Abstract
Background
Gastric cancer (GC) is a common malignant cancer with a poor prognosis. Ferroptosis has been shown to play crucial roles in GC development. Long non-coding RNAs (lncRNAs) is also associated with tumor progression in GC. This study aimed to screen the prognostic ferroptosis-related lncRNAs and to construct a prognostic risk model for GC.
Methods
Ferroptosis-related lncRNAs from The Cancer Genome Atlas (TCGA) GC expression data was downloaded. First, single factor Cox proportional hazard regression analysis was used to select seven prognostic ferroptosis-related lncRNAs from TCGA database. And then, the selected lncRNAs were further included in the multivariate Cox proportional hazard regression analysis to establish the prognostic model. A nomogram was constructed to predict individual survival probability. Finally, we performed quantitative reverse transcription polymerase chain reaction (qRT-PCR) to verify the risk model.
Results
We constructed a prognostic ferroptosis-related lncRNA signature in this study. Kaplan-Meier curve analysis revealed a significantly better prognosis for the low-risk group than for the high-risk group (P = 2.036e-05). Multivariate Cox proportional risk regression analysis demonstrated that risk score was an independent prognostic factor [hazard ratio (HR) = 1.798, 95% confidence interval (CI) =1.410–2.291, P < 0.001]. A nomogram, receiver operating characteristic curve, and principal component analysis were used to predict individual prognosis. Finally, the expression levels of AP003392.1, AC245041.2, AP001271.1, and BOLA3-AS1 in GC cell lines and normal cell lines were tested by qRT-PCR.
Conclusions
This risk model was shown to be a novel method for predicting prognosis for GC patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08975-2.
Keywords: Ferroptosis, Gastric cancer, Long non-coding RNA, Prognosis, Bioinformatics
Background
Gastric cancer (GC) is a common cancer. It was the third leading cause of cancer-related deaths in 2018 [1]. The incidence and mortality rates of GC have increased in China [2]. After surgery, multimodal therapies, including chemoradiation and chemotherapy, are utilized to prevent recurrence. Although such treatments have improved the survival rates of patients, patients with GC still have a survival rate of approximately 30% worldwide [3]. Therefore, novel biomarkers for GC need to be explored to predict the progression, prognosis, and response to treatment.
Ferroptosis is a non-apoptotic form of cell death [4]. Ferroptosis is associated with small molecules that target the antioxidant system or enzymes such as system xc − and glutathione peroxidase 4 (GPX4) [5]. Studies have shown that ferroptosis is associated with drug resistance [6, 7]. Zhang, H et al. found that ferroptosis promoted drug resistance in GC [8], while Shin, D. et al. indicated that Nrf2 inhibition reversed resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancers [9]. These results revealed that ferroptosis played an important role in cancer development.
Long non-coding RNAs (lncRNAs) are a class of transcripts longer than 200 nucleotides [10]. Increasing studies have shown that lncRNAs play a crucial role in tumor progression [11, 12]. Recently, molecular risk signatures as prognostic predictors of cancer progression were investigated, especially lncRNA signatures [13, 14]. However, the prognostic value of ferroptosis-related lncRNAs signature in GC has not been systematically explored.
In this study, we constructed a molecular signature model comprising four prognostic ferroptosis-related lncRNAs and assessed their prognostic ability for GC. This study showed that the prognostic ferroptosis-related lncRNA signature was a reliable prognostic predictor for GC. Moreover, we explored the correlation between clinical factors and the four prognostic ferroptosis-related lncRNAs in this risk model. Finally, four lncRNAs (AP003392.1, AC245041.2, AP001271.1 and BOLA3-AS1) were validated in GC cell lines.
Materials and methods
Data acquisition
A search was performed for the word “ferroptosis” in MSigDB (http://www.gsea-msigdb.org/gsea/msigdb/index.jsp) to download ferroptosis-related genes. The original transcriptome sequencing dataset and GC-clinical characteristics were obtained from TCGA database (https://portal.gdc.cancer.gov/). The survival time of all patients was ≥30 days.
Identification and correlation analysis
A total of 40 ferroptosis-related genes were downloaded from MSigDB v7.2. First, we selected all lncRNA expression data from TCGA database as shown in Table 1.
Table 1.
Ferroptosis-related lncRNAs in TCGA database
Pearson correlation analysis was performed to determine the correlation between ferroptosis-related genes and all lncRNA expression data of the samples. Finally, ferroptosis-related lncRNAs were identified based on the Pearson correlation coefficient and p values (|Cor Pearson | > 0.4, p < 0.001). The correlation between ferroptosis-related lncRNAs and clinical characteristics was analyzed using the R package “ggpubr.”
Prognostic risk signature construction
Single-variable Cox proportional risk regression analysis was performed to identify ferroptosis-related lncRNAs, which were significantly associated with OS in TCGA GC dataset. Multivariate Cox proportional risk regression analysis was then used to establish the prognostic model for GC. The results were plotted using a forest map with the R package “ggplot2.” The risk score for each patient was calculated using the risk formula: explncRNA1*coef lncRNA1 + explncRNA2*coef lncRNA2 + … + explncRNAi*coef lncRNAi [15]. Kaplan - Meier plotter was used to analyze the different OS times between the high-risk and low-risk groups using the R package “survival.”
PCA
PCA is a widely used tool for dimensionality reduction and feature extraction in the computer vision field [16]. The R package “scatterplot3d” was used to assess potential differences between the high-risk and low-risk groups.
Construction of nomogram
A nomogram was built according to all the independent prognostic factors using the R package “rms” (https://cran.rproject.org/web/packages/rms/index.html). A calibration plot curve analysis was performed to assess the consistency between the actual and predicted survival.
Cell culture and qRT-PCR
Human gastric epithelial cell line (GES-1) and GC cell lines (MKN-45 and AGS) were obtained from Tianjin Createch Biotechnology Co. LTD (Tianjin, China). All cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 1% penicillin-streptomycin (Invitrogen) and 10% fetal bovine serum (Invitrogen) at 37 °C and 5% CO2. qRT-PCR was performed according to the method described earlier [17]. Total RNA was extracted using TRIzol reagent (Invitrogen) and synthesized into cDNA using M-MLV reverse transcriptase (TaKaRa Bio, Japan) following the manufacturer’s instructions. qRT-PCR was performed using SYBR Green assay (Roche, Switzerland). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or U6 was utilized as an endogenous reference. The primers sequences are listed in Supplementary Table S1.
Statistical analysis
All data were analyzed using the R software (R version: 3.6.1) and the following packages: “limma,” “survival,” “Pheatmap,” “ggpubr,” and “survivalROC.” The OS difference was determined using the Kaplan - Meier analysis method and log-rank test. Statistical significance was set at p < 0.05.
Results
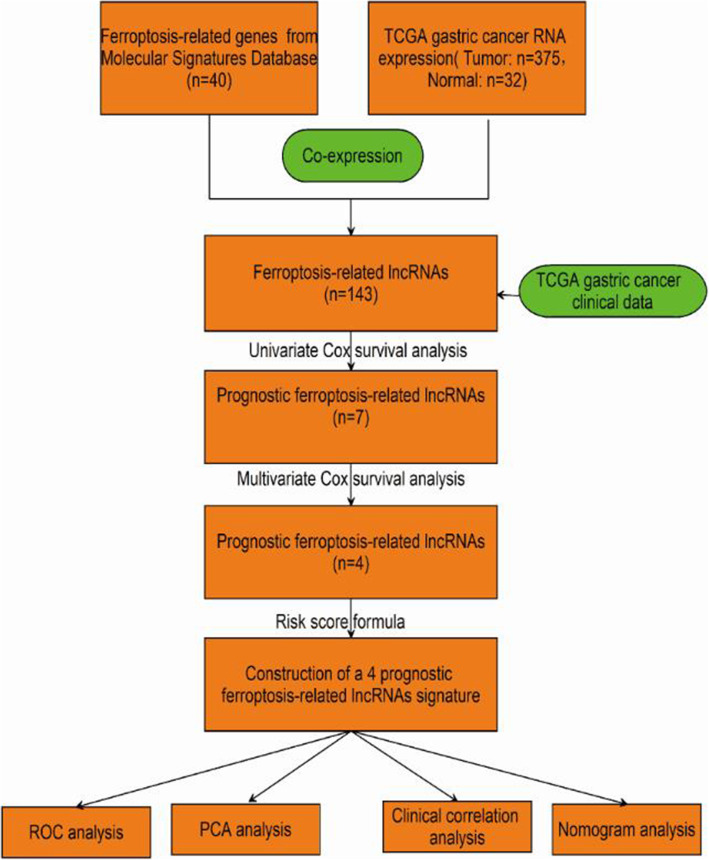
The workflow of the prognostic model analysis is illustrated in Fig. 1. In this study, we used the data of 407 GC patients from The Cancer Genome Atlas (TCGA) cohort (T = 375, N = 32).
Fig. 1.
Workflow of the prognostic risk model analysis
Ferroptosis-related lncRNA identification in TCGA cohort
Forty ferroptosis-related mRNAs were selected based on one ferroptosis gene set (WP_FERROPTOSIS) from the Molecular Signatures Database (MSigDB). A total of 173 ferroptosis-related lncRNAs were screened out, with 148 ferroptosis-related lncRNAs positively correlated and 25 ferroptosis-related lncRNAs negatively correlated with GC according to the co-expression observed in TCGA cohort.
Construction of prognostic ferroptosis -related lncRNA signature
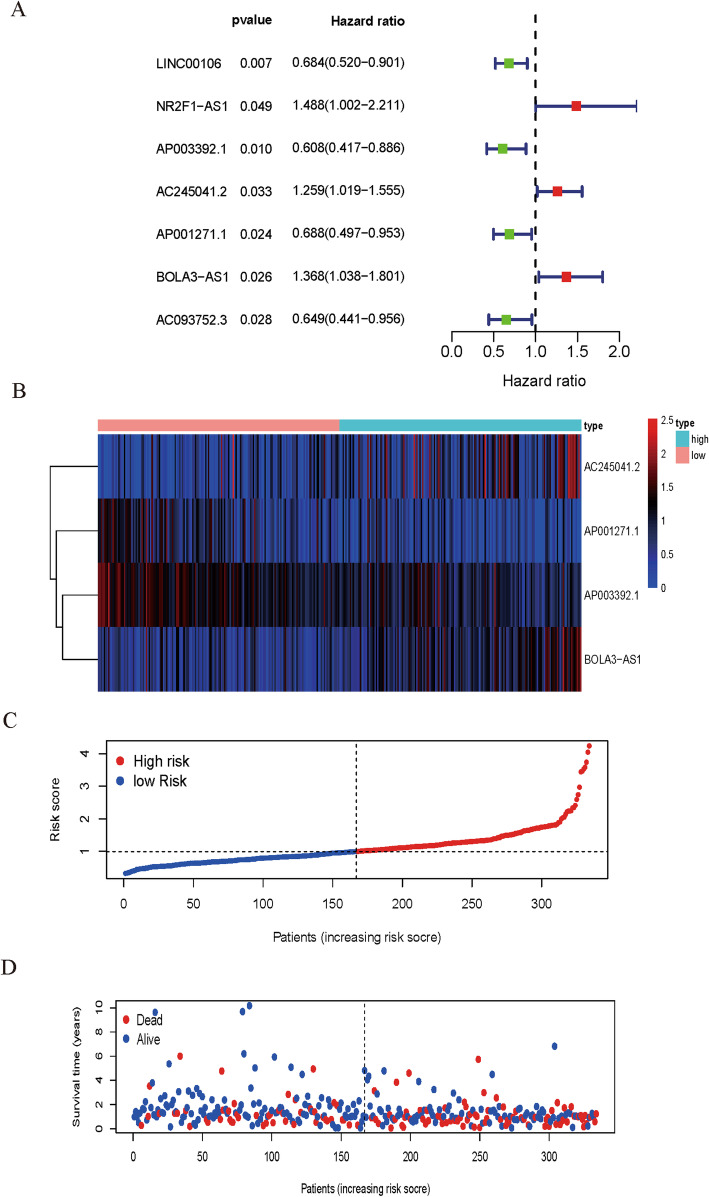
The clinical data of 334 GC patients were analyzed. Using univariate Cox regression analysis, seven prognostic ferroptosis-related lncRNAs were identified. The results were presented as a forest plot in Fig. 2A. Seven prognostic ferroptosis-related lncRNAs were further analyzed using multivariate Cox regression analysis. Finally, a 4-ferroptosis-related lncRNA signature model was established. The coefficients of each lncRNA were listed in Table 2.
Fig. 2.
Outcomes of the four ferroptosis-related-lncRNA model in all the samples. (A) HR and 95% CI of the seven top lncRNAs using univariate Cox regression. (B) The distribution of the four ferroptosis-related lncRNA expression profiles of patients in all samples. (C) The distributions of the risk scores in all samples. (D) The distribution of the follow-up time in all samples
Table 2.
The coefficients (coef) of the prognostic four ferroptosis -related lncRNAs
| id | coef | HR | HR.95 L | HR.95H | pvalue |
|---|---|---|---|---|---|
| AP003392.1 | −0.50932 | 0.600904 | 0.40146 | 0.899432 | 0.013322 |
| AC245041.2 | 0.235579 | 1.265641 | 1.02113 | 1.568701 | 0.031486 |
| AP001271.1 | −0.31336 | 0.73099 | 0.511205 | 1.045267 | 0.085919 |
| BOLA3-AS1 | 0.525606 | 1.691484 | 1.267559 | 2.257188 | 0.000356 |
The risk score was calculated as follows: Risk score = (− 0.509319782 × expression value of AP003392.1) + (0.235578881 × expression value of AC245041.2) + (− 0.313355736 × expression value of AP001271.1) + (0.525606422 × expression value of BOLA3-AS1). Heatmap library was used to evaluate the values of the risk scores. Based on the median risk score, we divided the patients into high-risk and low-risk groups and assessed the score’s ability. The patients were ranked from low to high according to the risk score. We have shown the population follow-up time and gene heat-map by ranking as well (Fig. 2B - D).
Correlation between the four lncRNAs and clinical features
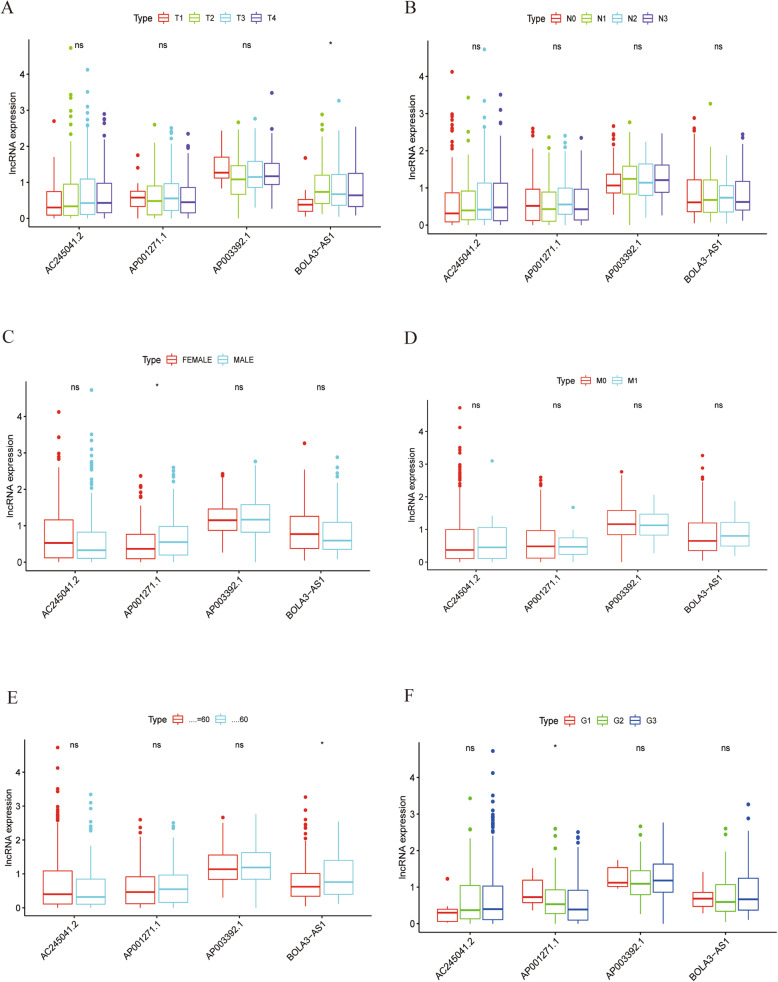
The correlation between the four prognostic ferroptosis-related lncRNA signatures and clinicopathological characteristics was assessed. The expression of BOLA3-AS1 was significantly associated with T and age (Fig. 3A, E). Moreover, AP001271.1 expression was significantly associated with gender and grade (Fig. 3C, F). However, the expression of none of the four lncRNAs was significantly associated with N and M (Fig. 3B, D).
Fig. 3.
Correlation between the four ferroptosis-related lncRNAs and clinical features. The relationship between the expression of the four ferroptosis-related lncRNAs and (A) T, (B) N, (C) gender, (D) M, (E) age, and (F) grade. NS: Not Significant, *: P < 0.05. Note: T: Tumor, classified into T1, T2, T3, T4; N: Node, classified into N1, N2, N3; M: metastasis, classified into M0, M1
Univariate and multivariate cox regression analyses of the ferroptosis-related lncRNA signature for GC
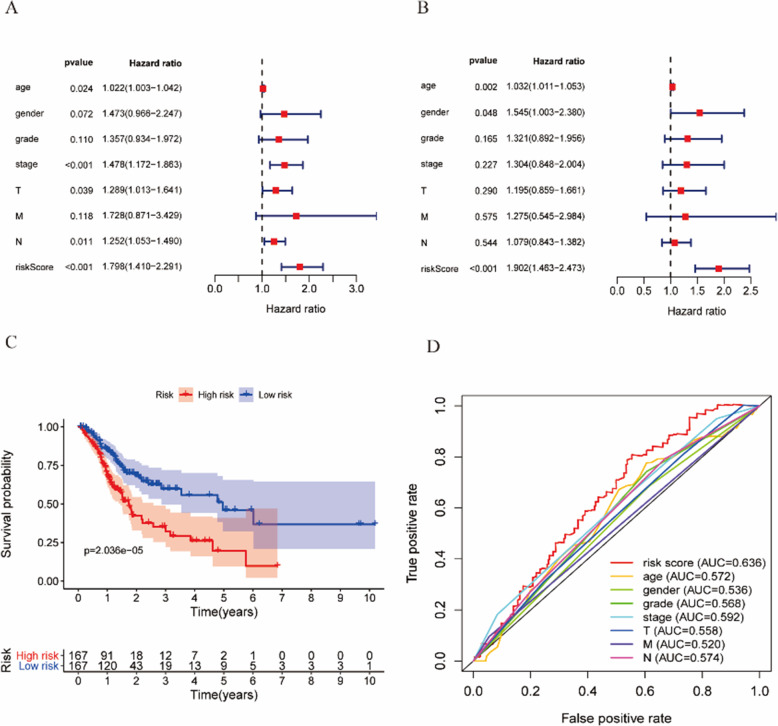
In the univariate Cox regression analysis, age, stage, T, N, and risk score were significantly associated with overall survival (OS; HR = 1.022, 95% CI = 1.003–1.042, P = 0.024; HR = 1.478, 95% CI = 1.172–1.863, P < 0.001; HR = 1.289, 95% CI = 1.013–1.641, P = 0.039; HR = 1.252, 95% CI = 1.053–1.490, P = 0.011; 0.001; HR = 1.798, 95% CI =1.410–2.291, P < 0.001; respectively, Fig. 4A). In the multivariate Cox regression analysis, risk score was an independent predictor of OS (HR =1.902, 95% CI = 1.463–2.473, P < 0.001; Fig. 4B). The survival curve shows that the low-risk group has a better survival period than that of the high-risk group (Fig. 4C). In this study, the AUC of the ROC curve was calculated for risk score (AUC = 0.636), age (AUC = 0.572), gender (AUC = 0.536), grade (AUC = 0.568), stage (AUC = 0.592), T (AUC = 0.558), M (AUC = 0.520), and N (AUC = 0.574). The AUC value of the risk score was higher than that of other clinical parameters, revealing the good predictive power of the Cox regression model for predicting the prognosis of GC patients (Fig. 4D).
Fig. 4.
Univariate and multiple regression analysis of the ferroptosis-related lncRNA signature for GC. Results of the (A) univariate and (B) Multivariate Cox regression analysis show the effects of clinical factors and risk score in all samples. Results of the (C) survival analysis show the prognosis of high-risk and low-risk patients. (D) The ROC for risk-score, age, grade, stage, T, N, and M with OS for GC cohorts
Differential analysis between high-risk and low-risk groups
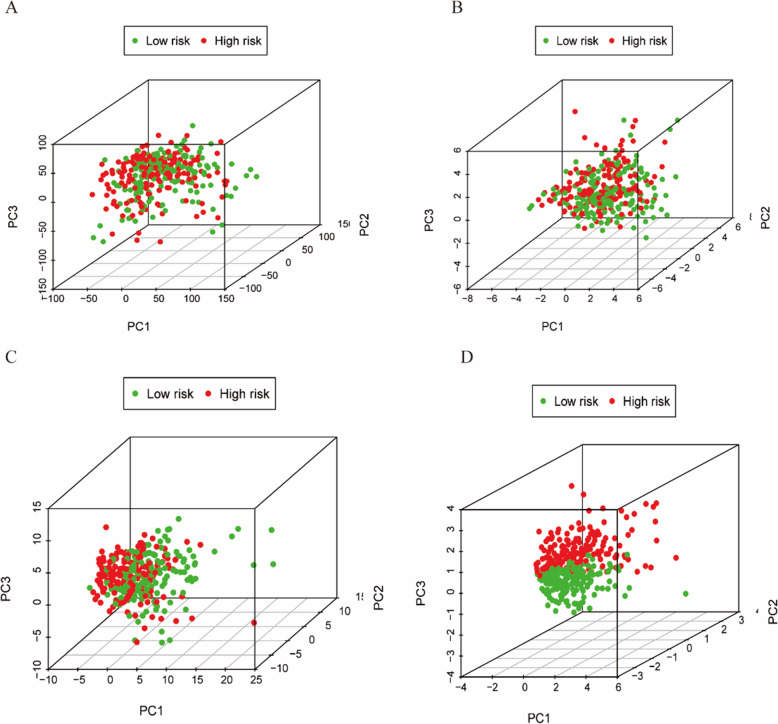
Results of principal component analysis (PCA) are shown in Fig. 5. There were no significant differences between the high-risk and low-risk groups in the expression of all examined genes (Fig. 5A), and ferroptosis-related genes (Fig. 5B), and lncRNAs (Fig. 5C); however, but there was a significant difference between the high-risk and low-risk groups in the expression of for the four lncRNAs (Fig. 5D) used in the prognostic model. This study revealed that the high-risk and low-risk groups were significantly different in terms of the ferroptosis signature.
Fig. 5.
Principal component analysis. Results of the principal component analysis between low-risk and high-risk groups based on the expression of all genes (A), ferroptosis-related genes (B), and lncRNAs (C), and the four lncRNAs of the prognostic model (D)
Construction of nomogram
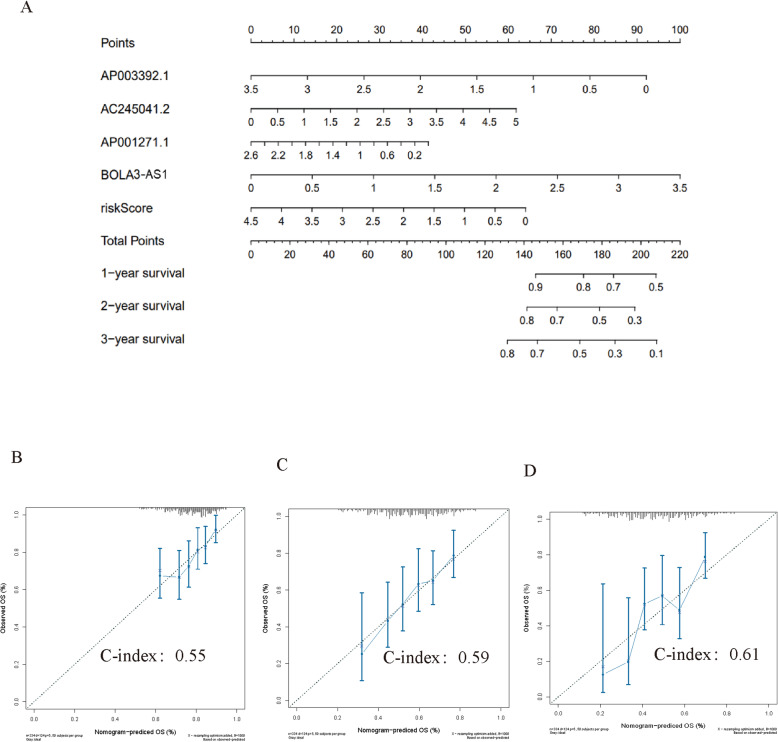
A nomogram was constructed to quantify the risk of each patient [18]. The nomogram was then used to predict the 1-year-, 2-year-, and 3-year OS by weighing AP003392.1, AC245041.2, AP001271.1, BOLA3-AS1, and the risk scores. The score assigned to each factor was proportional to its risk contribution to survival (Fig. 6A). The indications on the calibration curve matched well (Fig. 6B - D). The C-index of 1-year-, 2-year-, and 3-year OS was 0.55, 0.59, 0.61.
Fig. 6.
An individualized prediction model for determining the overall survival (OS) of GC patients. (A) Nomogram construction for predicting the 1-, 2- and 3- year OS of GC patients. (B - D) Calibration curve analysis
The expression of AP003392.1, AC245041.2, AP001271.1, and BOLA3-AS1 in GC
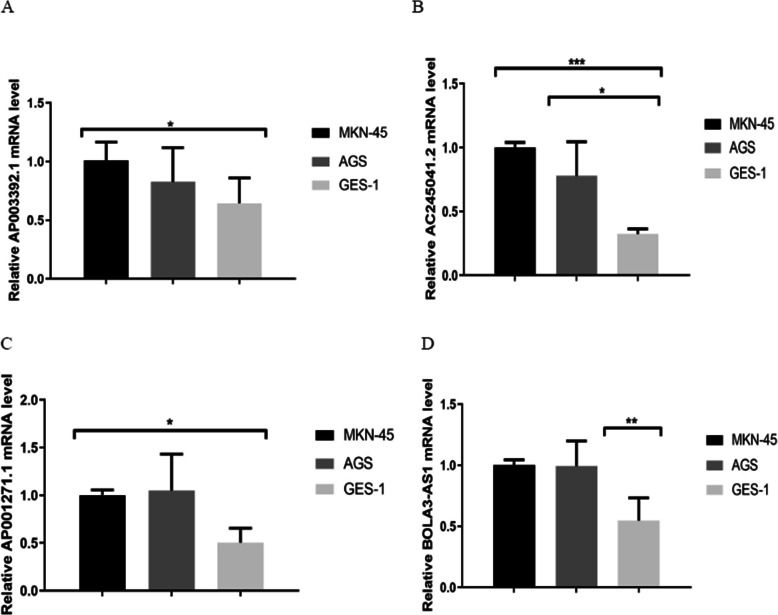
To further explore the expression of AP003392.1, AC245041.2, AP001271.1 and BOLA3-AS1, GC cell lines (MKN-45 and AGS) and human gastric epithelial cell lines (GES-1) were used to validate the expression levels of the four lncRNAs. Quantitative real-time PCR (qRT-PCR) analysis results showed that AP003392.1, AC245041.2, AP001271.1, and BOLA3-AS1 were differentially expressed in GC cell lines compared to that in gastric normal cell lines (Fig. 7). Moreover, these results showed that AP003392.1, AC245041.2, AP001271.1, and BOLA3-AS1 may play an important role in GC.
Fig. 7.
The expression of AP003392.1, AC245041.2, AP001271.1, and BOLA3-AS1 in GC cell lines
Discussion
The term ferroptosis was coined in 2012 to describe iron-dependent cell death [19]. Ferroptosis is increasingly recognized to be associated with the prognosis of patients with GC and other cancers [20–25]. Several studies have focused on the effect of ferroptosis on tumor development and treatment. Carbone, M. et al. found that regulating ferroptosis could be a new therapeutic approach in ovarian cancer [26], revealing the potential value of ferroptosis in guiding clinical decisions. Zhang et al. showed that ferroptosis was influenced by the differential regulation of transcription in liver cancer [27].
Recently, prognostic signatures, comprising mRNAs [28, 29], mRNA expression-based stemness index (mRNAsi) [30, 31], and microRNAs [32, 33] have been used to infer the prognosis of cancers. LncRNA signatures have been constructed to predict OS in many cancers [34–37]. Li, J. et al. identified a five-lncRNA signature that predicted the risk of tumor recurrence in breast cancer patients [38]. Zhu, X. et al. showed that long non-coding RNA signature could improve prognosis prediction in GC [39].
Previous studies have demonstrated that lncRNAs are closely related to ferroptosis [40–42]. Yang, Y et al. revealed that lncRNA ZFAS1 promoted ferroptosis via the miR-150-5p/SLC38A1 axis [43]. Wang et al. showed that nuclear lncRNA LINC00618 accelerates ferroptosis in a manner dependent on apoptosis [44]. However, the prognostic signature of ferroptosis-related lncRNAs in GC has rarely been explored.
In this study, we first identified 173 ferroptosis-related lncRNAs, and then, 7 prognostic ferroptosis-related lncRNAs were further analyzed using multivariate Cox regression analysis. Finally, a four -ferroptosis-related lncRNA signature model was established from TCGA datasets. We also demonstrated that four ferroptosis-related lncRNA signature was an independent risk factor for GC. This result indicated that the prognostic signature of the four ferroptosis-related lncRNAs could accurately predict the prognosis of GC patients.
Nomograms are widely used in prognostic prediction in oncology and medicine [45]. In the current study, a nomogram was constructed using AP003392.1, AC245041.2, AP001271.1, BOLA3-AS1, and the risk score. The nomogram showed a good performance in predicting 1-year, 2-year, and 3-year survival OS of GC patients, which may contribute to the promotion of individualized treatment of GC patients. To investigate the relationship between the four ferroptosis-related lncRNAs and clinical factors, we further analyzed the relationship between clinical features and the four lncRNAs. We found that the four prognostic ferroptosis-related lncRNAs were associated with T-staging, sex, age, and grade.
Interestingly, Wang, S et al. built a novel prognostic nomogram based on five lnc-RNAs in clear cell renal cell carcinoma, comprising AC026992.2, AC245041.2, LINC00524, LINC01956, and LINC02080 [46]. Previous work on lncRNA profiling also revealed that BOLA3-AS1 was associated with higher-risk myelodysplastic syndrome, which played an important role in the development of blood lineages such as platelets, erythrocytes, and myeloid cells [47]. However, the roles of AP003392.1 and AP001271.1 are yet unknown. To explore the expression levels of AP003392.1, AC245041.2, AP001271.1, and BOLA3-AS1, qRT-PCR analysis was applied. We found that the expression of AP003392.1, AC245041.2, AP001271.1, and BOLA3-AS1 was upregulated in GC cells lines. Thus, despite the important prognostic value of ferroptosis-related lncRNA signature identified in this study, future experiments on lncRNAs components are required to elucidate their roles in GC.
In conclusion, we demonstrated the prognostic value of a ferroptosis-related lncRNA signature, comprising AP003392.1, AC245041.2, AP001271.1, and BOLA3-AS1, thus providing a theoretical basis for ferroptosis-related targeted therapies.
Our study has some limitations. First, this was a retrospective study, and therefore it lacked novel clinical samples and data. Second, the mechanism and interrelationship among ferroptosis-related lncRNAs comprising this signature require further study. In conclusion, this study constructed a prognostic risk model consisting of four ferroptosis-related lncRNAs by analyzing the RNA-sequencing-based gene expression profiles of GC from TCGA database. This risk model has been established be independently associated with OS and facilitated the prediction of GC prognosis. However, the mechanisms underlying the interplay between ferroptosis and lncRNAs in GC require further investigation.
Conclusions
This risk model was shown to be a novel method for predicting prognosis for GC patients. Thus, the mechanisms underlying the interplay between ferroptosis and lncRNAs in GC require further investigation.
Supplementary Information
Additional file 1. The primers sequences of four ferroptosis-related lncRNAs.
Acknowledgments
Not applicable.
Authors’ contributions
JW and YZ wrote the main manuscript text and XG prepared Figs. 1, 2, 3, 4, 5, 6 and 7. All authors reviewed the manuscript. The authors read and approved the final manuscript.
Funding
This study was supported by the General Program of the National Natural Science Foundation of China (82070554) and Tianjin Health Commission Science and Technology Personnel Cultivation Project (KJ20103).
Availability of data and materials
The data supporting the findings of this study are available in the supplementary material of this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There are no conflicts of interest concerning the publishing of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianming Wei and Ye Zeng contributed equally to this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Eusebi LH, Telese A, Marasco G, Bazzoli F, Zagari RM. Gastric cancer prevention strategies: a global perspective. J Gastroenterol Hepatol. 2020;35(9):1495–1502. doi: 10.1111/jgh.15037. [DOI] [PubMed] [Google Scholar]
- 4.Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang R, Zhu S, Zeh HJ, Klionsky DJ, Tang D. BECN1 is a new driver of ferroptosis. Autophagy. 2018;14(12):2173–2175. doi: 10.1080/15548627.2018.1513758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64(2):488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, Zhang Q, Lin D, Ge S, Bai M, Wang X, Zhang L, Li H, Yang Y, Ji Z, Wang H, Ying G, Ba Y. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19(1):43. doi: 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- 10.Cao C, Sun G, Liu C. Long non-coding RNA SNHG6 regulates the sensitivity of prostate cancer cells to paclitaxel by sponging miR-186. Cancer Cell Int. 2020;20(1):381. doi: 10.1186/s12935-020-01462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz SU, Grote P, Herrmann BG. Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci CMLS. 2016;73(13):2491–2509. doi: 10.1007/s00018-016-2174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and Cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres-Bayona S, Aldaz P, Auzmendi-Iriarte J, Saenz-Antoñanzas A, Garcia I, Arrazola M, Gerovska D, Undabeitia J, Querejeta A, Egaña L, Villanúa J, Ruiz I, Sarasqueta C, Urculo E, Araúzo-Bravo MJ, Huarte M, Samprón N, Matheu A. PR-LncRNA signature regulates glioma cell activity through expression of SOX factors. Sci Rep. 2018;8(1):12746. doi: 10.1038/s41598-018-30836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang GW, Xue YJ, Wu ZY, Xu XE, Wu JY, Cao HH, Zhu Y, He JZ, Li CQ, Li EM, Xu LY. A three-lncRNA signature predicts overall survival and disease-free survival in patients with esophageal squamous cell carcinoma. BMC Cancer. 2018;18(1):147. doi: 10.1186/s12885-018-4058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen SH, Lin F, Zhu JM, Ke ZB, Lin TT, Lin YZ, Xue XY, Wei Y, Zheng QS, Chen YH, Xu N. An immune-related lncRNA prognostic model in papillary renal cell carcinoma: A lncRNA expression analysis. Genomics. 2020;113(1):531–540. doi: 10.1016/j.ygeno.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Mi JX, Zhang YN, Lai Z, Li W, Zhou L, Zhong F. Principal component analysis based on nuclear norm minimization. Neural Netw. 2019;118:1–16. doi: 10.1016/j.neunet.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Wei J, Wang J, Gao X, Qi F. Identification of differentially expressed circRNAs and a novel hsa_circ_0000144 that promote tumor growth in gastric cancer. Cancer Cell Int. 2019;19(1):268. doi: 10.1186/s12935-019-0975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu J, Sun M, Wang Y, Chen B. Identification and validation of an individualized autophagy-clinical prognostic index in gastric cancer patients. Cancer Cell Int. 2020;20(1):178. doi: 10.1186/s12935-020-01267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, III, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao S, Yu J, He W, Huang Q, Zhao Y, Liang B, Zhang S, Wen Z, Dong S, Rao J, Liao W, Shi M. Cysteine dioxygenase 1 mediates Erastin-induced Ferroptosis in human gastric Cancer cells. Neoplasia. 2017;19(12):1022–1032. doi: 10.1016/j.neo.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai Y, Zhang Z, Li J, Li W, Huang Z, Zhang C, Li X, Zhao J. STYK1/NOK correlates with ferroptosis in non-small cell lung carcinoma. Biochem Biophys Res Commun. 2019;519(4):659–666. doi: 10.1016/j.bbrc.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Nie J, Lin B, Zhou M, Wu L, Zheng T. Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2018;144(12):2329–2337. doi: 10.1007/s00432-018-2740-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Zhang Z, Wang M, Cao X, Qi J, Wang D, Gong A, Zhu H. Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des Dev Ther. 2019;13:2135–2144. doi: 10.2147/DDDT.S199459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Shi M, Ji J, Cai Q, Zhao Q, Jiang J, Liu J, Zhang H, Zhu Z, Zhang J. Stearoyl-CoA desaturase 1 (SCD1) facilitates the growth and anti-ferroptosis of gastric cancer cells and predicts poor prognosis of gastric cancer. Aging. 2020;12(15):15374–15391. doi: 10.18632/aging.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19(7):405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 26.Carbone M, Melino G. Stearoyl CoA desaturase regulates Ferroptosis in ovarian Cancer offering new therapeutic perspectives. Cancer Res. 2019;79(20):5149–5150. doi: 10.1158/0008-5472.CAN-19-2453. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Du L, Qiao Y, Zhang X, Zheng W, Wu Q, Chen Y, Zhu G, Liu Y, Bian Z, et al. Ferroptosis is governed by differential regulation of transcription in liver cancer. Redox Biol. 2019;24:101211. doi: 10.1016/j.redox.2019.101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comincini S, Manai F, Meazza C, Pagani S, Martinelli C, Pasqua N, Pelizzo G, Biggiogera M, Bozzola M. Identification of Autophagy-Related Genes and Their Regulatory miRNAs Associated with Celiac Disease in Children. Int J Mol Sci. 2017;18(2):18(2). doi: 10.3390/ijms18020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Z, Huang R, Chai R, Zhou X, Hu Z, Wang W, Chen B, Deng L, Liu Y, Wu F. Identification of an energy metabolism-related signature associated with clinical prognosis in diffuse glioma. Aging. 2018;10(11):3185–3209. doi: 10.18632/aging.101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Y, Wang Y, Cheng M, Huang C, Fan X. Weighted gene Coexpression network analysis of features that control Cancer stem cells reveals prognostic biomarkers in lung adenocarcinoma. Front Genet. 2020;11:311. doi: 10.3389/fgene.2020.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y, Xiao H, Cheng M, Fan X. Bioinformatics analysis reveals biomarkers with Cancer stem cell characteristics in lung squamous cell carcinoma. Front Genet. 2020;11:427. doi: 10.3389/fgene.2020.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yerukala Sathipati S, Ho SY. Identifying the miRNA signature associated with survival time in patients with lung adenocarcinoma using miRNA expression profiles. Sci Rep. 2017;7(1):7507. doi: 10.1038/s41598-017-07739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nziza N, Jeziorski E, Delpont M, Cren M, Chevassus H, Carbasse A, Mahe P, Abassi H, Joly-Monrigal P, Schordan E, Mangé A, Jorgensen C, Apparailly F, Duroux-Richard I. Synovial-Fluid miRNA Signature for Diagnosis of Juvenile Idiopathic Arthritis. Cells. 2019;8(12):8(12). doi: 10.3390/cells8121521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song P, Jiang B, Liu Z, Ding J, Liu S, Guan W. A three-lncRNA expression signature associated with the prognosis of gastric cancer patients. Cancer Med. 2017;6(6):1154–1164. doi: 10.1002/cam4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao Y, Fu Z, Zhang Y, Dong L, Zhang Y, Zhang Q, Li X, Liu J. A seven-lncRNA signature predicts overall survival in esophageal squamous cell carcinoma. Sci Rep. 2018;8(1):8823. doi: 10.1038/s41598-018-27307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Ren L, Yan X, Shan Y, Liu L, Zhou J, Kuang Q, Li M, Long H, Lai W. Identification of immune-related lncRNAs in periodontitis reveals regulation network of gene-lncRNA-pathway-immunocyte. Int Immunopharmacol. 2020;84:106600. doi: 10.1016/j.intimp.2020.106600. [DOI] [PubMed] [Google Scholar]
- 37.Zhang G, Fan E, Zhong Q, Feng G, Shuai Y, Wu M, Chen Q, Gou X. Identification and potential mechanisms of a 4-lncRNA signature that predicts prognosis in patients with laryngeal cancer. Hum Genomics. 2019;13(1):36. doi: 10.1186/s40246-019-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Wang W, Xia P, Wan L, Zhang L, Yu L, Wang L, Chen X, Xiao Y, Xu C. Identification of a five-lncRNA signature for predicting the risk of tumor recurrence in patients with breast cancer. Int J Cancer. 2018;143(9):2150–2160. doi: 10.1002/ijc.31573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu X, Tian X, Yu C, Shen C, Yan T, Hong J, Wang Z, Fang JY, Chen H. A long non-coding RNA signature to improve prognosis prediction of gastric cancer. Mol Cancer. 2016;15(1):60. doi: 10.1186/s12943-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu J, Xu F, Lu H. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 2020;260:118305. doi: 10.1016/j.lfs.2020.118305. [DOI] [PubMed] [Google Scholar]
- 41.Qi W, Li Z, Xia L, Dai J, Zhang Q, Wu C, Xu S. LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep. 2019;9(1):16185. doi: 10.1038/s41598-019-52837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, Shi Y, Chen L, Xiao D, Yu F, Wang X, Zhou H, Cao Y, Liu S, Yan Q, Tao Y, Zhang B. Correction to: Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2020;27(4):1447. doi: 10.1038/s41418-019-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Tai W, Lu N, Li T, Liu Y, Wu W, Li Z, Pu L, Zhao X, Zhang T, et al. lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging. 2020;12(10):9085–9102. doi: 10.18632/aging.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Chen X, Liu N, Shi Y, Liu Y, Ouyang L, Tam S, Xiao D, Liu S, Wen F, Tao Y. A nuclear Long non-coding RNA LINC00618 accelerates Ferroptosis in a manner dependent upon apoptosis. Mol Ther. 2020;29(1):263–274. doi: 10.1016/j.ymthe.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeong SH, Kim RB, Park SY, Park J, Jung EJ, Ju YT, Jeong CY, Park M, Ko GH, Song DH, Koh HM, Kim WH, Yang HK, Lee YJ, Hong SC. Nomogram for predicting gastric cancer recurrence using biomarker gene expression. Eur J Surg Oncol. 2020;46(1):195–201. doi: 10.1016/j.ejso.2019.09.143. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Chai K, Chen J. A novel prognostic nomogram based on 5 long non-coding RNAs in clear cell renal cell carcinoma. Oncol Lett. 2019;18(6):6605–6613. doi: 10.3892/ol.2019.11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szikszai K, Krejcik Z, Klema J, Loudova N, Hrustincova A, Belickova M, Hruba M, Vesela J, Stranecky V, Kundrat D, Pecherkova P, Cermak J, Jonasova A, Dostalova Merkerova M. LncRNA Profiling Reveals That the Deregulation of H19, WT1-AS, TCL6, and LEF1-AS1 Is Associated with Higher-Risk Myelodysplastic Syndrome. Cancers. 2020;12(10):12(10). doi: 10.3390/cancers12102726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The primers sequences of four ferroptosis-related lncRNAs.
Data Availability Statement
The data supporting the findings of this study are available in the supplementary material of this article.