Abstract
Background
The antimicrobial activities of some new oxazolidinones against slowly growing mycobacteria (SGM) have never been well evaluated.
Methods
We evaluate the in vitro susceptibility of 20 reference strains and 157 clinical isolates, pertaining different SGM species, against four oxazolidinones, ie, delpazolid, sutezolid, tedizolid and linezolid. In addition, the association of linezolid resistance and mutations in 23srRNA, rplC, rplD were also tested.
Results
Sutezolid presented the strongest antimicrobial activity against the clinical isolates of M. intracellulare than the other oxazolidinones, with MIC50 at 2 μg/mL and MIC90 at 4 μg/mL. MICs of sutezolid were usually 4- to 8-fold lower than these of linezolid against M. intracellulare and M. avium. The tested isolates of M. kansasii were susceptible to all of the four oxazolidinones. According to the multiple sequence alignment, novel 23srRNA mutations (A2267C and A2266G) in M. intracellulare and rplD mutations (Thr147Ala) in M. avium were identified in this study which have plausible involvement in rendering resistance against linezolid.
Conclusion
This study showed that sutezolid harbors the strongest inhibitory activity against M. intracellulare, M. avium and M. kansasii in vitro, which provided important insights on the potential clinical application of oxazolidinones for treating SGM infections.
Keywords: slowly growing mycobacteria, delpazolid, sutezolid, tedizolid, linezolid, antimicrobial activity
Introduction
Non-tuberculous mycobacteria (NTM) are opportunistic pathogens which are often associated with pulmonary infections, skin abscess, lymphadenitis, or disseminated infection. The prevalence of NTM infections has increased globally and has even surpassed tuberculosis (TB) in certain countries.1–4 NTM are broadly categorized as rapidly growing mycobacteria (RGM) or slowly growing mycobacteria (SGM), depending on their speed of growth. Among the SGM species, the Mycobacterium avium complex (MAC) is generally considered pathogenic and is mainly composed of M. avium and M. intracellulare. In China, M. intracellulare, M. avium and M. kansasii are the three highly prevalent SGM species.5 Except rifampicin and fluoroquinolone, SGM are generally resistant to other commonly used anti-TB drugs. Therefore, limited drug choice and prolonged treatment course for SGM infections underline the requirement to identify novel and potent antimicrobials reagents.
Linezolid (LZD), the first licensed oxazolidinone, manifests excellent antibacterial activities against the drug-resistant tuberculosis (DR-TB) and NTM infections.6–8 However, extended treatment with LZD contributes to high frequency of severe side effects which emphasizes the necessity of reconstructing the drug while keeping the potency and decreasing the potential side effects. Recently, three new next-generation oxazolidinones have been developed. Tedizolid (TZD) phosphate is a novel, potent oxazolidinone pro-drug with a broad range of activities against Gram-positive organisms, including mycobacteria.9 In contrast to LZD, longer half-life of TZD allows it to be administered orally once daily, facilitating its usage in prolonged treatment course. Current data indicates that TZD offers better tolerance and safety profile of long-term therapeutic regimes in mycobacterium infections.10 Sutezolid (SZD) (PNU-100480) is a thiomorpholinyl analog of LZD that showed superior efficacy against M. tuberculosis in a preliminary study.11 SZD does not appear to cause bone marrow suppression or prolongation of QT-interval when compared with LZD, although there are still concerns regarding the potential neurotoxicity and hepatotoxicity.12 Delpazolid (DZD) (LCB01-0371) has demonstrated broad-spectrum antimicrobial activities against Gram-positive pathogens in vitro and in animal infection model.13 DZD are currently being studied in Phase I clinical trials, its safety profile will have to be determined in future during the Phase III studies.14
To better understand the inhibitory activities of these three new-generation oxazolidinones against different SGM species, we determined the MICs of 20 SGM reference strains and 157 SGM clinical isolates collected in Beijing, China. Additionally, we investigated the reported LZD-resistance genes (including rplC, rplD and 23srRNA) in different SGM species to identify their potential relationships with oxazolidinone resistance.
Materials and Methods
Ethics Statement
As the study only concerned laboratory testing of mycobacteria without the direct involvement of human subjects, ethical approval was not sought.
Reference Strains and Clinical Isolates
The mycobacterial reference strains stored in the Bio-bank in Beijing Chest Hospital (Beijing, China) were used and their susceptibility to LZD, TZD, SZD and DZD in vitro was investigated. In addition, 20 SGM reference species were also used in the susceptibility test. These reference strains (Table 1) were obtained either from the American Type Culture Collection (ATCC) or from the German Collection of Microorganisms (DSM). One hundred and fifty-seven isolates of SGM were recruited in Beijing chest hospital from 2016 to 2019 that included 48 M. intracelluare, 41 M. avium, 42 M. kansasii. The species constitution of the remaining 26 isolates is presented in Supplementary Table S1.
Table 1.
MICs of LZD, TZD, SZD and DZD Against the Reference Strains of 20 SGM Species
| Strain by Type | Mycobacterium Species (Strain) | MIC (µg/mL) | |||
|---|---|---|---|---|---|
| LZD | TZD | SZD | DZD | ||
| ATCC 25276 | Mycobacterium asiaticum | 2 | 0.5 | 0.25 | 2 |
| ATCC 25291 | Mycobacterium avium | 1 | 0.5 | 0.125 | 1 |
| ATCC 51131 | Mycobacterium celatum | 8 | 2 | 0.5 | 4 |
| DSM44623 | Mycobacterium chimaera | 1 | 0.063 | 0.063 | 0.5 |
| ATCC 14470 | Mycobacterium gordonae | 0.5 | 0.125 | 0.25 | 0.5 |
| ATCC 13950 | Mycobacterium intracellulare | 4 | 32 | 0.5 | 16 |
| ATCC 12478 | Mycobacterium kansassi | 1 | 1 | 0.25 | 2 |
| ATCC 33013 | Mycobacterium komossense | 0.5 | 0.063 | 2 | 0.5 |
| ATCC 29571 | Mycobacterium malmoense | 4 | 2 | 0.25 | 1 |
| ATCC 927 | Mycobacterium marinum | 0.5 | 0.125 | 0.5 | 0.25 |
| ATCC 19530 | Mycobacterium nonchromogenicum | 0.5 | 0.03 | 0.5 | 0.13 |
| BAA-614 | Mycobacterium parascrofulaceum | 4 | 2 | 0.5 | 2 |
| ATCC 19981 | Mycobacterium scrofulaceum | 4 | 1 | 4 | 1 |
| ATCC 27962 | Mycobacterium shimoidei | 1 | 0.5 | 0.03 | 1 |
| ATCC 33027 | Mycobacterium sphagni | 0.25 | 0.03 | 0.5 | 0.25 |
| ATCC 25275 | Mycobacterium simiae | >32 | 8 | 2 | 8 |
| ATCC 35799 | Mycobacterium szulgai | 1 | 0.5 | 0.25 | 1 |
| ATCC 15755 | Mycobacterium terrae | 2 | 1 | 0.5 | 1 |
| ATCC 19423 | Mycobacterium ulcerans | 0.13 | 0.13 | 0.03 | 0.25 |
| ATCC 19250 | Mycobacterium xenopi | 4 | 2 | 0.25 | 4 |
The 157 SGM clinical strains were all isolated from tuberculosis suspected patients. The strains were classified as SGM preliminarily by growth test on p-nitrobenzoic acid containing medium. Subsequently, species identification was performed by sequence analysis of 16S rRNA, hsp65, 16–23S rRNA internal transcribed spacer and rpoB gene as described previously.15 All isolates were stored at −80°C and sub-cultured on LÖwenstein–Jensen (LJ) medium before performing a drug susceptibility test.
Minimal Inhibitory Concentration (MIC) Testing
LZD phosphate and TZD were purchased from Sigma-Aldrich and Toronto Research Chemicals, respectively. DZD and SZD were purchased from TargetMol,USA. Oxazolidinones were dissolved in dimethyl sulfoxide (DMSO) with the concentration of 2.56 mg/mL for the stock solution. Broth microdilution method was performed according to the guidelines of Clinical and Laboratory Standards Institute (CLSI).16 Cation-adjusted Mueller–Hinton broth (CAMHB) containing 5% OADC was used for MIC test. The inoculum was prepared with fresh culture grown on LJ medium. The concentrations of all the tested drugs ranged from 0.063μg/mL to 32μg/mL. Briefly, a bacterial suspension of 0.5 McFarland standard was prepared, diluted and then inoculated into 96-well microtiter plate to achieve final bacterial load of 105 colony forming unit (CFU) per well. The plates containing the remaining SGM were incubated at 37°C for 7 days except M. marinum which was incubated at 30°C. The MICs of each isolate were determined in triplicate. Fifty microliter Tween80 (5%) and 20μL AlamarBlue (Bio-rad) were added to each well and incubated for 24 h at 37 °C before assessing color development. A change from blue to pink or purple indicated bacterial growth.17 The MIC was defined as the lowest concentration of antibiotic that prevented a color change from blue to pink.
The breakpoint of LZD was adopted from the CLSI document M24-A2 (susceptible: ≤8 mg/L; intermediate susceptible: 16 mg/L; resistant: ≥32 mg/L).16 Since no well-recognized breakpoint has been proposed for TZD, SZD or DZD, the MIC distribution characterizations of them were analyzed.
Mutations Conferring Oxazolidinones Resistance and Protein Alignment
The homologous genes of reported linezolid-resistant genes, ie, rplC, rplD and 23S rRNA in the recruited isolates of SGM were amplified and sequenced. We used previously described primers for 23S rRNA18 and designed new primers for rplC and rplD amplifications. The primers used in this study are listed in Supplementary Table S2. The rplC and rplD of the reference strains of three SGM species, ie, M. avium (ATCC 25291), M. intracellulare (ATCC 13950) and M.kansasii (ATCC 12478) plus M. tuberculosis H37Rv (ATCC 27294) were also sequenced. Mutations were identified according to the outcome of the alignments against corresponding sequences of the reference strains. Multiple sequence alignment of the homologous proteins was performed using the Clustal Omega software. Structure-based multiple sequence alignment was performed with ESPript 3 based on the crystal structure of RplC and RplD protein of M. tuberculosis from the following URL:http://espript.ibcp.fr/ESPript/ESPript/.
Quality Control and Statistical Analysis
The MIC of a drug was defined as readable if there was an acceptable growth in both positive-control wells and no contamination occurred in the wells for that drug. The MIC for quality control strains H37Rv (ATCC 27294) was determined using each lot of the prepared microtiter plates, and the results for LZD were within the acceptable range 0.125 μg/mL-0.25 μg/mL. Descriptive analyses were conducted with the outcomes. Drug resistant rate for LZD was calculated, while for the other drugs without any well-recognized breakpoint, MIC50 and MIC90 were presented.
Results
MICs of LZD, TZD, SZD and DZD Against SGM Reference Strains
The MICs of the 20 reference strains to LZD, TZD, SZD and DZD are presented in Table 1. All of the four oxazolidinones exhibited antimicrobial activities in vitro against the recruited SGM reference stains. Eighteen of the 20 SGM species had MICs equal to or below 8 μg/mL for the four drugs. Only M. simiae had MIC of LZD greater than 32μg/mL. Generally, for a given isolate, the MIC values could be uniformly higher or uniformly lower for the four tested drugs when compared with other isolates. For the majority of the strains, the inhibitory activities of SZD and TZD were stronger than that of LZD.
MIC Distributions of the Clinical Isolates of M. intracellulare to LZD, TZD, SZD and DZD
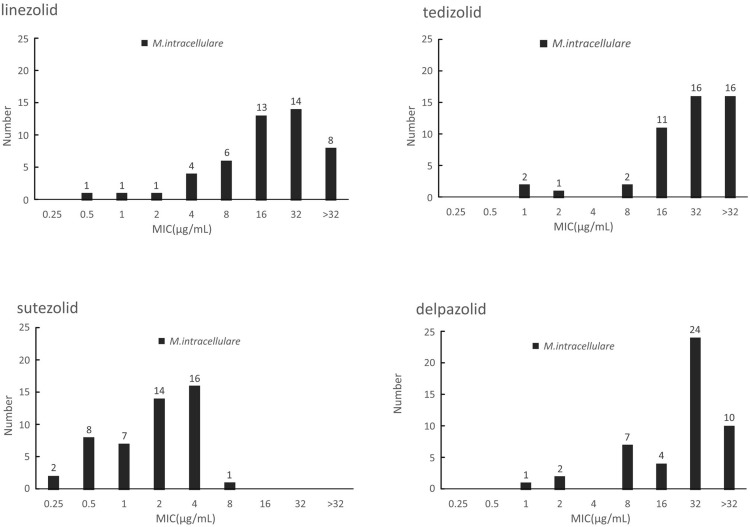
SZD showed the strongest activity against M. intracelulare with MIC50=2μg/mL and MIC90=4μg/mL among the tested oxazolidinones. MICs of SZD were generally 4- to 8-fold less than LZD for M. intracelulare whereas the MIC distribution patterns of TZD and DZD were similar to this of LZD. According to the CLSI resistance criteria for LZD against M. intracellulare (MIC > 16μg/mL), the resistant rates of the recruited clinical isolates of M. intracellulare in this study were 45.8% (22/48) whereas the MIC50 of TZD, SZD and DZD were 32μg/mL, 2μg/mL and 32μg/mL, and MIC90 were >32ug/mL, 4μg/mL and >32μg/mL, respectively. The outcomes are shown in Figure 1.
Figure 1.
The MIC distributions of M. intracellulare against LZD, TZD, SZD and DZD.
The MIC Distributions of the Clinical Isolates of M. avium to LZD, TZD, SZD and DZD
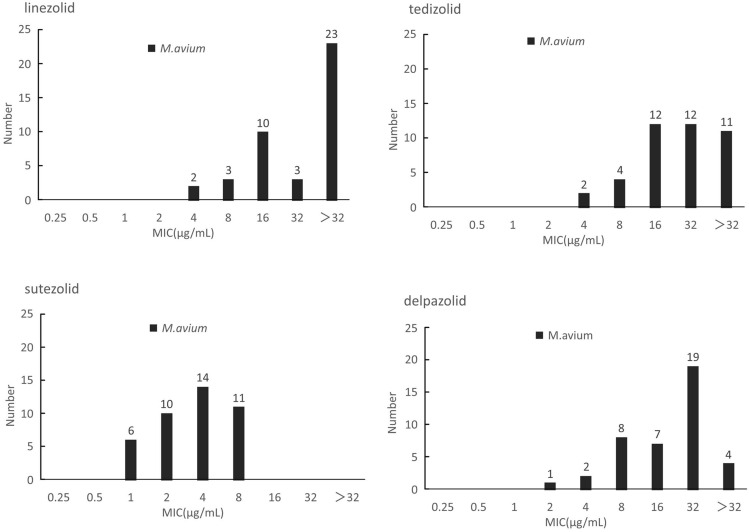
In contrast with M. intracellulare, M. avium presented similar susceptibility profiles to the four oxazolidinones. The in vitro inhibitory activity of SZD was significantly better than LZD, as indicated by its 4- to 8-fold lower MICs. According to the CLSI resistance criteria for LZD against M. intracellulare, the resistant rates of M. avium to LZD was 63.4% (26/41). The MIC50 of TZD, SZD and DZD were 32 μg/mL, 4 μg/mL and 32 μg/mL, whereas the MIC90 was >32ug/mL, 8μg/mL and 32μg/mL, respectively (Figure 2).
Figure 2.
The MIC distributions of M. avium against LZD, TZD, SZD and DZD.
The MIC Distributions of the Clinical Isolates of M. kansasii to LZD, TZD, SZD and DZD
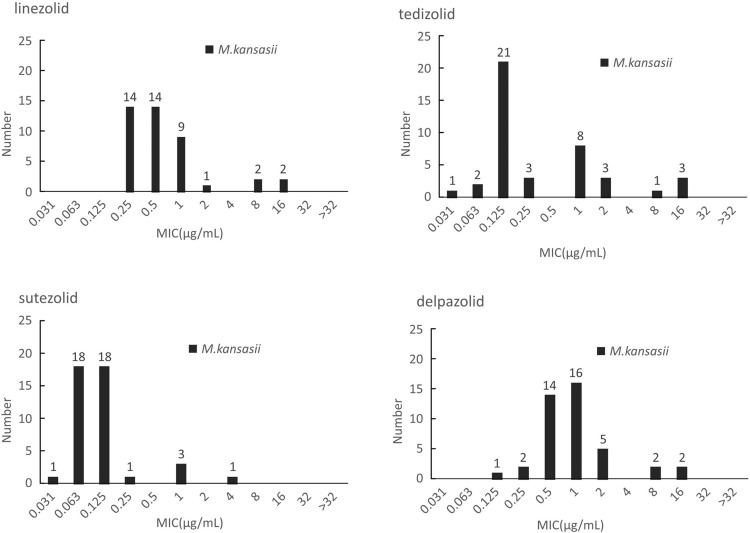
M. kansasii presented the lowest MIC distributions to the four oxazolidinones in contrast with both M. intracellulare and M. avium. Although none of the tested isolates had MIC greater than 16μg/mL, all of the 42 tested M. kansasii isolates showed susceptibility to LZD. MIC50 of TZD, SZD and DZD were 0.125μg/mL, 0.125 μg/mL and 1 μg/mL whereas MIC90 was 2 ug/mL, 0.25 μg/mL and 2 μg/mL, respectively. SZD presented the best in vitro inhibitory activities among these drugs with MICs 4- to 8-fold lower than these of LZD. In addition, MIC distribution pattern of DZD and LZD were similar whereas the MIC of TZD for a given isolate was generally half of the MIC for LZD. The outcomes are shown in Figure 3. The MIC outcomes for species with less than five isolates are presented in Table S2.
Figure 3.
The MIC distributions of M. kansasii against LZD, TZD, SZD and DZD.
Sequence Alternations in the Oxazolidinones Target Sites
The entire 23SrRNA, rplC, and rplD genes were sequenced for the identification of potential mutations associated with oxazolidinones resistance. The sequences of the tested clinical isolates of M. intracellulare, M. avium and M. kansasii were compared with their corresponding reference strains. For M. avium isolates, Thr147Ala in rplD was detected in 3 isolates with MIC of LZD≥16μg/mL, while no mutation in rplC was found. Furthermore, an insertion of guanine nucleotide at locus 2407 was found in 23SrRNA in an isolate with MIC of LZD >32μg/mL, while C2078T was found in 23SrRNA in one isolate with MIC of LZD=16μg/mL. For M. intracellulare isolates, Val75Met within the coding region of rplC was observed in two isolates with MIC=4μg/mL, while no mutation in rplD was detected. Interestingly, A2266G, A2266C and A2267C were detected in three LZD resistant M. intracellulare isolates (MIC≥32μg/mL), respectively. Besides, G1943A and T2420C were identified in both LZD resistance and susceptible M. intracellulare isolates (Table 2). For M. kansasii isolates, all MICs for LZD were ≤ 16μg/mL and no non-synonymous mutation was detected in rplC, rplD and 23SrRNA.
Table 2.
The MICs of LZD and rplC, rplD and 23srRNA Mutations Against M. avium and M. intracellulare Isolates
| MIC of LZD (μg/mL) | Species (NO.) | RplC | RplD | 23SrRNA |
|---|---|---|---|---|
| 0.5 | M. avium (0) | — | — | — |
| M. intracellulare (1) | WT(1) | WT(1) | WT(1) | |
| 1 | M. avium (0) | — | — | — |
| M. intracellulare (1) | WT(1) | WT(1) | T2420C(1) | |
| 2 | M. avium (0) | — | — | — |
| M. intracellulare (1) | WT(1) | WT(1) | T2420C(1) | |
| 4 | M. avium (2) | WT(2) | WT(2) | WT(2) |
| M. intracellulare (4) | WT(2) Val175Met(2) |
WT(4) | WT(3) G1943A/ T2420C(1) |
|
| 8 | M. avium (3) | WT(3) | WT(3) | WT(3) |
| M. intracellulare (6) | WT(6) | WT(6) | WT(5) A1929G/ T2420C(1) |
|
| 16 | M. avium (10) | WT(10) | WT (9) Thr147Ala (1) |
WT(9) C2078T(1) |
| M. intracellulare (13) | WT(13) | WT(13) | WT(7) T2420C(5) G1943A(1) |
|
| 32 | M. avium (3) | WT(3) | WT(2) Thr147Ala (1) |
WT(3) |
| M. intracellulare (14) | WT(14) | WT(14) | WT(8) T2420C(4) A2267C(1) A2266G(1) |
|
| >32 | M. avium (23) | WT(23) | WT(22) Thr147Ala (1) |
WT(22) Insa 2407G(1) |
| M. intracellulare (8) | WT(8) | WT(8) | WT(6) T2420C(1) A2266C(1) |
Note: aIns, insertion.
The protein sequences of RplC and RplD in different mycobacterial species are highly conserved. Therefore, we performed multiple sequence alignment of RplC and RplD homologues from different mycobacterial species for gaining an insight into the functional relevance of RplC and RplD mutations (Figures S1 and S2). RplD structure of M. tuberculosis was used as a model to map M. avium RplD mutation (PDB ID:5V7Q). The structure showed that Thr147 was located in a highly variable region, between β3 and α2, which suggests that this mutation could be related to LZD resistance. Next, we mapped the 23SrRNA functional mutations of M. intracellulare and M. avium. The results showed that A2267 and A2266 of M. intracellulare were close to the catalytic center, therefore, it is plausible that mutations of these two amino acids can also cause resistance. Furthermore, other mutations (such as C2407 and C2078) in M. avium were not in vicinity of the catalytic center, therefore, it is arguable that these mutations have less chance to be relevant in rendering resistance against LZD.
Discussion
Treatment of NTM infections is typically troublesome and has poor prognosis. Another major hinderance in successful treatment of NTM infections is the natural resistance of microbes to a wide range of antibiotics. Therefore, new and effective drugs are an urgent need for establishing a regimen with high efficacy for NTM therapy. LZD, the first widely used oxazolidinone, has demonstrated strong bactericidal activity against TB and certain NTM species in vitro and in vivo. Limited studies have reported that new oxazolidinones such as TZD and SZD possess promising activities against NTM in vitro.1,19 Our previous study showed that TZD harbors the strongest inhibitory activity against M. abscessus in vitro, while DZD offered the best inhibitory activity against M. fortuitum in vitro.20 Furthermore, TZD could inhibit the intracellular bacterial population of both M. avium and M. abscessus and had a concentration-dependent synergistic effect against amikacin and ethambutol.21 Since these new oxazolidinone drugs are currently not in clinical usage or have been recently adopted, therefore, well-recognized susceptibility testing methods for TZD, SZD and DTD have not been developed. Furthermore, the breakpoints to define drug resistance for antibiotics are still elusive. Overall, the MIC data of different SGM species against these new generation oxazolidinones remain scarce. In this study, the four tested oxazolidinones exhibited promising inhibitory activities in vitro against the recruited SGM reference stains. Eighteen out of the 20 SGM species had MICs ≤ 8 μg/mL for the four oxazolidinones. However, different species presented non-uniform susceptibility patterns.
The clinical isolates of M. intracelluare and M. avium both demonstrated high MIC90 for the tested oxazolidinone, with an exception of SZD. The MIC90 of SZD against M. avium and M. intracelulare was 8 μg/mL and 4 μg/mL, respectively. These observations were in concordance with the antibacterial activity against M. tuberculosis in vitro. SZD proved to be 1–2 orders of magnitude more effective than LZD.12 In addition, SZD has a less toxic and better safety profile than LZD as reported elsewhere.19 SZD also showed favorable pharmacokinetics as the Cmax of SZD and its major metabolite were 1.97 μg/mL and 7.05 μg/mL at a dose of 1200 mg QD. The latter proved to be more potent than SZD itself against extracellular tuberculosis.11,19 Therefore, SZD might be a better candidate than LZD for the treatment of MAC infections.
M. kansasii is the only NTM species which is susceptible to majority of the anti-TB drugs. The standard therapy regimen (including isoniazid, rifampin, ethambutol, with or without a macrolide) for pulmonary M. kansasii infections lasts for more than a year.16 Therefore, a short course regimen that may include newer antibiotics is often aspired. Recent study showed that TZD plus Rifampicin and moxifloxacin could potentially serve as a short-course chemotherapy regimen for M. kansasii treatment.22 In our study, SZD showed even stronger antibacterial activity against M. kansasii isolates in vitro than TZD as 41 out of the 42 tested M. kansasii isolates had MICs ≤ 1 μg/mL. Considering the better safety profile and favorable pharmacokinetic parameters, a regimen containing SZD to treat pulmonary M. kansasii infection would be very encouraging.
The breakpoint for clinical bacteria is mainly based on the bacterial activity in vitro and pharmacokinetic/pharmacodynamic (PK/PD) features. There has been no recommended breakpoint for different NTM species for TZD, SZD or DZD. Limited PK/PD studies have been done for these new oxazolidinones. Generally, all the oxazolidinones are well tolerated and dose-dependent. In contrast to LZD, SZD demonstrated superior bactericidal activity against Mycobacterium tuberculosis in hollow fiber, whole blood and mouse models.12 As the major metabolite of SZD, superior antibacterial activity has been reported for PNU-101603 than its prototype, since it provides better and early bactericidal activity against tuberculosis.11 In another study, a single 800 mg dose of DZD under fasting conditions acquired Cmax at 11.74 μg/mL.23 These PK/PD data can help in a preliminary assessment of the effectiveness of these next-generation oxazolidinones against SGM, and may also facilitate in defining appropriate breakpoints for these drugs.
LZD inhibits protein synthesis by binding with the 50S ribosomal subunit, which is composed of 5S and 23S rRNAs and 36 riboproteins (L1 through L36).24 Recently, the near-atomic structure of the 50S ribosomal subunit of M. tuberculosis bound with a potent LZD analog (LZD-114) was determined (PDB:5V7Q).25 It has been proved that LZD-114 also binds in the same pocket of ribosome with linezolid in other bacteria as well. In the Cryo EM map, both ribosomal protein L3 (encoded by rplC) and ribosomal protein L4 (encoded by rplD) were shown to be directly bound with the 23S ribosomal RNA, and located closely to the LZD binding site. This feature suggests that structural perturbation of the LZD binding site may result in reducing susceptibility to oxazolidinone. Furthermore, previous studies have demonstrated that mutations in rplC and rplD could lead to LZD in M. tuberculosis.8,26 Thr147Ala mutation detected in rplD of M. avium (Figure S2) is located in a variable site which may also be involved in rendering LZD resistance. Kim et al have previously detected a single Thr147Ala mutation in LZD resistant M. intracellulare isolate.18 Except A2267C/G and A2266G mutation in 23SrRNA gene in M. intracellulare, that was closer to the binding site of LZD, other mutations including C2078T and ins 2470G found in M. avium were far from the LZD-binding site. Overall, our results showed the novel 23srRNA mutations A2267C/G and A2266G in M. intracellulare and Thr147Ala in rplD in M. avium might cause LZD resistance.
In conclusion, this study has demonstrated that oxazolidinones have good in vitro inhibitory activities against the majority of enrolled SGM species. However, the activities of the four oxazolidinones were variable against different species. SZD showed the strongest antimicrobial activity against M. intracellulare and M. avium, while M. kansasii was very susceptible to all the four oxazolidinones. In addition, it is also plausible that the novel 23srRNA mutations A2267C and A2266G in M. intracellulare and Thr147Ala in rplD in M. avium are related with LZD resistance. The data provided important insights on the possible clinical applications of oxazolidinones to treat SGM infections.
Funding Statement
The Natural Science Fund of China (81672065,81802057,81930112), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201824) and Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20181602).
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Cowman S, van Ingen J, Griffith DE, Loebinger MR. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J. 2019;54(1):1900250. doi: 10.1183/13993003.00250-2019 [DOI] [PubMed] [Google Scholar]
- 2.Santin M, Barrabeig I, Malchair P, et al. Pulmonary infections with nontuberculous mycobacteria, Catalonia, Spain, 1994–2014. Emerg Infect Dis. 2018;24(6):1091–1094. doi: 10.3201/eid2406.172095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin C, Russell C, Soll B, et al. Increasing prevalence of nontuberculous mycobacteria in respiratory specimens from US-affiliated pacific island jurisdictions. Emerg Infect Dis. 2018;24(3):485–491. doi: 10.3201/eid2403.171301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brode SK, Marchand-Austin A, Jamieson FB, Marras TK. Pulmonary versus nonpulmonary nontuberculous Mycobacteria, Ontario, Canada. Emerg Infect Dis. 2017;23(11):1898–1901. doi: 10.3201/eid2311.170959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Liu P, Liu G, et al. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect. 2016;73(6):558–567. doi: 10.1016/j.jinf.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 6.Singh B, Cocker D, Ryan H, Sloan DJ. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2019;3:CD012836. doi: 10.1002/14651858.CD012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolhuis MS, Akkerman OW, Sturkenboom MGG, et al. Linezolid-based regimens for multidrug-resistant Tuberculosis (TB): a systematic review to establish or revise the current recommended dose for TB treatment. Clin Infect Dis. 2018;67(suppl_3):S327–S335. doi: 10.1093/cid/ciy625 [DOI] [PubMed] [Google Scholar]
- 8.Zong Z, Jing W, Shi J, et al. Comparison of in vitro activity and MIC distributions between the novel oxazolidinone delpazolid and linezolid against multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis in China. Antimicrob Agents Chemother. 2018;62(8). doi: 10.1128/AAC.00165-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compain F, Soroka D, Heym B, et al. In vitro activity of tedizolid against the Mycobacterium abscessus complex. Diagn Microbiol Infect Dis. 2018;90(3):186–189. doi: 10.1016/j.diagmicrobio.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 10.Salavert Lletí M, García-Bustos V, Morata Ruiz L, Cabañero-Navalon MD. Tedizolid: new data and experiences for clinical practice. Rev Esp Quimioter. 2021;34(Suppl 1):22–25. doi: 10.37201/req/s01.06.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallis RS, Dawson R, Friedrich SO, et al. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS One. 2014;9(4):e94462. doi: 10.1371/journal.pone.0094462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallis RS, Jakubiec W, Kumar V, et al. Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob Agents Chemother. 2011;55(2):567–574. doi: 10.1128/AAC.01179-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YS, Lim HS, Lee SH, Cho YL, Nam HS, Bae KS. Pharmacokinetics, pharmacodynamics, and tolerability of single-dose oral LCB01-0371, a novel oxazolidinone with broad-spectrum activity, in healthy volunteers. Antimicrob Agents Chemother. 2018;62(7). doi: 10.1128/AAC.00451-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellarès-Nadal J, Burgos J, Falcó V, Almirante B. Investigational and experimental drugs for community-acquired pneumonia: the current evidence. J Exp Pharmacol. 2020;12:529–538. doi: 10.2147/JEP.S259286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JC, Whang KS. Mycobacterium aquiterrae sp. nov., a rapidly growing bacterium isolated from groundwater. Int J Syst Evol Microbiol. 2017;67(10):4104–4110. doi: 10.1099/ijsem.0.002261 [DOI] [PubMed] [Google Scholar]
- 16.Clinical K. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard. 2nd ed. Wayne, PA: CLSI document M24-A2; 2011. [PubMed] [Google Scholar]
- 17.Coeck N, de Jong BC, Diels M, et al. Correlation of different phenotypic drug susceptibility testing methods for four fluoroquinolones in Mycobacterium tuberculosis. J Antimicrob Chemother. 2016;71(5):1233–1240. doi: 10.1093/jac/dkv499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Jhun BW, Moon SM, et al. Genetic mutations in linezolid-resistant Mycobacterium avium complex and Mycobacterium abscessus clinical isolates. Diagn Microbiol Infect Dis. 2019;94(1):38–40. doi: 10.1016/j.diagmicrobio.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 19.Bahuguna A, Rawat DS. An overview of new antitubercular drugs, drug candidates, and their targets. Med Res Rev. 2020;40(1):263–292. doi: 10.1002/med.21602 [DOI] [PubMed] [Google Scholar]
- 20.Wen S, Gao X, Zhao W, et al. Comparison of the in vitro activity of linezolid, tedizolid, sutezolid, and delpazolid against rapidly growing mycobacteria isolated in Beijing, China. Int J Infect Dis. 2021;109:253–260. doi: 10.1016/j.ijid.2021.06.055 [DOI] [PubMed] [Google Scholar]
- 21.Ruth MM, Koeken V, Pennings LJ, et al. Is there a role for tedizolid in the treatment of non-tuberculous mycobacterial disease? J Antimicrob Chemother. 2020;75(3):609–617. doi: 10.1093/jac/dkz511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava S, Wang JY, Magombedze G, et al. Novel short-course therapy and morphism mapping for clinical pulmonary Mycobacterium kansasii. Antimicrob Agents Chemother. 2021;65. doi: 10.1128/AAC.01553-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunwoo J, Kim YK, Choi Y, et al. Effect of food on the pharmacokinetic characteristics of a single oral dose of LCB01-0371, a novel oxazolidinone antibiotic. Drug Des Devel Ther. 2018;12:1707–1714. doi: 10.2147/DDDT.S155657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong W, Chochua S, McGee L, Jackson D, Klugman KP, Vidal JE. Mutations within the rplD gene of linezolid-nonsusceptible streptococcus pneumoniae strains isolated in the United States. Antimicrob Agents Chemother. 2014;58(4):2459–2462. doi: 10.1128/AAC.02630-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang K, Chang JY, Cui Z, et al. Structural insights into species-specific features of the ribosome from the human pathogen Mycobacterium tuberculosis. Nucleic Acids Res. 2017;45(18):10884–10894. doi: 10.1093/nar/gkx785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckert P, Hillemann D, Kohl TA, et al. rplC T460C identified as a dominant mutation in linezolid-resistant Mycobacterium tuberculosis strains. Antimicrob Agents Chemother. 2012;56(5):2743–2745. doi: 10.1128/AAC.06227-11 [DOI] [PMC free article] [PubMed] [Google Scholar]