Abstract
Objective
This study aims to explore the factors influencing the renal glucose threshold (RTG) in patients with newly diagnosed type 2 diabetes mellitus (T2DM).
Methods
A cross-sectional study was conducted on 1009 hospitalized patients with T2DM using stratified random sampling. Blood glucose was monitored using a dynamic blood glucose monitor to obtain the mean blood glucose (MBG), which is used to calculate the RTG. The factors influencing the RTG were then analyzed.
Results
The mean RTG in patients with newly diagnosed T2DM was 203.58 ± 55.22 mg/dl. The correlation between the RTG and the various variables was analyzed, and the results demonstrated that the RTG was correlated with the patient’s age (r = −0.14539, P = 0.0001); MBG (r = −0.35009, P = 0.0001); renal long neck (r = 0.16762, P = 0.0001); homeostatic model assessment for insulin resistance (r = −0.38322, P = 0.0001); homeostatic model assessment for beta-cell function (r = −0.22770, P = 0.0001); and the levels of glycated hemoglobin (HbA1c; r = 0.98994, P = 0.0001), blood urea nitrogen (r = −0.11093, P = 0.0004), creatinine (r = −0.26414, P = 0.0001), uric acid (r = −0.20149, P = 0.0001), total cholesterol (r = 0.13192, P = 0.0001), low-density lipoprotein (r = 0.12466, P = 0.0001), thyroid-stimulating hormone (r = −0.06346, P = 0.0460), beta-2 microglobulin (r = −0.08884, P = 0.0056), and 24-hour urine glucose (r = 0.32115, P = 0.0001). Multiple linear stepwise regression analysis revealed that the HbA1c, 24-hour urine glucose, estimated glomerular filtration rate (eGFR), D-dimer, and body mass index (BMI) should be included in the final model, and HbA1c had the greatest impact on the RTG followed in descending order by the 24-hour urine glucose, eGFR, D-dimer, and BMI (P < 0.05).
Conclusion
The RTG increases in most patients with newly diagnosed diabetes. The risk factors for the RTG are HbA1c, 24-hour urine glucose, eGFR, D-dimer, and BMI.
Keywords: newly diagnosed, type 2 diabetes mellitus, renal glucose threshold, continuous glucose monitoring
Introduction
The kidney plays an important role in the regulation of energy metabolism in type 2 diabetes mellitus (T2DM), and its regulatory effects on glucose metabolism include gluconeogenesis, glucose utilization, glomerular filtration, and proximal convoluted tubule reabsorption.1 The renal glucose threshold (RTG) refers to the plasma glucose concentration at which glucose begins to appear in the urine. The physiological relationship between the plasma glucose concentration and the maximum renal glucose reabsorption is a threshold relationship, but in practice, the RTG is variable. A study revealed that the RTG in patients with T2DM is related to age, duration of the disease, and the body mass index (BMI).2 But few studies have been conducted on the factors influencing the RTG in patients with newly diagnosed T2DM. The results from an Italian study showed the clinical usefulness of Glycated Albumin for the diagnosis of diabetes in Caucasian subjects at risk for diabetes.3 This study discusses the relevant contents to provide a more specific theoretical basis and medication guidance for clinical practice.
Subjects and Methods
Subjects
The stratified random sampling method was used to enroll 1009 patients with newly diagnosed T2DM who were admitted to the Tianjin First Central Hospital from January 2019 to April 2021, including 530 male and 479 female patients, with an average age of 58.08 ± 8.80 y. Exclusion criteria: Patients with diabetic ketoacidosis, nonketotic hyperosmolar coma, severe infection (especially urinary tract infection), acute cardiovascular or cerebrovascular accident, severe liver or kidney insufficiency, severe allergic reaction, drugs used to influence sugar metabolism, immunological diseases, pregnancy, acute complications of diabetes mellitus at admission to hospital, clinical evidence of renal injury (estimated glomerular filtration rate <90 mL/min, urinary microalbumin-creatinine ratio >30 mg/g, abnormal pathological results or abnormal biochemical components of urine, or abnormal imaging findings), anemia (diagnostic criterion for males is hemoglobin [Hb] < 120 g/L and females is Hb < 110 g/L); patients taking vitamin C, aspirin, and other drugs affecting urine sugar in the preceding month; and patients with a history of significant blood loss or receiving a blood transfusion within the preceding three months. This study was conducted with approval from the Ethics Committee of Tianjin First Central Hospital (No:2018N160KY), and all patients provided signed informed consent.
Research Methods
(1) Test index: The patient’s height, body mass, waist circumference, and hip circumference were measured, and the BMI, waist-to-hip ratio (WHR) and waist-to-height ratio (WtHR) were calculated. At rest, brachial artery pressure (BP) was measured in the sitting position. Blood urea nitrogen (BUN), blood creatinine, blood uric acid, triglyceride, total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), total protein, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were detected using the automatic biochemical analyzer. Glycated hemoglobin (HbA1c) was detected by high performance liquid chromatography, and the 24-hour urine glucose was detected using the Roche analyzer: 24hUGE (mg/mL) = 24-hour urine glucose (g) × 1000 ÷ 24×60. Radioimmunoassay was performed to determine the 24-hour urinary microalbumin (24hUMA). The oral glucose tolerance test (OGTT) and insulin release test: The subjects were fasted overnight for 8–12 hours and fasting blood samples were taken. Exactly 75 g of anhydrous glucose was dissolved in 250 mL of water, the subject drank the mixture within five minutes, and blood samples were taken at 30, 60, 120, and 180 min to measure blood glucose and C-peptide (CP) levels. The islet β-cell Phase I secretion function was evaluated using the modified insulin CP secretion function index: homeostatic model assessment (HOMA)-CP = 0.27 × CP 0 min ÷ (PG 0 min − 3.5) + 50, and the HOMA-insulin resistance (IR) index = (FPG × FIns) ÷ 22.5.
(2) Diagnostic criteria for obesity: normal weight is a BMI ≥ 18.5 and <24.0 kg/m2, overweight is a BMI ≥ 24 and <28.0 kg/m2, and obese is a BMI ≥ 28.0 kg/m2.
(3) The glomerular filtration rate (eGFR) was calculated using the CKD-EPI creatinine formula published in 2009:4
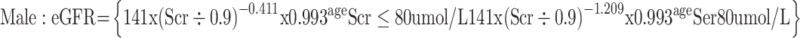 |
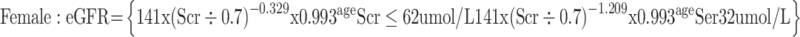 |
(4) The mean blood glucose: After admission, all patients wore a Medtronic continuous glucose monitoring system for dynamic blood glucose monitoring. The mean blood glucose (MBG) was calculated according to the area under the blood glucose curve.
(5) The RTG: A mathematical model for the clinical calculation of the RTG has been developed:2
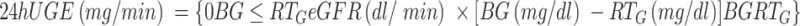 |
Statistical Methods
The data were analyzed using IBM SPSS 25.0. In the statistical description, the categorical data were expressed as frequencies (percentages), and the measurement data were expressed as mean ± standard deviation or median and interquartile range. The independent sample t-test was used to compare normally distributed count data between two groups, and the chi-squared test was used to compare categorical data. The RTG was grouped based on the quantile, and the baseline RTG was compared. Since the RTG was not normally distributed, it was logarithmically converted and then analyzed. The factors influencing the RTG were analyzed using multiple linear stepwise regression.
Results
General Statistics
The average age of the patients in the present study was 55.58 ± 12.23 y, RTG was 203.58 ± 55.22 mg/dl, and eGFR was 94.40 ± 24.02 mL/min/1.73m2. The RTG was grouped using the quartile method, and the general data were compared using the t-test (rank-sum test) and chi-squared test between the groups. As shown in Table 1, the results revealed that there was no significant difference in gender, smoking history, drinking history, WHR, WtHR, systolic BP, HOMA-β, D-Dimer, γ-glutamyl transpeptidase, BUN, HDLC, RTG, thyroid-stimulating hormone (TSH), thyroid peroxidase antibody, beta-2 microglobulin (β2-MG), and 24hUMA between the groups. As RTG increased, there were significant differences in age, the course of disease, BMI, diastolic BP, MBG, renal long neck, HOMA-IR, HbA1c, ALT, AST, Cr, UA, TC, low-density lipoprotein (LDLC), 24-hour urinary glucose, and 24-hour uric acid between the groups (P < 0.05; see Table 1).
Table 1.
Comparison of General Data of Patients with Different Renal Glucose Threshold Groups
| Index | Renal Glucose Threshold Grouping | |||
|---|---|---|---|---|
| <161.54 (N=724) | 161.54–195.13 195.1311221195.131 (N=485) |
195.13–236.48 | >236.48 | |
| Age* | 56.71±11.63 | 57.58±10.94 | 54.02±12.67 | 53.1±13.49 |
| Gender | ||||
| Male | 284 | 156 | 153 | 137 |
| Female | 172 | 95 | 98 | 114 |
| Smoking history | ||||
| Yes | 156 | 78 | 82 | 95 |
| No | 206 | 99 | 103 | 119 |
| Drinking history | ||||
| Yes | 219 | 108 | 122 | 138 |
| No | 148 | 79 | 68 | 83 |
| BMI* | 26.37±4.04 | 27.06±4.07 | 27.25±4.37 | 26.63±4.7 |
| Waist-to-hip ratio | 0.95±0.08 | 0.95±0.06 | 0.95±0.06 | 0.95±0.07 |
| Waist-to-height ratio | 0.56±0.06 | 0.57±0.06 | 0.57±0.06 | 0.57±0.07 |
| Systolic BP | 133.91±16.95 | 136.03±17.18 | 136.16±18.13 | 133.13±18.88 |
| Diastolic BP* | 80.02±10.57 | 81.6±11.03 | 82.12±10.63 | 80.68±11.64 |
| Mean blood glucose* | 9.36±2.16 | 10.09±2.89 | 10.64±2.54 | 11.1±3.21 |
| Renal long neck* | 10.51±0.81 | 10.49±0.76 | 10.67±0.87 | 10.75±0.88 |
| HOMA-IR* | 200.64±855.06 | 75.33±60.1 | 124.15±409.52 | 73.28±227.94 |
| HOMA-β | 6.82±20.9 | 4.71±5.58 | 7.53±25.06 | 4.03±9.53 |
| HbA1c* | 7.51±2.06 | 7.67±0.44 | 8.97±0.5 | 11.24±1.48 |
| D-Dimer | 0.46±1.29 | 0.42±1.1 | 0.38±1.15 | 0.35±0.37 |
| ALT* | 24.56±17.74 | 24.61±18.59 | 28.06±23.45 | 27.27±27.06 |
| AST* | 22.03±13.64 | 20.95±10.13 | 23.89±18.15 | 22.56±15.44 |
| GGT | 40.75±49.84 | 36.5±39.43 | 45.08±66.63 | 37.99±37.79 |
| BUN | 7.61±15.41 | 6.19±2.32 | 6.63±8.87 | 6.26±8.36 |
| Cr* | 89.47±79.31 | 76.63±35.84 | 72.72±30.82 | 67.36±37.02 |
| UA* | 354.43±100.11 | 332.37±91.37 | 325.98±93.01 | 311.71±94.14 |
| TC* | 5.12±1.36 | 5.02±1.22 | 5.2±1.19 | 5.56±1.61 |
| HDL | 1.16±0.4 | 1.16±0.42 | 1.13±0.27 | 1.23±0.6 |
| LDL* | 3.23±0.96 | 3.22±0.93 | 3.34±0.97 | 3.54±1.11 |
| TG | 2.35±2.39 | 2.24±1.7 | 2.49±2.25 | 2.57±2.67 |
| TSH | 5.97±24.09 | 4.91±15.46 | 4.19±12.06 | 3.41±8.41 |
| TPO-Ab | 25.31±62.3 | 36.75±95.71 | 43.75±103.1 | 28.45±72.13 |
| β2-MG | 0.62±1.78 | 0.6±2 | 0.37±1.17 | 0.64±1.78 |
| 24hUMA | 70.79±111.33 | 75.92±134.98 | 78.61±108.38 | 63.33±106.63 |
| 24-hour urine glucose* | 4.44±9.90 | 6.45±9.89 | 10.92±12.34 | 11.58±14.82 |
| 24-hour uric acid* | 587.46±240.17 | 593.41±232.77 | 682.07±528.09 | 586.9±243.32 |
Note: *It means that the difference between RTG groups under this variable grouping is statistically significant (*P<0.05).
Correlation Analysis Between the Variables
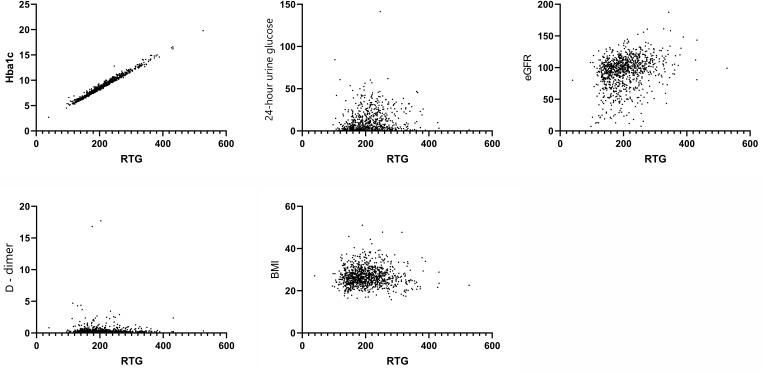
The correlation between the RTG and the other variables was analyzed, and the results demonstrated that the RTG was correlated with age (r = −0.14539, P = 0.0001), MBG (r = −0.35009, P = 0.0001), renal long neck (r = 0.16762, P = 0.0001), HOMA-IR (r = −0.38322, P = 0.0001), HOMA-β (r = 0.22770, P = 0.0001), HbA1c (r = 0.98994, P = 0.0001), BUN (r = −0.11093, P = 0.0004), Cr (r = −0.26414, P = 0.0001), UA (r = −0.20149, P = 0.0001), TC (r = 0.13192, P = 0.0001), LDLC (r = 0.12466, P = 0.0001), TSH (r = −0.06346, P = 0.0460), β2-MG (r = −0.08884, P = 0.0056), and 24-hour urine glucose (r = 0.32115, P = 0.0001; see Figure 1).
Figure 1.
The correlation between the RTG and HbA1c, 24-Hour urine glucose, eGFR, D-Dimer, and BMI.
Multiple Linear Stepwise Regression Analysis
Because the RTG was not normally distributed, it was first logarithmically converted and then the corresponding analysis was conducted. With RTG as a dependent variable and each biochemical index as an independent variable, the factors influencing the RTG were analyzed using multiple linear stepwise regression analysis. The results revealed that HbA1c, 24-hour urine glucose, eGFR, D-dimer, and BMI should be included in the final model (P < 0.05). HbA1c had the greatest impact on the RTG followed in descending order by 24-hour urine glucose, eGFR, D-dimer, and BMI (see Table 2).
Table 2.
Multiple Linear Stepwise Regression Analysis of Influencing Factors of RTG
| Model Coefficient | Non Standardized Coefficient | Standardization Coefficient β | t | Significance | |
|---|---|---|---|---|---|
| β | Standard Error | ||||
| Intercept term | 4.082 | 0.025 | 164.327 | 0.000 | |
| HbA1c | 0.129 | 0.001 | 1.021 | 88.474 | 0.000 |
| 24-hour urine glucose | −0.003 | 0.000 | −0.143 | −12.237 | 0.000 |
| eGFR | 0.001 | 0.000 | 0.056 | 4.661 | 0.000 |
| D-Dimer | −0.033 | 0.010 | −0.036 | −3.192 | 0.002 |
| BMI | 0.011 | 0.004 | 0.031 | 2.805 | 0.005 |
Discussion
T2DM is a chronic disease with an imbalance of energy homeostasis in the body, and multiple organs are involved in its pathogenesis including the pancreas, liver, fat, intestines, muscle tissue, and kidneys. The regulatory effects of the kidney on glucose metabolism include gluconeogenesis, glucose utilization, glomerular filtration, and proximal convoluted tubule reabsorption.1
In healthy individuals, the kidneys filter 160–180 g of glucose per day, and the glucose concentration in the ultrafiltration of the renal capsule is equal to that in the plasma, but under normal circumstances, there is almost no glucose in the urine,2 indicating that all the glucose has been reabsorbed. A micro-puncture experiment verified that the filtered glucose was reabsorbed in the proximal tubule, especially in the first half of the proximal tubule. There is a mechanism of SGLT on the apical membrane of the proximal tubular epithelial cells, and sodium and glucose in the tubular fluid are combined with transporters and transferred into cells, which is the secondary active transport. The glucose entering the cells is transported by the glucose transporter 2 on the basolateral membrane, such as the intercellular space, and SGLT2 located on the lumen side cell membrane of the proximal renal tubule is responsible for the reabsorption of 90% of the glucose in the renal tubules. To a certain extent, the reabsorption of glucose by the proximal tubules is limited, and when the blood glucose concentration reaches 180 mg/100 mL, some renal tubules reach the limit of glucose absorption and glucose begins to appear in urine. The plasma glucose concentration above measurable glycosuria occurred is called the RTg. The physiological relationship between the plasma glucose concentration and the maximum renal glucose reabsorption is a threshold relationship, but the RTG of each nephron is not identical. When the blood glucose concentration continues to increase, the glucose concentration in the urine also increases, and when the blood glucose concentration increases to 300 mg/100 mL, the reabsorption of glucose by all the renal tubules has reached or exceeded the maximum transport rate of glucose by the proximal tubules. At that time, the amount of glucose filtered per minute reaches the glucose reabsorption limit of both kidneys, and the urinary glucose excretion rate increases parallelly with the increase in the blood glucose concentration. The average limit of glucose reabsorption for healthy people in both kidneys is 375 mg/min for men and 300 mg/min for women.
However, clinically, differences have been found in urine glucose concentration at the same blood glucose level. One study4 showed that the RTG in T2DM was related to age, the course of disease, and the BMI, but the factors influencing the RTG in patients with newly diagnosed diabetes are still unclear. The ability of the renal tubules to change glucose reabsorption by regulating the expression of the SGLT2 changes dynamically with blood glucose levels. One study5 revealed that in diabetic animal models, the expression of the SGLT2s was upregulated, resulting in increased renal reabsorption of glucose, and causing malignant hyperglycemia. Previous studies revealed that in patients with early diabetic nephropathy, the expression of SGLT2 in the proximal renal tubular epithelial cells was upregulated, resulting in increased sodium reabsorption and reducing sodium ions passing through dense spots in the tubulospheric feedback mechanism. This resulted in high perfusion, high pressure, and high filtration of the glomerulus, with a compensatory hypertrophic hyperplasia of the renal tubular epithelial cells in diabetic patients, causing a significant increase in the expression of SGLT2, which increased the reabsorption of glucose and reduced the excretion of urinary glucose, thereby increasing the RTG.
The step-by-step hyperglycemic clamp procedure was performed to accurately measure the renal glucose excretion threshold.6 However, due to its complex operation, it is not suitable for widespread clinical application. Dynamic blood glucose monitoring can continuously and comprehensively reflect the average blood glucose of patients in real-time and can be used as the gold standard for calculating the RTG in clinical settings. In this study, dynamic blood glucose monitoring was performed to calculate the MBG of patients, which was used to calculate the RTG. A total of 128 patients with T2DM were included in the study conducted by Yue et al4 in which the mean RTG was 10.8 ± 1.2 mmol/L, and patients with increased RTG accounted for 58.33%. A multicenter study conducted in the United States, Germany, and South Korea demonstrated that the average 24-hour RTG of 116 patients with T2DM was 13.7 ± 1.7 mmol/L.7 The above studies showed that the RTG in patients with T2DM is generally elevated, but racial and regional differences were found. However, the sample size in the present study was small, and multicenter large sample studies are needed to confirm whether the RTG has racial and regional differences. This study revealed that the mean RTG in patients with newly diagnosed T2DM was 203.58 ± 55.22 mg/dl, which is higher than that of healthy people, suggesting that the increase in the RTG occurred in the early stage of diabetes.
By using the high glucose clamp test, Rave et al8 confirmed that the urinary glucose excretion rate of the kidney is directly proportional to the blood glucose level. A prediabetes study revealed that as time passed during the OGTT, the UGE increased with an increase in blood glucose.9 This study revealed that the RTG was positively correlated with HbA1c, and the RTG increased with an increase in eGFR and D-dimer in patients with newly diagnosed diabetes. An increase in D-dimer can promote platelet adhesion and aggregation; directly damage vascular endothelial cells; and deposit and form microcrystals in the renal capillary wall, resulting in renal microvascular stenosis or even occlusion. To a certain extent, the level of D-dimer reflects the extent of the renal injury, which suggests that D-dimer can be used as an indicator for predicting the risk of diabetic nephropathy and assisting in the early diagnosis of diabetic nephropathy.
Increasing BMI is also a risk factor for increasing RTG. Obesity aggravates the disorder of glucose and lipid metabolism, increases insulin resistance, and leads to an increase in blood glucose.10 In addition, obesity will increase the glomerular filtration rate and can directly increase renal tubular reabsorption, which may lead to an increased RTG in obese patients with diabetes.11 This study revealed that there was no correlation between the RTG and islet function in patients with newly diagnosed diabetes. But a previous study10 revealed that in prediabetes, the UGE was negatively correlated with islet function, and it is speculated that the determination of UGE (≥ 150 mg/min) in an OGTT can be used as a clinical index for the early detection of islet function injury.
In summary, this study revealed that the RTG increased in most patients with newly diagnosed diabetes suggests beneficial anti-hyperglycemic effects of SGLT2 at this stage. The risk factors for the RTG were HbA1c, 24-hour urine glucose, eGFR, D-dimer, and BMI. However, large sample multicenter cross-sectional studies are still needed to confirm the influencing factors for the RTG in the future and to provide clinical guidance for the treatment of diabetes mellitus.
Funding Statement
National Natural Science Foundation of China (NO. 82000763); Spring project of Tianjin First Central Hospital (NO.2019CF20); Spring project of Tianjin First Central Hospital (NO.CF201824).
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Tianjin First Central Hospital (No:2018N160KY). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for Publication
All participants signed a document of informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl. 2011;79:S1–S6. doi: 10.1038/ki.2010.509 [DOI] [PubMed] [Google Scholar]
- 2.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–142. doi: 10.1111/j.1464-5491.2009.02894.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellia C, Zaninotto M, Cosma C. Clinical usefulness of glycated albumin in the diagnosis of diabetes: results from an Italian study. Clin Biochem. 2018;54:68–72. doi: 10.1016/j.clinbiochem.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 4.Yue XD, Wang JY, Zhang XR, et al. Characteristics and impact factors of renal threshold for glucose excretion in patients with type 2 diabetes mellitus. J Korean Med Sci. 2017;32(4):621–627. doi: 10.3346/jkms.2017.32.4.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallon V, Gerasimova M, Rose MA, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306(2):F194–204. doi: 10.1152/ajprenal.00520.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36(10):3169–3176. doi: 10.2337/dc13-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sha S, Devineni D, Ghosh A, et al. Pharmacodynamic effects of canagliflozin, a sodium glucose co transporter 2 inhibitor, from a randomized study in patients with type 2 diabetes. PLoS One. 2014;9(9):e110069. doi: 10.1371/journal.pone.0105638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rave K, Nosek L, Posner J, et al. Renal glucose excretion as a function of blood glucose concentration in subjects with type 2 diabetes results of a hyperglycaemic glucose clamp study. Nephrol Dial Transplant. 2006;21(8):2166–2171. doi: 10.1093/ndt/gfl175 [DOI] [PubMed] [Google Scholar]
- 9.Ono Y, Ono S, Hinata T, et al. Usefulness of urinary glucose excretion after oral glucose tolerance testing to detect insulin secretion failure before the onset of diabetes mellitus. Endocr J. 2017;64(1):75–81. doi: 10.1507/endocrj.EJ16-0289 [DOI] [PubMed] [Google Scholar]
- 10.Zameni F, Bakhtiyari M, Mansournia MA, Ramezankhani A, Azizi F, Hadaegh F. Is incident type 2 diabetes associated with cumulative excess weight and abdominal adiposity? Tehran Lipid and Glucose Study. Diabetes Res Clin Pract. 2018;136:134–142. doi: 10.1016/j.diabres.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 11.D’Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–471. [DOI] [PubMed] [Google Scholar]