Abstract
Purpose of review:
The purpose of this review is to summarize the role of complement in regulating the removal of a target alloantigen following an incompatible red blood cell (RBC) transfusion, the formation of alloantibodies following RBC alloantigen exposure, and the development of hyperhemolysis in patients with sickle cell anemia.
Recent findings:
Recent studies demonstrate that complement can accelerate alloantibody-mediated removal of target alloantigens from the RBC surface following incompatible transfusion. Complement also influences alloantigen availability during developing alloimmune responses and serves as a unique mediator of CD4 T cell-independent alloantibody formation following RBC alloantigen exposure. Finally, alternative complement activation appears to play a key role in the development of acute hemolytic episodes in patients with sickle cell anemia, providing a potential druggable target to prevent acute complications in patients with this disease
Summary:
Recent studies suggest that complement can regulate a wide variety of processes germane to hematology, from transfusion complications to baseline hemolysis in patients with sickle cell anemia. As the role of complement in various disease processes becomes more fully understood, the ability to leverage recently developed complement modulating drugs will only continue to enhance provider’s ability to favorable intervene on many hematological diseases.
Keywords: Alloimmunization, complement, hyperhemolysis, transfusion, anemia
Introduction
Complement has its origins in the earliest aspects of host immunity, both with respect to immune evolution and discovery of host immunity itself. Jules Bordet, who would later win the Nobel Prize in Physiology and Medicine in 1919 for his work on complement, among other aspects of immunity, is credited with performing the critical experiments associated with complement’s discovery, where he identified the thermosensitive component of complement [1,2]. It was not until 1899 that Paul Ehrlich, who won the Nobel Prize in Physiology and Medicine in 1908, that the heat-labile alexin was renamed “complement” and the complementary heat-stable portion that interacts with complement as “amboceptor,” which we now know as antibodies. RBCs were intimately linked to these discoveries; before the components of complement were identified, the temperature-sensitive nature of complement was determined by storing guinea pig serum at various temperatures and observing its ability to hemolyze sheep red blood cells (SRBCs) [2].
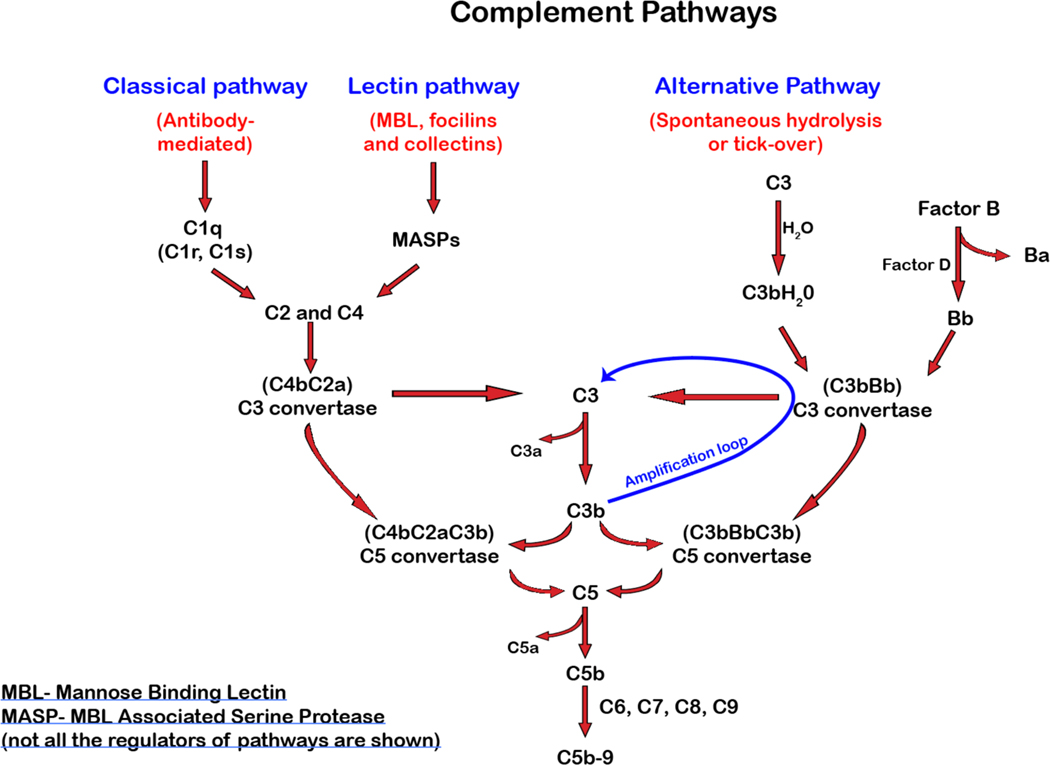
Complement activation can occur through three distinct, yet convergent cascades, the classical, lectin and the alternative or nonclassical pathways[3–5]. While each pathway is initiated by unique effector pathways, all converge on the formation of an enzymatic complex that converts complement component 3 (C3) into C3a and C3b[6]. The classical or antibody-driven pathway is marked by antibody engagement of C1q, which ultimately induces cleavage of C2 and C4. Exposure of thioesters following proteolysis results in the covalent attachment of C2a and C4b to the target cell surface and formation of the C3 convertase. While discovered much later, the lectin and alternative pathways are activated by the mannose binding lectin or spontaneous hydrolysis, respectively, which also results in the formation of C3 convertases that likewise cleaves C3 into C3a and C3b. Once activated, bound C3b can serve as an opsonin while also participating in the formation of a C5 convertase. Release of C3a and C5a by the C3 and C5 convertases, respectively, results in systemic consequences due to their anaphylatoxin activities[6–8]. Bound C5b recruits C6, C7 and C8, which as a complex, initiates the insertion of C9 into the plasma membrane as a C9 polymer that ultimately results in membrane attack complex (MAC) formation[9] (Figure 1).
Figure 1. Complement activation.
The complement cascade is initiated by one or more of the three pathways; classical, lectin, and the alternative pathway. Once a unique effector system initiates each pathway, they all converge to form a C3 convertase that cleaves C3 into membrane-bound C3b and an anaphylatoxin, C3a. C3b produced by any of the three pathways contributes to the amplification loop and cleaves more C3. C3b, along with the C3 convertase, forms a C5 convertase, which in turn cleaves C5 to form C5a, an anaphylatoxin, and C5b. The formation of C5b initiates the formation of the terminal complement complex (C5b9).
While many elegant studies resulted in the initial discovery and characterization of complement pathways, for many years, complement biology was sidelined within much of immunology and hematology. The renewed emphasis of complement biology largely stems from the advent of complement inhibitors[10], which have not only provided unique approaches to treat commonly recognized complement-related diseases, but as result of their expanded use, have also uncovered roles for complement in a wide variety of other diseases.
Complement in antibody-induced hemolysis and antigen modulation
Some of the earliest studies examining the consequences of antibody-antigen interactions used RBCs as a target surface. RBCs not only provided a useful model to study complement-mediated hemolysis due to the ease of obtaining large numbers of cells and relative simplicity of measuring membrane rupture (hemolysis), but antibody deposition on the RBCs surface and ensuing complement activation became quickly recognized as a key feature of hemolytic transfusion reactions. Indeed, detection of complement on the RBC surface continues to serve as an important diagnostic feature of blood bank evaluations when a hemolytic transfusion reaction is suspected. However, despite a long history of recognizing the possible role of complement in the pathophysiology of incompatible RBC transfusions, the consequence of complement activation on the RBCs surface is only beginning to be fully understood.
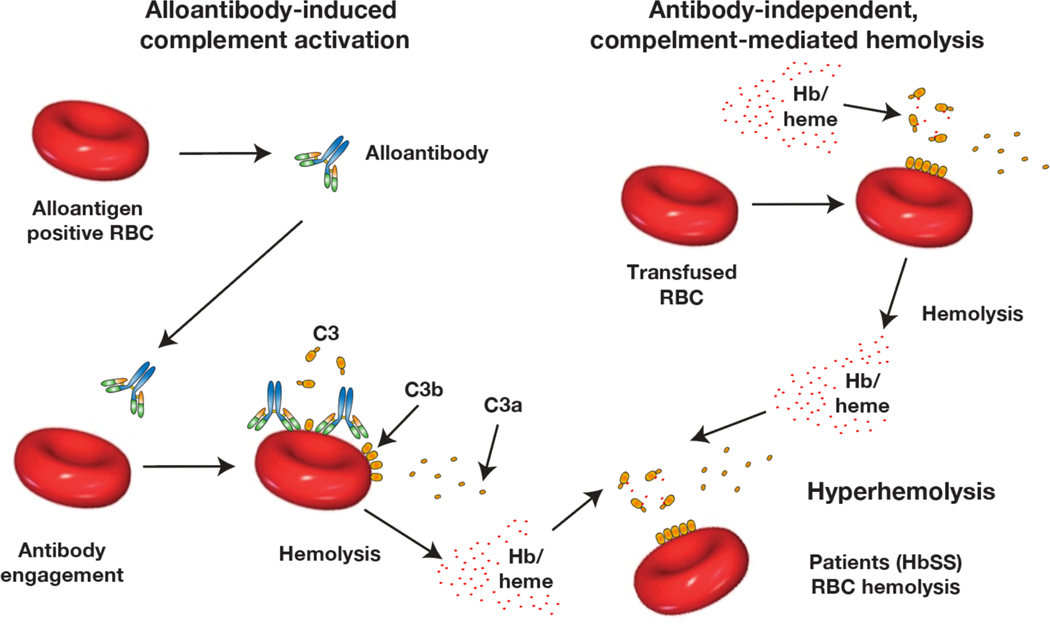
Alloantibody engagement of RBC surface alloantigens can initiate the classical complement pathway (Figure 2), resulting in the rapid release of C3a and C5a anaphylatoxins, formation of the membrane attack complex and extensive hemolysis[11]. However, hemolysis is not the inevitable outcome of antibody engagement and complement deposition. Complement regulatory proteins on the RBC surface, including CD55 and CD59, serve to inhibit complement activation following antibody engagement[12]. Rapid degradation of C3b into C3d, for example, prevents bound C3 fragments from serving as opsonins, facilitating further C3 activation or ultimately activating the C5-dependent MAC complex[12]. In addition to regulatory pathways that drive complement degradation once activated, complement deposition on the RBC surface also appears to trigger a relatively unique process recently termed antigen modulation[13]. In this setting, alloantibody engagement of an alloantigen and subsequent complement fixation can induce the selective removal of the target alloantigen. It should be noted that alloantibody-induced alloantigen removal can occur independent of complement, as studies examining RBCs that express the model HOD antigen (HEL, OVA and Duffy) have demonstrated that antigen removal can occur through complement independent, but Fc receptor-dependent pathways[14–16]. Additional studies suggest that removal of smaller portions of the HOD antigen (HEL) can actually occur completely independent of Fc gamma receptors or complement[17]. However, when complement is activated and plays a role in antigen modulation, such as following transfusion of RBCs expressing the KEL antigen, antigen removal is much more rapid than similar processes orchestrated by Fc gamma receptors[13,18,19]. This likely reflects the relatively rapid rate of complement deposition and ensuing engagement of a series of unique complement receptors. Complement-mediated antigen modulation does not appear to simply reflect decoration and therefore masking of the antigen by complement, as alloantibody-induced complement-dependent alloantigen modulation results in complete removal of the target antigen from the cell surface[20,21]. Ultimately, antigen modulation appears to render RBCs resistant to further alloantibody-induced hemolysis, even in the face of circulating anti-alloantigen antibodies[13,14,22,23]. However, fundamental questions remain regarding the role of complement in this process. These include what factors regulate antibody-induced hemolysis versus antigen modulation, the receptor(s) involved in mediating this pathway and the overall mechanism responsible for antigen removal independent of RBC hemolysis.
Figure 2: Complement can induce RBC hemolysis through antibody dependent and independent pathways.
Transfusion of incompatible RBCs can result in antibody engagement and subsequent complement activation, which in turn can result in hemolysis and attendant consequences. A variety of situations, including hemolytic transfusion reactions, can result in acute increases in heme release, which appears to drive alternative complement pathway activation. RBCs from patients with sickle cell anemia appear to be particularly sensitive to complement-induced hemolysis even following heme-mediated alternative complement pathway activation.
Complement and antibody development
While it was known that B cells have a membrane receptor that can engage C3, the possible role of complement in B cell biology initially remained unclear. Early studies examining the immune response to SRBCs revealed that removal of complement by cobra venom factor (CFV) depletion inhibited antibody formation[24]. Many studies have corroborated these initial findings, suggesting that complement plays a critical role in successful antibody formation following exposure to numerous antigens. However, not all antigens appear to require complement to induce antibody formation. Depletion of complement fails to impact the antibody response to streptococcus polysaccharide, suggesting that C3 may not play a role in the antibody response to T-cell independent antigens [24]. Complement engagement of the B cell surface occurs through ligation of complement receptors CR1 and 2 (CR1/2) and targeted deletion of the CR2 locus in mice results in decreased germinal center formation and antibody production [25,26]. Crosslinking of the B cell receptor (BCR) or CR1/2 with anti-IgM, anti-CR2 or anti-CR1 enhances calcium flux and increases 3H thymidine incorporation with CR2 and IgM, suggesting a key role for complement in the direct activation and proliferation of B cells [27,28].
While the direct role of complement in signaling B cell activation and antibody production has been studied extensively following exposure to infectious organisms and model antigens, despite the common evaluation of complement levels on RBCs following an incompatible transfusion, the role complement may play in actually shaping an immune response to RBC alloantigens had remained less clear. Given the role of complement in driving antibody formation against a wide variety of antigens, the potential role of complement in driving RBC alloimmunization appeared to be especially intriguing when considering that antibody formation following RBC transfusion often occurs in the absence of a known adjuvant, suggesting that early IgM antibody formation and subsequent complement activation may serve as surrogate for exogenous adjuvants. Consistent with this, models of alloantibody formation suggest that early IgM antibody formation occurs independent of CD4 T cells and is likely dictated by target antigen density[29,30]. These and other findings motivated several studies to define the role of complement in alloantibody formation. This was accomplished by coupling the model systems described above with complement deletion recipients to define the role of complement in alloimmunization. Using this approach, transfusion of HOD RBCs into C3 knock out (KO) recipients resulted in antibody formation that was found to be no different than the same transfusion into wild type (WT) recipients[20], suggesting that unlike antibody formation in response to many infectious challenges, anti-RBC alloantibody formation does not require complement activation. In contrast to HOD RBC-induced antibody formation, KEL RBCs not only can successfully drive antibody development in C3 KO recipients, but antibody formation in this setting is paradoxically increased when compared to similar transfusion into WT recipients[20]. These results suggest that following exposure to KEL, C3 may play an unexpected inhibitory role. Conflicting results have been reported for the role C3 plays in the formation of alloantibodies against other alloantigens, such as the clotting factor, fVIII, which is likewise capable of inducing alloantibody formation in the absence of adjuvant. Early studies demonstrated that depletion of complement in hemophilia A mice attenuates anti-fVIII antibody formation, while subsequent studies using C3 KO mice on a fVIII sufficient background failed to detect a similar role for complement in this process[31,32]. Genetic deletion of C3 and Fc gamma receptors did result in the diminution of anti-fVIII antibody formation, suggesting a complementary role for these antibody effector systems in anti-fVIII antibody development[32]. Differences in recipient backgrounds (hemophilia versus WT), complement removal strategy (CVF depletion versus C3 genetic deletion) or other variables altogether may contribute to these varied outcomes.
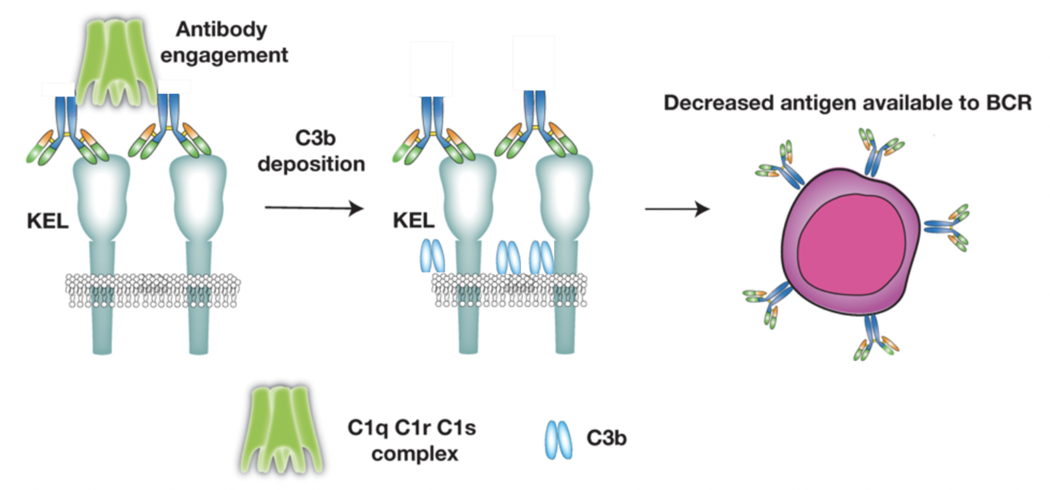
The ability of complement to regulate alloantibody formation in distinct ways following exposure to different alloantigens is intriguing and remains incompletely understood. Given the consequences of alloantibody engagement following an incompatible transfusion, several studies have examined the potential outcome of alloantibody engagement of alloantigen levels during an alloimmune response. Alloantibody engagement of the HOD antigen, for example, results in gradual reductions in the HEL antigen over time. However, despite low levels of complement deposition, decreases in the HEL antigen appear to occur independent of C3 or Fc gamma receptors[17,20]. In contrast, while KEL antigen levels also decrease during the developing anti-KEL immune response, the rate of antigen decline over time is significantly blunted following transfusion into C3 KO mice, despite higher anti-KEL antibody levels[20]. While soluble KEL may be predicted to similarly drive the humoral immune response following KEL RBC exposure, the impact of antigen density on the development of anti-KEL antibodies strongly suggests that surface-associated KEL is key in ligating the B cell receptor and therefore facilitating the ongoing immune responses[30]. These results suggest that C3-mediated removal of cell surface associated KEL antigen may attenuate an ongoing immune response by reducing surface antigen substrate (Figure 3).
Figure 3: Complement can accelerate target RBC alloantigen loss following alloantibody engagement.
The development of antibody during a primary immune response to RBC alloantigens can result in alloantibody engagement and subsequent complement fixation. Complement deposition appears to facilitate the selective removal of the target alloantigen, which may reduce alloantigen availability, thereby attenuating the ongoing immune response.
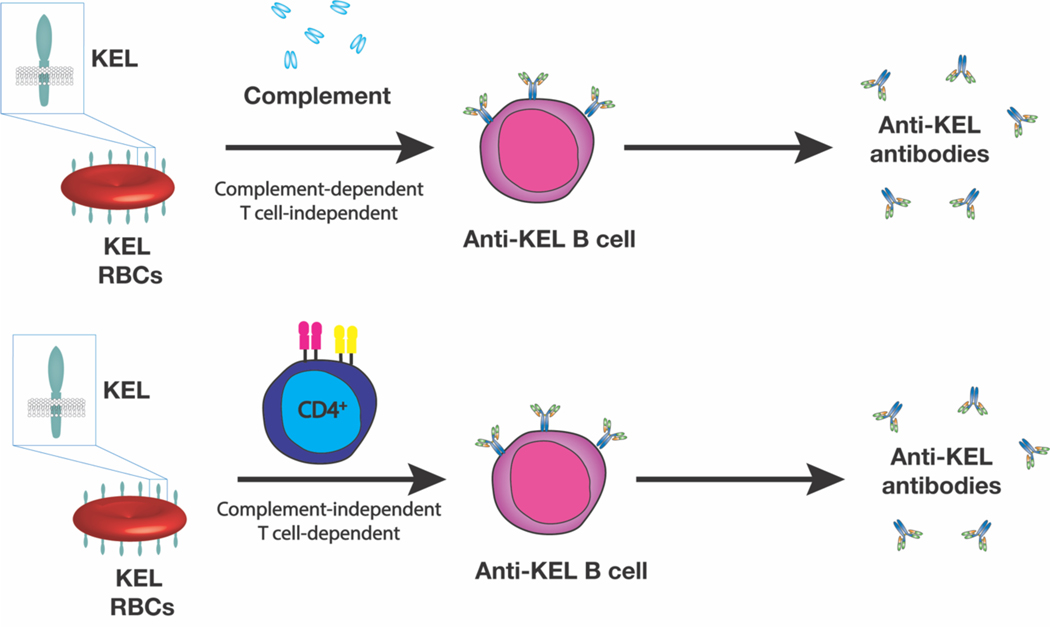
In addition to regulating antigen availability during a developing immune response, complement may actually play a key role in dictating what type of humoral response is engaged following RBC alloantigen exposure. RBC-induced alloimmunization has been thought to uniformly require CD4 T cells, an understandable paradigm given the traditional requirement of CD4 T cells and more specifically T follicular helper (TFH) cells in the development of IgG antibody responses. Appropriate activation of TFH cells and development of germinal center reactions is thought to be critical in the formation of long-lived plasma cells that are responsible for sustaining antibody formation long after alloantigen exposure. However, in contrast to the possible requirement of TFH in the development of alloantibodies, the KEL system provides a unique example of an alloantigen capable of inducing alloantibodies in the complete absence of CD4 T cells[33]. Indeed, KEL RBCs induce equivalent anti-KEL alloantibodies following transfusion into wild type, CD4 T cell depleted, MHC class II KO or TCRαβ KO recipients[34]. However, the factors capable of driving IgG class switching and overall antibody formation in the absence of CD4 T cells initially remained unknown. Subsequent studies demonstrated that in the absence of C3, KEL RBCs induces IgG anti-KEL antibody formation in a CD4 T cell dependent manner. Additional studies demonstrated that while KEL transfusion into WT mice results in IgG antibody formation in the absence of CD4 T cells, KEL RBC-induced alloantibody formation in CR1/2 KO recipients also requires CD4 T cells. Additional studies demonstrated that CR1/2 expression on B cells, not follicular dendritic cells, is required for complement-induced CD4 T cell independent IgG antibody formation[34] (Figure 4).
Figure 4: Complement induces CD4 T cell-independent alloantibody formation following exposure to KEL RBCS.
In the presence of complement, early alloantibody formation results in complement deposition on the RBC surface. RBC surface complement appears to engage CR1/2 on the B cell surface to induce B cell activation, class switching and differentiation into antibody secreting cells independent of CD4 T cell help. In the absence of complement, the antibody deposition does not result in RBC complement deposition and CR1/2 ligation. In this setting, CD4 T cells are required for IgG anti-KEL antibody formation.
The ability of C3 to regulate IgG antibody formation following KEL RBC exposure provides a unique example of a T cell independent antibody response and illustrates the distinct immune pathways alloantigens can engage following RBC transfusion. Most antigens are either intrinsically CD4 T cell-dependent or -independent. Indeed, antigens themselves are often described with respect to their requirement for CD4 T cells in the induction of an IgG antibody response. In contrast, following KEL RBC exposure C3 appears to act as an innate immune switch, allowing KEL RBCs to induce IgG antibody formation in the absence of CD4 T cells. The ability of complement to regulate B cells in this way not only reflects a novel pathway of humoral immunity, but also has significant clinical consequences when considering factors that dictate alloantibody responses following transfusion. Not all patients generate alloantibodies following RBC exposure[35]. While several studies have linked HLA antigens to alloantibody responses[36,37], HLA preferences do not exist for all antigens and are certainly not required for alloantibody formation even when HLA associations have been observed. The ability of complement to regulate antibody formation in the absence of CD4 T cells at least suggests that in some patients, alloantibody formation could bypass CD4 T cell and therefore HLA requirements, providing possible insight into a key variable that may influence antibody formation following exposure to allogenic RBC transfusion.
Complement in regulating hyperhemolysis
While alloantibodies can certainly activate complement with attendant consequences, recent studies have highlighted the key role of the alternative complement activation pathway in various hemolytic processes[38–40]. Although pathophysiology and role of complement pathways in hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinuria are well known [41,42], mechanisms underlying complement dysregulation resulting in host tissue damage are increasingly recognized in other diseases such as sickle cell disease (SCD), autoimmune hemolytic anemia, and malaria [43–45]. Heme is now recognized as an important driver of complement activation in SCD, where chronic hemolytic anemia is associated with an increase in plasma free hemoglobin and free heme. Merle et al. reported that patients with SCD nephropathy had deposits of C3 and C5b-9 (MAC or terminal complement complex) in the glomeruli and vascular endothelium of their kidneys. They hypothesized that plasma free heme could activate complement. Phenylhydrazine-induced intravascular hemolysis model in mice resulted in the activation of alternative complement pathway (ACP) propagated by free heme, and also by microvesicles released by red blood cells (RBC) from patients with SCD [43]. Heme was previously shown to activate Toll-like receptor 4 (TLR4) on endothelial cells thus contributing to the pathophysiology of vasoocclusion and acute lung injury in sickle mice [46,47]. Recent data has shown that heme/TLR4 mediated P-selectin expression on endothelial cells facilitates the deposition of C3b and C3(H2O); the hydrolyzed form provides a positive feedback loop to the ACP [48]. In addition to the conventional thinking of ACP being ‘constitutively active,’ these data suggest that it may be additionally ‘activated’ by free heme during states of chronic and exaggerated hemolysis.
While patients with chronic hemolytic disorders such as RBC membrane, enzyme, or hemoglobin disorders can experience excessive hemolysis during periods of stress such as infections, or oxidant drugs, the contribution of ACP in hyperhemolysis has been best described in patients with SCD. Increased activation of ACP in SCD was first reported in 1985 by Chudwin et al., and subsequently by many others [49,50]. The role of complement in hyperhemolysis is only becoming increasingly clear, with improved knowledge, availability, and understanding of complement testing nuances. Acute and chronic hemolytic transfusion reactions (AHTR and DHTR) have served as prime models to explore the mechanistic and therapeutic strategies underlying hyperhemolysis[51]. Hyperhemolysis-mediated activation of ACP was reported in a pediatric patient with SCD, presenting with multiple episodes of DHTR. As described earlier in a pre-clinical model, this patient case further supported the role ACP could play in acute organ injury [52]. Complement inhibition at C5 by eculizumab has shown to be beneficial in mitigating hyperhemolysis and improving organ function in the setting of DHTR [52–54] and other SCD complications associated with hyperhemolysis [55]. The extent to which free heme and complement activation contribute to the chronic organ injury seen in patients with SCD remains unclear. While sickle RBCs, other plasma factors, inflamed endothelium, and other yet unknown genetic factors could further contribute to this pathogenesis, it is becoming clear that during episodes of acute exacerbation in hemolysis as seen during DHTR, acute chest syndrome, vasoocclusion, exuberant activation of ACP from free heme can result in further hemolysis and activation of the feedback loop of complement pathways, leading to increased morbidity and mortality.
Conclusions
Complement can regulate a wide variety of processes directly relevant to hematology, from the development and consequences of alloantibody development to hyperhemolysis in SCD. In each of these situations, complement can play a variety of distinct roles, which are likely dictated by the distinct types of complement receptors and activation pathways involved. Most importantly, a wide variety of complement inhibitors are increasingly becoming available, which provides significant promise to favorably manipulate these pathways as additional role of complement in hematological diseases become apparent.
Key Points.
Complement mediates antigen removal following incompatible RBC transfusion
Complement regulates alloantigen availability during alloantibody responses
Complement induces CD4 T cell-independent alloantibody formation
Complement enhances acute hemolysis in patients with sickle cell anemia
Acknowledgements:
We thank Dr. Connie Arthur for careful reading on the manuscript.
Financial support: NIH/NHLBI K12 award to SC and R01 HL135575 and P01 HL132819 awarded to SRS.
Footnotes
Conflicts of interest: None
REFERENCES
- 1.Immunobiology of the Complement System: An Introduction for Research and Clinical Medicine. Orlando, Florida: Academic Press, Inc.; 1986. [Google Scholar]
- 2.Morrison LF: On the Origin and Nature of Alexin (Complement) in Guinea-Pig Blood. The Journal of Immunology 1922. [Google Scholar]
- 3.Walport MJ: Complement. Second of two parts. N Engl J Med 2001, 344:1140–1144. [DOI] [PubMed] [Google Scholar]
- 4.Walport MJ: Complement. First of two parts. N Engl J Med 2001, 344:1058–1066. [DOI] [PubMed] [Google Scholar]
- 5.Reis ES, Mastellos DC, Hajishengallis G, Lambris JD: New insights into the immune functions of complement. Nat Rev Immunol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambris JD: The multifunctional role of C3, the third component of complement. Immunol Today 1988, 9:387–393. [DOI] [PubMed] [Google Scholar]
- 7.Kang YS, Do Y, Lee HK, et al. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell 2006, 125:47–58. [DOI] [PubMed] [Google Scholar]
- 8.Nordahl EA, Rydengard V, Nyberg P, et al. Activation of the complement system generates antibacterial peptides. Proc Natl Acad Sci U S A 2004, 101:16879–16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller-Eberhard HJ: The membrane attack complex of complement. Annu Rev Immunol 1986, 4:503–528. [DOI] [PubMed] [Google Scholar]
- 10.Ricklin D, Mastellos DC, Lambris JD: Therapeutic targeting of the complement system. Nat Rev Drug Discov 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stowell SR, Winkler AM, Maier CL, et al. Initiation and regulation of complement during hemolytic transfusion reactions. Clin Dev Immunol 2012, 2012:307093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DD, Song WC: Membrane complement regulatory proteins. Clin Immunol 2006, 118:127–136. [DOI] [PubMed] [Google Scholar]
- 13.Girard-Pierce KR, Stowell SR, et al. A novel role for C3 in antibody-induced red blood cell clearance and antigen modulation. Blood 2013, 122:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stowell SR, Liepkalns JS, Hendrickson JE, et al. Antigen modulation confers protection to red blood cells from antibody through Fcgamma receptor ligation. J Immunol 2013, 191:5013–5025. [DOI] [PubMed] [Google Scholar]
- 15.Zimring JC, Cadwell CM, Chadwick TE, et al. Nonhemolytic antigen loss from red blood cells requires cooperative binding of multiple antibodies recognizing different epitopes. Blood 2007, 110:2201–2208. [DOI] [PubMed] [Google Scholar]
- 16.Zimring JC, Hair GA, Chadwick TE, et al. Nonhemolytic antibody-induced loss of erythrocyte surface antigen. Blood 2005, 106:1105–1112. [DOI] [PubMed] [Google Scholar]
- 17.Mener A, Patel SR, Arthur CM, Stowell SR Antibody-mediated immunosuppression can result from RBC antigen loss independent of Fcgamma receptors in mice. Transfusion 2019, 59:371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowell SR, Arthur CM, Girard-Pierce KR et al. Anti-KEL sera prevents alloimmunization to transfused KEL RBCs in a murine model. Haematologica 2015, 100:e394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier CL, Mener A, Patel SR, et al. Antibody-mediated immune suppression by antigen modulation is antigen-specific. Blood Adv 2018, 2:2986–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mener A, Arthur CM, Patel SR, et al. Complement Component 3 Negatively Regulates Antibody Response by Modulation of Red Blood Cell Antigen. Front Immunol 2018, 9:676. *This study demosntrates that antibody engagement and complement fixation during a developing immune response can reduce alloantigen availability, possibly influencing the magnitude of the ongoing immune response.
- 21.Liu J, Santhanakrishnan M, Natarajan P, et al. Antigen modulation as a potential mechanism of anti-KEL immunoprophylaxis in mice. Blood 2016, 128:31593168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan HC, Arthur CM, Thompson L, et al. Anti-RhD reduces levels of detectable RhD antigen following anti-RhD infusion. Transfusion 2018, 58:542–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan HC, Gerner-Smidt C, Nooka AK, et al. Daratumumab (anti-CD38) induces loss of CD38 on red blood cells. Blood 2017, 129:3033–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepys MB: Role of complement in induction of the allergic response. Nature 1972. [DOI] [PubMed] [Google Scholar]
- 25.Ahearn JM, Fischer MB, Croix D, et al. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity 1996, 4:251–262. [DOI] [PubMed] [Google Scholar]
- 26.Molina H, Holers VM, Li B, et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proceedings of the National Academy of Sciences of the United States of America 1996, 93:3357–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter RH, Spycher MO, Ng YC, et al. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. Journal of immunology (Baltimore, Md. : 1950) 1988, 141:457–463. [PubMed] [Google Scholar]
- 28.Fearon DT, Carter RH: The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annual review of immunology 1995, 13:127–149. [DOI] [PubMed] [Google Scholar]
- 29.Patel SR, Gibb DR, Girard-Pierce K, et al. Marginal Zone B Cells Induce Alloantibody Formation Following RBC Transfusion. Front Immunol 2018, 9:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur CM, Patel SR, Smith NH, et al. Antigen Density Dictates Immune Responsiveness following Red Blood Cell Transfusion. J Immunol 2017, 198:2671–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayes J, Ing M, Delignat S, et al. Complement C3 is a novel modulator of the anti-factor VIII immune response. Haematologica 2018, 103:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zerra PE, Arthur CM, Chonat S, et al. Fc Gamma Receptors and Complement Component 3 Facilitate Anti-fVIII Antibody Formation. Front Immunol 2020, 11:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel SR, Bennett A, Girard-Pierce K, et al. Recipient priming to one RBC alloantigen directly enhances subsequent alloimmunization in mice. Blood Adv 2018, 2:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mener A, Patel SR, Arthur CM, et al. Complement serves as a switch between CD4+ T cell-independent and -dependent RBC antibody responses. JCI Insight 2018, 3. *This study describes the ability of complement to induce CD4 T cell-independent alloantibody fomation following RBC transfusion.
- 35.Tormey CA, Hendrickson JE: Transfusion-related red blood cell alloantibodies: induction and consequences. Blood 2019, 133:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiaroni J, Dettori I, Ferrera V, et al. HLA-DRB1 polymorphism is associated with Kell immunisation. Br J Haematol 2006, 132:374–378. [DOI] [PubMed] [Google Scholar]
- 37.Noizat-Pirenne F, Tournamille C, Bierling P, et al. Relative immunogenicity of Fya and K antigens in a Caucasian population, based on HLA class II restriction analysis. Transfusion 2006, 46:1328–1333. [DOI] [PubMed] [Google Scholar]
- 38.Thein SL, Pirenne F, Fasano RM, et al. Hemolytic transfusion reactions in sickle cell disease: underappreciated and potentially fatal. Haematologica 2020, 105:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou ST, Alsawas M, Fasano RM, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv 2020, 4:327–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arthur CM, Chonat S, Fasano R, et al. Examining the Role of Complement in Predicting, Preventing, and Treating Hemolytic Transfusion Reactions. Transfus Med Rev 2019, 33:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill A, DeZern AE, Kinoshita T, Brodsky RA: Paroxysmal nocturnal haemoglobinuria. Nature Publishing Group 2017, 3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C: Haemolytic uraemic syndrome. The Lancet 2017, 390:1–16. [DOI] [PubMed] [Google Scholar]
- 43. Merle NS, Grunenwald A, Rajaratnam H, et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 2018, 3:1–18. *This study highlights the ability of heme released secondary to invascular hemolysis to activate the alternative complement pathway.
- 44.Berentsen S, Randen U, Tjonnfjord GE: Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am 2015, 29:455–471. [DOI] [PubMed] [Google Scholar]
- 45.Biryukov S, Stoute JA: Complement activation in malaria: friend or foe? Trends Mol Med 2014, 20:293–301. [DOI] [PubMed] [Google Scholar]
- 46.Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014, 123:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh S, Adisa OA, Chappa P, et al. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. The Journal of Clinical Investigation 2013, 123:4809–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merle NS, Paule R, Leon J, et al. P-selectin drives complement attack on endothelium during intravascular hemolysis in TLR-4/heme-dependent manner. Proceedings of the National Academy of Sciences 2019, 89:201814797–201814796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chudwin DS, Korenblit AD, Kingzette M, et al. Increased activation of the alternative complement pathway in sickle cell disease. Clinical immunology and immunopathology 1985, 37:93–97. [DOI] [PubMed] [Google Scholar]
- 50.Test ST, Woolworth VS: Defective regulation of complement by the sickle erythrocyte: evidence for a defect in control of membrane attack complex formation. Blood 1994, 83:842–852. [PubMed] [Google Scholar]
- 51.Vidler JB, Gardner K, Amenyah K, et al. Delayed haemolytic transfusion reaction in adults with sickle cell disease: a 5-year experience. Br J Haematol 2015, 169:746–753. [DOI] [PubMed] [Google Scholar]
- 52. Chonat S, Quarmyne M-O, Bennett CM, et al. Contribution of alternative complement pathway to delayed hemolytic transfusion reaction in sickle cell disease. Haematologica 2018:haematol.2018.194670–194614. *This study provides important correlative data suggesting that acute hemolytic episodes in patients with sickle cell anemia are marked by activation of the alternative complement pathway
- 53.Dumas G, Habibi A, Onimus T, et al. Eculizumab salvage therapy for delayed hemolysis transfusion reaction in sickle cell disease patients. Blood 2016, 127:1062–1064. [DOI] [PubMed] [Google Scholar]
- 54. Floch A, Morel A, Zanchetta-Balint F, et al. Anti-C5 antibody treatment for delayed hemolytic transfusion reactions in sickle cell disease. Haematologica 2020. **This study illustrates, in a large cohort of patients, the potential utility of complement inhibition in the treatment of patients experiencing delayed type transfusion reactions.
- 55.Chonat S, Chandrakasan S, Kalinyak KA, et al. Atypical haemolytic uraemic syndrome in a patient with sickle cell disease, successfully treated with eculizumab. British Journal of Haematology 2016, 175:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]