Abstract
Background
Approximately 80% of people with COVID-19 do not require hospitalization. Studies examining the outpatient experience have not tracked symptoms to resolution leading to unknown expected symptom duration. Our objectives were to (1) determine symptom duration among patients with COVID-19 who do not require hospitalization and (2) identify potential risk factors associated with prolonged symptom duration.
Design
This is a retrospective cohort study conducted across an academic healthcare system including adult patients with laboratory‐confirmed SARS-CoV-2 infection between March 18th and April 28th, 2020 who were not hospitalized. Symptom duration encompassed time from patient-reported symptom onset as documented in the chart until documented symptom resolution. We calculated the median symptom duration and tested if demographics, comorbidities, or reported symptoms were associated with symptom duration.
Key results
Of 294 patients meeting inclusion criteria, 178 (60.5%) had documented symptom resolution. The median [interquartile range (IQR)] symptom duration for included patients was 15 (8-24) days. No associations were found between comorbidities and symptom duration. Factors associated with prolonged symptom duration were presence vs lack of lower respiratory symptoms [median (IQR) 16.5 (10.75-33.5) vs 14.5 (7-21.75) days respectively, P < .001] and neurologic symptoms [median (IQR) 17 (9-28) vs 9.5 (4-17) days, P < .001] at disease onset.
Conclusions
The median symptom duration in outpatients is 15 days and over 25% of patients have symptoms longer than 21 days.
Key Words: Coronavirus infections, Risk factors, Ambulatory care, Recovery of function, Outpatients statistics and numerical data
Background
The novel coronavirus disease 2019 (COVID-19) is a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which has caused a global pandemic. While there are numerous studies examining the clinical course of patients with COVID-19 requiring hospitalization or critical care,1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 there is limited data describing the disease course in the outpatient population despite the fact that most patients with COVID-19 do not require hospitalization.15, 16, 17, 18, 19, 20, 21, 22, 23 Older age and the presence of specific medical conditions have previously been associated with illness severity among adults hospitalized with COVID-19.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 Both age and specific medical conditions have been associated with greater susceptibility to the disease and prolonged illness in outpatient populations, although data supporting these findings are limited.15 , 35, 36, 37 Several studies have described symptoms at presentation and used pre-specified time periods to check patient symptoms.15 , 18 , 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 However, to our knowledge, only two studies have followed subjects to symptom resolution,20 , 21 and no study has tested if symptoms at disease onset are predictive of symptom duration.
Objective
Our objectives were to (1) determine the symptom duration among patients with COVID-19 who do not require hospitalization and (2) identify potential risk factors associated with prolonged duration of symptoms.
Methods
Study design and participants
This retrospective cohort study was conducted across a United States academic healthcare system. The study was approved by the hospital institutional review board with a waiver of informed consent. We included adult patients diagnosed with COVID-19 in the outpatient setting (excluding the emergency department) between March 18, 2020 and April 28, 2020. Inclusion criteria were: (1) adult patients (ie, age ≥18 years); (2) reverse transcription polymerase chain reaction (RT-PCR) confirmed SARS-CoV-2 infection; and (3) primary care received within our healthcare system. We chose the study inclusion dates because during this period our institution limited testing to those with at least one symptom of COVID-19 and at least one risk factor—because of the limitations and the relative novelty of the disease, close follow up was possible and the standard of care. After April 28, 2020, restrictions were no longer placed on testing and thus close follow up was no longer feasible due to the high volume of testing. Symptoms were defined as cough, shortness of breath, or fever (T>100°F). Risk factors were defined according to Centers for Disease Control (CDC) as being a healthcare worker or first responder (emergency medical services, firefighters, police, etc.), resident of an institutional home setting (group home, barracks, etc.), having an age greater than 65 years, being in an immunocompromised state, pregnancy, chronic lung disease, congestive heart failure, end-stage renal disease/dialysis, uncontrolled diabetes, or vulnerable household contacts with these same risk factors as above. 55 Patients were excluded if they tested negative for COVID-19, if they had suspicious symptoms for COVID-19 but were not tested, if they were admitted to the hospital due to COVID-19, or if symptom duration could not be determined. Reasons for indeterminate symptom resolution were presence of symptoms at time of data collection, conflicting dates of symptom resolution recorded in the EMR, patient being lost to immediate follow up with documentation in the EMR that symptoms had resolved without an exact date, and patient lost to any follow up. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement.52
Data collection
Investigators reviewed the electronic medical record [EPIC (EPIC Systems Corporation, WI)] and abstracted data for each subject. A standardized data extraction form and predefined definitions of variables were used for all data collection (Appendix). The abstractors held periodic meetings to review coding rules and to monitor performance.53 We calculated the intraclass correlation coefficient to determine inter-observer agreement between the abstractors based on a 10% sample of cases selected at random.
Table 2.
Symptoms during COVID-19 Illness
| All Subjects (Percent) n = 294 | Included n = 178 | Excluded* n = 116 | P-value | |
|---|---|---|---|---|
| Symptoms at Illness Onset | ||||
| Cough | 201 (68.4%) | 117 (65.7%) | 84 (72.4%) | .22 |
| Fever > 100 degrees | 162 (55.1%) | 98 (55.1%) | 64 (55.2%) | .98 |
| Myalgias | 118 (40.1%) | 69 (38.8%) | 49 (42.2%) | .55 |
| Chills | 82 (27.9%) | 37 (20.8%) | 45 (38.8%) | <.001 |
| Headache | 78 (26.5%) | 45 (25.3%) | 33 (28.4%) | .54 |
| Fatigue | 68 (23.1%) | 41 (23%) | 27 (23.3%) | .96 |
| Rhinorrhea | 59 (20.1%) | 33 (18.5%) | 26 (22.4%) | .41 |
| SOB | 53 (18%) | 26 (14.6%) | 27 (23.3%) | .05 |
| Sore Throat | 50 (17%) | 28 (15.7%) | 22 (18.9%) | .47 |
| Anosmia | 46 (15.6%) | 28 (15.7%) | 18 (15.5%) | .96 |
| Diarrhea | 32 (10.9%) | 19 (10.7%) | 13 (11.2%) | .88 |
| Nausea | 24 (8.2%) | 15 (8.4%) | 9 (7.8%) | .83 |
| Sputum Production | 19 (6.4%) | 11 (6.2%) | 8 (6.9%) | .80 |
| Vomiting | 14 (4.8%) | 5 (2.8%) | 9 (7.8%) | .05 |
| DOE | 13 (4.4%) | 6 (3.4%) | 7 (6 %) | .27 |
| Conjunctivitis | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Symptoms at Any Point in Illness* | ||||
| Cough | 241 (82%) | 139 (78.1%) | 102 (87.9%) | .03 |
| Fever >100 degrees | 200 (68%) | 120 (67.4%) | 80 (68.9%) | .78 |
| Myalgias | 151 (51.4%) | 86 (48.3%) | 65 (56%) | .19 |
| Headache | 112 (38.1%) | 65 (36.5%) | 47 (40.5%) | .49 |
| Fatigue | 106 (36.1%) | 61 (34.3%) | 45 (38.8%) | .43 |
| SOB | 102 (34.7%) | 52 (29.2%) | 50 (43.1%) | .01 |
| Chills | 99 (33.7%) | 49 (27.5%) | 50 (43.1%) | .006 |
| Rhinorrhea | 79 (26.9%) | 44 (24.7%) | 35 (30.2%) | .30 |
| Anosmia | 76 (25.9%) | 49 (27.5%) | 27 (23.3%) | .41 |
| Diarrhea | 69 (23.5%) | 41 (23%) | 28 (24.1%) | .82 |
| Sore Throat | 66 (22.4%) | 37 (20.8%) | 29 (25%) | .39 |
| Nausea | 40 (13.6%) | 24 (13.5%) | 16 (13.8%) | .94 |
| DOE | 39 (13.3%) | 16 (8.9%) | 23 (19.8%) | .007 |
| Sputum Production | 35 (11.9%) | 20 (11.2%) | 15 (12.9%) | .66 |
| Vomiting | 14 (4.8%) | 5 (2.8%) | 9 (7.8%) | .05 |
| Conjunctivitis | 2 (0.7%) | 0 (0%) | 2 (1.7%) | .15 |
SOB, shortness of breath; DOE, dyspnea on exertion.
This is for symptoms that were present at least once in patient's chart during documented illness period.
We recorded demographics and medical comorbidities. Zip code data were collected and used as a surrogate for socioeconomic status. We defined low socioeconomic status as having a zip code within Camden City, NJ. Camden City was designated by the 2010 U.S. Census Bureau as the second poorest city with a population over 65,000 in the U.S. Camden families earned an average of $33,120 in 2019, which is far below the state median family income of $85,751.54
We recorded all documented patient-reported symptoms. We defined symptoms at disease onset as the first symptoms the patient identified as a change from baseline and symptoms during disease course as any symptom the patient identified from the change in their baseline to symptom resolution. For both symptoms at disease onset and symptoms during disease course we categorized symptoms as respiratory (cough, shortness of breath, and dyspnea on exertion), neurological (headache, myalgia, and fatigue), or gastrointestinal symptoms (nausea, vomiting, and diarrhea). During the data-collection period, the included pre-defined symptoms included were those identified by the CDC as being associated with COVID-19.55 In addition to the previously mentioned symptoms, these symptoms included a fever >100° Fahrenheit, chills, sputum production, sore throat, rhinorrhea, anosmia, conjunctivitis, myalgias, nausea, vomiting, and fatigue. We also included a category for other symptoms not included in this list.
Main measures
The primary outcome was symptom duration, defined as the number of days a patient had one or more symptoms associated with RT-PCR-confirmed SARS-CoV-2 infection. We used patient-reported onset of symptoms and symptoms were followed until documented resolution. The chart had to have explicit documentation of the date when patients reported their symptoms had ended. During our study, primary care offices and the public health department followed patients closely, and common practice at the time was to call patients every two days to monitor and document symptoms until resolution using a standardized question form. As the number of patients with COVID-19 grew, this follows up practice did decrease prior to the conclusion of the study dates and physicians often stopped calling patients when the majority of symptoms resolved and patients reported feeling well otherwise. We defined prolonged symptoms as symptoms lasting longer than the recommended isolation period of 10 days. We entered all data into a Research Electronic Data Capture (REDCap, Vanderbilt University, TN) database56 , 57 and exported the data into SPSS 22 (IBM, Armonk, NY) for analysis of the data.
Statistical analysis
We performed descriptive analyses using means and standard deviations (SD) or medians and interquartile ranges (IQR), depending on the data distribution, for continuous variables; we used frequencies with proportions for categorical data. We calculated the median (IQR) symptom duration for the entire cohort. We compared demographics and reported symptoms between included patients versus excluded patients.
Among included patients, we performed univariable analyses to test for potential predictors of symptom duration using the Mann-Whitney U test. Potential risk factors tested included symptoms documented at disease presentation, comorbidities, and zip codes within Camden City. The significance level was P ≤ .05. We then looked at clusters of lower respiratory, neurologic, and gastrointestinal symptoms to determine if an association with time to resolution existed. We defined the clusters as follows: lower respiratory (cough, shortness of breath, dyspnea on exertion); gastrointestinal (nausea, vomiting, diarrhea); and neurologic (myalgia, fatigue, headache). Our goal was to determine if having more symptoms at onset in a particular organ system would have greater predictability in symptom duration. We did not adjust for multiple comparisons because this second objective was an exploratory analysis.
Key results
Demographic data
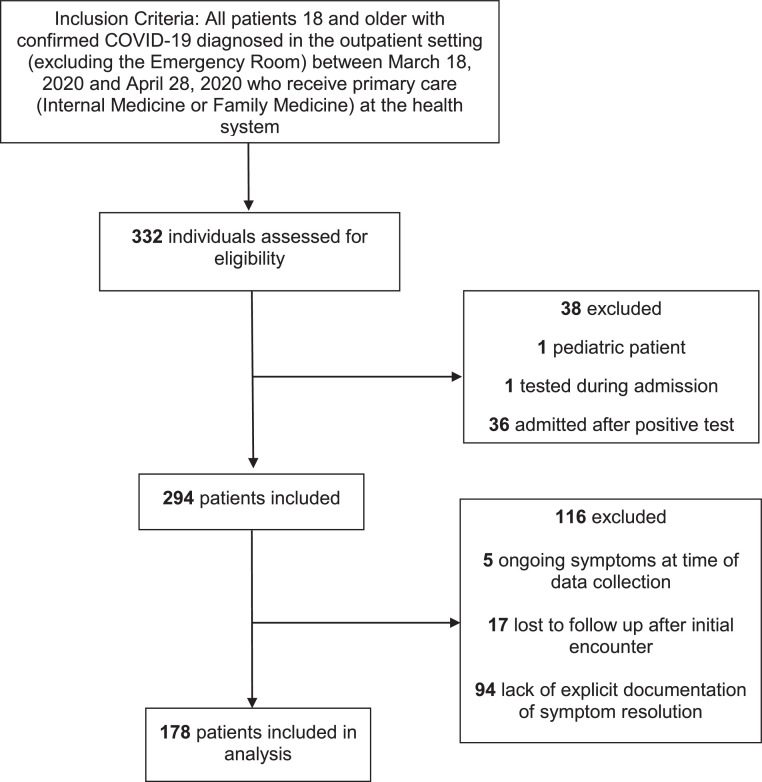
Of the 294 patients who met the inclusion criteria, symptom duration could be determined for 178 (60.5%) (Fig 1 ). The demographic data for the included cohort, as well as those excluded secondary to inability to determine symptom duration are displayed in Table 1 . The mean (SD) age was 47.2 (13.6) years. The majority of patients were women (57.9%). When stratifying by zip code we found that 26.4% of patients resided in Camden City. The majority of patients were Hispanic (37.1%), followed by Black non-Hispanic (27.5%) and White non-Hispanic (24.2%). We found only one significant difference in patient baseline characteristics: our included cohort was less likely to be non-Hispanic white.
Fig 1.
Flow diagram of inclusion criteria.
Table 1.
Demographic characteristics and comorbidities of patients
| Characteristic | All Subjects (Percent) n = 294 | Included n = 178 | Excluded* n = 116 | P-value |
|---|---|---|---|---|
| Sex | ||||
| Women | 174 (59.2%) | 103 (57.9%) | 71 (61.2%) | .569 |
| Age group (yrs) | .518 | |||
| 18-34 | 67 (22.8%) | 40 (22.5%) | 27 (23.3%) | |
| 35-49 | 88 (29.9%) | 50 (28.1%) | 38 (32.7%) | |
| 50-64 | 116 (39.5%) | 71 (39.9%) | 45 (38.8%) | |
| ≥65 | 23 (7.8%) | 17 (9.5%) | 6 (5.2%) | |
| BMI | .693 | |||
| Normal (18.5-24.9) | 46 (15.6%) | 28 (15.7%) | 18 (15.5%) | |
| Overweight (25-29.9) | 87 (29.6%) | 58 (32.6%) | 29 (25%) | |
| Class I Obesity (30-34.9) | 82 (27.9%) | 46 25.8% | 36 (31%) | |
| Class II Obesity (35-39.9) | 37 (12.6%) | 20 (11.2%) | 17 (14.7%) | |
| Class III Obesity (>40) | 38 (12.9%) | 23 (12.9%) | 15 (12.9%) | |
| Unknown | 4 (1.4%) | 3 (1.7%) | 1 (0.9%) | |
| Camden City Resident | 74 (25.2%) | 47 (26.4%) | 27 (23.3%) | .546 |
| Race/Ethnicity | ||||
| White/Non-Hispanic | 86 (29.3%) | 43 (24.2%) | 43 (37.1%) | .011 |
| Black/Non-Hispanic | 78 (26.5%) | 49 (27.5%) | 29 (25%) | .711 |
| Hispanic | 98 (33.3%) | 66 (37.1%) | 32 (27.6%) | .113 |
| Asian | 13 (4.4%) | 10 (5.6%) | 3 (2.6%) | .230 |
| Unknown | 19 (6.5%) | 10 (5.6%) | 9 (7.7%) | .466 |
| Comorbidities | ||||
| HTN | 120 (40.1%) | 75 (42.1%) | 45(38.8%) | .569 |
| Smoking | n = 293 | n = 177 | n = 116 | |
| Positive Smoking Hx | 89 (30.4%) | 59 (33.3%) | 30 (25.9%) | .174 |
| Current Smoker | 28 (9.6%) | 21 (11.9%) | 7 (6%) | .097 |
| DM1 | 2 (0.7%) | 1 (0.6%) | 1 (0.9%) | 1.000 |
| DM2 | 49 (16.4%) | 28 (15.7%) | 21 (18.1%) | .594 |
| Mild intermittent asthma | 25 (8.4%) | 13 (7.3%) | 12 (10.3%) | .361 |
| Moderate persistent asthma | 10 (3.3%) | 9 (5.1%) | 1 (0.9%) | .095 |
| Severe persistent asthma | 2 (0.7%) | 1 (0.6%) | 1 (0.9%) | 1.000 |
| CAD | 9 (3%) | 4 (2.2%) | 5 (4.3%) | .326 |
| Immunosuppressive condition | 8 (2.7%) | 4 (2.2%) | 4 (3.4%) | .716 |
| Cancer | 8 (2.7%) | 4 (2.2%) | 4 (3.4%) | .716 |
| COPD | 5 (1.7%) | 3 (1.7%) | 2 (1.7%) | 1.000 |
| COPD on home oxygen | 0 (0%) | 0 (0%) | 0 (0%) | – |
| HFrEF | 2 (0.7%) | 1 (0.6%) | 1 (0.9%) | 1.000 |
| HFpEF | 0 (0%) | 0 (0%) | 0 (0%) | – |
| PAD | 2 (0.7%) | 0 (0%) | 2 (1.7%) | .155 |
| Chronic steroid use | 2 (0.7%) | 2 (1.1%) | 0 (0%) | .521 |
| Liver cirrhosis | 1 (0.3%) | 1 (0.6%) | 0 (0%) | 1.000 |
| ESRD | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Hyperlipidemia | 40 (13.6%) | 26 (14.1%) | 14 (12.1%) | .535 |
| Anxiety | 30 (10.2%) | 17 (9.6%) | 13 (11.2%) | .647 |
| OSA | 24 (8.2%) | 15 (8.4%) | 9 (7.6%) | .838 |
| GERD | 24 (8.2%) | 18 (10.1%) | 6 (5.2%) | .131 |
| Depression | 21 (7.1%) | 11 (6.2%) | 10 (8.6%) | .427 |
| Allergic rhinitis | 14 (4.8%) | 10 (5.6%) | 4 (3.4%) | .393 |
| Pre-diabetes | 11 (3.7%) | 7 (3.9%) | 4 (3.4%) | 1.000 |
BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DM1, diabetes mellitus type 1; DM2, diabetes mellitus type 2; ESRD. End stage renal disease; HFpEF, heart failure with preserved ejection fraction; HTN, hypertension; IQR, interquartile range; HFrEF, heart failure with reduced ejection fraction; PAD, peripheral arterial disease; SD, standard deviation.
Excluded secondary to inability to determine symptom duration.
Clinical outcomes
Symptoms
The most common symptoms at presentation are presented in Table 2 . The most common symptoms at presentation were cough (65.7%), fever (55.1%), and myalgia (38.8%). Similarly, the most common symptoms at any point in symptom illness were cough (78.1%), fever (67.4%), and myalgia (48.3%).
Comorbidities
The most common comorbidity was hypertension (42.1%), followed by diabetes mellitus (16.3%) and mild intermittent asthma (7.3%) (Table 1). There were no reported comorbidities in 39 patients (21.9%).
Symptom duration
Inter-observer agreement among data abstractors was excellent for symptom duration [intraclass correlation coefficient = 0.97 (95% confidence interval 0.94-0.99)]. The mean symptom duration was 19 days, with a minimum of 0 days and a maximum of 93 days (SD 16.29). The median (IQR) symptom duration was 15 (8-24) days.
The following symptoms if present at any point during disease course were found to be associated with prolonged symptom duration: cough (P = .002), shortness of breath (P < .001), dyspnea on exertion (P = .004), myalgia (P < .001), and anosmia (P = .022). There was no statistically significant difference in time to resolution of symptoms with any of the demographic variables we included in the study (Table 3 ).
Table 3.
Time to resolution based on symptom versus no symptom
| Symptom | Median Time to Resolution (IQR) | P-value |
|---|---|---|
| Fever | 15 (8-24.75) | .922 |
| No Fever | 15 (8.75-24) | |
| Chills | 17 (11-26.5) | .118 |
| No Chills | 14 (7.5-23.5) | |
| Headaches | 17 (9-28.5) | .151 |
| No Headache | 15 (7.5-23) | |
| Sputum Production | 17 (14.25-30) | .288 |
| No Sputum Production | 14 (8-24) | |
| Sore Throat | 18 (11-26.5) | .1 |
| No Sore Throat | 14 (8-23) | |
| Rhinorrhea | 18 (8-29) | .077 |
| No Rhinorrhea | 14 (8-23) | |
| Diarrhea | 19 (11-34) | .006 |
| No Diarrhea | 14 (7-22) | |
| Nausea | 15.5 (8.25-34.25) | .425 |
| No Nausea | 15 (8-24) | |
| Vomiting | 21 (7.5-40) | .457 |
| No Vomiting | 15 (8-24) | |
| Anosmia | 17 (10-29) | .022 |
| No Anosmia | 14 (7-21.5) | |
| Myalgias | 18 (11.75-28) | <.001 |
| No Myalgias | 11.5 (6-21) | |
| Fatigue | 17 (9-27) | .075 |
| No Fatigue | 14 (7.5-22) | |
| SOB | 19.5 (13-35.75) | <.001 |
| No SOB | 13.5 (7-21.25) | |
| DOE | 29 (13.25-43.75) | .004 |
| No DOE | 14.5 (8-23) | |
| Cough | 17 (9-27) | .002 |
| No Cough | 9 (4-18) |
doe, dyspnea on exertion; IQR, interquartile range; sob, shortness of breath.
The median (IQR) symptom duration when patients had no lower respiratory symptoms at disease onset was 14.5 (7-21.75) days compared to 16.5 (10.75-33.5) days if patients had all three lower respiratory symptoms of cough, shortness of breath, and dyspnea on exertion (P < .001). Gastrointestinal predominant symptoms were not associated with symptom duration. The median (IQR) time to resolution when patients had no neurologic symptoms was 9.5 (4-17) days compared to 17 (9-28) days if patients had all three neurologic symptoms of myalgia, fatigue, and headache. Residence in a Camden City zip code was not associated with the number of symptoms or duration of symptoms associated with the illness. We also found no association between medical comorbidities and symptom duration (Table 4 ).
Table 4.
Time to resolution based on comorbidity versus no comorbidity
| Comorbidity* | Median Time to Resolution (IQR) | P-value |
|---|---|---|
| HTN | 17 (11-26) | .138 |
| No HTN | 14 (7-23) | |
| Smoking | 15 (11-24) | .804 |
| No Smoking | 15.5 (7.75-24.25) | |
| Diabetes Type 1 or 2 | 14 (9-21) | .710 |
| No Diabetes | 15 (8-25) | |
| Asthma | 17 (7-26) | .993 |
| No Asthma | 15 (9-24) | |
| CAD | 10 (3.5-13) | .074 |
| No CAD | 15 (8-24.5) | |
| Immunosuppressive condition | 15.5 (7.25-35.75) | .902 |
| No Immunosuppressive condition | 15 (8-24) | |
| Cancer | 15 (3.25-55.25) | .945 |
| No Cancer | 15 (8-24) |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; ESRD. End stage renal disease; PAD, peripheral arterial disease; HTN, hypertension.
The following comorbidities did not have enough data to report full IQR: COPD, heart failure, PAD, chronic steroid use, cirrhosis, ESRD.
Discussion
The outpatient experience of COVID-19 has been relatively undefined in the literature. This study found that the median symptom duration was 15 days which is longer than the 10 days the CDC recommends for isolation.58 This suggests persons with COVID-19 may remain symptomatic longer than their isolation period. Further, over 25% of patients had symptoms lasting longer than three weeks. We did not exclude patients with symptoms lasting longer than 4 weeks as long COVID-19 was not known at the time of our data analysis, but in our data analysis, only one patient met criteria for symptoms longer than 4 weeks with symptom resolution after 93 days.
Similar to other studies14 , 15 , 20 , 22 , 33 , 37 , 42, 43, 44 , 59 we found cough and fever to be the most common presenting symptoms at disease onset. We found fever to be the most common presenting symptom, unlike Blair et al20 However, similar to Blair et al,20 we also found cough to be the most predominant symptom ongoing. The lower respiratory cluster and the neurologic cluster were associated with the longest-lasting symptoms and the only symptoms associated with a prolonged duration of illness in the outpatient setting. Notably, gastrointestinal symptoms, constitutional symptoms such as fever, and upper respiratory symptoms (sore throat and rhinorrhea) had no association with symptom duration. Although numerous studies have published the likelihood of anosmia in mild COVID-19 cases,12 , 36 , 40 , 42 , 45 , 50 , 60 we found this to be a symptom in only 25.9% of cases. However, as this symptom was not as well-known during the initial months of the pandemic, clinicians may have failed to ask and document this symptom.
Also similar to other outpatient studies,14 , 17 , 20 , 34 , 35 we found the most common comorbidity to be hypertension (40%). In our cohort, the majority of subjects had a BMI >30 (77%). This is a greater prevalence than the general population (hypertension, 33% and BMI >30, 42%).61 , 62 Although it is possible that these comorbid conditions are risk factors for disease contraction in mild to moderate illness, this result is also possibly due to selection bias given that all subjects tested during this time period had to be high risk. However, while comorbidities such as hypertension and obesity are known risk factors for COVID-19 disease severity63, 64, 65 we did not find any of the tested comorbidities to be associated with prolonged symptom duration in outpatients.
Our results showing an association between lower respiratory symptoms and prolonged symptom duration among ambulatory patients are consistent with previous studies that found hospitalized patients have prolonged clinical courses in the presence of severe respiratory disease.25 , 27 , 28 , 30, 31, 32 , 35 , 37 , 38 While ongoing research on long COVID-19 must continue, our findings suggest that even for those who have symptom resolution, lower respiratory symptoms portend a longer recovery period. Our findings are of clinical importance to primary care providers as they can help provide guidance on informing patients what to expect of symptomatology and expected disease course of mild to moderate outpatient COVID-19 illness.
Limitations
This study has several important limitations. First, this study was a retrospective cohort study performed across a single healthcare system and 39.5% of potential subjects were excluded secondary to inability to determine symptom duration. However, in comparing our included and excluded cohorts, we found only one significant difference in patient baseline characteristics: our included cohort was less likely to be non-Hispanic white. This could decrease the generalizability to the general public. When interpreting our results, it is also important to note that our included cohort was less likely to report chills or shortness of breath compared to those excluded for lack of documented symptom duration. We are reassured in the accuracy of symptom duration given the excellent inter-observer agreement among data abstractors. Although patients’ and physicians’ interpretations of symptoms could have led to recall bias and errors in documenting both onset and termination of symptoms, given the high concern about COVID-19 during the study period, physicians were following up regularly in real time with their patients and using standard questions that mitigated this bias. Second, subjects included in the study may have been more ill that others due to the testing restrictions which were in place during the study period, and patients with lower risk and fewer symptoms may have a different symptom duration. However, 42% of included patients did not have co-morbidities, thereby supporting the generalizability of results. Therefore, these results are important for primary care physicians caring for similar symptomatic COVID-19 patients with regard to discussing the expected duration of symptoms.
Conclusion
We found the median (IQR) time to resolution of symptoms for COVID-19 in the outpatient setting was 15 (8-24) days, with greater than 25% of subjects having symptom duration greater than three weeks. The only predictors of prolonged symptom duration were lower respiratory symptoms or neurologic symptoms at onset. These results are of clinical importance to providers as they can help provide guidance when informing patients as to the expected disease course of mild to moderate outpatient COVID-19 illness.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2021.10.039.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Y, Tu L, Zhu P, et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am J Resp Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim ES, Chin BS, Kang CK, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the korean cohort study on COVID-19. J Korean Med Sci. 2020;35:e142. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang D, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. Jama. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clinical research ed) 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ (Clinical research ed) 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang G, Gong T, Wang G, et al. Timely diagnosis and treatment shortens the time to resolution of coronavirus disease (covid-19) pneumonia and lowers the highest and last CT scores from sequential chest CT. AJR Am J Roentgenol. 2020;215:367–373. doi: 10.2214/AJR.20.23078. [DOI] [PubMed] [Google Scholar]
- 14.Ramasamy K, Saniasiaya J, Abdul Gani N. Olfactory and Gustatory dysfunctions as a clinical manifestation of coronavirus disease 2019 in a Malaysian tertiary center. Ann Otol Rhinol Laryngol. 2020;130 doi: 10.1177/0003489420963165. [DOI] [PubMed] [Google Scholar]
- 15.Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020;26:861–868. doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- 17.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergquist SH, Partin C, Roberts DL, et al. Non-hospitalized adults with COVID-19 differ noticeably from hospitalized adults in their demographic, clinical, and social characteristics. SN Compr Clin Med. 2020;2:1349–1357. doi: 10.1007/s42399-020-00453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight D, Downes K, Munipalli B, et al. Symptoms and clinical outcomes of coronavirus disease 2019 in the outpatient setting. SN Compr Clin Med. 2021;3:247–254. doi: 10.1007/s42399-021-00746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blair JE, Gotimukul A, Wang F, et al. Mild to moderate COVID-19 illness in adult outpatients: Characteristics, symptoms, and outcomes in the first 4 weeks of illness. Medicine (Baltimore) 2021;100:e26371. doi: 10.1097/MD.0000000000026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettrone K, Burnett E, Link-Gelles R, et al. Characteristics and risk factors of hospitalized and nonhospitalized COVID-19 patients, Atlanta, Georgia, USA, March-April 2020. Emerg Infect Dis. 2021;27:1164–1168. doi: 10.3201/eid2704.204709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim GU, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26:948.e941–948.e943. doi: 10.1016/j.cmi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pullen MF, Skipper CP, Hullsiek KH, et al. Symptoms of COVID-19 Outpatients in the United States. Open Forum Infect Dis. 2020;7:ofaa271. doi: 10.1093/ofid/ofaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ (Clinical research ed) 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott SE, Zabel K, Collins J, et al. First Mildly Ill, Nonhospitalized Case of Coronavirus Disease 2019 (COVID-19) Without Viral Transmission in the United States-Maricopa County, Arizona, 2020. Clin Infect Dis. 2020;71:807–812. doi: 10.1093/cid/ciaa374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirtana J, Kumar A, Kumar SS, et al. Mild COVID-19 infection-predicting symptomatic phase and outcome: A study from AIIMS, New Delhi. J Family Med Prim Care. 2020;9:5360–5365. doi: 10.4103/jfmpc.jfmpc_1610_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Han X, Alwalid O, et al. Baseline characteristics and risk factors for short-term outcomes in 132 COVID-19 patients with diabetes in Wuhan China: A retrospective study. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flaherty GT, Hession P, Liew CH, et al. COVID-19 in adult patients with pre-existing chronic cardiac, respiratory and metabolic disease: a critical literature review with clinical recommendations. Trop Dis Travel Med Vaccines. 2020;6:16. doi: 10.1186/s40794-020-00118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann-Podczaska A, SR Al-Saad, Karbowski LM, Chojnicki M, Tobis S, Wieczorowska-Tobis K. COVID 19 - clinical picture in the elderly population: a qualitative systematic review. Aging Dis. 2020;11:988–1008. doi: 10.14336/AD.2020.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12:12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Y, Lu Y, Xia L, et al. Analysis of 2019 novel coronavirus infection and clinical characteristics of outpatients: An epidemiological study from a fever clinic in Wuhan, China. J Med Virol. 2020;92:2758–2767. doi: 10.1002/jmv.26175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun H, Jain A, Leone MJ, et al. COVID-19 outpatient screening: a prediction score for adverse events. medRxiv. 2020 [Google Scholar]
- 41.Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020;10:821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clemency BM, Varughese R, Scheafer DK, et al. Symptom Criteria for COVID-19 Testing of Heath Care Workers. Acad Emerg Med. 2020;27:469–474. doi: 10.1111/acem.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapostolle F, Schneider E, Vianu I, et al. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern Emerg Med. 2020;15:813–817. doi: 10.1007/s11739-020-02379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joffily L, Ungierowicz A, David AG, et al. The close relationship between sudden loss of smell and COVID-19. Braz J Otorhinolaryngol. 2020;86:632–638. doi: 10.1016/j.bjorl.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmerman RK, Nowalk MP, Bear T, et al. Proposed Clinical indicators for efficient screening and testing for covid-19 infection from classification and regression trees (CART) analysis. Hum Vaccin Immunother. 2021;17:1109–1112. doi: 10.1080/21645515.2020.1822135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zayet S, Klopfenstein T, Mercier J, et al. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection. 2021;49:361–365. doi: 10.1007/s15010-020-01442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, Pinto MD, Borelli JL, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: looking for clarity in the haze of the pandemic. medRxiv [Preprint] 2021 doi: 10.1101/2021.03.03.21252086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mancuso P, Venturelli F, Vicentini M, et al. Temporal profile and determinants of viral shedding and of viral clearance confirmation on nasopharyngeal swabs from SARS-CoV-2-positive subjects: a population-based prospective cohort study in Reggio Emilia, Italy. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makaronidis J, Firman C, Magee CG, et al. Distorted chemosensory perception and female sex associate with persistent smell and/or taste loss in people with SARS-CoV-2 antibodies: a community based cohort study investigating clinical course and resolution of acute smell and/or taste loss in people with and without SARS-CoV-2 antibodies in London, UK. BMC Infect Dis. 2021;21:221. doi: 10.1186/s12879-021-05927-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodruff A, Walsh KL, Knight D, Irizarry-Alvarado JM. COVID-19 infection: Strategies on when to discontinue isolation, a retrospective study. Am J Infect Control. 2020;48:1032–1036. doi: 10.1016/j.ajic.2020.06.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 53.Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996;27:305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 54.Camden, New Jersey. City-Data Web site. https://www.city-data.com/city/Camden-New-Jersey.html. Published 2019. Accessed February 27, 2021.
- 55.CDC. Clinical Care Guidance for Healthcare Professionals about Coronavirus (COVID-19). COVID-19 Web site. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html. Published 2020. Updated October 9, 2020. Accessed March 14, 2021.
- 56.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.CDC. Discontinuation of Isolation for Persons with COVID-19 Not in Healthcare Settings. Centers for Disease Control and Prevention. COVID-19 Web site.https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html. Published 2021. Updated February 18, 2021. Accessed March, 7, 2021.
- 59.Gandhi RT, Lynch JB, Del Rio C. Mild or Moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 60.Boscolo-Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146:729–732. doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Statistics NCfH. Hypertension. CDC. FastStats Web site. https://www.cdc.gov/nchs/fastats/hypertension.htm. Published 2021. Updated January 25, 2021. Accessed February 28, 2021.
- 62.Statistics NCfH. Adult Obesity Facts. CDC. FastFacts Web site. https://www.cdc.gov/obesity/data/adult.html. Published 2018. Updated February 11, 2021. Accessed February 28, 2021.
- 63.CDC. Underlying Medical Conditions Associated with High Risk for Severe COVID-19: Information for Healthcare Providers. COVID-19 Web site. Published 2021. Updated May 13, 2021. Accessed August 15, 2021.
- 64.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.