Abstract
Objective
Previous studies reported a poor outcome in patients with coronavirus 2019 (COVID-19) undergoing cardiac surgery. Complications most frequently described were respiratory failure, renal failure, and thromboembolic events. In their recent experience, the authors observed a very high incidence of bleeding complications. The purpose of the study was to investigate a possible significant correlation between perioperative COVID-19 infection and hemorrhagic complications compared to non-COVID-19 patients.
Design
Single-center, observational, retrospective, matched case-control (1:2) study involving patients who underwent open-heart cardiac surgery from February 2020 and March 2021 with positive perioperative diagnosis of COVID-19 infection, matched with patients without COVID-19 infection.
Setting
Cardiac surgery unit and intensive care unit of a university tertiary center in a metropolitan area.
Participants
In the study period, 773 patients underwent cardiac surgery on cardiopulmonary bypass (CPB). Among them, 23 consecutive patients had perioperative diagnosis of COVID-19 infection (study group). These patients were compared with 46 corresponding controls (control group) that matched for age, sex, body mass index, and Society of Thoracic Surgeons score.
Interventions
Open-heart cardiac surgery on CPB.
Measurements and Main Results
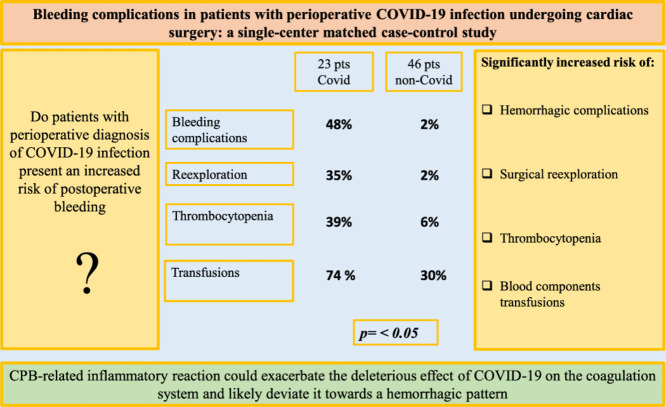
In the study group, 2 patients (9%) died in the intensive care unit from severe respiratory failure, shock, and multiple organ failure. In the study group, patients showed a significantly higher incidence of bleeding complications (48% v 2%, p = 0.0001) and cases of surgical reexploration for bleeding (35% v 2%, p = 0.0001), a higher incidence of severe postoperative thrombocytopenia (39% v 6%, p = 0.0007), and a higher need of blood components transfusions (74% v 30%, p = 0.0006). Chest tubes blood loss and surgical hemostasis time were markedly prolonged (p = 0.02 and p = 0.003, respectively).
Conclusions
A worrisome increased risk of early and late bleeding complications in COVID-19 patients was observed, and it should be considered when assessing the operative risk. CPB-related inflammatory reaction could exacerbate the deleterious effect of COVID-19 on the coagulation system and likely deviate it toward a hemorrhagic pattern.
Key Words: COVID-19, coronavirus, intensive care unit, cardiac surgery, cardiopulmonary bypass, bleeding
Graphical Abstract
THE SEVERE acute respiratory syndrome coronavirus-2 (SARS-COV-2) infection pandemic has markedly affected surgical activity and, in particular, cardiac surgery, worldwide.1, 2, 3, 4, 5, 6, 7 In many centers, the number of elective surgical cases decreased and patients undergoing surgery that could no longer be delayed experienced worse outcomes, with increased mortality rate and postoperative complications incidence.1 , 5 , 7, 8, 9 Among patients with coronavirus disease 2019 (COVID-19), the most frequent complications included interstitial pneumonia with respiratory failure and acute respiratory distress syndrome (ARDS), acute renal failure, and thromboembolic events.1 , 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 However, during surgery in patients with COVID-19, less perioperative bleeding was observed and has not been thoroughly investigated. Moreover, data regarding postoperative bleeding were limited.
Main endpoint of the present study was to evaluate if patients with perioperative COVID-19 who underwent cardiac surgery with cardiopulmonary bypass (CPB) presented an increased risk of hemorrhagic complications. More specifically, the authors considered as “hemorrhagic complications” the rate of surgical reexploration for bleeding (via mediastinal revision through full resternotomy or subxiphoid pericardial drainage), intracranial hemorrhage, and massive hemorrhagic pleural effusion requiring drainage.
The mortality rate, the incidence of severe thrombocytopenia, need for blood components transfusions and procoagulant drugs, length of surgical hemostasis time (SHT), blood loss from drains, and length of hospitalization also were analyzed.12, 13, 14, 15 The aim of this study was to evaluate whether possible inflammatory reactions associated with perioperative COVID-19 infection could contribute to coagulation derangements of CPB and, consequently, significantly increase the risk for hemorrhagic complications.
Methods
The present study was a retrospective, matched (1:2), case-control analysis. Data were acquired from an electronic database of patients undergoing elective, urgent, or emergency cardiac surgery requiring CPB. Patients underwent surgery in the authors’ cardiac surgery unit from February 2020 to March 2021. The study was approved by the Ethics Committee of the authors’ University Polyclinic (Approval number: 0051824/20; Protocol ID 3663). Given the retrospective nature of the study and the use of anonymized patient data, requirements for ad hoc patient consent were waived.
Due to the severity of the COVID-19 pandemic, in the authors’ region, surgical activity was markedly reduced in most hospitals, and patients with a need for cardiac surgery were compelled to concentrate in a few metropolitan centers, including the authors’ Polyclinic. Regardless, in the authors’ center an overall reduction of 87 cardiac surgery cases from 2019 to 2020 was recorded.
As a regional reference center for COVID-19 care, in the study period, this hospital admitted more than 4,800 patients with COVID-19 (more than 450 of these patients were hospitalized in the intensive care unit). Except for patients with type-A aortic dissection, the surgical indication of patients with COVID-19 was, in all instances, validated by the internal Institutional Heart Team and was deemed urgent or delayable (due to unstable angina or chest pain, heart failure, endocarditis, large aneurysm, or rapid aneurysmal growth).
Diagnosis of SARS-CoV-2 infection was confirmed via positive throat swab test and real-time polymerase chain reaction assay, which were routinely performed at prehospitalization, on admission, and daily during the postoperative course.
On admission, data regarding patient's medical history were carefully collected. Chest X-ray and chest computed tomography scan were performed when indicated. Personal protective equipment was provided to medical and paramedical health workers, and care staff was limited to essential professionals. Orotracheal intubation was performed mostly using a video laryngoscope. The postoperative intensive care unit and hospital ward for patients with COVID-19 were isolated from other hospital areas. According to the Health Authority directives, direct contact between patients and their family members was not permitted, and information regarding the patient's clinical condition was conveyed via telephone.
Surgeries were performed by 8 surgeons with an experience of conducting at least 200 procedures each. Patients underwent surgery through median sternotomy and central cannulation for CPB. For CBP priming, the extracorporeal circuit was filled with about 1,500 mL of Ringer's Lactate and about 80 to 100 mL of mannitol.
In the postoperative period, patients were mechanically ventilated using a volume-controlled ventilator and extubated usually 6-to-8 hours after surgery, in the absence of complications, after having reached satisfactory levels for blood gases in spontaneous breathing and adequate lung ventilation, as documented by chest X-ray.
During the postoperative course, red cell transfusion was administered when hemoglobin levels reached 6-to-7 g/dL and hematocrit was at 22%-to-24%. Platelets were transfused in patients with bleeding manifestations when the platelet count (PLTs) decreased to 80-to-100.000/μL. To improve hemostasis and increase filling pressure, fresh frozen plasma was administered, when deemed necessary. Mediastinal reexploration for bleeding was indicated in patients in whom the rate of blood loss was >300-to-400 mL for 1 hour, or >200-to-300 mL during the subsequent hours. Hemodynamic instability and hypotension refractory to medical therapy reinforced the indication for mediastinal revision.
In addition to the primary and secondary endpoints previously described, attempts were made to indirectly quantify the time taken by surgical maneuvers to achieve hemostasis (SHT). For this purpose, the time interval (in minutes) from the end of CBP (protamine administration) and the beginning of sternal closure was retrospectively evaluated, which are time intervals routinely recorded by the anesthesiologist.
Patient group matching was based on age (within ± 3 years), sex, body mass index (within 2 points) and STS (Society of Thoracic Surgeons) score (within 2 points). When multiple control candidates met the core-matching criteria, the choice was based on the date of cardiac surgery. The investigators were blinded to case outcomes during matching.
Statistical Analysis
The Kolmogorov-Smirnov test was used to evaluate the distribution of variables. Data with nonnormal distribution were assessed using the Mann-Whitney U test, and expressed as median and selected centile (25th-75th). Data with normal distribution were assessed using the Student's t test. Categorical variables were expressed as proportions, and compared using the χ2 test or Fisher's exact test, as appropriate. Differences with p < 0.05 were considered to be statistically significant. The crude odds ratio and corresponding 95% confidence interval were calculated for each clinically relevant variable. Variables that reached p < 0.1 in the univariate analysis were included in the multivariate logistic regression analysis. A stepwise selection procedure was used to select variables for inclusion in the final model. The Hosmer–Lemeshow goodness-of-fit test and receiver operating characteristic curve analysis were used to assess the goodness of the logistic final model. All statistical analyses were performed using SPSS version 21.0 (IBM Corporation, Armonk, NY) for Windows (Microsoft Corporation, Redmond, WA).
Results
A total of 826 consecutive patients underwent cardiac surgery at the authors’ Polyclinic, from February 2020 to March 2021, of whom 773 required CPB. Among these patients, age 68.7 ± 8.3 years, 19 (83%) were men and had a perioperative diagnosis of COVID-19. On admission, 6 (26%) patients were receiving acetylsalicylic acid, 3 (13%) received double-antiplatelet drugs, and 4 (17%) received oral anticoagulants. Of the 4 patients with COVID-19 at the time of surgery, 3 were receiving acetylsalicylic acid, and one was not receiving any therapy. In the control group, 13 (30%) patients were treated with acetylsalicylic acid (p = 0.3). Among patients undergoing elective procedures, medications were discontinued in a timely manner before surgery, and of those who underwent emergency surgery, only one was being treated with acetylsalicylic acid.
The study group was composed of patients with a perioperative diagnosis of COVID-19, confirmed by positive throat swab test results between 30 days before and 7 days after surgery. Four (17%) patients presented with COVID-19 infection (positive swab test result) on admission, 5 (22%) patients had a recent history (within 30 days) of COVID-19 infection with a negative swab test result on admission, and 14 (61%) patients had a COVID-19 infection that was detected early postoperatively (within 7 days of surgery). Only 1 patient with positive COVID-19 infection at the time of surgery underwent surgery for type-A aortic dissection repair.
As the time of positive test after the initial infection was variable, the exact beginning of the infection was not identifiable. Therefore, the authors considered COVID-19 patients all positive ones in the time period from 30 days before surgery up to 7 days after surgery. In so doing, the COVID-19 inflammatory reaction should have been present in all patients.
Symptomatic patients were identified and considered acute, all other patients were considered COVID-19 positive and were included in the COVID-19 series on the basis of the laboratory swab positivity. Indication for emergent/urgent surgery was related mainly to the cardiac disease requiring immediate or prompt surgery.
Among the 4 COVID-19 patients, only 1 patient exhibited typical coronavirus symptoms, such as severe dyspnea, asthenia, and fever, along with minor radiologic signs of interstitial pneumonia requiring noninvasive ventilation. On admission, none of the patients needed intubation and neither did any patients require perioperative extracorporeal membrane oxygenation support.
Cold crystalloid cardioplegia was administered to 14 (69%) patients, and mixed cold hematocrystalloid cardioplegia to 9 (39%) patients. Four (17%) patients underwent surgery via a ministernotomy approach. Patients with type-A aortic dissection underwent circulatory arrest with moderate hypothermia (25°C-28°C) and antegrade cerebral perfusion. As usual in the authors’ center, the pericardium was left open in all patients.
None of the patients exhibited hematologic disorders except for one with a congenital XI coagulation factor deficit, and one with a history of non-Hodgkin lymphoma, neither of whom tested positive for COVID-19 at the time of surgery. Preoperative laboratory investigations, blood count and coagulation profiles were normal in all patients.
COVID-19 group patients were compared with 46 corresponding controls with no history or evidence of COVID-19 (baseline characteristics are summarized in Table 1 ). There were no significant between-cohort differences in terms of preoperative characteristics (Table 1). In both groups, 3 (13%) surgeries were performed on an urgent-emergent basis (type A aortic dissection). STS score for morbidity and mortality was comparable (11 ± 6.9% v 8.1 ± 5.8, p = 0.3). CBP time in the study group was 152 ± 79 minutes (v 130 ± 45, p = 0.08), and aortic cross-clamp time was 106 ± 36 minutes (v 86 ± 27 min, p = 0.07). SHT was significantly prolonged in the study group (81 ± 56 v 64 ± 45 min, p = 0.0003) (Table 1).
Table 1.
Patients Characteristics and Operative Data
| Clinical Characteristics | Covid n = 23 | Non-Covid n = 46 | p Value |
|---|---|---|---|
| Age (mean ± SD), y | 68.7 ± 8.3 | 67 ± 12.6 | 0.1 |
| Female patients n (%) | 4 (17) | 11 (24) | 0.5 |
| BMI (mean ± SD), Kg/m2 | 26.6 ± 2 | 26.8 ± 3.4 | 0.1 |
| Hypertension, n (%) | 20 (87) | 36 (78.2) | 0.3 |
| Diabetes, n (%) | 9 (39) | 7 (15) | *0.02 |
| Dyslipidemia, n (%) | 11 (48) | 33 (72) | 0.05 |
| Smoking habit, n (%) | 9 (39) | 17 (37) | 0.8 |
| Renal failure, n (%) | 4(17) | 2 (4) | 0.06 |
| COPD, n (%) | 7 (30) | 6 (13) | 0.08 |
| Previous NV events, n (%) | 3 (13) | 1 (2) | 0.06 |
| PVD, n (%) | 6 (26) | 4 (9) | 0.05 |
| Emergent operation, n (%) | 3 (13) | 3 (6) | 0.3 |
| CABG isolated, n (%) | 7 (30) | 19 (41) | - |
| AAR, n (%) | 1 (4) | 2 (4) | - |
| AV surgery, n (%) | 1 (4) | 6 (13) | - |
| MV (± TV) surgery, n (%) | 5 (22) | 7 (15) | - |
| MV + AV (± TV) surgery, n (%) | 2 (9) | 0 (0) | - |
| AV or MV + CABG, n (%) | 0 (0) | 4 (9) | - |
| AAR + CABG, n (%) | 1 (4) | 2 (4) | - |
| AAR + AV surgery, n (%) | 3 (13) | 5 (11) | - |
| AVPL + AAR + CABG, n (%) | 2 (9) | 0 (0) | - |
| AVPL+ AAR + ER, n (%) | 1(4) | 1 (2) | - |
| Ministernotomy, n (%) | 4 (17) | 9 (19) | 0.8 |
| Infective endocarditis, n (%) | 1 (4) | 1 (2) | 0.6 |
| EF (mean ± SD) % | 56 ± 7 | 55 ± 8 | 0.4 |
| STS score-morbidity/mortality (mean ± SD) % | 11 ± 6.9 | 8.1 ± 5.8 | 0.3 |
| CPB time, (mean ± SD) min | 152 ± 79 | 130 ± 45 | 0.08 |
| Aortic cross-clamp time (mean ± SD) min | 106 ± 36 | 86 ± 27 | 0.07 |
| SHT (mean ± SD) min | 81 ± 56 | 64 ± 45 | *0.0003 |
| ACT pre-CPB (mean ± SD) s | 490 ± 87 | 483 ± 142 | 0.05 |
| ACT post-CPB (mean ± SD) s | 509 ± 140 | 425 ± 181 | 0.1 |
Abbreviations: AAR, ascending aorta replacement; ACT, activated clotting time; AV, aortic valve; BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; EF, ejection fraction; ER, emiarch replacement; MV, mitral valve; NV, neurovascular; PVD, peripheral vascular disease; TV, tricuspid valve; SD, standard deviation; SHT, surgical hemostasis time; STS score, Society of Thoracic Surgeons.
p < 0.05.
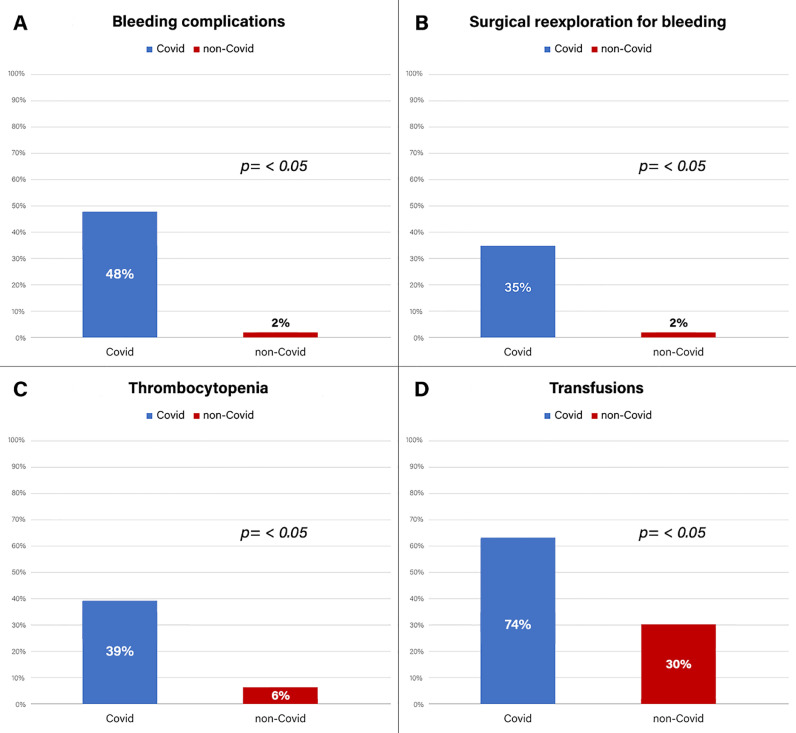
Postoperative data are summarized in Table 2 . In the study group, severe respiratory failure was observed in 6 (26%) patients, 2 (9%) of whom died from ARDS, cardiovascular shock, and multiple organ failure. Bleeding complications occurred in 11 (48%) patients. In 8 (35%) patients, surgical reexploration for bleeding was required, 4 of whom underwent reoperation at least once for late (after the postoperative day 6) hemorrhagic pericardial effusion and cardiac tamponade (Fig 1 ). Six patients underwent reoperation during their in-hospital postoperative course, and 2 were urgently rehospitalized for cardiac tamponade 2 weeks and 3 months after surgery, respectively. Four (17%) patients underwent multiple mediastinal reexplorations for recurrent bleeding, 3 of whom required 2 mediastinal revisions, and one required 3 revisions. In 1 patient, temporary mediastinal packing was performed with subsequent sternal closure. Among them, only 1 patient had positive COVID-19 diagnosis at the time of the first surgery. In summary, 13 surgical reexplorations were performed in 8 patients (8 mediastinal revisions through resternotomy and 5 through a subxiphoid approach). During mediastinal revision, no active hemorrhagic source was found, except for one type-A aortic dissection patient who had undergone multiple postoperative revisions and at the first reexploration exhibited diffuse mediastinal bleeding with a slight oozing (apparently not relevant) at the distal aortic anastomosis site.
Table 2.
Postoperative Results
| Postoperative Results | COVID-19 n = 23 | Non-COVID-19 n = 46 | p Value |
|---|---|---|---|
| Mortality, n (%) | 2 (9) | 0 (0) | - |
| Severe respiratory failure, n (%) | 6 (26) | 2 (4) | *0.008 |
| Hemorrhagic complications, n (%) | 11 (48) | 1 (2) | *0.00001 |
| Surgical re-exploration for bleeding (patients), n (%) | 8 (35) | 1 (2) | *0.0001 |
| Cardiac tamponade, n (%) | 4 (17) | 0 (0) | - |
| Multiple surgical re-explorations (patients), n (%) | 4 (17) | 0 (0) | - |
| Intracranial hemorrhage, n (%) | 1 (4) | 0 (0) | - |
| Pleural effusion requiring drainage, n (%) | 2 (9) | 0 (0) | - |
| Fluid loss from drains, (mean ± SD) mL | 1,535 ± 1.915 | 463 ± 164 | *0.02 |
| Rehospitalization, n (%) | 2 (9) | 2 (4) | 0.4 |
| Severe thrombocytopenia, n (%) | 9 (39) | 3 (6) | *0.0007 |
| Platelet count, *109/L, median (25th-75th) | 112(82-157) | 176 (139-207) | *0.01 |
| Fibrinogen, mg/dL, median (25th-75th) | 457 (331-675) | 467 (362-653) | 0.9 |
| APTT, s, median (25th-75th) | 42 (36-49) | 35 (31-37) | *0.01 |
| Patients transfused, n (%) | 17 (74) | 14 (30) | *0.0006 |
| Procoagulant drugs, n (%)† | 17 (74) | 9 (19) | *0.00001 |
| Sternal dehiscence, n (%) | 3 (13) | 1 (2) | 0.06 |
| Total intubation time, (mean ± SD) h | 13 ± 6 | 10 ± 2 | *0.01 |
| Acute renal failure, n (%) | 2 (9) | 0 (0) | - |
| Hemodialysis, n (%) | 1 (4) | 0 (0) | - |
| Hospital stay, (mean ± SD) d | 17 ± 2.5 | 9.2 ± 4.7 | *0.01 |
Abbreviations: APTT, activated partial thromboplastin time; SD, standard deviation.
p < 0.05.
Tranexamic acid, desmopressin, and fibrinogen.
Fig 1.
Incidence of bleeding complications, surgical reexploration for bleeding, severe postoperative thrombocytopenia, and need of blood components transfusion in COVID-19 and non-COVID-19 patients.
Two patients presented with massive serohemorrhagic pleural effusion requiring drainage 2 and 4 weeks after surgery. One patient experienced a right temporoparietal intracranial hemorrhage on postoperative day 27. None of these patients was COVID-19 positive at the time of surgery. Mean blood loss from drains was markedly high (1,535.25 ± 1,914 [450-7,465] mL). Mostly during the first week after surgery, 9 (39%) patients experienced severe thrombocytopenia (PLTs, <80 × 109 units/L), 20 (87%) patients experienced lymphocytopenia (<10% at leukocyte formula), and 7 (30%) patients exhibited prolonged prothrombin time (PT >13 seconds). D-dimer level, determined in 9 patients during the phase of active bleeding, was 6,221.4 (357-35,200) ng/mL.
The rate of surgical reexploration for bleeding in the control group, during the same enrollment period, was significantly lower (2% in the control group v 35% in the study group, p = 0.0001) (Fig 1). There was an increased need for blood-component transfusion in the study group. Seventeen (74%) patients received blood products (v 30% in the control group, p = <0.01). Red cell concentrates were used in 16 patients, PLTs concentrates in 4, and fresh frozen plasma in 13. Among transfused patients in the study group, the mean red cell concentrates, PLTs, and fresh frozen plasma administered per patient were 4.3 ± 3.7 units, 1.25 ± 0.5 units, and 1,745 ± 972 mL, respectively (Fig 1). Seventeen (74%) patients received procoagulant drugs (v 19%, p = 0.00001); 17 (74%) patients received tranexamic acid, 5 (22%) received desmopressin, and 2 (9%) received fibrinogen. In the study group, sternal dehiscence was observed in 3 (13%) patients. Complications in the study group had a significant influence on length of hospital stay (17 ± 2.5 v 9.2 ± 4.7 d, p = 0.01). In the multivariate logistic regression final model, SARS-CoV-2 infection and renal failure were the unique independent factors associated with postoperative bleeding complications (Table 3 ).
Table 3.
Logistic Regression Analysis of Factors Associated With the Postoperative Hemorrhagic Complications
| Variable | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| OR | p Value | OR | p Value | |
| SARS-CoV-2 infection | 24 | 0.004 | 25 | 0.01 |
| CPB time | 1.01 | 0.006 | — | — |
| Aortic cross-clamping time | 1 | 0.85 | ||
| PVD | 3.25 | 0.14 | ||
| Renal failure | 23 | <0.01 | 24 | 0.01 |
| Diabetes | 0.85 | 0.8 | ||
| COPD | 1.42 | 0.68 | ||
| Dyslipidemia | 0.18 | 0.02 | — | — |
| Platelet count, X 109 | 0.98 | 0.06 | — | — |
| Fibrinogen, mg/dL | 1 | 0.77 | ||
| Activated partial thromboplastin time, s | 1.04 | 0.09 | — | — |
NOTE. The variables dyslipidemia, CPB time, platelet count, and activated partial thromboplastin time were not included in the final multivariate model.
Abbreviations: COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; OR, odds ratio; PVD, peripheral vascular disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
The COVID-19 pandemic and its burden on national health systems have severely impacted services in many areas of medical care.2, 3, 4 In addition, in many cardiothoracic surgery centers, a severe reduction of surgical cases has been observed.1 , 4 , 7, 8, 9, 10 COVID-19 syndrome, putatively caused by coronavirus-2, frequently is characterized by acute respiratory failure with radiologic signs of interstitial pneumonia, ground glass opacities, and/or bilateral patchy shadowing. In some instances, ARDS and shock also may occur.11
A few studies investigating the clinical outcomes of patients with COVID-19 undergoing surgery reported increased morbidity and mortality.1 , 8, 9, 10 In 9 patients with COVID-19 undergoing cardiac surgery, Yates et al.1 reported a 44% mortality rate, mainly due to severe respiratory failure. SARS-CoV-2 is a pleiotropic virus that causes several different clinical syndromes and changes in laboratory parameters, including thrombocytopenia, lymphocytopenia, and a prolonged PT.10, 11
In addition to respiratory and renal complications, COVID-19 patients may develop coagulation disorders. An increased risk for thromboembolic events also has been documented.10, 11 However, little is known about the risk for perioperative bleeding complications in patients with COVID-19 undergoing cardiac surgery.
In this study, the authors observed a marked increase in the incidence of postoperative bleeding complications compared with that noted in non-COVID-19 patients. In the authors’ Intensive Care Unit, during the same period, the rate of overall surgical reexplorations for bleeding in non-Covid patients who had undergone CPB was 4% (30 out of 750 patients), consistent with previously reported results,12, 13, 14, 15 which was markedly lower than the rate of surgical reexplorations in the studygroup.
Among patients with perioperative COVID-19, the authors observed a high rate of surgical reexploration due to bleeding, with 1 case of intracranial hemorrhage, 2 cases of massive hemorrhagic pleural effusion, prolonged hemostasis time (ie, SHT), conspicuous and prolonged blood loss from drains, and extensive need for blood-component transfusions and procoagulant drugs.
Pathophysiologic Mechanisms
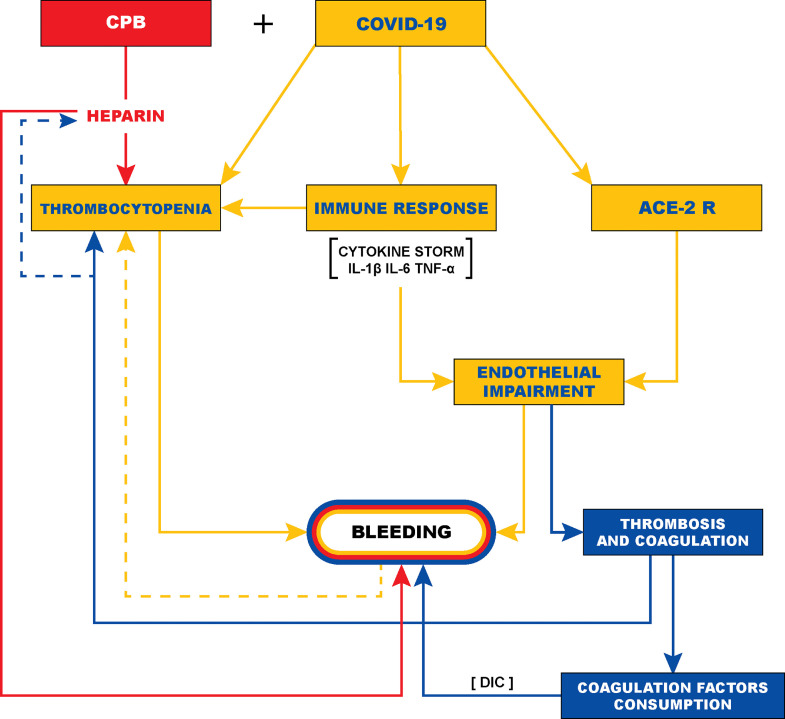
The pathophysiologic mechanisms responsible for the observed postoperative hemorrhage in these patients are not fully understood. As reported in the literature, COVID-19 generates excessive oxidative stress caused by a “cytokine storm” characterized by hyperexpression of proinflammatory mediators (interleukin-1 β, interleukin-6, and tumor necrosis factor-α) responsible for massive diffuse inflammation.11 , 16, 17, 18 An overactive immune response may lead to multiple systemic effects, including direct vascular and microvascular injury with vasculitis, swelling of endothelial cells, cellular apoptosis, necrosis, and severe endothelial damage.19, 20, 21 The angiotensin-converting enzyme II receptor expressed on the arterial and venous endothelium and arterial smooth muscle cells favors viral invasion of the vascular and microvascular tissue. This amplifies the endothelial damage, with increased permeability of the capillary wall, contributing to diffuse systemic bleeding, vasospasm, and possibly ARDS, and multiple organ failure.19, 20, 21, 22, 23, 24
(Fig 2 ). A similar mechanism has been described for intracranial hemorrhagic complications occurring spontaneously or after a biopsy procedure,25, 26, 27, 28 and is likely to occur with any form of bleeding during COVID-19. Furthermore, endothelial damage and diffuse bleeding could lead to thrombocytopenia, consumption of coagulation factors and fibrinolysis, with a possible clinical evolution progressing to acute disseminated intravascular coagulation, thus aggravating hemorrhagic complications.29 Moreover, direct virus-mediated thrombocytopenia has been widely described.11 , 30 In a multicentric analysis of 1,099 patients with laboratory-confirmed COVID-19, Guan et al.11 reported a thrombocytopenia rate of 36.2%. In this vicious cycle, the inflammatory storm with endothelial damage, associated with thrombocytopenia and possible fibrinolysis, may explain and justify the observed incidence of late bleeding events (Fig 2). The observed significant increase of bleeding complications among patients with COVID-19 undergoing cardiac surgery with CPB seems to support the hypotheses described.
Fig 2.
Possible pathophysiologic mechanism of postoperative bleeding in patients with COVID-19 undergoing cardiac surgery. ACE II-R, angiotensin-converting enzyme II receptor; CPB, cardiopulmonary bypass; DIC, disseminated intravascular coagulation.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30
In this study, all patients underwent CPB and, in contrast to that reported in other studies,1 , 5 , 7, 8, 9, 10 bleeding was the most frequently encountered complication. The most likely pathophysiologic mechanisms are, in addition to surgical trauma, CPB-related inflammatory reaction, interstitial edema, heparinization, hemodilution, hemolysis, and coagulation factor consumption, which may have contributed to precipitating postoperative hemorrhagic complications (Fig 2). CPB could exacerbate the deleterious effect of COVID-19 on the coagulation system, leading to its disruption and a deregulation trend toward a hemorrhagic pattern. For these reasons, patients with perioperative COVID-19 undergoing cardiac surgery appear to be more susceptible to postoperative bleeding requiring mediastinal surgical revision and a high number of blood component transfusions.
The authors observed an increased hemorrhagic risk in patients with active COVID-19 at the time of surgery or early postoperatively, and also in those with healed infection before the surgery, thus suggesting a possible persistent hyperinflammatory response even after the resolution of COVID-19.
Despite the introduction of vaccines, the problem of global disease spread does not appear to be resolved. In fact, a new increase in infections recently has been observed in Europe and worldwide, and COVID-19 still is considered to be a severe comorbidity among cardiac surgery patients.31, 32, 33 Based on the authors’ experience, it appears that the high risk for perioperative bleeding is associated with emergency surgery, such as aortic dissection, and also with elective surgery with no additional preoperative risk factors.
Patients with perioperative COVID-19 undergoing cardiac surgery experience a poor outcome, with a high rate of complications, including early and late postoperative bleeding. The aim of this study was to provide the authors’ experience. The authors are aware that the relatively small number of patients in this initial retrospective series, the possible relative inaccuracy of certain parameters such as SHT, and the lack of definitive information in the literature to support their conclusions may represent a limitation of the study. Another limitation of this study was that the authors included patients whose molecular diagnosis of COVID-19 was obtained in the postoperative period, and this theoretically could not have a direct influence on immediate postoperative bleeding, but rather on late hemorrhagic complications. Nevertheless, as the coronavirus incubation time remains uncertain in the literature, the authors selected a 7-day period to rule out postoperative SARS-COV-2 infection. Furthermore, the present study was carried out during the acute phase of COVID-19 outbreak in Italy. Therefore, there were no formal hypotheses being implemented to drive the sample size calculation, and the authors included the maximum number of patients who met the inclusion criteria during this critical period. However, given that the authors’ observations appeared to indicate a significantly increased risk for postoperative complications, this increased risk should be taken into consideration when assessing the operative risk for patients with a perioperative diagnosis of COVID-19.
Acknowledgments
Thank you to Prof. Massimo Fantoni and Dr. Federica Balducci. A very special thanks to Dr. Serena D'Avino and Dr. Francesco Ferraro for their highly valuable contribution to this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.jvca.2021.11.013.
Appendix. Supplementary materials
References
- 1.Yates MT, Balmforth D, Lopez-Marco A, et al. Outcome of patients diagnosed with COVID-19 in the early postoperative period following cardiac surgery. Interact Cardiovasc Thorac Surg. 2020;31:483–485. doi: 10.1093/icvts/ivaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Genio G, Merlino F, Tolone S, et al. Surgery at the time of COVID-19 pandemic: Initial evidence of safe practice. Br J Surg. 2020;107:e266. doi: 10.1002/bjs.11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyos Mejía L, Romero Román A, Gil Barturen M, et al. Thoracic surgery during the coronavirus disease 2019 (COVID-19) pandemic in Madrid, Spain: Single-centre report. Eur J Cardiothorac Surg. 2020;58:1991–1996. doi: 10.1093/ejcts/ezaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussain A, Khan H, Lopez-Marco A, et al. Cardiac surgery in patients with confirmed COVID-19 infection: Early experience. J Card Surg. 2020;35:1351–1353. doi: 10.1111/jocs.14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biondi Zoccai G, Landoni G, Carnevale R, et al. SARS-CoV-2 and COVID-19: Facing the pandemic together as citizens and cardiovascular practitioners. Minerva Cardioangiol. 2020;68:61–64. doi: 10.23736/S0026-4725.20.05250-0. [DOI] [PubMed] [Google Scholar]
- 6.Villa E, Saccocci M, Messina A, et al. COVID-19 and coronary artery disease: Selective and collaborative use of resources during public health crisis. G Ital Cardiol (Rome) 2020;21:360–363. doi: 10.1714/3343.33135. [DOI] [PubMed] [Google Scholar]
- 7.Hu X, Wang Y, Xia J, et al. Managements of 13 emergency cardiac surgeries under COVID-19 pandemic in a Sentinel Hospital. J Thorac Dis. 2020;12:6663–6669. doi: 10.21037/jtd-20-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubino AS, De Santo LS, Pisano A. Cardiac surgery practice during the COVID-19 outbreak: A multicenter national survey. Eur J Cardiothorac Surg. 2021;59:901–907. doi: 10.1093/ejcts/ezaa436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattouch K, Corrao S, Augugliaro E, et al. Cardiac surgery outcomes in patients with coronavirus disease 2019 (COVID-19): a case series report. J Thorac Cardiovasc Surg. 2020 doi: 10.1016/j.jtcvs.2020.09.138. On-line ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunaydin S, Stammers AH. Perioperative management of COVID-19 patients undergoing cardiac surgery with cardiopulmonary bypass. Perfusion. 2020;35:465–473. doi: 10.1177/0267659120941341. [DOI] [PubMed] [Google Scholar]
- 11.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford TC, Magruder JT, Grimm JC, et al. Planned versus unplanned reexplorations for bleeding: a comparison of morbidity and mortality. Ann Thorac Surg. 2017;103:779–786. doi: 10.1016/j.athoracsur.2016.06.096. [DOI] [PubMed] [Google Scholar]
- 13.Biancari F, Mikkola R, Heikkinen J, et al. Estimating the risk of complications related to re-exploration for bleeding after adult cardiac surgery: A systematic review and meta-analysis. Eur J Cardiothorac Surgery. 2012;41:50–55. doi: 10.1016/j.ejcts.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranucci M, Baryshnikova E, Castelvecchio S, et al. Major bleeding, transfusions, and anemia: The deadly triad of cardiac surgery. Ann Thorac Surg. 2013;96:478–485. doi: 10.1016/j.athoracsur.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aksöyek A, Tütün U, Ulus T, et al. Surgical drainage of late cardiac tamponade following open heart surgery. Thorac Cardiovasc Surg. 2005;53:285–290. doi: 10.1055/s-2005-837679. [DOI] [PubMed] [Google Scholar]
- 17.Iannaccone G, Scacciavillani R, Del Buono MG, et al. Weathering the cytokine storm in COVID-19: Therapeutic implications. Cardiorenal Med. 2020;10:277–287. doi: 10.1159/000509483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montone RA, Iannaccone G, Meucci MC, et al. Myocardial and microvascular injury due to Coronavirus disease 2019. Eur Cardiol. 2020;15:e52. doi: 10.15420/ecr.2020.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endothelitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iba T, Levy JH, Levi M, et al. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altschul DJ, Unda SR, de La Garza Ramos R, et al. Hemorrhagic presentations of COVID-19: Risk factors for mortality. Clin Neurol Neurosurg. 2020;198 doi: 10.1016/j.clineuro.2020.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degeneffe A, Bruneau M, Spitaels J, et al. Acute hemorrhage after intracerebral biopsy in COVID-19 patients: Report of 3 cases. World Neurosurg. 2020;141:157–161. doi: 10.1016/j.wneu.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong S, Liu P, Luo Y, et al. Pathophysiology of SARS-CoV-2 infection in patients with intracerebral hemorrhage. Aging (Albany NY) 2020;12:13791–13802. doi: 10.18632/aging.103511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cezar-Junior AB, Faquini IV, Silva JLJ, et al. Subarachnoid hemorrhage and COVID-19: Association or coincidence? Medicine (Baltimore) 2020;99:e23862. doi: 10.1097/MD.0000000000023862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taccheri T, Calabrese M, Arlotta G, et al. Anesthetic management of patients undergoing aortic dissection repair with suspected severe acute respiratory syndrome coronavirus disease 2019 (COVID-19) infection. J Cardiothorac Vasc Anesth. 2021;35:350–352. doi: 10.1053/j.jvca.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giamberti A, Caldaroni F, Varrica A, et al. Impact of COVID-19 pandemic on the Italian humanitarian congenital cardiac surgery activity: What no one tells you. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.705029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harky A, Harrington D, Nawaytou O, et al. COVID-19 and cardiac surgery: The perspective from United Kingdom. J Card Surg. 2021;36:1649–1658. doi: 10.1111/jocs.15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clifford SP, Ramirez J, Akca O, et al. Perioperative cardiac research considerations during the Coronavirus Disease 2019 (COVID-19) pandemic. J Cardiothorac Vasc Anesth. 2021;35:1573–1577. doi: 10.1053/j.jvca.2021.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.