Key Points
Question
How does the real-world performance of atezolizumab compare with nivolumab and docetaxel for the treatment of patients with advanced non–small cell lung cancer (NSCLC) who did not respond to platinum-based chemotherapy?
Findings
This comparative effectiveness study of 3336 patients with advanced NSCLC found that the immune checkpoint inhibitors atezolizumab and nivolumab did not significantly differ in their association with overall survival (OS) in these patients. Atezolizumab was associated with a significantly longer OS compared with docetaxel.
Meaning
This study found that atezolizumab was associated with superior OS compared with docetaxel and similar OS compared with nivolumab in a real-world cohort of patients with advanced NSCLC who previously received platinum-based chemotherapy.
This comparative effectiveness study examines atezolizumab, nivolumab, and docetaxel in patients with advanced non–small cell lung cancer (NSCLC) resistant to platinum-based chemotherapy.
Abstract
Importance
Evidence regarding real-world effectiveness of therapies for patients with advanced non–small cell lung cancer (NSCLC) whose tumors are resistant to platinum-based chemotherapy is lacking.
Objective
To compare the effectiveness of the immune checkpoint inhibitors atezolizumab (programmed cell death ligand 1 inhibitor) and nivolumab (programmed cell death 1 inhibitor) and the chemotherapy drug docetaxel in patients with advanced NSCLC resistant to platinum-based chemotherapy.
Design, Setting, and Participants
This comparative effectiveness study compared patients aged 18 years or older with advanced NSCLC who initiated atezolizumab, docetaxel, or nivolumab and who had previously been exposed to platinum-based chemotherapy using nationally representative real-world data from more than 280 US cancer clinics. Patients were followed-up from May 2011 to March 2020. Data analysis was performed between April and June 2021. Comparisons of interest were between atezolizumab vs docetaxel and atezolizumab vs nivolumab.
Exposures
Initiation of atezolizumab, nivolumab, or docetaxel monotherapy.
Main Outcome and Measures
The main outcome was overall survival (OS).
Results
A total of 3336 patients (mean [SD] age, 67.1 [9.49] years; 1820 [54.6%] men and 1516 [45.4%] women) were assessed in the main analysis, including 206 patients receiving atezolizumab, 500 receiving docetaxel, and 2630 receiving nivolumab. Patients receiving atezolizumab were older than those treated with docetaxel (mean age [SD], 68.3 [9.4] years vs 65.6 [9.5] years), and were more likely to have been treated in an academic setting (39 patients [18.9%]) than those receiving docetaxel (49 patients [9.8%]) and nivolumab (128 patients [4.9%]). After adjustment for baseline characteristics, atezolizumab was associated with a significantly longer OS compared with docetaxel (adjusted hazard ratio [aHR], 0.79; 95% CI, 0.64-0.97). No significant difference in OS was observed between atezolizumab and nivolumab (aHR, 1.07; 95% CI, 0.89-1.28). These findings were consistent across all patient subgroups tested, and robust to plausible deviations from random missingness for Eastern Cooperative Oncology Group performance status in real-world data (eg, the tipping point for loss of a significantly beneficial effect for atezolizumab vs docetaxel was achieved if patients in the docetaxel group missing baseline Eastern Cooperative Oncology Group performance status had a mean performance status of 1.43 higher than expected).
Conclusions and Relevance
In this comparative effectiveness study, atezolizumab was superior to docetaxel and matched nivolumab in prolonging OS in a real-world cohort of patients with advanced NSCLC who previously received platinum-based chemotherapy.
Introduction
Lung cancer remains the leading cause of cancer deaths globally.1 Non–small cell lung cancer (NSCLC) is the most common form of lung cancer and accounts for 85% of all lung cancer cases in the United States.2 Most patients with advanced NSCLC present with locally advanced or metastatic disease at diagnosis, which has poor prognosis,3 and therefore require systemic therapy. Therapeutic advances in advanced NSCLC have been fueled by the identification of driver variants in EGFR, ALK, and ROS1 genes and development of targeted therapies, resulting in improvements in outcomes for some patient populations with advanced NSCLC.2,4 Until recently, chemotherapy with platinum doublet was the standard first-line option for most patients with advanced NSCLC who did not have these genetic drivers or were not tested for them5,6 and remains the first choice in many parts of the world. However, most patients’ disease progresses with platinum-based chemotherapy and requires additional lines of therapy.7 The cytotoxic chemotherapeutic agent docetaxel has been a key second-line therapy option for these patients.8
Unlike cytotoxic chemotherapy drugs, therapies based on immune checkpoint inhibitors (ICIs) selectively activate the host immune response and can therefore lead to more specific, durable, and adaptable antitumor responses.9,10,11 Immunotherapy with ICIs has shown significant improvements in overall survival (OS) in broad populations of patients with advanced NSCLC who previously experienced progression with platinum-based chemotherapy.2,4 Nivolumab is a human monoclonal antibody against programmed cell death 1 (PD-1) approved by the Food and Drug Administration in 2015 for this indication based on results from phase 3 trials CheckMate-01712 and CheckMate-057,13 which showed improved OS compared with docetaxel regardless of PD-1 expression. Atezolizumab is a newer ICI, a fully humanized, engineered monoclonal antibody against programmed cell death ligand 1 (PD-L1) that is also approved as monotherapy for treatment of patients with advanced NSCLC who previously experienced progression with platinum-based chemotherapy, based partly on results from the global phase 3 randomized OAK trial14, which reported a significant improvement in OS with atezolizumab treatment compared with docetaxel (hazard ratio [HR], 0.73; 95% CI, 0.62-0.87) irrespective of PD-L1 expression. Currently, a head-to-head comparison of the real-world effectiveness of atezolizumab, nivolumab, and docetaxel is lacking.
To fill this evidence gap, we compared OS for patients with prior exposure to platinum-based chemotherapy who initiated atezolizumab vs docetaxel or atezolizumab vs nivolumab, using data from real-world US clinical practices. We also report mortality HRs for specific patient subgroups and provide a rigorous assessment of the impact of missing data for confounders, including Eastern Cooperative Oncology Group (ECOG) performance status (PS), which were missing for a large proportion of eligible patients at baseline.
Methods
This comparative effectiveness study was approved by the Copernicus Group institutional review board with a waiver of informed consent because the data were deidentified. This report adheres to the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guideline and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study Population
At the date of initiation (index date) of atezolizumab, docetaxel, or nivolumab monotherapy, eligible patients were aged 18 years or older, diagnosed with locally advanced and/or metastatic NSCLC after January 1, 2011, and had previously been exposed to a platinum-based chemotherapy drug for treatment of advanced NSCLC (Figure 1). Race was self-reported by patients and captured in the electronic health records. It was assessed in this study because it has been found to be independently associated with lung cancer incidence and mortality, in part, as a result of differences in genetic susceptibility.15 At index date, eligible patients had not been previously exposed to atezolizumab, docetaxel, or nivolumab, ie, eligible treatment strategies were consistent with initiation. All actual treatment regimens received in routine practice that were consistent with initiation were permitted; initial dosing and decisions about treatment frequency and duration were left to the discretion of the physician and the patient. Patients were excluded if they had previously been exposed to PD-1, PD-L1, or CTLA-4 immunotherapies, including pembrolizumab. Patients were followed-up until the cutoff date of March 1, 2020, to account for the COVID-19 pandemic. Only patients with at least 6 months of potential follow-up were included in this study. In accordance with best practices (eg, Khozin et al10), patients with a gap of 90 days or longer between diagnosis and first visit or medication administration were excluded. The following subgroups were analyzed by restriction of eligibility criteria to examine if any significant effect heterogeneity was present: (1) patients with no documented ALK, ROS1, or EGFR variants or previous treatment with a targeted agent against ALK, ROS1, or EGFR; (2) only patients with index date in 2015 or later; (3) patients with stage IIIB cancer or higher at diagnosis; and (4) only patients with nonsquamous histological subtypes.
Figure 1. Flowchart of Patient Selection.
A total of 206 patients in the atezolizumab group, 500 patients in the docetaxel group, and 2630 patients in the nivolumab group had complete data for baseline confounders, and only these were assessed for the main analyses, except when using multiple imputation, in which case the full cohort of 269 patients in the atezolizumab group, 966 patients in the docetaxel group, and 3910 patients in the nivolumab group was used. NSCLC indicates non–small cell lung cancer, PD-(L)1, programmed death cell (ligand) 1.
Patient groups were included from the US nationwide electronic health record (EHR)–derived Flatiron Health database, which is a longitudinal database comprising deidentified patient-level structured and unstructured data curated via technology-enabled abstraction originating from approximately 280 cancer clinics and approximately 800 sites of care in the US.16,17
Quantitative Bias Assessment for Missing Values for Confounders
To assess sensitivity of results to missing data assumptions, HRs were computed under 3 scenarios. First, we assumed baseline confounder data were missing completely at random using complete case analysis in which patients with a missing value for 1 or more baseline confounders were excluded. Complete-case analysis was used as the default method, unless otherwise specified. Second, we assumed baseline confounder data were missing at random (MAR) using multiple imputation of missing data for baseline confounders. Third, we assumed ECOG PS were missing not at random. To account for the robustness of our findings to the large amounts of missing ECOG PS, we used multiple imputation with δ adjustment, in which missing data for baseline confounders were imputed under the assumption that patients with a missing ECOG PS in the comparator with atezolizumab could have been poorer than expected under MAR and therefore explained away some of the observed differences, or lack thereof, in outcomes.
MAR and missing not at random analyses required multiple imputation, which was performed using chained equations with the R package mice version 3.14.18 For multiple imputation, 20 imputed data sets were generated to account for uncertainty and random error in the imputation of missing values. We chose 20 data sets to balance computational efficiency with theoretical guidelines for multiple imputation from Graham et al,19 given the proportion of missing values in our data. Imputed mean matching and logistic regression were used to impute continuous and dichotomous variables except ECOG PS, which used ordered proportional-odds logistic regression. For congeniality, all variables used in propensity score estimation and Cox regression were included in multiple imputation, including outcome variables. Mean observed ECOG PS at any time and hemoglobin levels were included as auxiliary variables to improve imputation accuracy. HRs and SEs were computed for each imputed data set separately and then pooled using Rubin rules to account for intra– and inter–data set variance.20
For δ adjustments,21,22 δ was an additive term applied to the ordered logistic regression model for ECOG PS representing
 |
For the adjustments, fixed constant values of δ of 1, 0, −1, −2, −3, −4, and −5 were added to the ordered logistic regression imputation model for ECOG PS. Positive values for δ probabilistically shifted imputed ECOG PS to be more favorable than expected under MAR (ie, assigning a lower ECOG PS than imputed given other measured covariates) for those missing ECOG PS. Conversely, a negative δ randomly shifted imputed ECOG PS to be poorer than expected under MAR. Twenty data sets were multiply imputed for each setting of the δ parameter. For interpretability of results, instead of the log-odds defined by δ, we report the resulting mean shift in imputed ECOG PS for each setting of δ.
Statistical Analysis
The primary end point was overall survival, defined as the time from index date to death due to any cause. Patients without a recorded date of death were censored at the date of their last visit, or March 1, 2020, whichever was earlier. The treatment effect (association) of interest was the average treatment effect in the overall population or defined subgroups of initiating atezolizumab vs docetaxel or atezolizumab vs nivolumab. HRs were estimated using Cox proportional hazards models with an indicator for the treatment group to identify population-level outcomes.
To adjust for differences in baseline characteristics between treatment groups, patients were weighted by the inverse probability of treatment received using propensity weights estimated using a logistic regression model (eAppendix in the Supplement).23,24 Large (>10) propensity weights were trimmed at the 99th percentile to reduce variance, and propensity weight distributions were visually assessed for overlap and reported. Preweighting and postweighting balances in covariates were assessed using standardized mean differences (SMDs), in which a standardized mean difference of 0.1 or less implied negligible imbalance. To estimate the average treatment effect, inverse probability of treatment weights wi for patient i were
 |
for the treated group and
 |
for the reference group, in which
was the estimated probability of treatment initiation given measured covariates Xi. For propensity-weighted Cox models, robust SEs were calculated using a sandwich estimator of variance to derive 95% CIs. For the overall population, in addition to HR, propensity-weighted Kaplan-Meier curves with robust 95% CIs and P values from log-rank tests were reported as measures of absolute risk, and differences in mean restricted survival time (RMST) at month 40 (maximum follow-up time for atezolizumab) were reported as an additional measure of average treatment effect with bootstrapped 95% CIs. Two-sided P < .05 implied statistical significance. All analyses were performed in R statistical programming language version 4.1.1 (R Project for Statistical Computing). Data were analyzed from April to June 2021.
Results
Baseline Demographic and Clinical Characteristics
A total of 3336 patients (67.1 [9.49] years; 1820 [54.6%] men and 1516 [45.4%] women) were assessed, including 206 patients receiving atezolizumab, 500 receiving docetaxel, and 2630 receiving nivolumab. Patients in the atezolizumab group, compared with those in the docetaxel group, were older (mean [SD] age, 68.30 [9.39] years vs 65.57 [9.48] years) and more likely to be White (174 patients [84.5%] vs 379 patients [75.8%]) and to have been treated in an academic, rather than a community, setting (39 patients [18.9%] vs 49 patients [9.8%]) (Table 1).
Table 1. Unadjusted Baseline Characteristics by Treatment Group.
| Characteristic | Atezolizumab (n = 206) | Docetaxel (n = 500) | SMD | Nivolumab (n = 2630) | SMD |
|---|---|---|---|---|---|
| Follow-up duration, median (IQR), mo | 29.7 (18.4-37.2) | 69.4 (41.2-79.5) | NA | 41.4 (26.4-51.8) | NA |
| Age, mean (SD), 7 | 68.30 (9.39) | 65.57 (9.48) | 0.289 | 67.28 | 0.108 |
| Sex, No. (%) | |||||
| Women | 97 (47.1) | 232 (46.4) | 0.014 | 1187 (45.1) | 0.039 |
| Men | 109 (52.9) | 268 (53.6) | 0.014 | 1442 (54.9) | 0.039 |
| Race, No. (%) | |||||
| Asian | 7 (3.4) | 8 (1.6) | 0.295 | 50 (1.9) | 0.249 |
| White | 174 (84.5) | 379 (75.8) | 2031 (77.2) | ||
| Othera | 25 (12.1) | 113 (22.6) | 549 (20.9) | ||
| Smoking status, none, No. (%) | 23 (11.2) | 39 (7.8) | 0.115 | 274 (10.4) | 0.024 |
| Histological characteristics, No. (%) | |||||
| Nonsquamous | 144 (69.9) | 359 (71.8) | 0.042 | 1715 (65.2) | 0.102 |
| Not otherwise specified | 6 (2.9) | 16 (3.2) | 97 (3.7) | ||
| Squamous | 56 (27.2) | 125 (25.0) | 818 (31.1) | ||
| Stage, No. (%) | |||||
| <III | 20 (9.7) | 33 (6.6) | 0.228 | 229 (8.7) | 0.065 |
| III | 46 (22.3) | 77 (15.4) | 535 (20.3) | ||
| IV | 140 (68.0) | 390 (78.0) | 1866 (71.0) | ||
| ECOG PS, No. (%) | |||||
| 0 | 46 (22.3) | 73 (14.6) | 0.201 | 562 (21.4) | 0.087 |
| 1 | 110 (53.4) | 289 (57.8) | 1329 (50.5) | ||
| ≥2 | 50 (24.3) | 138 (27.6) | 739 (28.1) | ||
| Prior lines, mean (SD), No. | 1.35 (0.77) | 1.46 (0.77) | 0.146 | 1.31 (0.70) | 0.048 |
| Time from diagnosis to index, mean (SD), mo | 15.08 (15.62) | 11.57 (10.56) | 0.263 | 14.06 (14.56) | 0.068 |
| Prior EGFR therapy, No. (%) | 16 (7.8) | 36 (7.2) | 0.022 | 166 (6.3) | 0.057 |
| Comorbidities, mean (SD), No. | 0.41 (0.77) | 0.25 (0.54) | 0.231 | 0.32 (0.61) | 0.128 |
| Insured, No. (%) | 116 (56.3) | 223 (44.6) | 0.236 | 1488 (56.6) | 0.005 |
| Metastasis ICD codes, mean (SD)b | 2.74 (9.72) | 1.37 (7.22) | 0.16 | 1.22 (5.12) | 0.195 |
| CNS metastases present, No. (%) | 37 (18.0) | 75 (15.0) | 0.08 | 350 (13.3) | 0.128 |
| Liver metastases, No. (%) | 22 (10.7) | 22 (4.4) | 0.24 | 191 (7.3) | 0.12 |
| Treated in community practice, No. (%) | 167 (81.1) | 451 (90.2) | 0.263 | 2502 (95.1) | 0.445 |
| Prior ALK/ROS1 therapy, No. (%) | 2 (0.4) | 4 (1.9) | 0.144 | 11 (0.4) | 0.141 |
| Recorded ALK rearrangement present, No. (%) | 1 (0.5) | 0 (0) | 0.099 | 14 (0.5) | 0.007 |
| Recorded ROS1 variant, No. (%) | 3 (1.5) | 2 (0.4) | 0.11 | 6 (0.2) | 0.135 |
| Recorded EGFR variant, No. (%) | 12 (5.8) | 15 (3.0) | 0.138 | 110 (4.2) | 0.075 |
| Recorded PD-L1 positivity, No. (%) | 12 (5.8) | 2 (0.4) | 0.316 | 104 (4.0) | 0.087 |
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; ICD, International Classification of Diseases; NA, not applicable; PD-L1, programmed cell death ligand 1; PS, performance status; SMD, standardized mean differences.
Other race included Black or African American, Hispanic or Latino, or other (not specified).
Both International Classification of Diseases, Ninth Revision, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, were used.
Imbalances were generally smaller between patients treated with atezolizumab and nivolumab, except for practice type. Patients in the nivolumab group were less likely to be treated in an academic setting (128 patients [4.9%]) and to be White (2031 patients [77.2%) and had lower counts of comorbidities and metastases (Table 1).
Baseline ECOG PS was missing for 53 patients (19.7%) in the atezolizumab group, 435 patients (45.0%) in the docetaxel group, and 1071 patients (27.4%) in the nivolumab group. Besides ECOG PS, information on race was missing for approximately 5% to 6% of patients, and smoking history and cancer stage had less than 1% missingness. After weighting, sufficient balance was achieved across all baseline variables for both comparisons (eTable in the Supplement), and treatment propensities showed favorable overlap in their distributions (eFigure in the Supplement).
Comparison of Overall Survival
Compared with docetaxel, atezolizumab was associated with significantly longer survival in the overall population (adjusted HR [aHR], 0.79; 95% CI, 0.64-0.97; P = .02) and across all subgroups analyzed, including patient subgroups with stage IIIB or IV cancer at diagnosis or nonsquamous histological characteristics (Table 2). Balance was not achievable among patients with squamous histological characteristics. The unadjusted HR for OS in atezolizumab vs docetaxel was 0.73 (95% CI, 0.60-0.88). Consistent with this HR, Kaplan-Meier curves showed a significant difference in survival (Figure 2), and a significant improvement in RMST of 3.27 (95% CI, 1.14-5.97) months was observed at month 40 for atezolizumab in the overall population. After excluding patients with ROS1-positive NSCLC and those who had EGFR or ALK variants but did not receive any prior targeted therapy against EGFR or ALK, aHR was 0.81 (95% CI, 0.66-0.99).
Table 2. Unadjusted Sample Sizes and aHRs for Comparisons of Atezolizumab vs Docetaxel and Atezolizumab vs Nivolumab in the Overall Eligible Population and Within Subgroups Examined.
| Comparison | Complete analysis | Multiple imputation | Index date ≥2015 | Cancer stage at diagnosis ≥IIIB | Non-squamous histology | No ALK/EGFR/ROS1 variants or prior targeted therapies |
|---|---|---|---|---|---|---|
| Sample sizes | ||||||
| Docetaxel | 206 | 269 | 206 | 175 | 144 | 185 |
| Nivolumab | 500 | 966 | 166 | 446 | 359 | 453 |
| Atezolizumab | 2630 | 3910 | 2624 | 2249 | 1715 | 2401 |
| Atezolizumab vs docetaxel | ||||||
| aHR (95% CI) | 0.79 (0.64-0.97) | 0.80 (0.67-0.95) | 0.75 (0.59-0.97) | 0.77 (0.61-0.98) | 0.70 (0.54-0.90) | 0.78 (0.62-0.97) |
| P value | .02 | .01 | .03 | .03 | .005 | .02 |
| Atezolizumab vs nivolumab | ||||||
| aHR (95% CI) | 1.07 (0.89-1.28) | 1.08 (0.92-1.27) | 1.08 (0.9-1.29) | 1.05 (0.87-1.28) | 0.94 (0.75-1.18) | 1.08 (0.89-1.32) |
| P value | .47 | .33 | .43 | .61 | .59 | .41 |
Abbreviation: aHR, adjusted hazard ratio.
Figure 2. Kaplan-Meier Curves for Atezolizumab vs Docetaxel and Atezolizumab vs Nivolumab.

There were no significant differences in OS between atezolizumab and nivolumab in the overall population (aHR, 1.07; 95% CI, 0.89-1.28; P = .47) and across subgroups analyzed (Table 2). The unadjusted HR for atezolizumab vs nivolumab was 1.04 (95% CI, 0.88-1.24). The difference in RMST at month 40 was −0.68 (95% CI, −2.86 to 1.58) months. After excluding patients who had ROS1-positive NSCLC and those who had EGFR or ALK variants but did not receive prior targeted therapy, the aHR was 1.11 (95% CI, 0.92-1.33).
Sensitivity analysis using the administrative cutoff date of October 1, 2020, instead of March 1, 2021, and dropping the 90-day gap exclusion criterion resulted in aHRs of 0.79 (95% CI, 0.66-0.95) for the docetaxel comparison and 1.05 (95% CI, 0.89-1.25) for the nivolumab comparison, which were consistent with our conclusions. Owing to the low prevalence of reported variants in EGFR, ALK, and ROS1 and prior exposure to ALK or ROS1 inhibitors (Table 1), we adjusted for these separately as a sensitivity analysis. After adjustment for these confounders, aHRs were 0.78 (95% CI, 0.64-0.96) for the docetaxel comparison and 1.08 (95% CI, 0.90-1.29) for the nivolumab comparison. Further adjustment for recorded PD-L1 positivity as an additional covariate also did not affect the aHRs (atezolizumab vs docetaxel: aHR, 0.79; 95% CI, 0.64-0.98; atezolizumab vs nivolumab: aHR, 1.08; 95% CI, 0.90-1.30). Our results were also not affected if the not otherwise specified histological subtype was included as its own individual category instead of being grouped with squamous histological subtype (atezolizumab vs docetaxel: aHR, 0.77; 95% CI, 0.63-0.95; atezolizumab vs nivolumab: aHR, 1.06; 95% CI, 0.88-1.28).
Robustness to Partially Missing Confounder Data
With multiple imputation, estimated HRs were almost completely identical to complete-case analysis (atezolizumab vs docetaxel: aHR, 0.80; 95% CI, 0.67-0.95; P = .01; atezolizumab vs nivolumab: aHR, 1.08; 95% CI, 0.92-1.27; P = .33) (Table 2). To identify the distribution of missing ECOG PS that would be required to nullify the estimated HRs, we performed a tipping point analysis by assuming that ECOG PS among patients in the docetaxel or nivolumab groups in real-world data could have been worse than expected under the assumption of random missingness.
For the docetaxel comparison, the tipping point corresponding to loss of statistical significance of a beneficial effect for atezolizumab was identified at a δ of −4 (Figure 3), or a mean shift of +1.43 to the imputed ECOG PS in the docetaxel group. At this tipping point, 3 patients (0.8%) in the docetaxel arm were imputed to have an ECOG PS of zero and 258 patients (59.2%) had an ECOG PS of 3 or 4; in the observed data, these figures were 80 patients (15.1%) with ECOG PS of 0 and 29 patients (5.5%) with ECOG PS of 3 or 4. No tipping points in our conclusions were identified for the atezolizumab vs nivolumab comparison (Figure 3).
Figure 3. Tipping Point Analysis for Varying δ Shifts Applied to Missing Baseline Eastern Cooperative Oncology Group (ECOG) Performance Status.
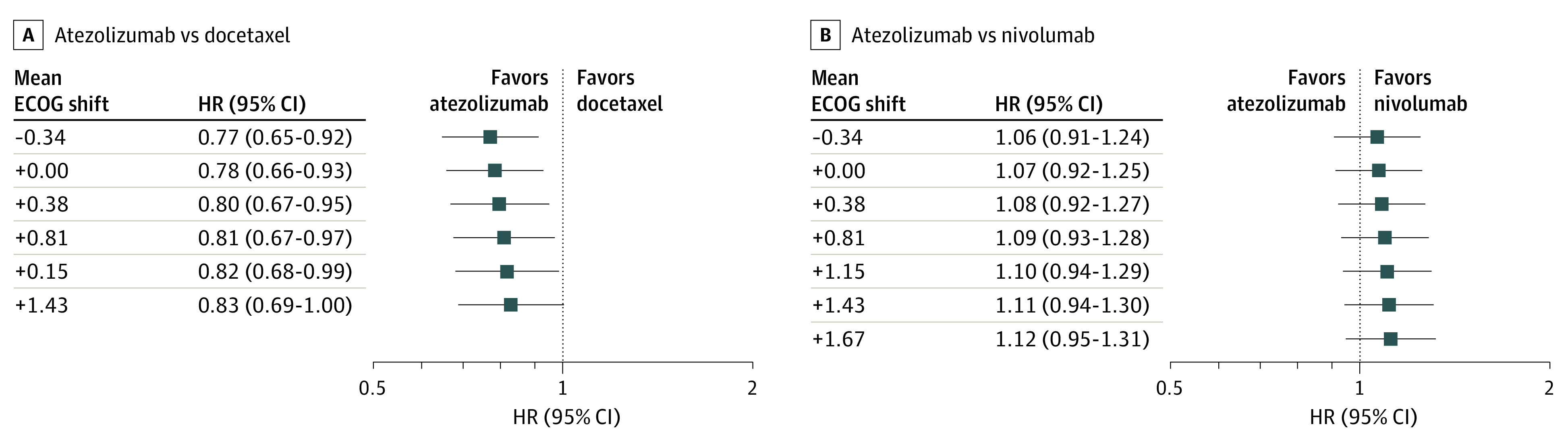
Negative values of δ imply exponentially increasing odds of patients in the comparator with atezolizumab having poorer baseline ECOG performance status than expected under random missingness, leading to a positive-valued mean ECOG performance status shift. HR indicates hazard ratio.
Discussion
In this comparative effectiveness study of advanced patients with NSCLC who had previously received platinum-based chemotherapy with or without variants in EGFR, ALK, or ROS1, we compared OS among patients initiating atezolizumab, docetaxel, or nivolumab. To our knowledge, this is the first analysis of OS in a large nationally representative real-world cohort of patients with advanced NSCLC with platinum-resistant cancer. In the absence of a randomized clinical trial, real-world EHR data can be used to provide evidence of performance of licensed therapies in routine clinical practice and help bridge the gap between treatment efficacy and effectiveness.25 For the rapidly evolving therapeutic landscape in advanced NSCLC, real-world evidence from head-to-head comparisons can help identify effectiveness associated with real-world use to guide treatment decisions about patient subgroups most likely to benefit in a timely manner.
Baseline patient characteristics were largely balanced between atezolizumab and docetaxel or nivolumab treatment groups. Larger differences were observed between atezolizumab and docetaxel arms, possibly because docetaxel has largely been replaced by ICIs in recent years in clinical guidelines for this indication.5 Sufficient balance was achieved across all covariates after propensity weighting. Atezolizumab was associated with significantly prolonged OS compared with docetaxel. The HR of 0.76 (95% CI, 0.58-1.00) from the final results of the POPLAR trial and 0.78 (95% CI, 0.68-0.89) from the final results of the OAK trials,26 both comparing atezolizumab and docetaxel among patients with ECOG PS of 0 or 1, which were consistent with our findings in the overall population of an HR of 0.79 (95% CI, 0.64-0.97). The significant benefit for atezolizumab was observed across all subgroups tested, indicating lack of significant treatment effect heterogeneity among these subgroups. Consistent with prior indirect comparisons of atezolizumab and nivolumab using meta-analysis of results from randomized trials27,28 or in a limited sample of real-world patients,29 we found no significant differences in OS between atezolizumab and nivolumab in the overall population or in any subgroup examined.
In this study, we also examined the extent to which missing ECOG PS could bias our findings. We focused on ECOG PS because baseline ECOG PS was missing for a large proportion of patients (between 20% and 45%), and as an important prognostic variable, had a large scope for confounding, and because it may not be uniformly recorded across clinical practices. We tested the assumption that baseline ECOG PS could have been worse than expected under random missingness in the comparator with atezolizumab, which could have led to confounded results. Using δ adjustments to apply an additive shift to imputed ECOG PS in the docetaxel arm, we identified a tipping point for loss of a statistically significant OS benefit for atezolizumab at a δ of −4, or a mean shift of +1.43 ECOG PS. This corresponded to a 95% reduction in the number of patients with imputed ECOG PS of 0 and a 984% increase for ECOG PS of 3 or 4 among those missing baseline ECOG PS compared with non missing data in the docetaxel arm. We expect that such a distribution is implausible to occur either by chance or systematically. No tipping points were identified for a change in our conclusions about the atezolizumab vs nivolumab comparison, possibly because of the lower fraction of missing ECOG PS in these treatment groups (20% and 27%). Strengths of this study include a large, real-world cohort of patients with cancer in the US, with rich information on exposures, OS, and confounders, as well as a rigorous sensitivity analysis for missing data on confounders.
Limitations
This study has some limitations. First, as with any observational study, there is the possibility of unmeasured confounding. Given that we generally observed relatively minor imbalances in measured covariates between treatment groups, we do not expect major systematic unmeasured confounding. We also assumed that diagnoses defined using information from claims data, such as driver variants, comorbidities, or metastases, were not differently recorded between treatment groups even though they may be underrecorded in general or over time. We expect that these are justified assumptions. Although performance status is often associated with therapy choice in clinical trials, it is not frequently reported in routine clinical care. Patient performance status should be regularly assessed in routine clinical practice in the care of patients with cancer to assist with therapy planning for patients but also for such applicability of trial data in real-world patients.
Note that treatment outcomes were defined as HRs with respect to treatment initiation without adjustment for adherence or postbaseline covariates, which is consistent with an intention-to-treat analysis but should be interpreted appropriately.30 Furthermore, although OS was not statistically significantly different between the atezolizumab and nivolumab treatment groups, we may be underpowered to detect a difference if it truly exists in the population and would require larger sample sizes.
Conclusions
This comparative effectiveness study assessed a large, real-world cohort of patients with advanced NSCLC who previously did not respond to platinum-based chemotherapy. Atezolizumab was associated with longer OS compared with docetaxel and was on par with nivolumab, supporting current clinical guidelines for systemic therapy for patients with advanced NSCLC in the US.5
eAppendix. Baseline Covariates
eTable. Propensity-Weighted Baseline Patient Characteristics for Atezolizumab vs Docetaxel and Atezolizumab vs Nivolumab
eFigure. Distribution of Treatment Propensity for Atezolizumab vs Docetaxel and Atezolizumab vs Nivolumab
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Duma N, Santana-Davila R, Molina JR. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623-1640. doi: 10.1016/j.mayocp.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 3.Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200. doi: 10.1016/j.lungcan.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 4.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446-454. doi: 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- 5.Masters GA, Temin S, Azzoli CG, et al. ; American Society of Clinical Oncology Clinical Practice . Systemic therapy for stage IV non–small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(30):3488-3515. doi: 10.1200/JCO.2015.62.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31(8):1039-1049. doi: 10.1200/JCO.2012.45.3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6(2):233-241. doi: 10.1513/pats.200809-110LC [DOI] [PubMed] [Google Scholar]
- 8.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095-2103. doi: 10.1200/JCO.2000.18.10.2095 [DOI] [PubMed] [Google Scholar]
- 9.Kaufman HL, Atkins MB, Subedi P, et al. The promise of Immuno-oncology: implications for defining the value of cancer treatment. J Immunother Cancer. 2019;7(1):129. doi: 10.1186/s40425-019-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khozin S, Miksad RA, Adami J, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer. 2019;125(22):4019-4032. doi: 10.1002/cncr.32383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214. doi: 10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non–small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schabath MB, Cress D, Munoz-Antonia T. Racial and Ethnic Differences in the Epidemiology and Genomics of Lung Cancer. Cancer Control. 2016;23(4):338-346. doi: 10.1177/107327481602300405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv. Preprint posted online January 13, 2020. https://arxiv.org/abs/2001.09765
- 17.Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. Preprint posted online May 30, 2020. doi: 10.1101/2020.03.16.20037143 [DOI]
- 18.Buuren SV, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Statist Software. 2011;(45)3:1-68. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 19.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206-213. doi: 10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- 20.Van Buuren S. Flexible Imputation of Missing Data. 2nd ed. CRC Press; 2018. doi: 10.1201/9780429492259 [DOI] [Google Scholar]
- 21.Leacy FP, Floyd S, Yates TA, White IR. Analyses of sensitivity to the missing-at-random assumption using multiple imputation with delta adjustment: application to a tuberculosis/HIV prevalence survey with incomplete HIV-status data. Am J Epidemiol. 2017;185(4):304-315. doi: 10.1093/aje/kww107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezvan PH, Lee KJ, Simpson JA. Sensitivity analysis within multiple imputation framework using delta-adjustment: application to longitudinal study of Australian Children. Longit Life Course Stud. 2018;9(3):259-278. doi: 10.14301/llcs.v9i3.503 [DOI] [Google Scholar]
- 23.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichler H-G, Abadie E, Breckenridge A, et al. Bridging the efficacy-effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nat Rev Drug Discov. 2011;10(7):495-506. doi: 10.1038/nrd3501 [DOI] [PubMed] [Google Scholar]
- 26.Mazieres J, Rittmeyer A, Gadgeel S, et al. Atezolizumab versus docetaxel in pretreated patients with NSCLC: final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. J Thorac Oncol. 2021;16(1):140-150. doi: 10.1016/j.jtho.2020.09.022 [DOI] [PubMed] [Google Scholar]
- 27.Passiglia F, Galvano A, Rizzo S, et al. Looking for the best immune-checkpoint inhibitor in pre-treated NSCLC patients: An indirect comparison between nivolumab, pembrolizumab and atezolizumab. Int J Cancer. 2018;142(6):1277-1284. doi: 10.1002/ijc.31136 [DOI] [PubMed] [Google Scholar]
- 28.Tan PS, Aguiar P Jr, Haaland B, Lopes G. Comparative effectiveness of immune-checkpoint inhibitors for previously treated advanced non–small cell lung cancer—a systematic review and network meta-analysis of 3024 participants. Lung Cancer. 2018;115:84-88. doi: 10.1016/j.lungcan.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 29.Weis TM, Hough S, Reddy HG, Daignault-Newton S, Kalemkerian GP. Real-world comparison of immune checkpoint inhibitors in non-small cell lung cancer following platinum-based chemotherapy. J Oncol Pharm Pract. 2020;26(3):564-571. doi: 10.1177/1078155219855127 [DOI] [PubMed] [Google Scholar]
- 30.Hernán MA, Hernández-Díaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9(1):48-55. doi: 10.1177/1740774511420743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Baseline Covariates
eTable. Propensity-Weighted Baseline Patient Characteristics for Atezolizumab vs Docetaxel and Atezolizumab vs Nivolumab
eFigure. Distribution of Treatment Propensity for Atezolizumab vs Docetaxel and Atezolizumab vs Nivolumab



