Abstract
SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, osteitis) is an inflammatory disorder of the bone, skin, and joints. We describe a family with multiple affected members who segregate a SAPHO syndrome–like phenotype, and we report the results of neutrophil studies and candidate gene analysis. We obtained written informed consent and a family history and reviewed medical records. We collected DNA and sequenced candidate genes, and we performed functional studies on neutrophils isolated from the proband and her mother. The pedigree segregated chronic osteomyelitis and cutaneous inflammation in a pattern that suggested an autosomal-dominant disorder. No coding sequence mutations were detected in PSTPIP1, PSTPIP2, LPIN2, SH3BP2, or NCF4. Analysis of neutrophil function in the proband, including nitroblue tetrazolium tests, myeloperoxidase assays, neutrophil chemotaxis, and neutrophil chemotaxis assays, revealed no identifiable abnormalities. However, an abnormality in the luminol, but not the isoluminol, respiratory burst assays following stimulation with phorbol myristate acetate (PMA) was detected in neutrophils isolated from the affected proband. Internal oxidant production was also reduced in the proband and her mother when neutrophils were treated with fMLP with or without platelet-activating factor, PMA alone, or tumor necrosis factor a alone. This family segregates a disorder characterized by chronic inflammation of the skin and bone. Functional differences in neutrophils exist between affected individuals and controls. The biologic significance of this defect remains unknown. Identification of the gene defect will help identify an immunologic pathway that, when dysregulated, causes inflammation of the skin and bone.
Autoinflammatory diseases are genetic disorders in which the innate immune system is involved in the development of recurrent bouts of unexplained inflammation (1). Chronic recurrent multifocal osteomyelitis (CRMO) is an autoinflammatory syndrome of the bone (2). CRMO presents with multifocal, culture-negative osteomyelitis often accompanied by palmar–plantar pustulosis, psoriasis vulgaris, or inflammatory bowel disease (2). SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, osteitis) is a related disorder that occurs primarily in adults, in which CRMO is often a subphenotype (3). It is unclear whether CRMO and SAPHO syndrome are distinct clinical entities, but the phenotypic similarities (osteitis, pustular skin rashes) suggest a similar pathophysiology (3). Both disorders are rare, with most reports from Europe and North America. However, reports from Asia and South America suggest a global distribution.
There is evidence that CRMO has a genetic contribution, and 2 genes have been identified that, when mutated, cause chronic osteomyelitis, including LPIN2 in Majeed syndrome and Pstpip2 in murine chronic multifocal osteomyelitis (2). Majeed syndrome is a rare, autosomal-recessive disorder presenting with early-onset CRMO, recurrent fevers, and congenital dyserythropoietic anemia with or without Sweet syndrome (2). In addition, a susceptibility locus for human CRMO has been reported on chromosome 18q (2). With the exception of CRMO found in the context of Majeed syndrome, no genetic abnormalities have been identified in human CRMO or SAPHO syndrome.
The immunologic basis of SAPHO syndrome remains poorly characterized. Neutrophils appear to play an important role in disease manifestations, with neutrophilic pseudoabscesses seen in skin lesions and neutrophilic infiltration in the bone (3). A comprehensive evaluation of neutrophil function in individuals with SAPHO syndrome has not been reported. We describe a family with multiple affected members who segregate a SAPHO syndrome–like phenotype, and we report the results of neutrophil studies and candidate gene analysis.
CASE REPORTS
Proband (IV-9).
The patient, a 15-year-old Caucasian girl, came to the pediatric rheumatology clinic for evaluation of steroid-responsive, recurrent osteomyelitis and chronic pustular rash. The patient’s symptoms had begun 1 year before, when she had developed forefoot pain and swelling without accompanying fever. Initial radiographs were normal, but 1 month later, an osteolytic lesion was seen in the distal second metatarsal joint, prompting referral to orthopedics. Magnetic resonance imaging (MRI) revealed edema in the right second proximal phalanx and second distal metatarsal joint and adjacent soft tissues. The patient had a normal white blood cell (WBC) count and erythrocyte sedimentation rate (ESR). An open-bone biopsy revealed a mixed inflammatory infiltrate consistent with osteomyelitis, yet stains and cultures for bacteria, fungi, and mycobacteria were negative. She was treated with antibiotics for months without improvement. Four months later, she returned with pain in the fourth and fifth metatarsophalangeal joints in the opposite foot. Radiographs were normal, and no MRI or bone scan was performed. She also developed polyarthralgia and a recurrent, sterile, pustular rash on her extremities, trunk, and face that histologically demonstrated lichenification and neutrophilia. She also had scarring acne and oral ulcers. She was treated with oral corticosteroids, which resulted in improvement in her bone pain, joint pain, and skin lesions.
Medical history.
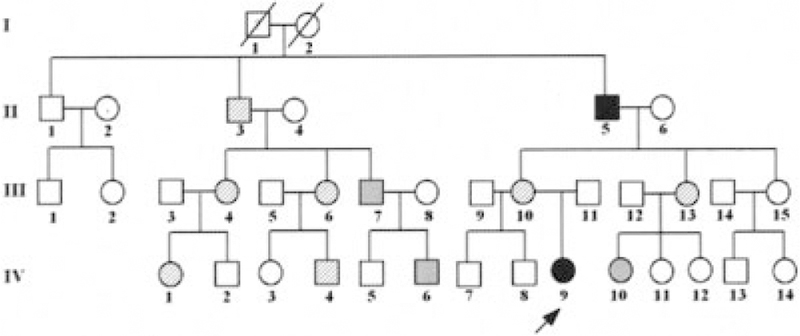
The patient’s medical history was remarkable for osteomyelitis in the distal right tibia and fibula at the age of 4 years, which was not associated with fever or elevated WBC count. Her ESR was 22 mm/hour. Her mother had been told that her condition was due to Staphylococcus aureus, but the patient’s synovial fluid was sterile, and we could not locate bone culture results. She received 6 weeks of intravenous antibiotics but continued to have chronic ankle pain. Between the ages of 6 years and 14 years, she had recurrent oral ulcers, a dental abscess, a thigh abscess, severe scarring acne, back pain localized to the T12 and L1 vertebrae, and a persistently swollen submental lymph node. Histology of the node demonstrated prominent mixed germinal and interfollicular cortical hyperplasia; stains and cultures were negative for bacteria, fungi, and mycobacteria. Her family history was positive for recurrent pustulosis and severe acne in her mother and for severe acne, recurrent folliculitis, and recurrent osteomyelitis in her maternal grandfather (Figure 1).
Figure 1.
Pedigree. The arrow points to the proband. Solid symbols denote individuals with both chronic multifocal osteomyelitis and skin inflammation. Hatched symbols denote individuals with skin inflammation. Gray symbols denote individuals with other inflammatory disorders (see last paragraph of Case Reports).
Clinical findings.
On physical examination, the patient was afebrile and had a moderate-to-severe scarring acneiform rash on her face, chest, and back. She had a pustular rash predominantly on her scalp and legs that spared her palms and soles, and she had pain and mild swelling over the right second metacarpophalangeal joint. A diagnosis of SAPHO syndrome was made at that time. At followup, a dual x-ray absorptiometry scan was performed, yielding a T score of –3.84 in the left femoral neck. Pamidronate at a dosage of 0.8 mg/kg every 6 months was instituted to improve her bone mass and as a treatment for her inflammatory bone lesions. Both her skin and bone symptoms improved dramatically after pamidronate infusions were instituted.
Immunologic assessment.
Laboratory tests revealed an antinuclear antibody titer of 1:320 in the absence of antibodies to extractable nuclear antigens. Complement studies yielded normal findings. The patient’s peripheral blood eosinophil counts ranged from 500/mm2 to 1,050/mm2. Quantitative immunoglobulins revealed minimally elevated IgG, IgM, and IgA levels. Her IgE levels were persistently elevated, ranging from 1,000 IU/ml to 1,988 IU/ml. A hypersensitivity screen yielded negative results for Aspergillus species, Mycobacterium faeni, pigeon serum, Thermoactinomyces candidus, and T hermoactinomyces vulgaris. Tests to determine lymphocyte populations, performed twice, revealed no gross abnormalities. Serologic testing for human immunodeficiency virus yielded negative results.
Nitroblue tetrazolium assays yielded normal results for 2 different specimens in 2 different laboratories. As part of a prior clinical evaluation, specialized neutrophil studies were performed in Dr. Nauseef’s laboratory at the University of Iowa (Iowa City, IA). Normal amounts of myeloperoxidase were detected by immunohistochemistry and by a functional enzyme assay. NADPH oxidase activity was normal when measured using ferricytochrome c on polymorphonuclear neutrophils (PMNs) stimulated with serum-opsonized zymosan (20 particles per PMN) or phorbol myristate acetate (PMA; 100 ng/ml). Ferricytochrome c reduction was quantified after 10 minutes of stimulation at 37°C. The proband’s neutrophils produced 94% and 111% of control levels of oxidant in response to PMA and serum-opsonized zymosan, respectively. Immunoblots of PMNs separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis reportedly showed normal amounts and sizes of p47phox, p67phox, gp91phox, and p22phox, suggesting a functionally and structurally normal NADPH oxidase.
Proband’s mother (III-10).
In the proband’s mother, recurrent pustulosis and severe acne began in childhood. Treatment included 25 weeks of isotretinoin without improvement; antibiotics occasionally provided mild improvement. Her skin lesions consistently improved with oral corticosteroids. She also reported recurrent bone pain in multiple locations, which has not been evaluated.
Proband’s maternal grandfather (II-5).
In the proband’s maternal grandfather, cutaneous symptoms included severe scarring acne, recurrent folliculitis, psoriasis, eczema, and skin abscesses. As an adult, he was diagnosed as having infectious osteomyelitis on several occasions and had cultures that were positive for coagulase-negative Staphylococcus and Trichophyton rubrum. He was treated for extended periods with antibiotics and antifungal therapies without improvement and underwent resection of his distal toe and subsequently had a below-the-knee amputation. Over many years, he had multiple affected areas noted on bone scan and MRI and had bone biopsies that revealed granulomatous inflammation with fibrosis and chronic inflammation. After years of treatment, he continued to be intermittently symptomatic, but no further biopsies were performed and no further antimicrobials were given.
Other phenotypes per family history (Figure 1).
Severe scarring acne and/or pustulosis was found in family members II-3, III-4, III-6, III-10, III-13, IV-1, and IV-4. Parry-Romberg syndrome (a rare form of linear scleroderma involving the face, resulting in hemifacial atrophy) was found in family member III-4. Noneosinophilic colitis was found in family member IV-4. Alopecia areata was found in family members III-7 and IV-6. Probable immune-mediated thrombocytopenia was found in family member IV-10.
MATERIALS AND METHODS
Written informed consent was obtained from each participating family member. A detailed family history was obtained, a pedigree was constructed, medical records were reviewed, and blood or saliva was obtained for DNA isolation.
DNA was extracted from whole blood or saliva by standard methods. The genomic sequences of PSTPIP1 (NM_003978), PSTPIP2 (NM_024430), LPIN2 (NM_014646), SH3BP2 (NM_003023), and NCF4 (neutrophil cytosolic factor 4 [40-kd] isoform 1, also known as p40phox NM_000631 and NM_013416) were obtained from public databases (http://ncbi.nlm.nih.gov, http://genome.ucsc.edu). Primers were designed using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The coding regions and splice sites were amplified by polymerase chain reaction (PCR) from genomic DNA, run on agarose gel, cut, and recovered by column purification using standard methods. Sequencing was performed in both directions on an automated ABI sequencer (Applied Biosystems, Foster City, CA) using DyeTerminator chemistry. Primer sequences and PCR conditions are available upon request from the corresponding author. Peripheral blood neutrophils were purified from human blood with the use of Polymorphprep (Nycomed, West Midlands, UK) as described previously (4).
Internal and external oxidant production was determined using the luminol and isoluminol reporter assays in black 96-well plates in EGM-2MV medium (Cambrex, Walkersville, MD) (5). To detect external oxidant, 0.5 mM isoluminol and 4 units of horseradish peroxidase (Sigma, St. Louis, MO) were added to the EGM-2MV. To detect internal oxidant, 1 mM luminol and 50 units of superoxide dismutase (Sigma) were added to the EGM-2MV. Next, 3.1 ng/ml, 6 ng/ml, or 31 ng/ml of PMA and 5 × 105 patient neutrophils or 5 × 105 control neutrophils were added to the plate. Luminescence was detected on a Tecan SpectraFluor Plus fluorometer (Tecan, Durham, NC) every 90 seconds for 1.5 hours. These assays were repeated on at least 4 different occasions using 4 different control samples with similar results.
Additionally, oxidant production was assessed using dihydrorhodamine 123 (6). Briefly, 2 × 105 neutrophils were incubated with 1 µM dihydrorhodamine 123 (Molecular Probes, Eugene, OR) for 5 minutes at 37°C. Cells were added to 70 µl plasma stimulated with Dulbecco’s phosphate buffered saline (Mediatech, Herndon, VA) and 30 µl autologous plasma reserved from the purification step which also contained 1 nM fMLP, 0.1 µM platelet-activating factor (PAF), 5 ng/ml PMA (all from Sigma), or 100 ng/ml recombinant human tumor necrosis factor α (TNFα; BioSource International, Camarillo, CA). Cells were incubated at 37°C for 30 minutes and iced immediately. Fluorescence was then read on a FACScan instrument (BD Biosciences, San Jose, CA) with excitation at 488 nm with an argon laser and emission measured at 525 nm.
RESULTS
Genes known to cause autoinflammatory bone disorders including Majeed syndrome (LPIN2), murine CRMO (Pstpip2), and cherubism (SH3BP2) were chosen as candidate genes. Due to the acne and other inflammatory skin lesions, we also analyzed the PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, and acne) gene PSTPIP1. Finally, we searched for mutations in NCF4 (p40phox) to complete the evaluation of the components of the NADPH oxidase. No variations in the coding regions or splice sites of LPIN2, NCF4, PSTPIP1, PSTPIP2, or SH3BP2 segregated with the phenotype.
Given the pustular rash and recurrent osteitis, a primary neutrophil defect was believed to be a diagnostic possibility; therefore, assays were performed to assess respiratory function and migration. No abnormalities in chemotaxis were detected in the patient’s neutrophils utilizing Transwell assays and live imaging. Neutrophil phagocytosis was determined to be normal using the Vybrant phagocytosis assay (Invitrogen, San Diego, CA). To determine external and internal respiratory function, we performed a plate reader–based assay using isoluminol and luminol as previously described (7). Interestingly, we observed a substantial defect in internal oxidative burst in response to treatment with the activator PMA (Figure 2). In response to treatment with PMA, we observed a robust external burst, as indicated by isoluminol, in both patient and control neutrophils. However, despite normal internal oxidative burst in the control neutrophils treated with PMA, the patient’s neutrophils showed a reproducible diminution in internal oxidative burst despite escalating concentrations of PMA.
Figure 2.
Respiratory burst in patient and control neutrophils in response to increasing doses of phorbol myristate acetate (PMA). The luminol and isoluminol assays can be used to differentiate extracellular oxidant production and intracellular oxidant production by primary neutrophils. Primary neutrophils were purified using Polymorphprep and treated with increasing doses of PMA (3.1 ng/ml, 6 ng/ml, and 31 ng/ml). A and B, Area under the burst curve for internal and external oxidant production by the luminol assay (A) or isoluminol assay (B) in patient and control neutrophils at varying doses of PMA. Values are the mean ± SEM of 4 experiments. * = P < 0.05 versus control neutrophils, by analysis of variance. C, Representative burst curve for both luminol and isoluminol after stimulation with 31 ng/ml of PMA in patient and control neutrophils. Control neutrophils isolated from healthy donors exhibit robust oxidant production both internally (luminol) and externally (isoluminol), while patient neutrophils exhibit a defect in only internal oxidant production. Shown in C are the results of 1 experiment, representative of >4 experiments performed.
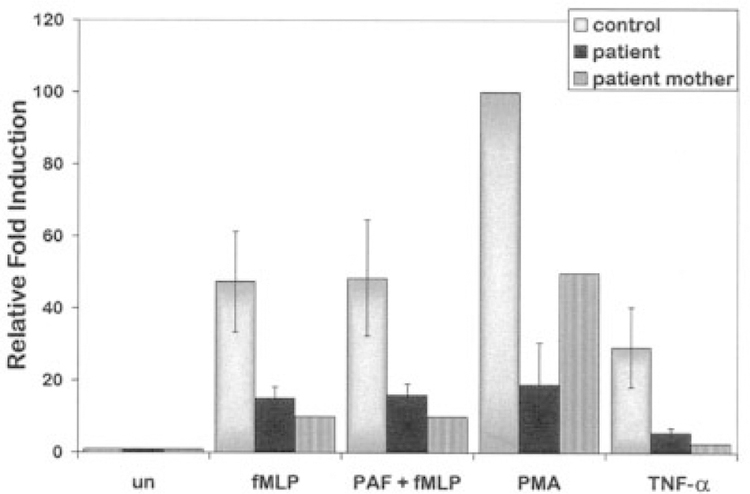
To further characterize respiratory burst function, we performed a more quantitative flow cytometry–based assay to detect oxidant production in patient and control neutrophils. In accordance with our findings in the plate-based assay, we observed a significant reduction in oxidant production in patient neutrophils compared with control neutrophils when neutrophils were treated with mediators of inflammation including fMLP, PAF plus fMLP, PMA, or TNFα. This difference was reproducible in 3 separate experiments (Figure 3). Interestingly, we also observed reduced internal oxidative burst in neutrophils isolated from the patient’s mother, who has a similar SAPHO-like phenotype (Figure 3). Taken together, these findings suggest that patients with SAPHO syndrome may have an intrinsic neutrophil defect and reduced capacity to generate internal oxidant.
Figure 3.
Flow cytometry analysis of oxidant production in neutrophils. Shown is the relative oxidative burst in neutrophils isolated from the patient and from her mother and in control neutrophils treated with fMLP, platelet-activating factor (PAF) plus fMLP, phorbol myristate acetate (PMA), or tumor necrosis factor α (TNFα). Data are the results from 3 experiments with the patient’s neutrophils and 1 experiment with neutrophils isolated from the patient’s mother. Values are the mean ± SEM of 3 experiments for the patient. Neutrophils from both the patient and her mother showed reduced oxidative burst compared with control neutrophils (P < 0.05 by analysis of variance). un = unstimulated neutrophils.
DISCUSSION
This family has a steroid-responsive inflammatory condition with a phenotype most reminiscent of SAPHO syndrome, with pustulosis, severe acne, and osteitis. Although the proband and her grandfather had osteomyelitis with bone cultures that reportedly grew various organisms at some point in the disease course, it is likely that these were contaminants or, in the case of the proband, a presumed diagnosis. There was no response to appropriate antimicrobials in either individual, yet corticosteroids provided prompt resolution of symptoms.
The responses to antiinflammatory medications suggest that this family has an autoinflammatory disorder, and the pedigree suggests an autosomal-dominant disorder with variable penetrance. A candidate gene analysis of genes known to cause sterile, noninfectious osteitis (LPIN2, PSTPIP2, SH3BP2) and severe acne (PSTPIP1) did not yield a causative mutation. While this does not definitively exclude these genes, since regulatory regions were not studied, it suggests that another, as-yet-unidentified gene is involved in producing the phenotype in this family.
Neutrophils play a prominent role in the clinical manifestations seen in several of the autoinflammatory disorders including SAPHO syndrome. For instance, in PAPA syndrome, neutrophilic infiltration into the tissues causes the bulk of the phenotypic features (8). Our findings suggest an intrinsic defect in neutrophil function characterized by impaired production of internal oxidant with relatively normal production of extracellular oxidant in response to treatment with different inflammatory agents. Interestingly, the patient and her mother had no history of recurrent bacterial infections, suggesting that their functional responses to bacterial infection are normal.
The functional significance of impaired internal oxidant production in the setting of normal response to pathogens is not clear. However, recent studies suggest that the production of internal and external oxidant are separable and differentially regulated by treatment with antihistamines (9). Higher concentrations of PMA were insufficient to bring the patient’s internal oxidative burst to normal levels, demonstrating an inability to produce a normal burst independent of the strength of signal. Our data support the hypotheses that SAPHO syndrome is a disorder of innate immunity and that it has a genetic basis.
Currently, there are no available laboratory tests to screen for SAPHO syndrome, and our findings suggest that this test may be used to aid in the diagnosis of patients with SAPHO syndrome. Additional studies in larger cohorts of patients with SAPHO syndrome are planned to determine the generalizability of our findings.
Acknowledgments
Supported in part by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS] grant R03-AR-051130-01) and the Children’s Miracle Network. Dr. El-Shanti’s work was supported by the NIH (NIAMS grant R21-AR-053924), the Children’s Miracle Network (grant 1615), and a Carver Medical Research Initiative grant. Dr. Huttenlocher is recipient of a Burroughs Wellcome Foundation Clinical Scientist Award.
REFERENCES
- 1.Masters SL, Lobito AA, Chae J, Kastner DL. Recent advances in the molecular pathogenesis of hereditary recurrent fevers. Curr Opin Allergy Clin Immunol 2006;6:428–33. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson PJ, El-Shanti HI. Autoinflammatory bone disorders. Curr Opin Rheumatol 2007;19:492–8. [DOI] [PubMed] [Google Scholar]
- 3.Rohekar G, Inman RD. Conundrums in nosology: synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome and spondylarthritis. Arthritis Rheum 2006;55:665–9. [DOI] [PubMed] [Google Scholar]
- 4.Lokuta MA, Nuzzi PA, Huttenlocher A. Analysis of neutrophil polarization and chemotaxis. Methods Mol Biol 2007;138:211–29. [DOI] [PubMed] [Google Scholar]
- 5.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods 1999;232:3–14. [DOI] [PubMed] [Google Scholar]
- 6.Rothe G, Emmendorffer A, Oser A, Roesler J, Valet G. Flow cytometric measurement of the respiratory burst activity of phagocytes using dihydrorhodamine 123. J Immunol Methods 1991;138: 133–5. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Suzuki K, Kudo S, Yamada M, Kowatari K, Umeda T, et al. Effect of decaglycerol mono-oleate on chemiluminescence of human neutrophils. Luminescence 1999;14:327–30. [DOI] [PubMed] [Google Scholar]
- 8.Wise CA, Bennett LB, Pascual V, Gillum JD, Bowcock AM. Localization of a gene for familial recurrent arthritis. Arthritis Rheum 2000;43:2041–5. [DOI] [PubMed] [Google Scholar]
- 9.Jancinova V, Drabikova K, Nosal R, Rackova L, Majekova M, Holomanova D. The combined luminol/isoluminol chemiluminescence method for differentiating between extracellular and intracellular oxidant production by neutrophils. Redox Rep 2006;11: 110–6. [DOI] [PubMed] [Google Scholar]