Abstract
Ethnopharmacological relevance
Keguan-1, a new traditional Chinese medicine (TCM) prescription contained seven Chinese herbs, is developed to treat coronavirus disease 19 (COVID-19). The first internationally registered COVID-19 randomised clinical trial on integrated therapy demonstrated that Keguan-1 significantly reduced the incidence of ARDS and inhibited the severe progression of COVID-19.
Aim of the study
To investigate the protective mechanism of Keguan-1 on ARDS, a lipopolysaccharide (LPS)-induced acute lung injury (ALI) model was used to simulate the pathological state of ARDS in patients with COVID-19, focusing on its effect and mechanism on ALI.
Materials and methods
Mice were challenged with LPS (2 mg/kg) by intratracheal instillation (i.t.) and were orally administered Keguan-1 (low dose, 1.25 g/kg; medium dose, 2.5 g/kg; high dose, 5 g/kg) after 2 h. Bronchoalveolar lavage fluid (BALF) and lung tissue were collected 6 h and 24 h after i.t. administration of LPS. The levels of inflammatory factors tumour necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-1β, keratinocyte-derived chemokine (KC or mCXCL1), macrophage inflammatory protein 2 (MIP2 or mCXCL2), angiotensin II (Ang II), and endothelial cell junction-associated proteins were analysed using ELISA or western blotting.
Results
Keguan-1 improved the survival rate, respiratory condition, and pathological lung injury; decreased the production of proinflammatory factors (TNF-α, IL-6, IL-1β, KC, and MIP2) in BALF and the number of neutrophils in the lung tissues; and ameliorated inflammatory injury in the lung tissues of the mice with LPS-induced ALI. Keguan-1 also reduced the expression of Ang II and the adhesion molecule ICAM-1; increased tight junction proteins (JAM-1 and claudin-5) and VE-cadherin expression; and alleviated pulmonary vascular endothelial injury in LPS-induced ALI.
Conclusion
These results demonstrate that Keguan-1 can improve LPS-induced ALI by reducing inflammation and pulmonary vascular endothelial injury, providing scientific support for the clinical treatment of patients with COVID-19. Moreover, it also provides a theoretical basis and technical support for the scientific use of TCMs in emerging infectious diseases.
Keywords: COVID-19, Keguan-1, ALI, Inflammation, Pulmonary vascular endothelial injury
Graphical abstract
Abbreviations
- TCM
traditional Chinese medicine
- ARDS
acute respiratory distress syndrome
- RCT
randomised clinical trial
- LPS
lipopolysaccharide
- ALI
acute lung injury
- BALF
bronchoalveolar lavage fluid
- COVID-19
coronavirus disease 19
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- HPLC-MS
high-performance liquid chromatography-tandem mass spectrometry
- DXM
dexamethasone
- TNF-α
tumour necrosis factor alpha
- IL-6
interleukin-6
- IL-1α
interleukin-1alpha
- IL-1β
interleukin-1beta
- KC
keratinocyte-derived chemokine
- MIP2
macrophage inflammatory protein 2
- Ang II
angiotensin II
- PBS
phosphate-buffered saline
- PMSF
phenylmethanesulfonyl fluoride
- PaCO2
CO2 partial pressure
- PaO2
O2 partial pressure
- SO2
oxygen saturation
- ACE2
angiotensin-converting enzyme II
- ICAM-1
intercellular adhesion molecule-1
1. Introduction
Coronavirus disease 19 (COVID-19), caused by the highly infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterised by a long incubation period, high infectivity, and general susceptibility among various age groups (Wei et al., 2020; Yang et al., 2020b; S. Zhang et al., 2020a). The COVID-19 pandemic has become a severe public health crisis worldwide since December 2019. As of July 26, 2021, more than 194 million people have been reported to be infected by SARS-CoV-2 and over 4 million people worldwide have died due to SARS-CoV-2 infection (data compiled by Johns Hopkins University). Studies have shown that approximately 80% of COVID-19 patients showed mild and flu-like symptoms, such as fever, headache, dry cough, shortness of breath, and fatigue, which are generally self-limited. However, severe patients could develop ARDS characterised by dyspnea and low blood oxygen saturation, which may further progress into multiple organ failure, or even death (Karmakar et al., 2021; B. Zhang et al., 2020). Currently, the treatment for ARDS is relatively limited. If the disease progresses to a severe stage, the mortality rate might reach as high as 40%–70% (Tay et al., 2020). The key to COVID-19 management is to prevent the occurrence of ARDS and to control the disease progression from mild to severe.
COVID-19 has emerged as a pandemic and public health crisis due to a lack of specific antiviral drugs and vaccinations for treatment and prevention. Traditional Chinese medicine (TCM) has a long history and plays an indispensable role in preventing and treating several epidemic diseases (Ni et al., 2020; Ren et al., 2020). TCMs have shown the unique advantage of having a rapid response to new outbreaks, a major feature of epidemic prevention and control in China. TCMs have been used to control infectious diseases for many years because they can treat a disease before its onset, with treatment determination based on syndrome differentiation and multi-target intervention. It has been reported that patients with SARS-CoV-2 infection have benefited from comprehensive TCM treatment and Western medicine (Hu et al., 2021; Yang et al., 2020a; Zhao et al., 2021). At the beginning of the SARS-CoV-2 outbreak, we developed TCM prescriptions to treat COVID-19 based on clinical experience and molecular docking technology. Thereafter, we developed a therapeutic plan for integrating traditional Chinese and Western medicine for the treatment of SARS-CoV-2-infected patients. Based on the similarities and differences between COVID-19 and SARS in terms of disease characteristics, clinical symptoms, TCM syndrome, and the law of transmission and transformation, the clinical team initially proposed the prevention and treatment of COVID-19 based on the Yinqiao powder (yinqiaosan), Sangju drink (sangjuyin), and Sanren decoction (sanrentang). In addition, 46 Chinese herbal active ingredients were detected using molecular docking, which could act on the binding region of the spike (S) protein of SARS-CoV-2 and angiotensin-converting enzyme II (ACE2). These ingredients mainly belong to ten Chinese herbal medicines, including Lonicera japonica Thunb. (Jinyinhua), Morus alba L. (Sangye), and Prunus armeniaca L. var. ansu Maxim. (Kuxingren). After combining the results of clinical and basic research, we developed the TCM therapeutic prescription named Keguan-1 with the following seven Chinese herbs: Lonicera japonica Thunb. (Jinyinhua), Forsythia suspensa (Thunb.) Vahl (Lianqiao), Morus alba L. (Sangye), Chrysanthemum morifolium Ramat. (Juhua), Coix lacryma-jobi L. var. mayuen (Roman.) Stapf (Yiyiren), Fritillaria thunbergia Miq. (Zhebeimu), and Prunus armeniaca L. var. ansu Maxim. (Kuxingren). A randomised clinical trial (RCT; NCT04251871) was conducted to determine the efficacy of integrated traditional Chinese and Western medicines for the effect of Keguan-1 in the COVID-19 treatment. The results showed that the incidence of ARDS decreased from 26.1% to 4.2% (P < 0.05) following Keguan-1 treatment, as compared with the control group, and Keguan-1 effectively prevented the progression to severe disease (Wang et al., 2020). The results of the RCT established that Keguan-1 significantly reduced the incidence of ARDS, providing strong clinical evidence that TCMs could be a valuable strategy for COVID-19 treatment.
This study focused on the inhibitory effect of Keguan-1 on ARDS and explored the possible mechanism of inhibition of inflammation, improvement of vascular endothelial injury, and elucidation of the entire intervention characteristics of Keguan-1, which will provide the scientific support for the clinical treatment of COVID-19 (Huppert et al., 2019; Nile et al., 2020; Varga et al., 2020). Lipopolysaccharide (LPS)-induced acute lung injury (ALI) simulates the key pathological process seen during ARDS, including inflammatory protein secretion, neutrophil infiltration, and changes in endothelial injury (Dagvadorj et al., 2015; Ma et al., 2014; Reis Gonçalves et al., 2012). Therefore, the LPS-induced ALI mouse model was applied to explore the underlying mechanism of action of Keguan-1.
2. Materials and methods
The data on COVID-19 infections and deaths is compiled by the Johns Hopkins University (https://coronavirus.jhu.edu/map.html).
2.1. Reagents
Keguan-1 was obtained from the Fifth Medical Center of the PLA General Hospital. In our previous study, we conducted a quality control assessment by analysing the relative number of standard compounds using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS). Hence, the quality of Keguan-1 was guaranteed (Wang et al., 2020). LPS (Escherichia coli 055:B5) was purchased from Sigma-Aldrich (St. Louis, MO, USA); dexamethasone (DXM), from HARVEYBIO (Beijing, China); Formalin fixative solution, from Yili Fine Chemicals Co., Ltd. (Beijing, China); the tumour necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-1α ELISA kits, from Dakewe Biotech Co., Ltd (Shenzhen, China); the IL-1β ELISA kit, from R&D Systems (Minneapolis, MN, USA); the keratinocyte-derived chemokine (KC or mCXCL1) and macrophage inflammatory protein 2 (MIP2 or mCXCL2) ELISA kits and Annexin V-PE/7-AAD apoptosis kit, from MultiSciences (Lianke) Biotech Co., Ltd (Hangzhou, China); the Angiotensin (Ang) II ELISA kit, from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); the BCA protein assay kit, from Thermo Fisher Scientific (Waltham, MA, USA); FITC anti-mouse Ly6G and APC anti-mouse F4/80 antibodies, from Biolegend (San Diego, CA, USA); intercellular adhesion molecule-1 (ICAM-1) and claudin-5 antibodies, from Abcam (Cambridge, UK); JAM-1, VE-cadherin, and GAPDH antibodies, from Santa Cruz Biotechnology (Santa Cruz, CA, USA); and Evans blue stain, from Sigma-Aldrich.
2.2. Animals and experimental procedures
Healthy male C57BL/6 mice (7–8-week-old, weighing 20 ± 2 g) were obtained from SPF (Beijing) Biotechnology Co., Ltd., with the certificate number SCXK (Jing) 2019–0010. The animals were housed at 25 ± 2 °C with a relative humidity of 50% ± 5% under a 12 h light/dark cycle and were given a standard laboratory diet and water. The experimental procedures were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals of China and the Institutional Animal Care and Use Committee of the Fifth Medical Center of PLA General Hospital. All animal studies were approved by the Committee on the Ethics of Animal Experiments of the Fifth Medical Center of the PLA General Hospital.
Mice were anaesthetised using intraperitoneal isoflurane. During the following procedures, the mice were spontaneously breathing (Reis Gonçalves et al., 2012; Soni et al., 2016). Mice in the LPS and LPS + Keguan-1/DXM groups were administered LPS (2 mg/kg, 0.8 mg/mL) suspended in saline solution by intratracheal instillation (i.t.), whereas those in the control and Keguan-1 groups were administered an equivalent volume of saline solution in the same manner. After 2 h, the animals were orally administered with Keguan-1. The clinical daily dose of Keguan-1 is 19.4 g/day for patients, and the dosages of Keguan-1 used in mice were calculated according to the recommended human–mouse conversion ratio (Nair and Jacob, 2016; Zhou et al., 2021). Three different final doses were selected: low dose (1.25 g/kg), medium dose (2.5 g/kg), and high dose (5 g/kg). These doses were equivalent to 0.5 × , 1 × , and 2 × the clinical dose. Intraperitoneal injection of DXM was used as a positive control (Brotherton et al., 2020; Ledford, 2020; Chaomin Wu et al., 2020; Zhang et al., 2017). Bronchoalveolar lavage fluid (BALF) and lung tissue were collected 6 h and 24 h after i.t. administration of LPS. For the survival study, a lethal dose of LPS (50 mg/kg) was administered to the mice, and the survival rate was recorded every 12 h up to 72 h after LPS administration.
2.3. Examination of vital signs and blood gas analysis
The anal temperature was recorded using an anal thermometer. For the blood gas analysis, blood was drawn from the abdominal aorta, anticoagulated with heparin lithium, and investigated using a blood gas analyser (Epoc blood analysis, USA).
2.4. Histological staining of lung tissue and immunofluorescence staining for Ly6G
After 6 h and 24 h of LPS administration, the lungs were collected and fixed with 4% paraformaldehyde, dehydrated using a graded ethanol series, embedded in paraffin wax, cut into 5-mm-thick slices, and stained with haematoxylin and eosin. The histopathological features of the tissues were assessed using a light microscope. The lung injury scores ranged from 0 to 4 and were evaluated by two pathologists in a blinded manner (INHAND scoring standard: 0 = no abnormality; 1 = lesions are not obvious, between normal and abnormal; 2 = lesions can be observed but not serious; 3 = the lesions are significant and may become more serious; 4 = lesions occurring in large areas of tissues and organs). The severity of ALI was quantified by adding the individual scores of each category. The sections used for immunofluorescence were incubated with rabbit antibody (1:300) against Ly6G after being blocked with bovine serum albumin and then incubated with a fluorescent secondary antibody. Images were captured using a digital camera connected to a microscope. Quantification of immunofluorescence staining was performed using the HALO analysis software. The HALO analysis software is a digital pathological image analysis platform based on artificial intelligence learning launched by Indica Labs in the United States. The instructions for use are as follows: enter the HALO analysis software, set the target area for measurement in images, apply the Indica Labs – HighPlex FL module automatically identified and set all red cells on tissue sections as positive and blue nuclei as other cells. After analysing each section, the positive cell number and the total cell number was obtained, and the percentage of positive cells (number of positive cells/total number of cells × 100) was calculated.
2.5. Measurement of proinflammatory mediator and Ang Ⅱ levels in BALF
The mice were euthanised under deep anaesthesia after 6 h and 24 h i.t. administration of LPS. The trachea was exposed and cannulated using a 22-gauge angiocatheter. Both the lungs were lavaged with three separate 0.5 mL volumes of ice-cold phosphate-buffered saline (PBS). The BALF was centrifuged at 420 × g for 10 min at 4 °C to pellet the cell fraction, and the supernatant was prepared to analyse the total protein, cytokine, and chemokine levels. The cell pellet was resuspended in 500 μL of cold PBS, and the total cell count was determined using a haemocytometer. BALF protein levels were quantified using a BCA protein assay kit.
The concentrations of the cytokines, including TNF-α, IL-6, IL-1β, IL-1α, KC, and MIP2, and Ang II in the cell-free BALF were measured using commercially available ELISA kits according to the manufacturer's instructions. The absorbance values were measured at 450 nm using an ELISA reader (Bio-Rad, USA).
2.6. Flow cytometry
BALF cells were collected from the individual mice and probed with anti-F4/80, Annexin V-PE, and 7AAD. The percentages of annexin V and 7AAD double-positive cells (necrotic cells) were determined. A total of 1–10 × 105 cells were collected and washed with PBS. The cells were suspended in 500 μL binding buffer, and 10 μL of anti-F4/80 was added to each tube. After gentle vortexing, the cells were incubated at room temperature away from light for 10 min. Thereafter, 5 μL Annexin V-PE and 10 μL 7AAD were added to each tube, incubated at room temperature without light for 5 min, and washed with PBS. After staining and washing, the cells were resuspended in PBS, and flow cytometry was conducted. The cells were analysed using a Cyan flow cytometer and analysed using the Flow.Jo10 software package (both from BD Biosciences).
2.7. Vascular permeability
Lung permeability was determined by assessing the tissue accrual of Evans blue. Animals were administered 30 mg/kg Evans blue by tail vein injection 2 h before the lungs were harvested. The lungs were perfused with 5 mL PBS and homogenised in 1 mL PBS before washing twice. Evans blue was extracted using 1 mL formamide at 60 °C for 24 h. The supernatant was separated by centrifugation at 5000 × g for 30 min. The optical density of the supernatant was measured spectrophotometrically at 620 nm. Evans blue dye concentration was calculated against a standard curve and was presented as μg of Evans blue dye per gram of tissue.
2.8. Western blotting analysis
After 24 h of LPS administration, the lungs were homogenised in a tissue protein extraction solution (RIPA) containing 1% proteinase inhibitor phenylmethanesulfonyl fluoride (PMSF). After centrifugation, the protein concentration in the supernatant was determined using a BCA protein assay kit following the manufacturer's instructions. Equal amounts of protein were subjected to gel electrophoresis according to standard procedures. The blots were then incubated with primary antibodies at 4 °C overnight and then probed with secondary antibodies conjugated with horseradish peroxidase. GAPDH was used as the loading control. The membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies and were then visualised using an enhanced chemiluminescence reagent (Millipore, Massachusetts, USA). Image J software was used for gray value analysis. GAPDH was used as the internal control to calculate the gray scale ratio for each protein band in relation to internal control bands.
2.9. Statistical analyses
All data are shown as the mean ± SEM or the mean ± SD. Statistical comparisons were performed using an unpaired, 2-tailed t-test between the two groups. One-way ANOVA, followed by least significant difference (LSD) post hoc test, was used to assess the differences of multi-groups with GraphPad Prism 8.4.2 (GraphPad Software). Statistical significance was set at P < 0.05.
3. Results
3.1. Effect of Keguan-1 on the survival rate and arterial blood gas in LPS-Induced ALI mouse model
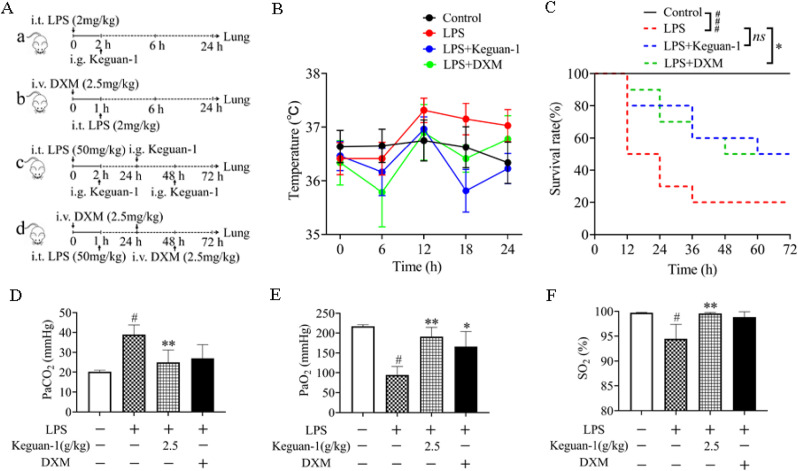
Stable ALI mice were successfully constructed by i.t. administration of 2 mg/kg LPS. The ALI mice were orally administered with Keguan-1 at 2 h after LPS exposure or intraperitoneally injected with DXM (a positive control) at 1 h before LPS challenge. BALF and lung tissue were collected at 6 h and 24 h. To assess the effect of Keguan-1 on the survival rate of ALI, the mice were treated with a lethal dose of LPS (50 mg/kg). At 2 h, 24 h, and 48 h after i.t. -administration of LPS, the mice were orally administered with Keguan-1, or injected with DXM at 1 h before LPS exposure and at 24 h, 48 h after LPS administration (Fig. 1 A). By measuring the anal temperature of the ALI mice, we found that Keguan-1 reduced the body temperature of ALI mice to normal levels (Fig. 1B). The survival curve results indicated that the survival rate of ALI mice was 20% (Fig. 1C). After intragastric administration of 2.5 g/kg Keguan-1, the survival rate reached 50%, which was the same as that in the LPS + DXM group and was higher than that in the LPS group (Fig. 1C). After 6 h of LPS (2 mg/kg) treatment, aortic blood was analysed using a blood gas analyser. Keguan-1 significantly reduced the CO2 partial pressure (PaCO2) and increased the O2 partial pressure (PaO2) and oxygen saturation (SO2) in the ALI mouse model (Fig. 1D–F), suggesting that Keguan-1 could improve the respiratory condition of ALI mice. These results indicate that Keguan-1 has a protective effect on ALI mice by improving the survival rate and respiratory condition.
Fig. 1.
Effects of Keguan-1 on survival rate and arterial blood gas concentration in LPS-induced ALI mouse model. (A) Schematic representation of ALI induction with LPS. The doses and time points of drug administration are indicated by arrows. a. The doses and time points of Keguan-1 administration in ALI mice; b. the doses and time points of DXM administration in ALI mice; c. the doses and time points of Keguan-1 and DXM administration in ALI mice for the survival study. (B) Anal temperature. (C) Survival rates were calculated at 12, 24, 36, 48, and 72 h after LPS challenge. Survival rate between the two groups was statistically analysed. (D–F) Arterial blood gas. Arterial partial pressure of carbon dioxide (PaCO2) (D), O2 partial pressure (PaO2) (E), O saturation (SO2) (F). The data are presented as the mean ± SEM. #P < 0.05 and ###P < 0.001 vs. control; ∗P < 0.05 and ∗∗P < 0.01 vs. LPS group.
3.2. Keguan-1 alleviates histological changes in the LPS-Induced ALI mouse model
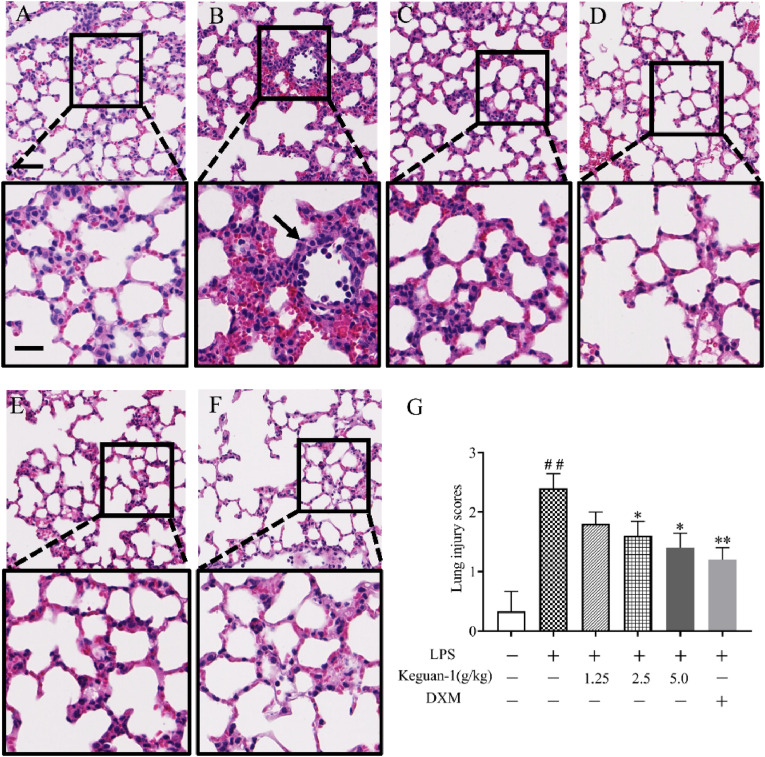
The histopathology of the lung changed significantly 24 h after LPS stimulation, with lymphocytes and a few neutrophils infiltrating the locally widened alveolar septum and perivascular areas, alveolar wall thickening, alveolar space congestion, and alveolar oedema, eventually leading to microvascular hyperpermeability. Treatment with Keguan-1 alleviated these effects (Fig. 2 ). These results suggest that Keguan-1 has a protective effect against ALI.
Fig. 2.
Effects of Keguan-1 on the histological changes in LPS-induced ALI mice. Representative images of mice lung tissue stained using HE after 24 h of LPS administration. (A) Control; (B) LPS; (C) LPS + 1.25 g/kg Keguan-1; (D) LPS + 2.5 g/kg Keguan-1; (E) LPS + 5.0 g/kg Keguan-1; (F) LPS + 2.5 mg/kg DXM. (G) Lung injury scores of mice in each group. The upper panel is low magnification (scale bar = 100 μm) of the images, the areas inside the boxes are shown in the lower panel at high magnification (scale bar = 50 μm). The data are presented as the mean ± SEM. ##P < 0.01 vs. control; ∗P < 0.05 and ∗∗P < 0.01 vs. LPS group.
3.3. Keguan-1 reduces the proinflammatory mediator levels in the BALF of the ALI mice after LPS treatment
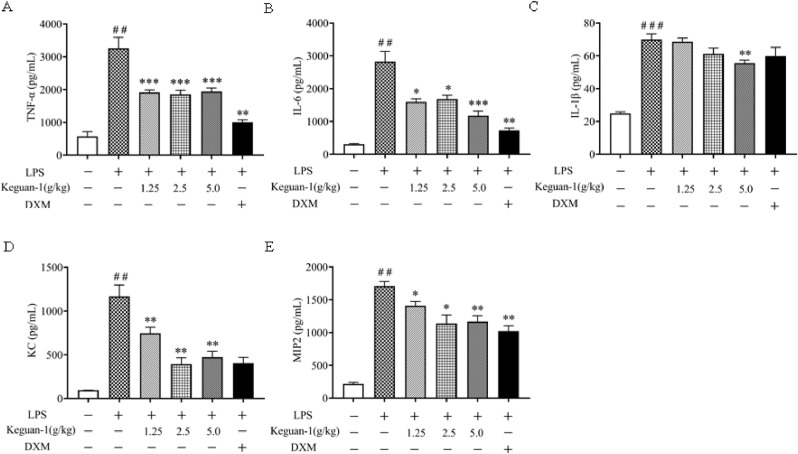
The levels of proinflammatory cytokines (TNF-α, IL-6, and IL-1β) and chemokines (KC and MIP2) were examined in BALF to evaluate the effect of Keguan-1 on LPS-induced inflammation. Compared to those in control, the levels of TNF-α, IL-6, IL-1β, KC, and MIP2 in BALF were significantly elevated in mice after LPS exposure. Keguan-1 significantly reduced the production of TNF-α, IL-6, KC, and MIP2 (Fig. 3 A–E). However, Keguan-1 was less effective in inhibiting IL-1β production. These results suggest that Keguan-1 could reduce the inflammatory response of lung tissue after ALI in mice.
Fig. 3.
Effects of Keguan-1 on the production of cytokines and chemokines in the lungs of the LPS-induced ALI mouse model. The concentrations of TNF-α (A), IL-6 (B), IL-1β (C), KC (D), and MIP2 (E) in the BALF. The data are presented as the mean ± SEM. ##P < 0.01 and ###P < 0.001 vs. control; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. LPS group.
3.4. Effect of Keguan-1 on neutrophils and alveolar macrophages in the lung tissue of LPS-Induced ALI mouse model
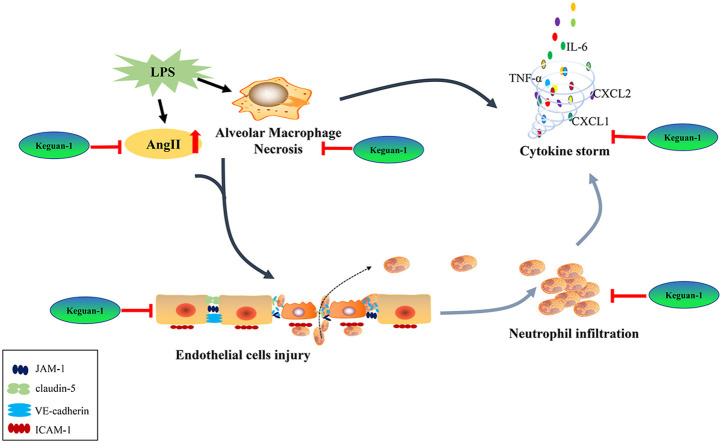
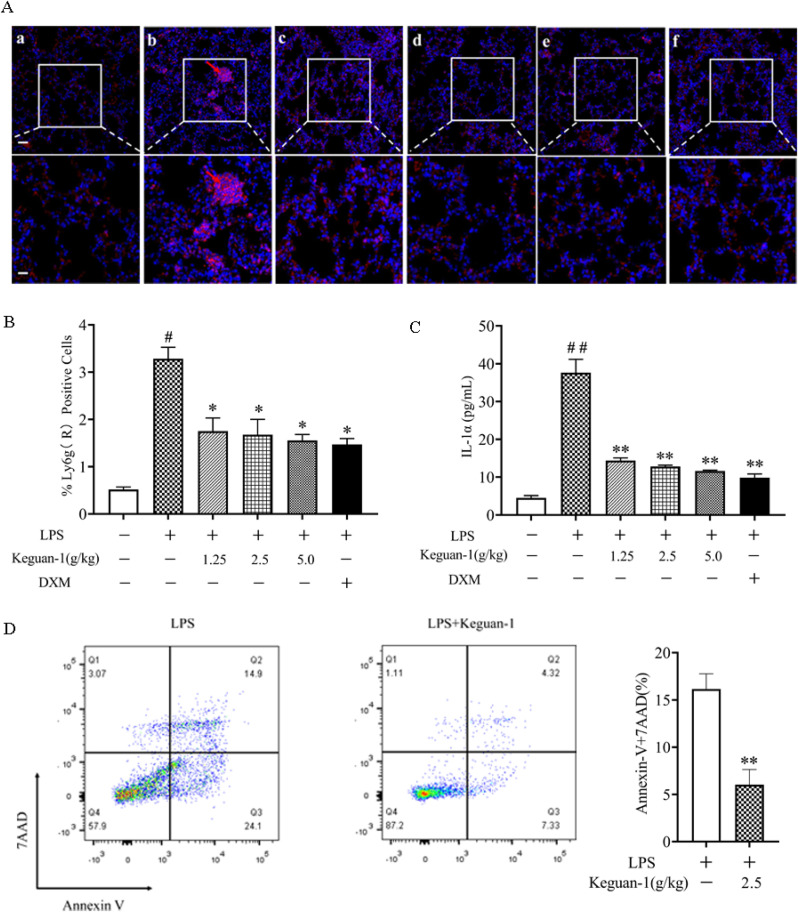
Neutrophil recruitment into the lungs is a hallmark of early ALI. Previous studies have shown that alveolar macrophage necrosis causes the release of IL-1α, activates vascular endothelial cells, and leads to increased vascular permeability (Dagvadorj et al., 2015), facilitating the entry of a large number of neutrophils into the lung and inducing a strong inflammatory response. Immunofluorescence staining showed that a large number of Ly6G+ neutrophils existed in the lungs at 6 h post-LPS administration. Keguan-1 treatment reduced the number of Ly6G+ neutrophils that infiltrated the lung tissue (Fig. 4 A and B). Thereafter, we examined whether Keguan-1 decreased alveolar macrophage necrosis during LPS-induced ALI. BALF cells were isolated and analysed using flow cytometry for F4/80 (macrophages), Annexin V (apoptotic and necrotic cells), and 7AAD (necrotic cells). The results showed that after Keguan-1 treatment, alveolar macrophage necrosis significantly decreased in the LPS-induced ALI mouse model (Fig. 4D). Consistent with this finding, the level of IL-1α in BALF was attenuated by treatment with Keguan-1 (Fig. 4C), suggesting a positive role of Keguan-1 in alleviating the lung injury induced by LPS.
Fig. 4.
Effects of Keguan-1 on neutrophils and alveolar macrophage in the LPS-induced ALI mouse model. (A) Representative immunofluorescent staining images for neutrophils in lung tissue at 6 h after LPS stimulation (Red, Ly6G staining; Blue, DAPI staining). (a) Control; (b) LPS; (c) LPS + 1.25 g/kg Keguan-1; (d) LPS + 2.5 g/kg Keguan-1; (e) LPS + 5.0 g/kg Keguan-1; (f) LPS + 2.5 mg/kg DXM. The upper panel is low magnification (scale bar = 100 μm) of the images, the lower panel is high magnification image (scale bar = 50 μm). (B) Statistical result of the number of Ly6G+-positive cells. (C) The concentration of IL-1α in BALF. (D) BALF cells were probed with anti-F4/80 and stained with annexin V and 7AAD. The number of necrotic cells was determined by using flow cytometry. The data are presented as the mean ± SEM. #P < 0.05 and ##P < 0.01 vs. control; ∗P < 0.05 and ∗∗P < 0.01 vs. LPS group.
3.5. Effect of Keguan-1 on pulmonary vascular endothelial injury in the LPS-Induced ALI mouse model
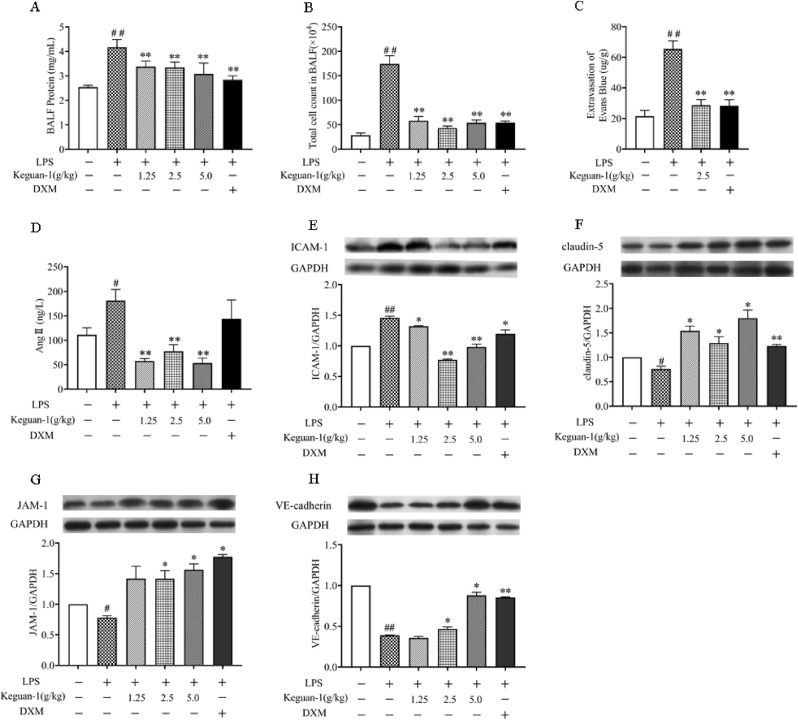
Thereafter, we examined the effect of Keguan-1 on pulmonary vascular endothelial injury. The expression levels of total protein content, total cell counts in BALF, and Evans blue extracted from lung tissue were measured at 6 h after LPS administration (Fig. 5 A–C). Keguan-1 significantly reduced the exudation of inflammatory protein/cells and Evans blue extracted, indicating that Keguan-1 has the potential to reduce microvascular permeability in lung tissue of ALI mice. To explore the protective effect of Keguan-1 on pulmonary vascular endothelial cells, we examined the expression of ICAM-1, claudin-5, JAM-1, and VE-cadherin, all of which are associated with the function of vascular endothelial cells (Dagvadorj et al., 2015; Ma et al., 2014). The expression of ICAM-1 increased after 24 h of LPS administration and was attenuated by treatment with Keguan-1. Moreover, Keguan-1 evidently increased claudin-5, JAM-1, and VE-cadherin expression after 24 h of LPS treatment.
Fig. 5.
Effects of Keguan-1 on endothelial injury in the LPS-induced ALI mouse model. (A) The BALF protein concentration. (B) The BALF total cell count. (C) The levels of Evans blue extracted from lungs. (D) The concentration of Ang II in BALF. (E–H) The expression of ICAM-1 (E), claudin-5 (F), JAM-1 (G), and VE-cadherin (H) in mice lungs as determined by using western blotting. The data are presented as the mean ± SEM. #P < 0.05 and ##P < 0.01 vs. control; ∗P < 0.05 and ∗∗P < 0.01 vs. LPS group.
Several studies have shown that elevated levels of Ang II may induce vasoconstriction, fibrosis, and inflammation (Amraei and Rahimi, 2020; Bourgonje et al., 2020). To determine whether Ang II is involved in the Keguan-1-mediated protective effect during LPS-induced ALI, we evaluated the concentration of Ang II in lung tissue using ELISA. As a result, the concentration of Ang II was markedly decreased in the lungs of Keguan-1-treated mice compared to that in the lungs of the LPS group.
These results suggest that Keguan-1 improves pulmonary vascular permeability and endothelial injury by regulating the expression and secretion of adhesion molecules in vascular endothelial cells. Keguan-1 also plays a protective role against pulmonary injury by reducing pulmonary vasoconstriction and Ang II levels and improving pulmonary circulation resistance.
4. Discussion
ARDS is a life-threatening condition of ALI characterised by bilateral pulmonary infiltrates, severe hypoxemia, and non-cardiogenic pulmonary oedema. It is thought to be a major cause of death in patients with COVID-19 (Berlin et al., 2020; Nile et al., 2020). The pathogenesis of ARDS is related to the hyperinflammatory response and endothelial injury (Huppert et al., 2019). Our previous clinical studies showed that Keguan-1 significantly reduced the incidence of ARDS in patients with severe COVID-19 and inhibited the progression to severe disease (Wang et al., 2020). Thus, this study aims to explore the effect and mechanism of Keguan-1 on ARDS in LPS-induced ALI model. In this study, we observed that Keguan-1 significantly decreased alveolar macrophage necrosis in an LPS-induced ALI model, thereby inhibiting the activation of vascular endothelial cells and reducing neutrophil infiltration. These results suggest that Keguan-1 can reduce the inflammatory response, inhibit endothelial cells injury, and alleviate severe pulmonary dysfunction, which may be an important mechanism for reducing the prevalence of ARDS in patients with COVID-19.
Some studies have indicated that excessive and uncontrolled inflammatory responses in the lung are essential for ALI/ARDS, which could first recruit neutrophils to induce the production of multiple inflammatory mediators and activate effector cells (Grommes and Soehnlein, 2011; Zhou et al., 2012). In ALI, neutrophils are first sequestered in the pulmonary microvessels following stimulation by many inflammatory mediators (such as LPS, TNF, and IL-1) and adhere to the endothelium where they become activated. In addition, neutrophils travel out of the pulmonary vascular bed and continue to activate and release a series of injury mediators that can cause diffuse alveolar damage, ultimately leading to ALI (Kolaczkowska and Kubes, 2013). Therefore, neutrophil infiltration is thought to play a key role in the progression of ALI/ARDS. In addition, studies have shown that LPS initiates pulmonary inflammation by inducing necrosis in alveolar macrophages, and subsequent endothelial cell activation allows neutrophil recruitment into the lungs and destroys the alveolar-capillary barrier (Dagvadorj et al., 2015). In this study, the results showed that Keguan-1 significantly decreased alveolar macrophage necrosis in BALF and neutrophilic infiltration in lung tissues of ALI mice, subsequently inhibiting the expression of inflammatory mediators, including TNF-α, IL-6, IL-1β, KC, and MIP2. These results suggest that Keguan-1 can alleviate ARDS by reducing the inflammatory response.
Inhibiting the binding of SARS-CoV-2 S protein to host ACE2 has been shown to be effective in preventing viral entry, whereas inhibiting 3ClPro activity can effectively block the replication and synthesis of RdRp and other key proteins of the virus, thus resulting in the loss of viral intracellular replication ability (Canrong Wu et al., 2020; Xian et al., 2020). Based on molecular docking technology, we previously found that several Chinese medicinal components in Keguan-1 had potential inhibitory effects on several key proteins in the viral life cycle. The binding of the S protein to ACE2 could be inhibited by Lonicera japonica Thunb. (Jinyinhua), Forsythia suspensa (Thunb.) Vahl (Lianqiao), Morus alba L. (Sangye), Chrysanthemum morifolium Ramat. (Juhua), and Fritillaria thunbergia Miq. (Zhebeimu) in the Keguan-1 formula. Moreover, some components of Lonicera japonica Thunb. (Jinyinhua), Forsythia suspensa (Thunb.) Vahl (Lianqiao), and Morus alba L. (Sangye) can inhibit 3ClPro (Niu et al., 2020). Therefore, Keguan-1 may have direct antiviral effects. In addition, a previous RCT demonstrated that Keguan-1 significantly reduced the incidence of ARDS in patients with COVID-19 (Wang et al., 2020). Based on these findings, we propose that Keguan-1 may reduce the incidence of ARDS development in COVID-19 patients by inhibiting the inflammatory response, which warrants further investigation.
Numerous studies have shown that the mortality associated with SARS-CoV-2 was caused by the virus itself and the resulting inflammatory cytokine storm and the microvascular dysfunction in the patients (Wei et al., 2020; S. Zhang et al., 2020b). When vascular injury is caused by SARS-CoV-2, especially in vulnerable patients with pre-existing endothelial dysfunction, the original microvascular dysfunction can be aggravated, which results in excessive vasoconstriction, organ ischaemia inflammation, and related tissue oedema. These responses could accelerate the disease progression and increase the mortality of patients with COVID-19 (Bilinska et al., 2020; Varga et al., 2020). As SARS-CoV-2 targets lung cells by binding to ACE2, unopposed Ang II accumulation occurs, leading to increased vascular permeability, oedema, inflammation, and fibrosis (Beyerstedt et al., 2021; Bilinska et al., 2020; Bourgonje et al., 2020; Walls et al., 2020). It has been reported that the concentration of Ang II in the blood of COVID-19 patients is higher than that in healthy controls and is positively correlated with SARS-CoV-2 load and disease severity (Liu et al., 2020). In this study, Keguan-1 significantly downregulated the Ang II levels in the lung tissue of ALI mice, thereby mitigating the pulmonary vascular injury caused by its elevated levels.
In addition, vascular endothelial cells could express receptors of several inflammatory mediators, including LPS, TNF-α, and IL-1 (Dagvadorj et al., 2015). Inflammatory factors released by a viral infection directly activates endothelial cells by binding to the receptors, thus disrupting adhesion protein secretion on endothelial cells, and increasing the adhesion of inflammatory mediators (such as neutrophils, monocytes, and macrophages) to blood vessels, and finally leads to vascular endothelial injury (Dagvadorj et al., 2015). ICAM-1, tight junction proteins (claudin-5 and JAM-1), and VE-cadherin play important roles in the immune response. ICAM-1 has been considered as the most important adhesion molecule for inflammatory cells to gather at the inflammatory site. ICAM-1 mediates vascular endothelial injury induced by white blood cells by promoting the adhesion of white blood cells to endothelial cells (Ma et al., 2014). Downregulated expression of claudin-5 and JAM-1 increases vascular permeability, making it easier for inflammatory cells to penetrate through vascular endothelial cells, leading to inflammatory cells infiltrating the lung tissue and causing tissue damage (Ma et al., 2014). The main function of VE-cadherin is to maintain and regulate endothelial barrier function and endothelial permeability (Dagvadorj et al., 2015). In this study, Keguan-1 reduced the expression of endothelial cell adhesion molecule ICAM-1 and increased the expression of tight junction proteins (claudin-5 and JAM-1), and VE-cadherin in ALI mice, thus improving pulmonary vascular permeability, reducing vascular endothelial injury, and playing a protective role in the pulmonary microvascular injury. In conclusion, Keguan-1 may systematically improve pulmonary microvascular injury and prevent ARDS by reducing the expression of ICAM-1, and increasing the expression of claudin-5, JAM-1, and VE-cadherin in vascular endothelial cells.
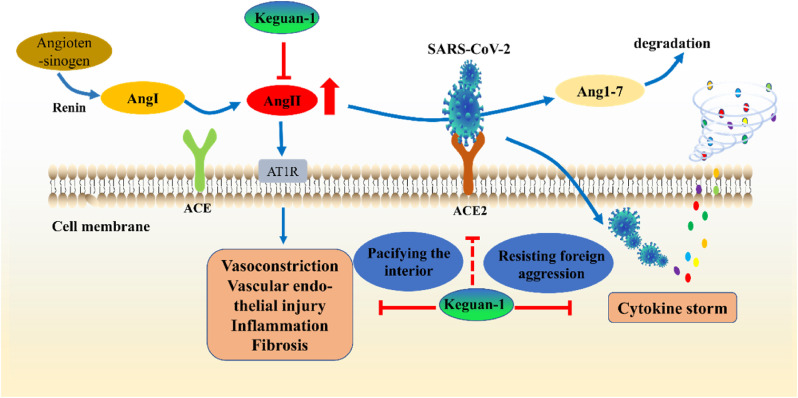
Our previous RCT study demonstrated that Keguan-1 significantly reduced the incidence of ARDS in patients with COVID-19, prevented the progression to severe disease (Wang et al., 2020). Based on molecular docking technology, we previously found that several Chinese medicinal components in Keguan-1 might also exert potential antiviral effects by targeting several key proteins in the viral life cycle (Niu et al., 2020). In this study, we demonstrated that Keguan-1 could improve LPS-induced ALI by ameliorating inflammation and pulmonary vascular endothelial injury to improve LPS-induced ALI. Based on the above-mentioned research results, we first proposed the hypothesis of “resisting foreign aggression and pacifying the interior” (Fig. 6 ): Keguan-1 can “resist foreign aggression” by directly inhibiting SARS-CoV-2 and the inflammation caused by the virus and “pacify the interior” by regulating pulmonary vascular endothelial injury, and then realise the comprehensive treatment of the patients with COVID-19 via the effect on both the pathogen and host, which is the unique advantage and characteristic of TCMs in preventing and treating new complex diseases. Although the antiviral effect and the substances and mechanisms of Keguan-1 in the prevention and treatment of ARDS need to be further studied, the hypothesis of “resisting foreign aggression and pacifying the interior” provides guidance for the clinical application of TCMs in preventing and treating COVID-19 and other emerging infectious diseases, and it is also instructive and meaningful to other diseases.
Fig. 6.
The mechanism of Traditional Chinese medicines (TCMs) against COVID-19. Keguan-1 can “resist foreign aggression” by directly inhibiting SARS-CoV-2 and the inflammation caused by the virus and “pacify the interior” by regulating pulmonary vascular endothelial injury, and then realise the comprehensive treatment of patient with COVID-19 via the effect on both the pathogen and host, which is the unique advantage and characteristic of TCMs in preventing and treating new complex diseases. The solid red line represents the regulation of Keguan-1 on pulmonary vascular endothelial injury and cytokine storm, and the red dotted line represents the potential inhibition of Keguan-1 on the binding of SARS-CoV-2 S protein to host ACE2. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Zhaofang Bai: conducted the study and wrote the manuscript, they contributed equally to this work as co-first authors, participated in research design. Pengyan Li: conducted the study and wrote the manuscript, they contributed equally to this work as co-first authors. Jincai Wen: Formal analysis. Yanzhong Han: Formal analysis. Yuanyuan Cui: Formal analysis. Yongfeng Zhou: Formal analysis. Zhuo Shi: Formal analysis. Shuaishuai Chen: Formal analysis. Qiang Li: Formal analysis. Xu Zhao: Formal analysis. Zhongxia Wang: provided technical assistance. Ruisheng Li: provided technical assistance. Yuming Guo: provided technical assistance. Xiaoyan Zhan: provided technical assistance. Guang Xu: provided technical assistance. Kaixin Ding: provided technical assistance. Jiabo Wang: participated in research design. Xiaohe Xiao: participated in research design.
Acknowledgments
This study has been funded by COVID-19 Emergency Research project of PLA General Hospital (Number:BWA20J006).
References
- Amraei R., Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9:1–18. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N. Engl. J. Med. 2020;383:2451–2460. doi: 10.1056/nejmcp2009575. [DOI] [PubMed] [Google Scholar]
- Beyerstedt S., Casaro E.B., Rangel É.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D.M.E., van der Voort P.H.J., Mulder D.J., van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton H., Usuf E., Nadjm B., Forrest K., Bojang K., Samateh A.L., Bittaye M., Roberts C.A., d'Alessandro U., Roca A. Dexamethasone for COVID-19: data needed from randomised clinical trials in Africa. Lancet Glob. Heal. 2020;8:e1125–e1126. doi: 10.1016/S2214-109X(20)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagvadorj J., Shimada K., Chen S., Jones H.D., Tumurkhuu G., Zhang W., Wawrowsky K.A., Crother T.R., Arditi M. Lipopolysaccharide induces alveolar macrophage necrosis via CD14 and the P2X7 receptor leading to interleukin-1α release. Immunity. 2015;42:640–653. doi: 10.1016/j.immuni.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes J., Soehnlein O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Guan W. jie, Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z., hua Zhong, shan N. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert L.A., Matthay M.A., Ware L.B. Pathogenesis of acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2019;40:31–39. doi: 10.1055/s-0039-1683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar D., Lahiri B., Ranjan P., Chatterjee J., Lahiri P., Sengupta S. 2021. Road Map to Understanding Sars-Cov-2 Clinico-Immunopathology and Covid-19 Disease Severity. Pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Ledford H. Shown to prevent. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li Jinxiu, Li Jianming, Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.Q., Pan C.S., Yang N., Liu Y.Y., Yan L., Sun K., Wei X.H., He K., Xiao M.M., Fan J.Y., Han J.Y. Posttreatment with Ma-Xing-Shi-Gan-ang, a Chinese medicine formula, ameliorates lipopolysaccharide-induced lung microvessel hyperpermeability and inflammatory reaction in rat. Microcirculation. 2014;21:649–663. doi: 10.1111/micc.12144. [DOI] [PubMed] [Google Scholar]
- Nair A., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7:27. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Chen L., Huang X., Han C., Xu Jianrong, Zhang H., Luan X., Zhao Y., Xu Jianguang, Yuan W., Chen H. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm. Sin. B. 2020;10:1149–1162. doi: 10.1016/j.apsb.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Wang R.L., Wang Z.X., Zhang P., Bai Z.F., Jing J., Guo Y.M., Zhao X., Zhan X.Y., Zhang Z.T., Song X.A., Qin E.Q., Wang J.B., Xiao X.H. Rapid establishment of traditional Chinese medicine prevention and treatment of 2019-nCoV based on clinical experience and molecular docking. Zhongguo Zhongyao Zazhi. 2020;45:1213–1218. doi: 10.19540/j.cnki.cjcmm.20200206.501. [DOI] [PubMed] [Google Scholar]
- Reis Gonçalves C.T., Reis Gonçalves C.G., de Almeida F.M., dos Santos Lopes F.D.T.Q., dos Santos Durão A.C.C., dos Santos F.A., da Silva L.F.F., Marcourakis T., Castro-Faria-Neto H.C., Vieira R.D.P., Dolhnikoff M. Protective effects of aerobic exercise on acute lung injury induced by LPS in mice. Crit. Care. 2012;16:R199. doi: 10.1186/cc11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J. ling, Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni S., Wilson M.R., O'Dea K.P., Yoshida M., Katbeh U., Woods S.J., Takata M. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax. 2016;71:1020–1029. doi: 10.1136/thoraxjnl-2015-208032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. bo, Wang Z. xia, Jing J., Zhao P., Dong J., hui Zhou, feng Y., Yang G., Niu M., Zhao X., Jiang T., jun Bi, feng J., Xu Z., Zhang P., Wu D., Bai Z., fang Guo, ming Y., Yu S., miao Sun, qiang Y., Zhang Z. teng, Zhan X. yan, Li P. yan, Ding J. biao, Zhao P. fei, Song X. ai, Tang J., yuan He, chu D., Chen Z., Qin E., qiang Wang, lin R., Xiao X. he. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin. J. Integr. Med. 2020;26:648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z.Y., Geng Y.J., Huang J., Qian H.Y. Pathogenesis and management of myocardial injury in coronavirus disease 2019. Eur. J. Heart Fail. 2020 doi: 10.1002/ejhf.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Chaomin, Chen X., Cai Y., Xia J., Zhou Xing, Xu S., Huang H., Zhang L., Zhou Xia, Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou Xin, Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Canrong, Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian Y., Zhang J., Bian Z., Zhou H., Zhang Z., Lin Z., Xu H. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm. Sin. B. 2020;10:1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Lu Q. Bin, Liu M.J., Wang Y.X., Zhang A.R., Jalali N., Dean N.E., Longini I., Elizabeth Halloran M., Xu B., Zhang X.A., Wang L.P., Liu W., Fang L.Q. 2020. Epidemiological and Clinical Features of the 2019 Novel Coronavirus Outbreak in China. medRxiv. [DOI] [Google Scholar]
- Zhang B., Zhou X., Qiu Y., Song Y., Feng F., Feng J., Song Q., Jia Q., Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., Zhang Shenghui, Fan Z., Dong J., Yuan Z., Ding Z., Zhang Y., Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13 doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., Zhang Shenghui, Fan Z., Dong J., Yuan Z., Ding Z., Zhang Y., Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13:1–22. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Luo Z., Bi A., Yang W., An W., Dong X., Chen R., Yang S., Tang H., Han X., Luo L. Compound edaravone alleviates lipopolysaccharide (LPS)-induced acute lung injury in mice. Eur. J. Pharmacol. 2017;811:1–11. doi: 10.1016/j.ejphar.2017.05.047. [DOI] [PubMed] [Google Scholar]
- Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F., Tu D., Ge G., Zheng Y., Shi T., Xu X., Zhao S., Yang Y., Zhang W. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2021;85:153315. doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Song Y., Liu X., Zhang C., Li F., Hu R., Huang Y., Ma W., Song K., Zhang M. Si-Wu-Tang facilitates ovarian function through improving ovarian microenvironment and angiogenesis in a mouse model of premature ovarian failure. J. Ethnopharmacol. 2021;280:114431. doi: 10.1016/j.jep.2021.114431. [DOI] [PubMed] [Google Scholar]
- Zhou X., Dai Q., Huang X. Neutrophils in acute lung injury. Front. Biosci. 2012;17:2278–2283. doi: 10.2741/4051. [DOI] [PubMed] [Google Scholar]