollutants toward the hypochlorous acid. It is reported that this chlorine species can attack functional groups such as phenols, activated aromatic rings, aliphatic and aromatic amines, sulfur reduced forms, amides, and alcohol moieties present in the organic pollutants [17].
CLX has a thioether, two amides, and a chlorophenyl isoxazole group; whereas, DFC contains an aniline-type structure. In turn, LOS has a biphenyl-tetrazole structure and an alcohol moiety. All these functional groups do the target pharmaceuticals susceptible to transformation by the electrogenerated HOCl. This active chlorine species presents the following reaction rates order: amines > thioethers > aromatic activated systems > amides [17]. Thus, DFC has a secondary amine and an activated aromatic ring, making this analgesic more reactive than LOS and CLX to the HOCl. Indeed, it is reported that the interaction of DFC with active chlorine leads to chlorinations of both the amine and the activated aromatic ring, as primary transformation pathways [57]. In the case of LOS, the electrogenerated HOCl could react with the biphenyl group, which is highly susceptible to chlorination [58]. Meanwhile, HOCl may transform CLX through oxidation of thioether, opening of the β-lactam ring, and breakdown of the central amide [38].
3.1.3. UVC/H2O2 process
The UVC/H2O2 process includes the action of light of 254 nm, hydrogen peroxide, and radicals. Then, to establish the routes that participate in the degradation of the pharmaceuticals in this process, the effects on the pollutants of UVC or H2O2 individually, and the combination UVC/H2O2 were determined (Fig. 3). The hydrogen peroxide alone did not degrade the pharmaceuticals significantly (elimination lower than 3% after 20 min of treatment). In contrast, at 20 min of exposure, the treatment with the sole UVC light degraded 95.2, 81.2, and 33.5% of DFC, CLX, and LOS, respectively. In turn, at the same time, the UVC/H2O2 process eliminated 98.4, 84.2, and 65.7 % of DFC, CLX, and LOS, respectively. These results indicated that in the UVC/H2O2 system, the photodegradation by the UVC light, especially for DFC and CLX, had the main contribution. In the case of LOS, the participation of the hydroxyl radical is particularly significant.
Fig. 3.
Treatment of the pharmaceuticals by the UVC/H2O2 process. Inset: removal of the pharmaceuticals by the individual components of the process. Experimental conditions: [Pharmaceutical] = 43.38 μmol L-1, [H2O2] = 500 µmol L-1, UVC light = 60 W, pH = 6.1.
The ultraviolet spectra for DFC, CLX, and LOS show they can absorb light at 254 nm (Fig. S6), due to the presence of aromatics rings, pi-conjugated systems, and heteroatoms [59]. In fact, the high participation of the light in the UVC/H2O2 process suggests that the differences in the degradation observed in Fig. 3 can be related to the diversity in the chemical structure of pharmaceuticals, which strongly affects the photodegradation of pollutants [60], [61].
3.2. Selectivity of the processes in the degradation of the pharmaceuticals in simulated fresh urine
After determining the main action routes of the three tested processes in the degradations of the pharmaceuticals, the treatment of these pollutants in simulated fresh urine was assessed. Then, to evaluate the selectivity of the process toward the pharmaceuticals elimination in the complex matrix, the ratio between the pseudo-fist-order degradation rate constants (Rk: k in simulated fresh urine/ k in distilled water) was calculated [13]. The Rk parameter is also an indicator of the inhibitory effect induced by the components of synthetic urine on the degradation of pharmaceuticals.
Fig. 4 shows the Rk values for each pharmaceutical under the different oxidation processes. Under the tested conditions, the electrochemical systems presented the lowest Rk values, followed by the UVC/H2O2 system and being ultrasound the most selective processes. To rationalize the high selectivity of the sonochemical system in the simulated fresh urine (Fig. 4), we must consider both the hydrophobic/hydrophilic nature of the substances and the degradation route of the pharmaceuticals in the fresh urine matrix. In fact, the matrix components (inorganic anions and organic matter in the simulated urine) could alter not only the kinetic but also the degradation routes of the processes.
Fig. 4.
Selectivity of the considered oxidation process toward the degradation of pharmaceuticals in simulated fresh urine. Experimental conditions: [Pharmaceutical] = 43.38 μmol L-1, pH = 6.1, acoustic power = 26.6 W and frequency = 375 kHz for the sonochemical process; BDD anode, Zr cathode, and J = 1.88 mA cm−2 for the electrochemical process (EO); [H2O2] = 500 µmol L-1, and UVC light = 60 W for the UVC/H2O2 system.
As the target pollutants are very hydrophobic (as denoted by their high Log Kow values, Table S1), they are mainly placed in the interfacial zone (see Section 3.1), and their degradations are low interfered by the components of the synthetic fresh urine (which have hydrophilic nature, as evidenced from their Log Kow close to zero or negative, see Table S1). Thus, because the hydrophilic components of the simulated urine matrix are mainly placed in the bulk of the solution, and our hydrophobic pharmaceuticals are in the interfacial zone (where there is a high concentration of the sonogenerated HO●), the degradation of the target pollutants by ultrasound is highly selective.
Regarding the inhibition by the simulated urine matrix components during the application of UVC/H2O2 for the removal of the tested chlorinated pharmaceuticals, an interesting phenomenon was observed (Fig. 4). The degradation of CLX and DFC in urine was slightly affected, while the elimination of LOS suffered an important inhibition. This behavior can be understood by two possible effects of the urine components: the shielding of the UVC light and the scavenging of hydroxyl radicals.
According to Fig. S7, a low shielding effect of the simulated urine matrix components was evidenced in all cases. In fact, the comparison of the UVC action on the three pharmaceuticals in synthetic fresh urine and distilled water had Rk values between 0.76 and 0.85. The results are consistent with the very high contribution of UVC light in the degradation of CLX and DFC, and the significant participation of radicals in the case of LOS. Thus, inorganic anions such as chloride or bicarbonate, and organic substances like urea and acetate, present in the simulated urine matrix, can compete for the radicals, which negatively affect the elimination of pharmaceuticals where the driven force for their degradation is mainly lead by the radicals, such as is the case of LOS.
In the case of the electrochemical process, in which, the indirect route via active chlorine species is the main degrading factor, the very strong inhibition (or low selectivity) in the elimination of pharmaceuticals (Fig. 4), could be understood through the interaction of electrogenerated HOCl and the components of the synthetic fresh urine. Indeed, this was experimentally confirmed by the lower active chlorine species accumulated in urine compared with distilled water (Fig. S8). The urine matrix has high concentrations of urea and ammonium (Table 1), and these components easily react with the active chlorine species (Eqs. (2), (3)) [17], [31]. Then, these matrix substances induce a strong competition on the degradation of the target pharmaceuticals, which explains the very low values of Rk for the electrochemical process.
| (2) |
| (3) |
On the other hand, to better reflect the selectivity of each process, and make the proper comparison among these treatment systems, an average of the Rk values for the three pharmaceuticals was proposed. The average was calculated using Eq. (4). It can be noted that under the considered experimental conditions, ultrasound had an average of selectivity (0.83) higher than that for UVC/H2O2 (0.69) and electrochemical oxidation (0.01), thus showing a superior capacity of the sonochemical system to degrade the target pharmaceuticals in the synthetic fresh urine at the tested experimental conditions. As the sonochemical process presented the highest selectivity, the following sections were focused on ultrasound.
| (4) |
3.3. Primary transformations and changes in the biological activity of pollutants under ultrasonic action
To analyze the effects of the sonochemical process beyond the degradation of the pharmaceuticals, the capability of this process to modify the biological activity was evaluated. For this purpose, it was considered the case of the antibiotic CLX. The evolution of the antimicrobial activity (AA) during CLX treatment in the synthetic fresh urine was followed. As seen in Fig. 5, the sonochemical process led to the elimination of AA after 75 min of sonication of CLX in the urine matrix.
Fig. 5.
Antimicrobial activity (AA) evolution for CLX in synthetic fresh urine. Experimental conditions: [Pharmaceutical] = 43.38 μmol L-1, acoustic power = 26.6 W, frequency = 375 kHz, pH = 6.1.
To understand the elimination of AA, it is important to consider both the primary transformations induced by the sonochemical treatment and the identification of the moieties on CLX more reactive toward HO•. Concerning the primary transformation products, it is reasonable to expect that they are similar in distilled water and fresh urine, because the sonochemical process degraded the target pharmaceuticals close to the cavitation bubble, with very low interference of the simulated fresh urine components (as demonstrated in the previous sections).
Three primary transformation products have been identified for the sonochemical treatment of CLX in distilled water (Table 3). Two products come from attacks of the sonogenerated HO• to the antibiotic on the β-lactam moiety (to generate the isomers P1 and P2 [62]). This ring is highly reactive, due to the strained nature of this ring that does labile the carbonyl-nitrogen bond [16]. A third product was generated from the cleavage of the central secondary amide (P3). The nitrogen in the central amide has its electron pair more available to react with HO• because of the inductive and resonant effects generated by the oxazolyl substituent (Fig. S9). Such transformations agree with the DFT analysis for CLX (see the end of Table 3), which shows that for this antibiotic, the region more susceptible to electrophilic attacks of radicals is the penicillin core (i.e., β-lactam, thioether, and central amine).
Table 3.
Primary transformation products and DFT analyses for CLX.
| Substance | Chemical structure |
|---|---|
| P1* | 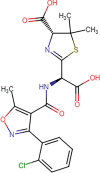 |
| P2 |  |
| P3 | 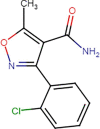 |
| Optimized structure of CLX |  |
| HOMO** for CLX |  |
The three primary products reported for CLX had no the β-lactam ring, which is a determinant moiety for the activity (inhibition of the cell wall synthesis) against the bacteria [64], [65]. Thereby, the cleavage or loss of the β-lactam ring explains the AA elimination observed in Fig. 5.
To better support the AA elimination through the chemical transformations, predictions of the biological activity for CLX and its primary products were performed using the PASS software [41]. Then, the probability of being active (Pa) for the parent antibiotic and its primary transformation products was determined (Table 4). It can be noted that Pa values corresponding to activities such as anti-infective, cell wall synthesis inhibitor, and β-lactamase inhibitor, for the three primary products were lower than those for CLX, which is coherent with the experimental results about the decrease of AA observed in Fig. 5.
Table 4.
Predictions of biological activity for CLX and its primary products.
| Biological activity+ | Pa for CLX | Pa for P1 | Pa for P2 | Pa for P3 |
|---|---|---|---|---|
| Anti-infective | 0.946 | 0.561 | 0.561 | 0.695 |
| β-lactamase inhibitor | 0.699 | 0.289 | 0.289 | 0.188 |
| Cell wall synthesis inhibitor | 0.498 | 0.224 | 0.224 | 0.173 |
| Antibiotic Penicillin-like | 0.313 | 0.016 | 0.016 | 0.000 |
The predictions of biological activity were carried out on the PASS software (free online version) [41].
For LOS, DFC, and their primary products (taken from the literature), predictions of the changes in the biological activity were also performed. Before discuss the results obtained from the PASS software, it is important to mention the primary transformations induced by ultrasound on LOS and DFC. In our previous work on LOS, it was found that the sonochemical action generates three products coming from imidazole ring rupture (LDP1, LDP2, and LDP3), several isomers of biphenyl hydroxylation (LDP4a-f), one product of alcohol moiety oxidation (LDP5), and products of hydroxylation/oxidation of alkyl chain on LOS (LDP6 and LDP7, see Table S2) [36]. Table S3 presents the Pa values for LOS and its primary products. It must be mentioned that the standard structure nucleus of the sartan-type antihypertensives, which is based on two aromatic rings bonded to a tetrazole group, is essential in the activity of losartan, i.e., for the interaction with angiotensin II and AT1 receptors [66]. From Table S3, it can be noted that all the considered products had Pa values slightly lower than LOS, which is reasonable considering that these products retain the active nucleus. These results are in agreement with a previous work on molecular docking for products of valsartan (another sartan antihypertensive structurally related with LOS) that contains the biphenyl-tetrazole moiety, having activity toward angiotensin AT1 receptor [66]. However, if the action of ultrasound is prolonged in time, the primary products of LOS could also be degraded by the action of radicals, and the biological activity of the further products may be decreased significantly.
Concerning DFC, its sonolysis can produce a dichloroaniline (DP1), an indolinone intermediate (DP2) by water elimination, two chlorinated N-phenylanilines (DP3, and DP4) formed through a decarboxylation, and hydroxylated derivatives (DP5, and DP6) (Table S4) [67], [68]. Table S5 presents the Pa values for DFC and its primary products. We should mention that DFC interacts with cyclooxygenases through the carboxylic group mainly [69]. It can be noted that all the primary products had lower Pa values than DFC. Additionally, DP1-DP4 (which had no carboxylic moiety) presented lower Pa than DP5-DP6. This indicates that the sonochemical treatment can decrease the biological activity associated with DFC.
After the assessment of biological activity modifications, the feasibility of the ultrasound system to degrade pharmaceuticals in actual urine was tested. The sonochemical treatment of a real sample (Table S6) containing the antibiotic CLX was performed (Fig. 6). Moreover, the CLX degradation in the real urine using the UVC/H2O2 process, which also showed high selectivity in the simulated urine (Fig. 4), was also assessed (Fig. S10). Fig. 6 compares the degradation of the antibiotic in distilled water, simulated fresh urine, and real urine. It can be noted that in the actual urine matrix, ∼43% of CLX was degraded after 90 min of sono-treatment. Although the degradation in the actual matrix was slower than in distilled water or simulated urine, the sonochemical process removed the antibiotic partially. This contrasts with the observed for the UV/H2O2 system (which degrading action was completely inhibited in the real urine (Fig. S10)). Such results indicate that even for the elimination of CLX in an actual urine sample, the sonochemical process was less affected (i.e., it was more selective) than the UVC/ H2O2 system.
Fig. 6.
Comparison of CLX degradation in distilled water, simulated fresh urine, and real urine by ultrasound. Inset: evolution of antimicrobial activity (AA) during treatment of CLX in real urine. Experimental conditions: [CLX] = 43.38 μmol L-1, acoustic power = 26.6 W, frequency = 375 kHz.
The complete inhibition of the UVC/H2O2 process to degrade CLX can be associated with the very high absorbance at 254 nm of the actual urine matrix (Table S6). This limits the availability of the UVC photons to cleavage H2O2, or directly degrade the target pollutant [13]. In turn, the sonodegradation of CLX in the real urine was slower than that observed in distilled water or simulated urine because of the higher complexity of the actual matrix. In Table S6, it is indicated that the real urine has a higher content of organic substances (total organic carbon: 7300.28 mg L-1) than the other two matrices, and they compete with CLX by the sonogenerated radicals [70]. Furthermore, it is reported that real urine has some surfactant substances, which can interfere the CLX degradation.
In general, proteins, polypeptides, some amino-acids (e.g., methionine and tyrosine), or amphiphilic metabolites (e.g., primary or secondary bile salts), present in the real urine naturally, have surfactant functions [71]. Thereby, such surfactant substances may be placed close to or inside the cavitation bubble [72], [73], [74], thus decreasing the CLX degradation rate regarding the simulated urine or distilled water. In fact, the Rk value for the treatment of CLX in real urine was calculated (being this 0.13), indicating that the selectivity was affected by the complexity of the actual matrix.
It is very important to mention that the selectivity is influenced by both intrinsic properties of the processes (e.g., the action routes involved in the pollutants degradation) and experimental parameters/conditions (e.g., the concentration of reagents, light power, ultrasound waves frequency, or even the matrix). Thereby, the Rk values of the processes could be increased by changing the operational parameters, and then the optimization of the processes can be convenient for this purpose. Indeed, we recommend that future works perform the optimization of operational parameters and the use of complementary analyses such as the life cycle assessment (which deals with the environmental impacts) to obtain a better/more robust comparison among the considered processes. It can be expected that even under optimized conditions, due to the intrinsic properties, the sonochemical process (where the hydroxyl radical, abundant in the interfacial zone, is more prone to interact with the target pharmaceuticals and lower interferences by the urine matrix components occur than in the other two systems, as previously shown) would be the most selective one to eliminate the considered pollutants.
On the other hand, despite the interference by the matrix components in the real urine, the sono-treatment of CLX removed ∼ 20% of the AA in the actual urine (inset in Fig. 6). This last fact denotes a positive effect, to limit the proliferation of antibiotic resistance, of the sonochemical treatment of the antibiotic in the real urine matrix. Additionally, we must mention that the ultrasound system does not require the addition of an external precursor of radicals as needed by the UVC/H2O2 process. Moreover, as the UVC/H2O2 involves the direct action of UVC on the pollutants, this degradation route can generate toxic products, whereas ultrasound leads to the formation of non-toxic substances [13]. In turn, the electrochemical treatment has the electrogenerated HOCl as the main degradation route, and this species can induce the formation of organochlorinated compounds or evolve toward chlorate and perchlorate [75]. These last species have a negative environmental impact due to their toxicity [4], [76], [77]. Thereby, all these aspects plus the above-discussed results make the sonochemical system as an option more suitable than UVC/H2O2 and electrochemical oxidation to degrade pharmaceuticals in urine.
Lastly, the viability of the sonochemical technology from an energy point of view (due to this parameter has a high weight in the operating costs of processes [15]) was evaluated. Then, the specific electrical energy consumption (SEC) was calculated using Eq. (5) (where P is the electric power in kW, t represents the time in h, V is the treated volume in L, and Co is the initial concentration of the pollutant, and Ct is the concentration at a selected time in g L-1, [75]).
For the treatment of the target pollutants in the simulated urine matrix for 1 h using the sonochemical process, the SEC parameter had values of 153.39, 108.10, and 105.39 kWh g−1, for DFC, LOS, and CLX, respectively (Table S7). Moreover, the SEC value for the degradation of CLX in the actual urine was 345.84 kWh g−1. These SEC values are high and represent a concern for the scaling up of the sonochemical process. However, these SEC values could be acceptable for niche applications at small volumes; e.g., the point of use of ultrasound for treating urine (which has a median value of 1.4 L/person/day [78]) loaded with pharmaceuticals.
| (5) |
We can mention that the information about fundamental aspects (such as degradation routes, matrix effect, primary transformations, and their impact on the biological activity) could be extrapolated/used in other systems based on cavitation phenomena such as the hydrodynamic cavitation processes, which are applicable at a large scale. Also, it must be remarked that future works should perform the sono-treatment of plausible binary mixtures of the pharmaceuticals (e.g., cloxacillin and diclofenac or losartan and diclofenac). Additionally, to provide a wider panorama about the elimination of pollutants in urine, future works using optimized systems, several actual matrices from diverse patients, and other pharmaceuticals (even mixtures) are needed.
4. Conclusions
The comparison of ultrasound, UVC/H2O2, and electrochemical oxidation processes in terms of selectivity for the treatment of the three target pharmaceuticals indicated that the sonochemical process was superior due to the lowest interference of the components in the simulated fresh urine. The high average selectivity for the sonochemical system was related to the degradation of the chlorine-containing pharmaceuticals by HO• at the interface of the cavitation bubble. Nevertheless, when the ultrasonic process was used for the treatment of cloxacillin in a real sample of urine the efficiency of degradation decreased (probably by the interference of surfactant substances in this matrix), and consequently, the selectivity was diminished.
The sonochemical process induced transformations that can decrease the biological activity of these pharmaceuticals. In fact, the ultrasound action eliminated AA after sonication of CLX in urine, which was correlated well with the opening of its β-lactam ring and oxidation of the thioether (the moieties most prone to electrophilic attacks by the sonogenerated radicals). Interestingly, although the sono-degradation of CLX in the actual urine was less efficient than in the synthetic matrix, this process was less affected than UVC/H2O2. Finally, the calculation of SEC for the sonochemical treatment of CLX, DFC, and LOS showed values that could be acceptable for niche applications at small volumes, such as the point of use for treating fresh urine loaded with these pharmaceuticals.
CRediT authorship contribution statement
Efraím A. Serna-Galvis: Investigation, Conceptualization, Software, Formal analysis, Writing – original draft, Writing – review & editing. John F. Guateque-Londoño: Investigation, Methodology, Writing – original draft. Javier Silva-Agredo: Methodology, Writing – review & editing. Jazmín Porras: Formal analysis, Funding acquisition, Writing – review & editing. Yenny Ávila-Torres: Software, Formal analysis, Supervision, Writing – review & editing. Ricardo A. Torres-Palma: Conceptualization, Resources, Formal analysis, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank MINCIENCIAS COLOMBIA (before named COLCIENCIAS), by the financial support through the project No. 111577757323. Researchers from Grupo de Investigación en Remediación Ambiental y Biocatálisis (GIRAB) acknowledge to Universidad de Antioquia UdeA the support provided through “PROGRAMA DE SOSTENIBILIDAD. E. A. Serna-Galvis thanks MINCIENCIAS COLOMBIA his Posdoctoral fellowship during November 2020-October 2021 (Convocatoria 848 de 2019), and UNIREMINGTON the support provided through project 4000000287 (Contract No. 80740-680-2020). J. F. Guateque-Londoño thanks MINCIENCIAS COLOMBIA the grant “Jóvenes investigadores por la Paz 2018- J19-18-1, 5381”.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105814.
Contributor Information
Yenny Ávila-Torres, Email: yenny.avila@udea.edu.co.
Ricardo A. Torres-Palma, Email: ricardo.torres@udea.edu.co.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Küster A., Adler N. Pharmaceuticals in the environment: scientific evidence of risks and its regulation. Philos. Trans. R. Soc. B. 2014;369(1656):20130587. doi: 10.1098/rstb.2013.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard P.H., Muir D.C.G. Identifying new persistent and bioaccumulative organics among chemicals in commerce. III: Byproducts, impurities, and transformation products. Environ. Sci. Technol. 2013;47(10):5259–5266. doi: 10.1021/es4004075. [DOI] [PubMed] [Google Scholar]
- 3.Fang W., Ravindar L., Rakesh K.P., Manukumar H.M., Shantharam C.S., Alharbi N.S., Qin H.-L. Synthetic approaches and pharmaceutical applications of chloro-containing molecules for drug discovery: A critical review. Eur. J. Med. Chem. 2019;173:117–153. doi: 10.1016/j.ejmech.2019.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naumann K. Influence of Chlorine Substituents on Biological Activity of Chemicals. Adv. Synth. Catal. 1999;341:417–435. doi: 10.1002/(sici)1521-3897(199907)341:5<417::aid-prac417>3.0.co;2-a. [DOI] [Google Scholar]
- 5.Kümmerer K. Antibiotics in the aquatic environment – A review – Part I. Chemosphere. 2009;75(4):417–434. doi: 10.1016/j.chemosphere.2008.11.086. [DOI] [PubMed] [Google Scholar]
- 6.Kümmerer K. The presence of pharmaceuticals in the environment due to human use - present knowledge and future challenges. J. Environ. Manage. 2009;90(8):2354–2366. doi: 10.1016/j.jenvman.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Daughton C.G., Ruhoy I.S. Environmental footprint of pharmaceuticals: The significance of factors beyond direct excretion to sewers. Environ. Toxicol. Chem. 2009;28:2495–2521. doi: 10.1897/08-382.1. [DOI] [PubMed] [Google Scholar]
- 8.Winker M., Faika D., Gulyas H., Otterpohl R. A comparison of human pharmaceutical concentrations in raw municipal wastewater and yellowwater. Sci. Total Environ. 2008;399(1-3):96–104. doi: 10.1016/j.scitotenv.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Winker M., Tettenborn F., Faika D., Gulyas H., Otterpohl R. Comparison of analytical and theoretical pharmaceutical concentrations in human urine in Germany. Water Res. 2008;42(14):3633–3640. doi: 10.1016/j.watres.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R., Sun P., Boyer T.H., Zhao L., Huang C.-H. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS. Environ. Sci. Technol. 2015;49(5):3056–3066. doi: 10.1021/es504799n. [DOI] [PubMed] [Google Scholar]
- 11.Cotillas S., Lacasa E., Herraiz M., Sáez C., Cañizares P., Rodrigo M.A. The Role of the Anode Material in Selective Penicillin G Oxidation in Urine. ChemElectroChem. 2019;6(5):1376–1384. doi: 10.1002/celc.v6.510.1002/celc.201801747. [DOI] [Google Scholar]
- 12.S. Giannakis M. Jovic N. Gasilova M. Pastor Gelabert S. Schindelholz J.-M. Furbringer H. Girault C. Pulgarin Iohexol degradation in wastewater and urine by UV-based Advanced Oxidation Processes (AOPs): Process modeling and by-products identification J. Environ. Manage. 195 (2017) 174 185 https://doi.org/https://doi.org/10.1016/j.jenvman.2016.07.004. [DOI] [PubMed]
- 13.Guateque-Londoño J.F., Serna-Galvis E.A., Ávila-Torres Y., Torres-Palma R.A. Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water. 2020;12:3398. doi: 10.3390/w12123398. [DOI] [Google Scholar]
- 14.Rayaroth M.P., Aravind U.K., Aravindakumar C.T. Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ. Chem. Lett. 2016;14(3):259–290. doi: 10.1007/s10311-016-0568-0. [DOI] [Google Scholar]
- 15.Mahamuni N.N., Adewuyi Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: a review with emphasis on cost estimation. Ultrason. Sonochem. 2010;17(6):990–1003. doi: 10.1016/j.ultsonch.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Serna-Galvis E.A., Montoya-Rodríguez D.M., Isaza-Pineda L., Ibáñez M., Hernández F., Moncayo-Lasso A., Torres-Palma R.A. Sonochemical degradation of antibiotics from representative classes-Considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason. Sonochem. 2018;50:157–165. doi: 10.1016/j.ultsonch.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Deborde M., von Gunten U. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: A critical review. Water Res. 2008;42(1-2):13–51. doi: 10.1016/j.watres.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Zhou C., Gao N., Deng Y., Chu W., Rong W., Zhou S. Factors affecting ultraviolet irradiation/hydrogen peroxide (UV/H2O2) degradation of mixed N-nitrosamines in water. J. Hazard. Mater. 2012;231–232:43–48. doi: 10.1016/j.jhazmat.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Giraldo-Aguirre A.L., Serna-Galvis E.A., Erazo-Erazo E.D., Silva-Agredo J., Giraldo-Ospina H., Flórez-Acosta O.A., Torres-Palma R.A. Removal of β-lactam antibiotics from pharmaceutical wastewaters using photo-Fenton process at near-neutral pH. Environ. Sci. Pollut. Res. 2018;25(21):20293–20303. doi: 10.1007/s11356-017-8420-z. [DOI] [PubMed] [Google Scholar]
- 20.Farzaneh H., Loganathan K., Saththasivam J., McKay G. Selectivity and competition in the chemical oxidation processes for a binary pharmaceutical system in treated sewage effluent. Sci. Total Environ. 2021;765:142704. doi: 10.1016/j.scitotenv.2020.142704. [DOI] [PubMed] [Google Scholar]
- 21.Dbira S., Bensalah N., Ahmad M.I., Bedoui A. Electrochemical oxidation/disinfection of urine wastewaters with different anode materials. Materials (Basel). 2019;12(8):1254. doi: 10.3390/ma12081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R., Yang Y., Huang C.-H., Li N.a., Liu H., Zhao L., Sun P. UV/H2O2 and UV/PDS Treatment of Trimethoprim and Sulfamethoxazole in Synthetic Human Urine: Transformation Products and Toxicity. Environ. Sci. Technol. 2016;50(5):2573–2583. doi: 10.1021/acs.est.5b0560410.1021/acs.est.5b05604.s001. [DOI] [PubMed] [Google Scholar]
- 23.Gu Q., Burt V.L., Dillon C.F., Yoon S. Trends in antihypertensive medication use and blood pressure control among united states adults with hypertension: The national health and nutrition examination survey, 2001 to 2010. Circulation. 2012;126:2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 24.Lonappan L., Brar S.K., Das R.K., Verma M., Surampalli R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity - A review. Environ. Int. 2016;96:127–138. doi: 10.1016/j.envint.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Cortez F.S., Souza L.da.S., Guimarães L.L., Almeida João.E., Pusceddu F.H., Maranho L.A., Mota L.Gonçalves., Nobre C.R., Moreno B.B., Abessa D.M.de.S., Cesar A., Santos A.R., Pereira C.D.S. Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil) Sci. Total Environ. 2018;637-638:1363–1371. doi: 10.1016/j.scitotenv.2018.05.069. [DOI] [PubMed] [Google Scholar]
- 26.Bayer A., Asner R., Schüssler W., Kopf W., Weiß K., Sengl M., Letzel M. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ. Sci. Pollut. Res. 2014;21(18):10830–10839. doi: 10.1007/s11356-014-3060-z. [DOI] [PubMed] [Google Scholar]
- 27.Elmolla E.S., Chaudhuri M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010;173(1-3):445–449. doi: 10.1016/j.jhazmat.2009.08.104. [DOI] [PubMed] [Google Scholar]
- 28.Cha J.M., Yang S., Carlson K.H. Trace determination of beta-lactam antibiotics in surface water and urban wastewater using liquid chromatography combined with electrospray tandem mass spectrometry. J. Chromatogr. A. 2006;1115:46–57. doi: 10.1016/j.chroma.2006.02.086. [DOI] [PubMed] [Google Scholar]
- 29.Gurke R., Rößler M., Marx C., Diamond S., Schubert S., Oertel R., Fauler J. Science of the Total Environment Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant. Sci. Total Environ. 2015;532:762–770. doi: 10.1016/j.scitotenv.2015.06.067. [DOI] [PubMed] [Google Scholar]
- 30.Patel M., Kumar R., Kishor K., Mlsna T., Pittman C.U., Mohan D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019;119(6):3510–3673. doi: 10.1021/acs.chemrev.8b00299. [DOI] [PubMed] [Google Scholar]
- 31.Amstutz Véronique, Katsaounis A., Kapalka A., Comninellis C., Udert K.M. Effects of carbonate on the electrolytic removal of ammonia and urea from urine with thermally prepared IrO2 electrodes. J. Appl. Electrochem. 2012;42(9):787–795. doi: 10.1007/s10800-012-0444-y. [DOI] [Google Scholar]
- 32.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Torres-Palma R.A. Sonochemical degradation of the pharmaceutical fluoxetine: Effect of parameters, organic and inorganic additives and combination with a biological system. Sci. Total Environ. 2015;524–525:354–360. doi: 10.1016/j.scitotenv.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 33.Camargo-Perea A.L., Serna-Galvis Efraím.A., Lee J., Torres-Palma R.A. Understanding the effects of mineral water matrix on degradation of several pharmaceuticals by ultrasound: Influence of chemical structure and concentration of the pollutants. Ultrason. Sonochem. 2021;73:105500. doi: 10.1016/j.ultsonch.2021.105500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serna-Galvis E.A., Silva-Agredo J., Giraldo-Aguirre A.L., Flórez-Acosta O.A., Torres-Palma R.A. High frequency ultrasound as a selective advanced oxidation process to remove penicillinic antibiotics and eliminate its antimicrobial activity from water. Ultrason. Sonochem. 2016;31:276–283. doi: 10.1016/j.ultsonch.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Kimura T., Sakamoto T., Leveque J.-M., Sohmiya H., Fujita M., Ikeda S., Ando T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996;3(3):S157–S161. doi: 10.1016/S1350-4177(96)00021-1. [DOI] [Google Scholar]
- 36.Serna-Galvis E.A., Isaza-Pineda L., Moncayo-Lasso A., Hernández Félix, Ibáñez M., Torres-Palma R.A. Comparative degradation of two highly consumed antihypertensives in water by sonochemical process. Determination of the reaction zone, primary degradation products and theoretical calculations on the oxidative process. Ultrason. Sonochem. 2019;58:104635. doi: 10.1016/j.ultsonch.2019.104635. [DOI] [PubMed] [Google Scholar]
- 37.Antonin V.S., Santos M.C., Garcia-segura S., Brillas E. Electrochemical incineration of the antibiotic ciprofloxacin in sulfate medium and synthetic urine matrix. Water Res. 2015;83:31–41. doi: 10.1016/j.watres.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 38.Serna-Galvis E.A., Silva-Agredo J., Giraldo A.L., Flórez O.A., Torres-Palma R.A. Comparison of route, mechanism and extent of treatment for the degradation of a β-lactam antibiotic by TiO2 photocatalysis, sonochemistry, electrochemistry and the photo-Fenton system. Chem. Eng. J. 2016;284:953–962. doi: 10.1016/j.cej.2015.08.154. [DOI] [Google Scholar]
- 39.Raghavachari K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. 1999:361–363. doi: 10.1007/978-3-662-10421-7_60. [DOI] [Google Scholar]
- 40.Tomasi J., Mennucci B., Cammi R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005;105(8):2999–3094. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
- 41.W2D Team - PharmaExpert, PASS online, (2021). http://www.pharmaexpert.ru/passonline/index.php (accessed May 2, 2021).
- 42.Adewuyi Y.G. Sonochemistry : Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001;40:4681–4715. doi: 10.1021/ie010096l. [DOI] [Google Scholar]
- 43.Cheng J., Vecitis C.D., Park H., Mader B.T., Hoffmann M.R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in landfill groundwater: Environmental matrix effects. Environ. Sci. Technol. Ronmental. 2008;42(21):8057–8063. doi: 10.1021/es8013858. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Y., Pétrier C., David Waite T. Kinetics and mechanisms of ultrasonic degradation of volatile chlorinated aromatics in aqueous solutions. Ultrason. Sonochem. 2002;9(6):317–323. doi: 10.1016/S1350-4177(02)00085-8. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez N.A., Rodriguez-freire L., Keswani M., Sierra-Alvarez R. Degradation of perfluoroalkyl and polyfluoroalkyl. Environ. Sci. 2016:975–983. doi: 10.1039/c6ew00150e. [DOI] [Google Scholar]
- 46.Yasman Y., Bulatov V., Gridin V.V., Agur S., Galil N., Armon R., Schechter I. A new sono-electrochemical method for enhanced detoxification of hydrophilic chloroorganic pollutants in water. Ultrason. - Sonochemistry. 2004;11(6):365–372. doi: 10.1016/j.ultsonch.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Villegas-Guzman P., Silva-Agredo J., Giraldo-Aguirre A.L., Flórez-Acosta O., Petrier C., Torres-Palma R.A. Enhancement and inhibition effects of water matrices during the sonochemical degradation of the antibiotic dicloxacillin. Ultrason. Sonochem. 2015;22:211–219. doi: 10.1016/j.ultsonch.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Siddique M., Farooq R., Price G.J. Synergistic effects of combining ultrasound with the Fenton process in the degradation of Reactive Blue 19. Ultrason. - Sonochemistry. 2014;21(3):1206–1212. doi: 10.1016/j.ultsonch.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Torres R.A., Pétrier C., Combet E., Moulet F., Pulgarin C. Bisphenol A Mineralization by Integrated Ultrasound-UV-Iron (II) Treatment. Environ. Sci. Technol. 2007;41(1):297–302. doi: 10.1021/es061440e. [DOI] [PubMed] [Google Scholar]
- 50.NHI, PubChem, (2021). https://pubchem.ncbi.nlm.nih.gov/ (accessed February 28, 2021).
- 51.Tran N., Drogui P., Brar S.K. Sonochemical techniques to degrade pharmaceutical organic pollutants. Environ. Chem. Lett. 2015;13(3):251–268. doi: 10.1007/s10311-015-0512-8. [DOI] [Google Scholar]
- 52.Loos G., Scheers T., Van Eyck K., Van Schepdael A., Adams E., Van Der Bruggen B., Cabooter D., Dewil R. Electrochemical oxidation of key pharmaceuticals using a boron doped diamond electrode. Sep. Purif. Technol. 2018;195:184–191. doi: 10.1016/j.seppur.2017.12.009. [DOI] [Google Scholar]
- 53.Rosman N., Salleh W.N.W., Azuwa M., Jaafar J., Ismail A.F., Harun Z. Hybrid membrane filtration-advanced oxidation processes for removal of pharmaceutical residue. J. Colloid Interface Sci. 2018;532:236–260. doi: 10.1016/j.jcis.2018.07.118. [DOI] [PubMed] [Google Scholar]
- 54.Wu M., Zhao G., Li M., Liu L., Li D. Applicability of boron-doped diamond electrode to the degradation of chloride-mediated and chloride-free wastewaters. J. Hazard. Mater. 2009;163(1):26–31. doi: 10.1016/j.jhazmat.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 55.Costa C.R., Montilla F., Morallón E., Olivi P. Electrochemical oxidation of acid black 210 dye on the boron-doped diamond electrode in the presence of phosphate ions: Effect of current density, pH, and chloride ions. Electrochim. Acta. 2009;54(27):7048–7055. doi: 10.1016/j.electacta.2009.07.027. [DOI] [Google Scholar]
- 56.de Araújo D.M., Sáez C., Martínez-Huitle C.A., Cañizares P., Rodrigo M.A. Influence of mediated processes on the removal of Rhodamine with conductive-diamond electrochemical oxidation. Appl. Catal. B Environ. 2015;166–167:454–459. doi: 10.1016/j.apcatb.2014.11.038. [DOI] [Google Scholar]
- 57.Soufan M., Deborde M., Legube B. Aqueous chlorination of diclofenac: Kinetic study and transformation products identification. Water Res. 2012;46(10):3377–3386. doi: 10.1016/j.watres.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 58.WEINGARTEN HAROLD. Chlorination of Biphenyl. J. Org. Chem. 1961;26(11):4347–4350. doi: 10.1021/jo01069a040. [DOI] [Google Scholar]
- 59.Challis J.K., Hanson M.L., Friesen K.J., Wong C.S. A critical assessment of the photodegradation of pharmaceuticals in aquatic environments: defining our current understanding and identifying knowledge gaps. Environ. Sci. Process. Impacts. 2014;16:672–696. doi: 10.1039/c3em00615h. [DOI] [PubMed] [Google Scholar]
- 60.Serna-Galvis E.A., Ferraro F., Silva-Agredo J., Torres-Palma R.A. Degradation of highly consumed fluoroquinolones, penicillins and cephalosporins in distilled water and simulated hospital wastewater by UV254 and UV254/persulfate processes. Water Res. 2017;122:128–138. doi: 10.1016/j.watres.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 61.Kawabata K., Sugihara K., Sanoh S., Kitamura S., Ohta S. Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A,-B and-C irradiation. J. Toxicol. Sci. 2013;38(2):215–223. doi: 10.2131/jts.38.215. [DOI] [PubMed] [Google Scholar]
- 62.Trovó A.G., Pupo Nogueira R.F., Agüera A., Fernandez-Alba A.R., Malato S. Degradation of the antibiotic amoxicillin by photo-Fenton process – Chemical and toxicological assessment. Water Res. 2011;45(3):1394–1402. doi: 10.1016/j.watres.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 63.Wacławek S. Do we still need a laboratory to study advanced oxidation processes ? a review. Ecol Chem Eng S. 2021;28:11–28. doi: 10.2478/eces-2021-0002. [DOI] [Google Scholar]
- 64.A. Gringauz, Antimicrobial Drugs I, in: Introd. to Med. Chem., First, Wiley-VCH, New York, 1997: pp. 191–263.
- 65.Konaklieva M. Molecular targets of β-lactam-based antimicrobials: Beyond the usual suspects. Antibiotics. 2014;3:128–142. doi: 10.3390/antibiotics3020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinosa-Barrera P.A., Delgado-Vargas C.Andrés., Martínez-Pachón D., Moncayo-Lasso A. Using computer tools for the evaluation of biodegradability, toxicity, and activity on the AT1 receptor of degradation products identified in the removal of valsartan by using photo-electro-Fenton process. Environ. Sci. Pollut. Res. 2021;28(19):23984–23994. doi: 10.1007/s11356-020-11949-9. [DOI] [PubMed] [Google Scholar]
- 67.Hartmann J., Bartels P., Mau U., Witter M., Tümpling W.v., Hofmann J., Nietzschmann E. Degradation of the drug diclofenac in water by sonolysis in presence of catalysts. Chemosphere. 2008;70(3):453–461. doi: 10.1016/j.chemosphere.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 68.Ziylan A., Dogan S., Agopcan S., Kidak R., Aviyente V., Ince N.H. Sonochemical degradation of diclofenac: Byproduct assessment, reaction mechanisms and environmental considerations. Environ. Sci. Pollut. Res. 2014;21(9):5929–5939. doi: 10.1007/s11356-014-2514-7. [DOI] [PubMed] [Google Scholar]
- 69.Rowlinson S.W., Kiefer J.R., Prusakiewicz J.J., Pawlitz J.L., Kozak K.R., Kalgutkar A.S., Stallings W.C., Kurumbail R.G., Marnett L.J. A Novel Mechanism of Cyclooxygenase-2 Inhibition Involving Interactions with Ser-530 and Tyr-385 *. J. Biol. Chem. 2003;278(46):45763–45769. doi: 10.1074/jbc.M305481200. [DOI] [PubMed] [Google Scholar]
- 70.Papoutsakis S., Afshari Z., Malato S., Pulgarin César. Elimination of the iodinated contrast agent iohexol in water, wastewater and urine matrices by application of photo-Fenton and ultrasound advanced oxidation processes. J. Environ. Chem. Eng. 2015;3(3):2002–2009. doi: 10.1016/j.jece:2015.07.002. [DOI] [Google Scholar]
- 71.Khitan Z.J., Glassock R.J. Foamy urine is this a sign of kidney disease? Clin. J. Am. Soc. Nephrol. 2019;14(11):1664–1666. doi: 10.2215/CJN.06840619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singla R., Grieser F., Ashokkumar M. Kinetics and mechanism for the sonochemical degradation of a nonionic surfactant. J. Phys. Chem. A. 2009;113(12):2865–2872. doi: 10.1021/jp808968e. [DOI] [PubMed] [Google Scholar]
- 73.Yang L., Rathman J.F., Weavers L.K. Degradation of alkylbenzene sulfonate surfactants by pulsed ultrasound. J. Phys. Chem. B. 2005;109(33):16203–16209. doi: 10.1021/jp0523221. [DOI] [PubMed] [Google Scholar]
- 74.Singla R., Grieser F., Ashokkumar M. The mechanism of sonochemical degradation of a cationic surfactant in aqueous solution. Ultrason. Sonochem. 2011;18(2):484–488. doi: 10.1016/j.ultsonch.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 75.Gonzaga I.M.D., Moratalla A., Eguiluz K.I.B., Salazar-Banda G.R., Cañizares P., Rodrigo M.A., Saez C. Outstanding performance of the microwave-made MMO-Ti/RuO2IrO2 anode on the removal of antimicrobial activity of Penicillin G by photoelectrolysis. Chem. Eng. J. 2021;420:129999. doi: 10.1016/j.cej.2021.129999. [DOI] [Google Scholar]
- 76.Sijimol M.R., Jyothy S., Pradeepkumar A.P., Chandran M.S.S., Ghouse S.S., Mohan M. Review on Fate, Toxicity, and Remediation of Perchlorate. Environ. Forensics. 2015;16(2):125–134. doi: 10.1080/15275922.2015.1022914. [DOI] [Google Scholar]
- 77.Weaver R.J. Some Responses of the Bean Plant To Chlorate and Perchlorate Ions. Plant Physiol. 1942;17(1):123–128. doi: 10.1104/pp.17.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rose C., Parker A., Jefferson B., Cartmell E. The characterization of feces and urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015;45(17):1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








