Abstract
The purpose of this survey is to explore changes in the management of COVID-19 during the first versus the second wave, with particular emphasis on therapies, antibiotic prescriptions, and elderly care. An internet-based questionnaire survey was distributed to European Society of Clinical Microbiology and Infectious Diseases (ESCMID) members. Therapeutic approach to patients with mild-to-moderate (PiO2/FiO2 200–350) and severe (PiO2/FiO2 < 200) COVID-19, antibiotic use, and reasons for excluding patients from the intensive care unit (ICU) were investigated. A total of 463 from 21 countries participated in the study. Most representatives were infectious disease specialists (68.3%). During the second wave of pandemic, physicians abandoned the use of hydroxychloroquine, lopinavir/ritonavir, and azithromycin in favor of dexamethasone, low-molecular weight heparin (LMWH), and remdesivir in mild-to-moderate COVID-19. In critically ill patients, we detected an increased use of high-dose steroids (51%) and a decrease in tocilizumab use. The use of antibiotics at hospital admission decreased but remained high in the second wave. Age was reported to be a main consideration for exclusion of patients from ICU care by 25% of responders; a third reported that elderly were not candidates for ICU admission in their center. The decision to exclude patients from ICU care was based on the individual decision of an intensivist in 59.6% of cases. The approach of physicians to COVID-19 changed over time following evidence accumulation and guidelines. Antibiotic use at hospital admission and decision to exclude patients from ICU care remain critical aspects that should be better investigated and harmonized among clinicians.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-021-04377-1.
Keywords: COVID-19, SARS-CoV-2, Antibiotics, Elderly, Intensive care unit, Long COVID
Introduction
Many countries around the world experienced a two-wave pattern of COVID-19 pandemic, with a first wave during the spring of 2020 followed by the current second wave in late summer and autumn. [1] New treatment options have been tested since the first wave, leading to several changes in the management of patients with COVID-19 during the second versus the first wave. [2] Some practices adopted during the first wave were abandoned because of a lack of evidence, [3] while others, such as steroids, were confirmed in the armamentarium against SARS-CoV-2 according to published randomized controlled trials. [4, 5] Controversies continue to exist about some therapeutic strategies, such as the use of immunomodulators (interleukin-6 and interleukin-6 inhibitors, JAK inhibitors) [6–8] and convalescent plasma, [9–11] and their optimal timing. Moreover, several aspects, such as the management of elderly patients, the decision to exclude patients from intensive care unit (ICU), or the clinical decision to start antibiotics, are unmet clinical needs that may increase the risk of inter- and intra-hospital variability in the management of COVID-19 patients.
The aim of our questionnaire survey was to explore the differences in the management of hospitalized patients with COVID-19 between the second versus the first wave in Europe, with particular emphasis to therapeutic strategies and management of elderly patients.
Methods
Survey design and definitions
This study was an internet-based questionnaire survey on the management of hospitalized patients with COVID-19 in different European countries. The survey was designed according to the methodological recommendations for surveys. [12] The questionnaire was developed by the European Study Group for Infections in the Elderly (ESGIE) executive committee and tested for content validity by the project coordinators. The questionnaire contained closed-ended questions: 15 questions about the management of COVID-19 plus additional 5 questions regarding responder’s information. The complete questionnaire is reported as Supplementary Material. Briefly, information about specialty and country of participants, size of hospital, and number of treated patients with COVID-19 was requested. Questions on therapeutics commonly used during the first versus the second wave of the COVID-19 pandemic were also submitted to participants. Among these, we investigated drugs targeting the virus (protease inhibitors as lopinavir/ritonavir and darunavir/cobicistat, RNA polymerase inhibitors as remdesivir, endosomal acidification inhibitors as hydroxychloroquine), the host immune response (steroids, tocilizumab, anakinra, JAK inhibitors as baricitinib, azithromycin), passive immunization (convalescent plasma), and supportive therapeutic interventions as thromboprophylaxis or antibiotic therapy. [13] The questions were split according to the severity of the disease and more than one answer was allowed. COVID-19 was classified as mild to moderate (PiO2/FiO2 200–350 mmHg) or severe (PiO2/FiO2 < 200 mmHg) according to thresholds used for Berlin definition of acute respiratory distress syndrome surveys. [14] Three questions targeted long-term COVID-19 and its follow-up. Finally, the specific management of elderly patients and the impact of age on the clinical decision to exclude patients from ICU were investigated.
The use of steroids was classified in low and high dose based on the cutoff of 1 mg/kg/day of methylprednisolone or equivalents. [5] The low-molecular weight heparin (LMWH) dosage was classified as prophylactic (enoxaparin 40–60 mg daily) or therapeutic (enoxaparin 40–60 mg twice daily). [15] Concerning the use of immunomodulators, the range of PaO2/FiO2 ratio and the biological parameters (C-reactive protein, IL-6, D-dimers, ferritin, and lymphocytes count) guiding prescription were asked. Concerning antibiotics, the frequency and the use of procalcitonin to guide their prescription was investigated. Infections in patients with COVID-19 were considered coinfections, if diagnosed within the first 24 h of COVID-19 hospital admission, or superinfections, if diagnosis occurred > 48 h after admission for COVID-19. [16]
Sample selection
The target survey population comprised physicians who treated COVID-19 patients since the start of the pandemic, including infectious disease (ID) or internal medicine specialists, geriatricians, and intensivists. Microbiologists were not a priori excluded. Considering the specific topic of the survey, we planned to receive response only by physicians who had experience in treating COVID-19 patients.
Survey administration
The survey was distributed using the Google Form platform. The survey link was sent as part of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Weekly Newsletter to ESCMID members, but access to the survey was not limited by ESCMID membership. The distribution was passive and indirect, not by personal emails. The distribution list for the newsletter send-out includes just over 21,000 email addresses (ESCMID members and registered users), with a mean of 5000 engagements (opening and clicking link). Due to internal ESCMID rules, the newsletter was sent once and no further attempts to contact subjects were made. The survey was promoted by social media. There were no incentives. All responses were anonymous.
Statistical analysis
Response rate was calculated as the number of subjects from which an answer was recorded (responders) of the total number of physicians who received the questionnaire survey. Simple counts and proportions were calculated for the survey responses. These were based on the number of responders answering each question. Data were analyzed using the number of completed responses per item as the denominator.
Results
The survey was administered between 9 February and 30 March 2021. A total of 463 invited physicians from 21 countries participated in the study. Considering the number of engagements (5000) of ESCMID newsletters, the response rate was 9.3%. The vast majority of respondents were ID specialists (316/463, 68.3%), followed by geriatricians (68/463, 14.7%) and internal medicine physicians (50/463, 10.8%), who had experience in taking care of COVID-19 patients (having treated over 200 patients in 2020) and mainly practicing in large hospitals (with more than 300 beds). The complete results of the survey were reported as Supplementary Material.
Treatment of mild-to-moderate COVID-19 between the first and the second wave
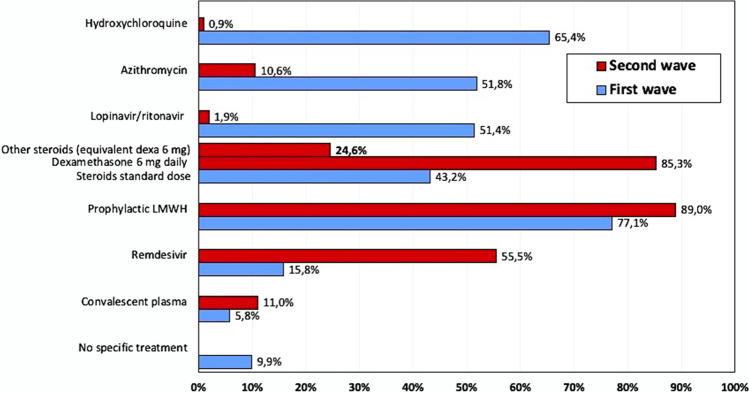
During the first wave of COVID-19 pandemic, prophylactic dose of LMWH and hydroxychloroquine were the most common therapies administered to patients with mild-to-moderate COVID-19 (77.1% and 65.4%, respectively) (Fig. 1). A high proportion of physicians also prescribed azithromycin (51.8%) and lopinavir/ritonavir (51.4%) during the first wave.
Fig. 1.
Therapies of patients with mild-to-moderate COVID-19 (PiO2/FiO2 200–350) during the first versus the second wave of the pandemic. *The use of steroids was classified in low dose and high dose of based on the cutoff of 1 mg/kg/d of methylprednisolone or equivalents (WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, JAMA. 2020;324:1330–1341). **LMWH prophylactic dose: enoxaparin 40–60 mg daily
During the second wave of the pandemic, the use of hydroxychloroquine, lopinavir/ritonavir, and azithromycin in mild-to-moderate COVID-19 dramatically decreased (0.9%, 1.9%, and 10.6%, respectively), and the vast majority of physicians prescribed steroids in the form of dexamethasone 6 mg daily (85.3%) or other steroids at equivalent dosage (24.6%). The use of prophylactic LMWH increased in the second wave and that of remdesivir increased from 15.8 during the first to 55.5% during the second wave. Convalescent plasma was rarely prescribed (5.8% and 11% in the first vs the second wave, respectively).
Treatment of severe COVID-19 between the first and the second wave
The treatment of patients with severe COVID-19 is illustrated in Fig. 2. During the first wave of COVID-19 pandemic, half of clinicians (51.6%) used tocilizumab for the treatment of these patients. This proportion decreased to 31.3% during the second phase of the pandemic. The use of other immunomodulators, such as anti-IL 1 and JAK inhibitors, remained limited during both waves. During the second wave, 51% of physicians prescribed high dose of steroids. With regard to the use of LMWH, about one half of physicians prescribed a prophylactic dose and another half therapeutic one. These proportions did not significantly differ between the first and the second wave. Convalescent plasma use was limited (13.8% vs 11.9% in the first vs the second wave, respectively).
Fig. 2.
Therapies of patients with severe COVID-19 (PiO2/FiO2 < 200) during the first versus the second wave of the pandemic. *The use of steroids was classified in low dose and high dose of based on the cutoff of 1 mg/kg/d of methylprednisolone or equivalents. **LMWH prophylactic dose: enoxaparin 40–60 mg daily; LMWH therapeutic dose: 40–60 mg twice daily
Antibiotic prescriptions
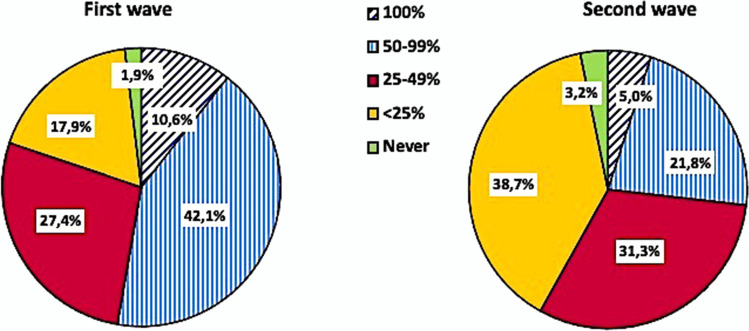
Fifty-three percent of physicians declared having prescribed at least one antibiotic at hospital admission during the first wave of the COVID-19 pandemic (Fig. 3). This proportion of physicians decreased to 26.8% during the second wave. Third-generation cephalosporins were the most prescribed antibiotics (51%), followed by beta-lactams/beta-lactamases inhibitors (39.3%), macrolides (6%), fluoroquinolones (3%), and carbapenems (0.6%).
Fig. 3.
Use of antibiotic therapy at hospital admission during the first versus the second wave of the COVID-19 pandemic
About one third (34.1%) of physicians did not consider procalcitonin values when starting antibiotic therapy both at hospital admission and during the hospitalization.
Long COVID-19 symptoms
Results about long COVID-19 symptoms are reported in Supplementary Tables. A total of 34.3% of physicians reported that 10–20% of their patients experienced long COVID-19 symptoms and an additional 26.6% that 20–30% of them had long COVID-19. The most common reported symptoms were excessive fatigue/exhaustion (81%), muscle fatigue (61.6%), breathlessness (52.3%), loss of taste and smell (44.7%), and cognitive issues such as “brain fog” (44.1%).
Management of elderly patients with COVID-19
The majority of physicians did not treat elderly patients with COVID-19 using the same therapeutic approach as for younger adults (Fig. 4): 33.7% of them excluded patients > 75 years old from ICU care, 15.3% paid more attention to potential drug-related adverse events, while 7.8% did not prescribe any immunomodulator. In contrast, 43.2% of them used the same therapeutic approach for elderly and non-elderly patients.
Fig. 4.
Treatment approach of elderly patients (> 75 years old) compared to younger adult patients
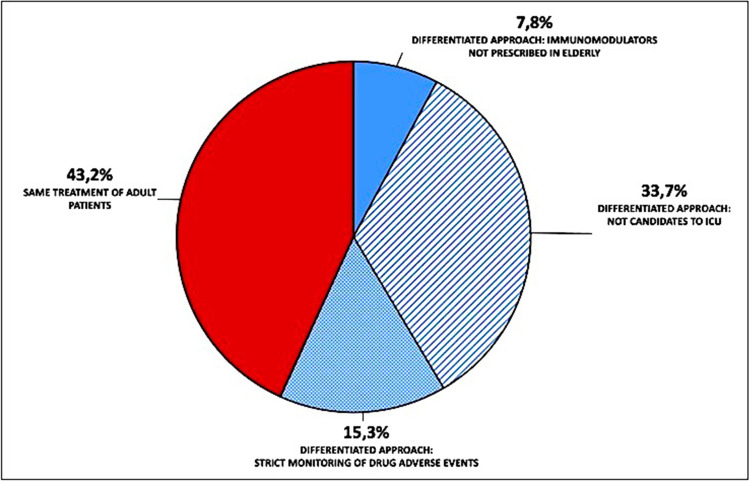
As shown in Fig. 5, the decision to exclude patients from ICU care was based on the individual decision of an intensivist in 59.6% or according to patients’ age in 25.1% of cases. Clinical frailty scale was used only by 24.2% and 19.9% of physicians, respectively.
Fig. 5.
Parameter used to exclude patients from ICU care
Discussion
This survey collecting results from 21 countries provided interesting information on the management of patients with COVID-19 over time. Our study reveals several important findings in the care and support of hospitalized patients with COVID-19.
First, we found that the therapeutic management of patients with COVID-19 greatly changed from March 2020 to September 2020. On one hand, several drugs, such as hydroxychloroquine, failed to demonstrate clinical efficacy. [3, 17] Conversely, dexamethasone became the standard of care in patients with COVID-19 who required supplementary oxygen therapy following the publication of the RECOVERY trial. [4] Evidence modified the clinical approach of physicians who faced with patients infected with SARS-CoV-2. As a matter of fact, we found that steroids, prophylactic LMWH and remdesivir were prescribed in mild-to-moderate COVID-19 by the majority of clinicians during the second wave, following the accumulating evidence and adhering to international guidelines and recommendations. [18]
Next, we found that a high proportion of physicians prescribed high dosages of steroids and therapeutic LMWH in patients with severe COVID-19. The use of steroids at dosage higher than 6 mg of dexamethasone (used in the RECOVERY trial) is debated. Dexamethasone 20 mg/day from day 1 to 5 and 10 mg/day from day 6 to 10 in severe COVID-19 showed no clinical benefit [19] and steroids may be also associated with potential devastating complications, such as COVID-19-associated pulmonary aspergillosis and mucormycosis. [19, 20] Similarly, the use of LMWH at a therapeutic dosage is still controversial. Although LMWH reduced mortality in patients with COVID-19, [21] the recommended type, dose, duration, and timing of anticoagulant have not been determined yet and adverse events such as major bleeding and spontaneous retroperitoneal hematoma have been reported. [21] It should be mentioned that the use of therapeutic dosages of unfractionated heparin did not show any benefit in critically ill patients with COVID-19 and the trial was prematurely stopped for futility. [22]
Although results of early randomized clinical trials were controversial, recent large randomized controlled trials (REMAP-CAP and RECOVERY) demonstrated a meaningful mortality benefit in patients receiving IL-6 inhibitors. [23, 24] IL-6 inhibitors are now recommended for patients with severe or critical COVID-19 by the Guidelines of The Italian Society of Infectious diseases, UK COVID-19 guidelines as well as the Infectious Diseases Society of America and those from NIH. [25] Surprisingly, our survey highlighted a reduction in the use of tocilizumab during the second wave. This could be related to several factors: the higher number of studies investigating tocilizumab during the first wave compared to the second one might have allowed physicians to treat more patients (both mild and severe COVID-19) in the context of clinical trials; the negative results from randomized clinical trials in mild-to-moderate COVID-19 may have influenced the use of tocilizumab during the second wave.
We are able to underline a decrease in the antibiotic prescription during the second wave in comparison to the first one. However, the use of antibiotics at hospital admission still remains high, despite the low prevalence of bacterial co-infection which is reported to be less than 5% in recent cohorts. [26, 27] The gap between the prevalence of bacterial infection and the frequency of antibiotic prescribing highlights the risk of antibiotic misuse and may result in increased selective pressure for antimicrobial resistance. [28] Antimicrobial stewardship programs and rational use of surrogate biomarkers, such as procalcitonin, should be urgently implemented to reduce consequences of multidrug-resistant bacteria outbreaks in hospitalized patients with COVID-19. [29]
Another observation arising from our survey is the different approach used in elderly versus adult patients with COVID-19. One third of the physicians declared that elderly patients were not candidate to the ICU care in their hospitals. The main reason to exclude them from ICU care was an individual decision by an intensivist, while comorbidity scores, such as clinical frailty scale (CFS), were less used to take this decision. ICU triage of patients is challenging and controversial in pandemics when resources are overwhelmed. Age was the second most used criterion for ICU exclusion, primarily because advanced age appears strongly associated with poorer outcomes. [30] However, the allocation of patients based only on age may generate ethical concerns and should not be the only determining factor in ICU triage decisions. [29] Since CFS correlates with outcome in patients with COVID-19, [31–33] the UK National Institute for Health and Care Excellence (NICE) advocated the use of CFS in clinical decision for elderly patients with COVID-19. [34] CFS could be useful to better stratify the risk of mortality among elderly patients and to better allocate them. Moreover, a multidisciplinary team, including also physicians, ID, and geriatricians, may support the challenging clinical decision to exclude a patient from ICU care to “make care rational and not to ration care.” [31] Trials addressing this population’s care are urgently needed. [35]
Importantly, it has been demonstrated that anti-SARS-CoV-2 monoclonal antibodies reduce the risk of hospitalization in high-risk non-hospitalized patients with COVID-19.[36] Thus, the outpatient therapy including intravenous or intramuscular anti-SARS-CoV-2 monoclonal antibodies may represent a promising therapeutic option to avoid hospitalization and its consequence, especially in elderly frail patients. [37]
This survey has some limitations. As for any survey-based study, the data are self-reported and have not been validated. Since the majority of responders were from large institutions, a selection bias may be recognized and strategies used in small centers may be under-represented. The response rate was low; nevertheless, we assume low risk for non-response error, assuming that physicians replying the survey were those with interest in the survey’s questions, probably representative of those treating considerable numbers of COVID-19 patients. This may be due to several reasons: (1) it was conducted during a pandemic, which may have reduced the response rate; (2) it was sent to all ESCMID members, but not all cared for COVID-19 patients, and had experience in this field; (3) it was sent through a passive distribution method, the ESCMID newsletter, which many physicians may have not read and discarded. Moreover, since some countries are more represented than others, this may have limited the generalizability of our results. Finally, the survey did not take into account the difference in the spread of variants of concern during the first and the second wave.
In conclusion, our survey showed a high heterogeneity in the treatment of severe COVID-19 with a considerable proportion of clinicians using high dose of steroids and a high rate of antibiotic prescription showing an urgent need for implementing antibiotic stewardship principles. Finally, the goals of decision to exclude elderly patients from ICU care remain a crucial dilemma. We plead for a more individualized approach taking into account not only age but also frailty and a multidisciplinary evaluation including geriatricians.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank for its contribution to the ESCMID Study Group for the Infections in the Elderly (ESGIE)—The ESGIE Executive Committee is represented by Marco Falcone, Italy (Chair); Gaetan Gavazzi, France (Science Officer); Dafna Yahav, Israel (Secretary); and Virginie Prendki, Switzerland (Treasurer).
Author contribution
GT, CM, and MF formulated the questions; DY, MP, MT, GG, CM, VP, and MF reviewed the questions and approved the final version of the survey; DY, VP, and MF interacted with the ESCMID and organized the distribution of the survey to ESCMID members; GT analyzed the data; GT and MF wrote the manuscript; all the authors revised the manuscript and approved the final version.
Data availability
All data are available following request to the corresponding author.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable (the participation into the survey was voluntary and anonymous and all participants declared their consent to participate and publish results of the survey).
Consent to participate
All participants declared their consent to participate.
Consent for publication
All participants declared their consent to publish the data from the survey.
Conflict of interest
The authors declare no competing interests.
Footnotes
Virginie Prendki and Marco Falcone share the last position
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Contou D, Fraissé M, Pajot O, Tirolien JA, Mentec H, Plantefève G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care. 2021;25:3. doi: 10.1186/s13054-020-03449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azoulay E, de Waele J, Ferrer R, Staudinger T, Borkowska M, Povoa P, et al. International variation in the management of severe COVID-19 patients. Crit Care. 2020;24:486. doi: 10.1186/s13054-020-03194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehra MR, Ruschitzka F, Patel AN. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395:1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020;324:1330–41. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.REMAP-CAP Investigators. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stebbing J, Sánchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2020;7:eabe4724. doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulcini C, Leibovici L. CMI Editorial Office. CMI guidance for authors of surveys. Clin Microbiol Infect. 2016;22:901–02. doi: 10.1016/j.cmi.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Fragkou PC, Belhadi D, Peiffer-Smadja N, Moschopoulos CD, Lescure FX, Janocha H, et al. ESCMID Study Group for Respiratory Viruses. Review of trials currently testing treatment and prevention of COVID-19. Clin Microbiol Infect. 2020;26:988–98. doi: 10.1016/j.cmi.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 15.Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165–186. doi: 10.1007/s11239-015-1315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falcone M, Tiseo G, Giordano C, Wirth D, Cuker A, Wittkowsky AK. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76:1078–1084. doi: 10.1093/jac/dkaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fihn SD, Perencevich E, Bradley SM. Caution needed on the use of chloroquine and hydroxychloroquine for Coronavirus Disease 2019. JAMA Netw Open. 2020;3:e209035. doi: 10.1001/jamanetworkopen.2020.9035. [DOI] [PubMed] [Google Scholar]
- 18.Mussini C, Falcone M, Nozza S, Sagnelli C, Parrella R, Meschiari M, et al. Therapeutic strategies for severe COVID-19: a position paper from the Italian Society of Infectious and Tropical Diseases (SIMIT) Clin Microbiol Infect. 2021;27:389–395. doi: 10.1016/j.cmi.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947. doi: 10.1016/j.ejphar.2021.173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmadikia K, Hashemi SJ, Khodavaisy S, Getso MI, Alijani N, Badali H et al (2021) The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 64:798–808 [DOI] [PMC free article] [PubMed]
- 21.Falcone M, Tiseo G, Barbieri G, Galfo V, Russo A, Virdis A, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:ofaa563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The REMAP-CAP, ACTIV-4a, ATTACC Investigators, Zarychanski R (2021) Therapeutic anticoagulation in critically ill patients with Covid-19 – Preliminary Report. MedRxiv Posted March 12, 2021
- 23.REMAP-CAP Investigators. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute of Health (NIH). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/interleukin-6-inhibitors/. Accessed 28 Oct 2021 [PubMed]
- 26.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. COVID-19 Researchers Group. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–8. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langford BJ, So M, Raybardhan S, Leung V, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcone M, Tiseo G, Nicastro M, Leonildi A, Vecchione A, Casella C, et al. Cefiderocol as rescue therapy for Acinetobacter baumannii and other carbapenem-resistant Gram-Negative infections in ICU patients. Clin Infect Dis. 2021;72:2021–2024. doi: 10.1093/cid/ciaa1410. [DOI] [PubMed] [Google Scholar]
- 29.Huttner BD, Catho G, Pano-Pardo JR, Pulcini C, Schouten J. COVID-19: don't neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis EG, Breckons M, Lee RP, Dotchin C, Walker R. Rationing care by frailty during the COVID-19 pandemic. Age Ageing. 2021;50:7–10. doi: 10.1093/ageing/afaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprung CL, Joynt GM, Christian MD, Truog RD, Rello J, Nates JL. Adult ICU triage during the coronavirus disease 2019 pandemic: who will live and who will die? recommendations to improve survival. Crit Care Med. 2020;48:1196–1202. doi: 10.1097/CCM.0000000000004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung C, Flaatten H, Fjølner J, Bruno RR, Wernly B, Artigas A, et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021;25:149. doi: 10.1186/s13054-021-03551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong E, Chan M, Tan HN, Lim WS. COVID-19: use of the clinical frailty scale for critical care decisions. J Am Geriatr Soc. 2020;68:E30–E32. doi: 10.1111/jgs.16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Critical Care. 2020. www.nice.org.uk/guidance/ng159. Accessed April 6, 2020 [PubMed]
- 35.Prendki V, Tau N, Avni T, Falcone M, Falcone M, Huttner A, Kaiser L, et al. ESCMID Study Group for Infections in the Elderly (ESGIE). A systematic review assessing the under-representation of elderly adults in COVID-19 trials. BMC Geriatr. 2020;20:538. doi: 10.1186/s12877-020-01954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Razonable RR, Pawlowski C, O'Horo JC, Arndt LL, Arndt R, Bierle DM, et al (2021) Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine 101102 [DOI] [PMC free article] [PubMed]
- 37.Falcone M, Tiseo G, Valoriani B, Barbieri C, Occhineri S, Mazzetti P, et al (2021) Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe covid-19 and role of variants of concern. Infect Dis Ther. 1–10 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available following request to the corresponding author.
Not applicable.