Abstract
Serologic testing of residual blood samples from 812 children from a hospital in New Orleans, LA, between March and May 2020, demonstrated a SARS-CoV-2 seroprevalence of 6.8% based on S and N protein IgG; Black and Hispanic children, and children living in zip codes with lower household incomes were over-represented.
Abbreviations: SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; ELISA, enzyme-linked immunosorbent assay
Keywords: SARS-CoV-2, COVID-19, Seroprevalence, Children
Introduction
Children with acute coronavirus disease 2019 (COVID-19) typically have milder symptoms than adults, thus it is likely that more children are infected than tested [1]. SARS-CoV-2 virus shed by asymptomatic, pre-symptomatic, or mildly symptomatic subjects has likely contributed to the spread of the virus [2]. High level shedding has been demonstrated in infected children, even those who are asymptomatic; therefore, children may be efficient vectors of transmission [3], [4], [5]. There is a critical and urgent need to understand the levels of past and current infection in children, and their roles in viral transmission. Serological studies have largely been limited to adults. Our objective was to define SARS-CoV-2 seroprevalence in children seeking medical care during the first Stay at Home Order in New Orleans, Louisiana, from March through May 2020.
Methods
With approval of the Tulane Institutional Review Board we collected residual blood samples from the regional children's hospital in Orleans Parish, Louisiana between March 18th-May 15th, 2020, during which a Stay-at-Home Order was in place. Samples were from children ≤18 years of age who had blood drawn as part of their care in the hospital or ambulatory clinics. Residual samples were released seven days after blood draw. Serum or plasma was isolated, heat inactivated for 30 min at 56 °C, and stored at −20 °C until analyzed. All samples were deidentified and tested blinded in regard to SARS-CoV-2 infection status or clinical presentation at the time of blood collection.
We screened serum samples at 1:100 dilution for IgG antibodies to SARS-CoV-2 Spike (S) protein using a direct coat ELISA with background subtraction as previously described [6]. A plasmid for expression of stable pre-fusion trimeric S protein (La Jolla Institute for Immunology) was used to produce recombinant stabilized S protein and then purified [8,9]. Cut off optical density (OD) values (positive value >0.4) were calculated based on testing of >100 pre-COVID-19 samples and calibrated using an NCI Seronet Human SARS-CoV-2 Serology Standard (https://frederick.cancer.gov/initiatives/seronet/serology-standard). Samples testing positive to the S protein ELISA were then tested for antibodies to Nucleocapsid (N) protein using a commercial N protein ELISA according to the manufacturers protocol (Zalgen Labs, Germantown, MD) (Fig. 1 A). Finally, we validated positive samples using a commercially purchased ELISA Kantaro SARS-CoV-2 IgG Antibody RUO kit (R&D Systems) following kit instructions. Serologic assays were performed blinded to all clinical and demographic data.
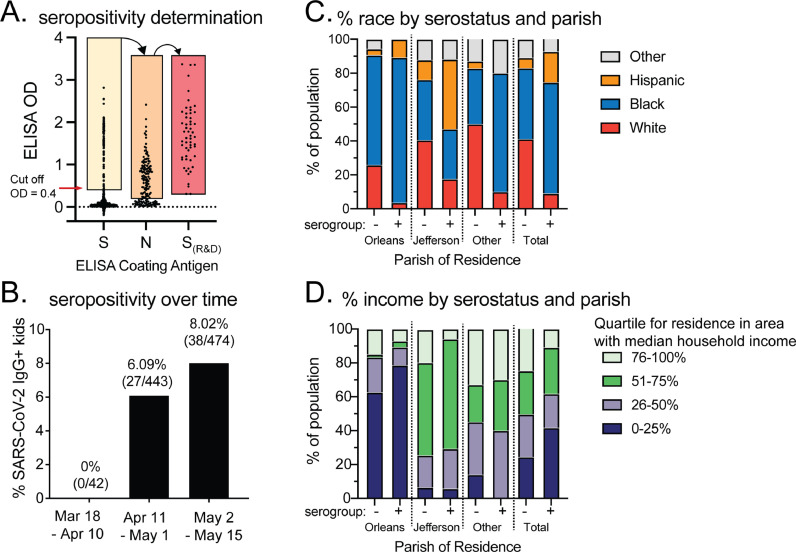
Fig. 1.
A) Three-step ELISA process to determine presence of IgG to SARS-CoV-2 spike and nucleocapsid protein. B) Seropositivity in blood samples from pediatric subjects by 3-week intervals. C) % racial group within Orleans, Jefferson, and other parishes by serostatus. D) % in income quartiles within Orleans, Jefferson, and other parishes by serostatus.
Demographics were abstracted from electronic medical records. Median household income for the patient's zip code of residence was obtained from the U.S. Census Bureau (2014–2018 averaged values) or a commercial vendor (U.S. Census Bureau data (2018) https://www.cubitplanning.com/)). Parishes (counties) with >200 subjects were identified. Statistical analysis was performed using GraphPad v7 and IBM SPSS (v.25). Categorical measures are presented as percentages and continuous measures as medians and interquartile ranges. Analysis between seronegative and seropositive groups was performed using Chi squared tests.
Results
A total of 1690 residual blood samples from 812 unique pediatric subjects were obtained from a children's hospital in New Orleans from March 18th to May 15th, 2020. Subjects were from 41 of 64 parishes (e.g. counties) within Louisiana. Most patients were from Jefferson (27.4%) or Orleans (27.3%) parishes.
The population was 50.4% female and 49.6% male. The age range was 2 days to 18 years with a median age of 11 years (interquartile range (IQR), 4–15 years). Our cohort included 43.4% children who identified as Black non-Hispanic, 39.1% as white non-Hispanic, 6.9% as Hispanic, and 10.6% who identified as other races or race/ethnicity was not listed.
Overall, 112 of the 1690 samples tested positive for IgG antibodies to our in-house S protein ELISA, resulting in 55 of 812 unique subjects (6.8%) testing positive. Of the 55 patients seropositive to the S protein, all 55 were also positive using the N protein ELISA. Total seroprevalence increased over time from March 18th to May 15th (Fig. 1B). There were no statistically significant differences in seroprevalence by sex or age. There were significant differences in seroprevalence by race (p < 0.001), parish (p < 0.001), and residence in an area with lower household incomes (estimated by zip code) (p = 0.014). Black and white children were equally represented in the cohort, but Black children accounted for 65.5% of seropositive subjects, while white children represented 9.1% of the seropositive subjects. Hispanic children of all races comprised 6.9% of all subjects, but 18.2% of seropositive subjects (Fig. 1C).
Despite equal numbers of children from Orleans and Jefferson Parish in the cohort, children living in Orleans Parish accounted for 50.9% of seropositive children, while children from Jefferson Parish accounted for 30.9%. Of seropositive subjects, 41.8% lived in a zip code with a median household income in the bottom quartile (<$36,939). The distribution of subjects in the seronegative sample was equal across income levels (Fig. 1D). Parishes had different compositions of seropositive children by race and residency in a zip codes with specific household income levels (Fig. 1C-D).
Discussion
We demonstrated 6.8% SARS-CoV-2 seroprevalence in children receiving medical care in New Orleans shortly after the global pandemic was declared. A study of adults in the general population of New Orleans observed a 7.8%-point prevalence in May 2020, similar to the 8% prevalence we observed from May 2 through May 15 (Fig. 1B) [7]. Our findings suggest that children were equally susceptible to infection with SARS-CoV-2 as adults in this area, despite fewer reported cases. Residents of Orleans Parish, while only about one fourth of the total study population, accounted for half (50.9%) of the positive cases. Orleans Parish was an early epicenter of COVID-19, with one of the highest per capita rates of infections in the country in March.
Our results implicate neighborhood income level as an indicator of risk for infection. Children residing in zip codes with median household income in the lowest quartile accounted for 25.7% of the total cohort, but 41.8% of positive cases, while children residing in zip codes with median household income in the highest quartile accounted for 23.7% of the total cohort but only 10.9% of positive cases. The disparity is particularly evident in Orleans Parish (Fig. 1D). This is consistent with preliminary data suggesting an initial higher rate of SARS-CoV-2 in lower-income neighborhoods [8]. Our results also indicate racial disparity in infections, with Black children and Hispanic children more frequently seropositive than white children during the time frame analyzed; this held true particularly for Black children in Orleans parish, and Hispanic children in Jefferson parish (Fig. 1C). This mirrors existing literature which has shown racial disparities in early pandemic infection rates, including a study of adults in New Orleans from March 1st – April 11th.
This study was limited to children obtaining medical care early in the pandemic, thus over-representing children with more complicated medical conditions, and may not have been representative of the general population. Additionally, our methodology required children to have mounted an antibody response to both the SARS-CoV-2 S and N protein, missing children who have been infected but failed to produce antibodies to the N-protein only. We do not know the clinical background of the patients in our study. It can be inferred that many of the patients have chronic conditions or are immunocompromised which are further limitations to this study. This may impact of results as some medical procedures may impact the serostatus of the sample. Last, we cannot rule out nosocomial transmission accounting for increased seroprevalence.
The overall seroprevalence of 6.8% in children seeking medical care from March 18th through May 15th suggests children were as susceptible to SARS-CoV-2 infection as adults in our region early in the pandemic. Also, existing racial and socioeconomic disparities seen in adults are reflected in the children tested in our study. The durability of the antibody response and the protection it confers to children both from disease and transmission remains unclear, and further studies are needed. COVID-19 vaccines are not currently approved for use in children under 12, and the proportion of this demographic in infected populations is increasing, particularly with new viral variants [9]. Variability in regional SARS-CoV-2 pediatric infections during the study period may impact spread of newer, highly infectious viral variants. However, vaccination of children is likely critical to mitigate SARS-CoV-2 outbreaks and protect children from serious potential complications of COVID-19.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health (NIH) under Award Number U54CA260581 (J.E.R, J.S.S, B.E.N, K.J.Z, M.L.D. and S.S.D) and NIH Award Number P20 GM103629 (K.J.Z). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank our department chairs Drs. Chad Steele and Samir El-Dahr for their financial support, Dr. Joshua Yukich for insightful comments, and Children's Hospital New Orleans (CHNOLA) and the CHNOLA Clinical Trials Center and clinical laboratory staff for their significant contributions.
References
- 1.Bialek S., Gierke R., Hughes M., McNamara L.A., Pilishvili T., Skoff T. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. Morbidity Mortal. Wkly. Rep. 2020;69:422. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelvin A.A., Halperin S. COVID-19 in children: the link in the transmission chain. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kam K-q, Yung C.F., Cui L., Tzer Pin Lin R., Mak T.M., Maiwald M., et al. A well infant with coronavirus disease 2019 with high viral load. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han M.S., Choi E.H., Chang S.H., Jin B.-.L., Lee E.J., Kim B.N. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson J.E., Hastie K.M., Cross R.W., Yenni R.E., Elliott D.H., Rouelle J.A., et al. Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits. Nat. Commun. 2016;7:11544. doi: 10.1038/ncomms11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feehan A.K., Fort D., Garcia-Diaz J., Price-Haywood E.G., Velasco C., Sapp E., et al. Seroprevalence of SARS-CoV-2 and infection fatality ratio, Orleans and Jefferson Parishes, Louisiana, USA, May 2020. Emerg. Infect. Dis. 2020;26:2766–2769. doi: 10.3201/eid2611.203029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalatbari-Soltani S., Cumming R.G., Delpierre C., Kelly-Irving M. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J. Epidemiol. Community Health. 2020 doi: 10.1136/jech-2020-214297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day M. Covid-19: more young children are being infected in Israel and Italy, emerging data suggest. BMJ. 2021;372:n383. doi: 10.1136/bmj.n383. [DOI] [PubMed] [Google Scholar]