In PNAS, Malonis et al. (1) isolate and characterize several human monoclonal antibodies (MoAbs) that neutralize infection with arthritogenic alphaviruses. Alphaviruses represent a large group of positive-strand RNA viruses, some of which cause infections in humans. They are widely distributed, are transmitted by mosquitoes, and have been clinically classified as encephalitic and arthritogenic, according to the most prevalent symptoms in human and animal infections. Among alphaviruses, Chikungunya virus (CHIKV) is a frequent cause of outbreaks in different geographic locations and is characterized by fever and joint inflammation, often accompanied by persistent joint pain and arthralgia for several months after initial infection that can lead to joint immobilization (2). CHIKV is transmitted by Aedes mosquitoes, and, although most infections take place in the tropical and subtropical regions, outbreaks and local transmission of CHIKV have been known to occur in Europe and the United States too. The expansion of the geographical distribution of the Aedes vector has been a major contributor to the increases in CHIKV infections during the last decades (3). Other arthritogenic alphaviruses known to infect humans are Mayaro virus (MAYV), Ross River virus (RRV), and O’nyong-nyong virus (ONNV), present in South America, the South Pacific, and Africa, respectively. Currently, despite their burden on human health, there are no available specific treatments or vaccines against any alphaviruses.
The use of pathogen-specific antibodies to treat viral infections is an old therapeutic strategy that still is successfully used, together with vaccination, to prevent postexposure development of rabies (4). Although the use of immune globulin to treat viral infections lost momentum during the last century in favor of specific antiviral drugs, the development of novel technologies that allow the rapid identification of MoAbs from circulating human B cells has resulted in a revival of antibody therapy to treat viral infections. Today, it is possible to identify and produce large numbers of human MoAbs from convalescent individuals, characterize their neutralizing activities, and manufacture those most inhibitory to hopefully successfully treat specific viral infections (5). This allows the rapid development of antibody-based therapies when outbreaks of new viruses takes place. For example, antibody therapy has been successfully developed for Ebola virus (6, 7) and severe acute respiratory syndrome coronavirus 2 infections (8), and, at this moment, such therapies, even though expensive and not always efficacious, continue to be one of the preferred treatments for infection with these emerging viruses. Immune escape and the need of an early time window during infection to make a better impact remain potential problems associated with MoAb therapy of viral infection. Nevertheless, due to the long life of antibodies, antibody-based therapy remains a promising strategy to prophylactically protect high-risk groups for several months against viral infections, especially for those individuals that do not respond well to vaccination (if there is an existing vaccine).
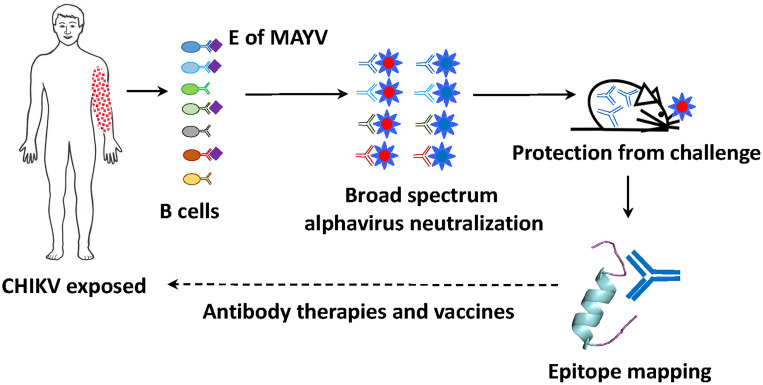
An important consideration for the isolation of protective MoAbs from convalescent human donors is the use of the right viral immunogen as a bait to clone relevant virus-specific B cells. In the PNAS study (1), Malonis et al. used a soluble, stabilized, and conformational relevant recombinant antigen derived from the surface receptor-binding protein of MAYV, containing the relevant antigenic sites recognized by neutralizing antibodies. This antigen was used to isolate single B cells from patients who previously experienced CHIKV infection, resulting in an enrichment of single B cells cross-reactive to both MAYV and CHIKV. A single donor gave rise to 71 MoAb sequences from single B cell clones, 33 of which demonstrated strong binding to both MAYV and CHIKV glycoproteins when expressed. Of these, five had strong neutralizing activities against a variety of arthritogenic alphaviruses, including MAYV, CHIKV, RRV, and ONNV. Mapping of these broadly cross-neutralizing antibodies revealed binding to distinct regions of a specific domain of the CHIKV glycoprotein, the B domain of the E2 peptide, highly conserved among related alphaviruses. When two of these antibodies were passively administered to mice that were infected with CHIKV or MAYV, treated animals had decreased viral replication and joint swelling, suggesting possible therapeutic properties (Fig. 1).
Fig. 1.
Genomics serology to inform therapies and vaccines for arthritogenic alphaviruses. Circulating B cells binding to the ectodomain of the glycoprotein of MAYV in a convalescent donor previously exposed to CHIKV infection are single-cell cloned, and their encoded antibodies are sequenced and expressed, and tested for broadly neutralizing activity in vitro and in vivo against multiple alphavirus. Those with neutralizing properties are being used to map the recognized conserved epitope/epitopes to which they bind. This information may lead to antibody-based therapeutics and inform vaccine antigen design for the treatment or prevention of arthritogenic alphavirus infections.
In PNAS, Malonis et al. isolate and characterize several human monoclonal antibodies (MoAbs) that neutralize infection with arthritogenic alphaviruses.
It is of interest that these antibodies, despite their protective efficacy, and in contrast to most of the broadly neutralizing antibodies against the glycoprotein of HIV (9, 10), showed little affinity maturation, and were very close to their germ line sequences. This not only increases their potential to be used for the treatment of alphavirus infections but also suggests that, given the right immunogen corresponding to the B domain of the E2 protein in its natural conformation, vaccinated people will quickly induce broad neutralizing antibodies against alphavirus. Thus, not only do the antibodies identified by Malonis et al. (1) offer some potential clinical applications for the prevention or treatment of alphavirus infections, but they also might illuminate the path toward a broadly protective arthritogenic alphavirus vaccine. Broadly neutralizing antibodies against multiple strains of influenza viruses have also being described in the past, and their mapping to the stalk of the HA viral glycoprotein (11, 12) has spurred the pursuit of broadly neutralizing influenza vaccines based on the use of immunogens that stimulate high levels of antibodies against the conserved HA stalk (13). Similar approaches could be applied for alphavirus vaccines. Nevertheless, more research is still needed to demonstrate the translational aspects of these studies.
In any case, the results depicted in the Malonis et al. PNAS article (1) offer a glimpse into the conserved regions containing protective epitopes of arthritogenic alphavirus infection. Kim et al. (14) have also recently isolated and characterized two human pan-alphaviruses that protect from both arthritogenic and encephalitic alpahaviruses but do not neutralize in vitro, suggesting different mechanisms of protection in vivo than the ones described in Malonis et al. These studies expand our knowledge of the vulnerabilities of this group of important human pathogens.
Footnotes
The author declares no competing interest.
See companion article, “Near-germline human monoclonal antibodies neutralize and protect against multiple arthritogenic alphaviruses,” 10.1073/pnas.2100104118.
References
- 1. Malonis R. J., et al., Near-germline human monoclonal antibodies neutralize and protect against multiple arthritogenic alphaviruses. Proc. Natl. Acad. Sci. U.S.A. 118, e2100104118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goupil B. A., Mores C. N., A review of Chikungunya virus-induced arthralgia: Clinical manifestations, therapeutics, and pathogenesis. Open Rheumatol. J. 10, 129–140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kraemer M. U. G., et al., Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus . Nat. Microbiol. 4, 854–863 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu S., Guo C., Rabies control and treatment: From prophylaxis to strategies with curative potential. Viruses 8, E279 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker L. M., Burton D. R., Passive immunotherapy of viral infections: ‘Super-antibodies’ enter the fray. Nat. Rev. Immunol. 18, 297–308 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiu X., Kobinger G. P., Antibody therapy for Ebola: Is the tide turning around? Hum. Vaccin. Immunother. 10, 964–967 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mulangu S., et al.; PALM Writing Group; PALM Consortium Study Team, A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 381, 2293–2303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor P. C., et al., Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 21, 382–393 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein F., et al., Antibodies in HIV-1 vaccine development and therapy. Science 341, 1199–1204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. West A. P. Jr., et al., Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell 156, 633–648 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sui J., et al., Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16, 265–273 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekiert D. C., et al., Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nachbagauer R., et al., A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 27, 106–114 (2021). [DOI] [PubMed] [Google Scholar]
- 14. Kim A. S., et al., Pan-protective anti-alphavirus human antibodies target a conserved E1 protein epitope. Cell 184, 4414–4429.e19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]