Abstract
The advent of microfluidic systems has led to significant developments in lab-on-a-chip devices integrating several functions onto a single platform. Over the years, these miniature devices have become a promising tool for faster analytical testing, displaying high precision and efficiency. Nonetheless, most microfluidic systems are not commercially available. Research is actually undergoing on the application of these devices in environmental, food, biomedical, and healthcare industries. The lab-on-a-chip industry is predicted to grow annually by 20%. Here, we review the use of lab-on-a-chip devices in the food sector. We present fabrication technologies and materials to developing lab-on-a-chip devices. We compare electrochemical, optical, colorimetric, chemiluminescence and biological methods for the detection of pathogens and microorganisms. We emphasize emulsion processing, food formulation, nutraceutical development due to their promising characteristics. Last, smart packaging technologies like radio frequency identification and indicators are highlighted because they allow better product identification and traceability.
Keywords: Lab-on-a-chip, Microfluidics, Food safety, Food processing, Food packaging, Analysis, Detection
Introduction
Rapid urbanization in the last few decades has driven the agro-food sector to undergo a massive transformation with the advent of new technologies and products. The rate at which chemical agents are used in agriculture and industry has risen rapidly with increasing demand for food. Although these chemicals have improved the efficiency of the processes, equal importance needs to be given to environmental and health impacts. Extensive production, usage of pesticides, and the presence of complex contaminants have led to issues in soil fertility, agricultural crop quality, and contaminated water (Ponnuchamy et al. 2021; Sridhar et al. 2022). These hazardous agents pose a serious threat to the environment and ecosystem with concerns arising on the safety of food. According to Food and Agricultural Organization, more than 1.3 billion tons of food gets thrown away and this wastage has increased by 50% (in weight) in recent years (Amicarelli et al. 2020). Significant amounts of food have also been wasted due to microbiological contamination at the post-harvest and consumer levels. The food contamination at any stage significantly impacts health and the economy (Sridhar et al. 2021c). Unsafe food commodities often remain unmonitored and pose a huge health risk for consumers, especially in developing countries. Thus, the detection and prevention of any food contamination and food spoilage should be addressed carefully to reduce the overall health risks as well as to improve productivity and quality of food.
With the rapidly evolving techno-economic landscapes, there has been a significant demand in food and agriculture sector to produce low-cost, environment-friendly, and sustainable technologies. Traditionally, analytical methods such as thin-layer chromatography. high-performance liquid chromatography, gas chromatography, and gas chromatography coupled with mass spectrometry have been used for toxicity and contaminant detection in various agricultural and food products (Guo et al. 2015). Although these techniques are reliable, the equipment space requirements and initial costs are high. In recent years, a rapid change in technological development has been observed aiming to deliver cost-effective solutions utilizing least raw materials with improved overall productivity. Researchers have been focusing on developing new technologies giving considerable attention to food quality, quantity, and safety. However, due to the complexity of agro-food systems, several drawbacks exist that need long-lasting innovations for creating new processes, tools, and technologies.
Advancements in manufacturing began with the development of several novel devices and components like microfluidic chips, microvalves, and micropumps (Min et al. 2004; Mark et al. 2010). This led to the advent of lab-on-a-chip (LOC) technology providing the basis for automation, cost, handling, and portability. LOC refers to a miniaturized device that allows one or more laboratory-scale operations, such as synthesis and analysis of chemicals, to run on a small scale within a handheld portable system. These devices have an overall size ranging from millimeters to a few centimeters with microfluidic channels suitable for handling tiny volumes of fluid samples. The devices are designed and fabricated to accurately and precisely handle fluids in the range of 10–6 to 10–9 L. There has been a huge demand for such technologies offering next-generation devices for different applications. However, only a fraction of the total fabricated devices manages to reach the market due to various reasons such as technical performance, user acceptability, and cost (Wongsrichanalai et al. 2007).
Rapid diagnostic tests are one of the most widely accepted and commercialized lab-on-a-chip technologies so far. These diagnostic devices work on the spot testing or lateral flow principle and are easy to operate. In recent years, significant advancements have been carried out in the development of smartphone-based chips, paper-based microfluidics, and miniature environmental monitoring systems (Hong et al. 2018; Marquez et al. 2019; Puangbanlang et al. 2019; Nelis et al. 2020; Fernández-Abedul 2021). The portable diagnostic test kits have been explored extensively in the healthcare sector and with pandemics like COVID-19, the requirements for accurate sample handling and analysis become even more crucial globally (Yang et al. 2018; Kim et al. 2019; Bhalla et al. 2020; Ghosh et al. 2020; Rasmi et al. 2021).
Apart from pathogen detection in bodily fluids, LOC devices have shown their versatility in the environmental and biomedical industry primarily for air quality detection and glucose measurements (Poenar 2019; Teymourian et al. 2020). When it comes to the food industry, major developments have been carried out in the detection of pathogens and pesticide levels in fruits, vegetables, and other packaged foods (Dooley et al. 2005; Chen et al. 2014; Hou et al. 2019). Studies have also involved efforts to dispense small amounts of liquid for simple pH measurements or sensing (Ude et al. 2015). The applicability of LOC technology in the food industry ranges from simple to complex systems. LOC devices have shown promise in food safety and pathogen control applications like detection of mycotoxins, bacteria, etc. (Wahyuni et al. 2019). However, they are not only limited to applications in detection and sampling. An evaluation was conducted to correlate different fluid flow parameters to produce a single emulsion in emulsion processing (Viza and Harding 2018). Investigation on formation, stability, and release characteristics for designing a one-step multiple emulsion system for encapsulation purposes has also been researched (Clegg et al. 2016). Additionally, applications of LOC technology in sensory studies in aroma and flavor testing have been explored (Ravi et al. 2013). It is noteworthy to mention that a lot of scope lies in related areas of food technology namely emulsion processing, nutraceutical developments, formulation, and packaging. However, these investigations are scattered and require more comprehensive research. With an increasing market growth and ever-changing landscape, the LOC industry is expanding rapidly (Rios et al. 2018). The food and nutraceutical industry would play a crucial role in integrating LOC in addition to biosensing and healthcare sectors. Until today, the market has dominated more in the research arena rather than business to consumer. The need of the hour lies in producing new materials and technologies for cost-effective fabrication methods for such microscale devices.
Review methodology
Bibliometric analysis is one of the useful ways to identify the area and scope of research. The analysis draws improved research output data making the scientists assess the future trends for a specific topic. In the present study, a bibliometric analysis was conducted using Scopus (https://www.scopus.com) and Dimensions (https://www.dimensions.ai/). Scopus is one of the most extensively used databases having wide coverage of journals and hence is chosen for analysis (Singh et al. 2021). However, in the last few years, new scholarly databases like Dimensions have provided possible alternatives to existing databases. They aim to offer high coverage, free cost, and unlimited accessibility and encompass a plethora of topics debated at a global level. Dimensions database is an open-source platform covering a wide range of topics compared to other traditional databases and is, therefore, explored to improve the ecosystem of scientific information (Orduña-Malea and López-Cózar 2018).
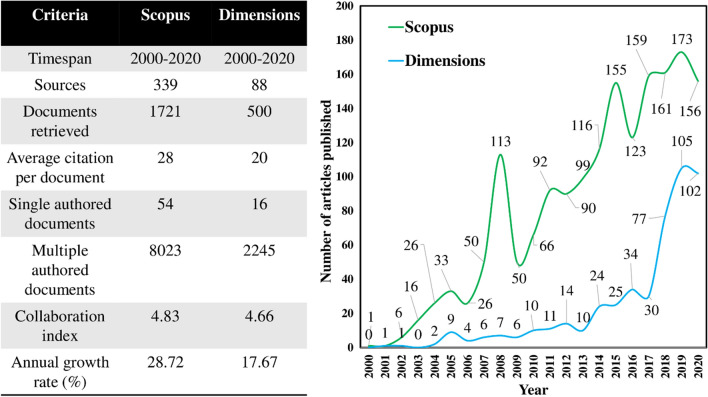
The data for the present work was extracted from Scopus and Dimensions on July 7, 2021, for the last two decades (2000–2020). Figure 1 illustrates the bibliometric analysis conducted to evaluate the growth trend of the two databases in the last two decades. As the present study involves a more focused approach toward the food industry, keywords relevant to lab-on-a-chip devices and the food industry were used. The results showed a higher number of documents being captured using Scopus (1721 documents) compared to Dimensions (500 documents). The possible reason for a higher number of documents in Scopus could be due to database popularity. The annual growth of publications for the topic investigated in this study for both the databases was on a positive scale (Scopus: 28.72%, Dimensions: 17.67%) implying the increasing demand for the utilization of lab-on-a-chip devices in food systems. Thus, the goal of this bibliometric analysis and review lies in helping researchers to evaluate the recent developments in the area of lab-on-a-chip technologies in the food industry.
Fig. 1.
Bibliometric analysis using Scopus and Dimensions for evaluating growth trends in the last two decades (2000–2020). Bibliometrics serves as an efficient tool for analyzing research trends in various scientific disciplines based on multiple criterions
The data extraction from the databases retrieved 1721 documents and 500 documents from Scopus and Dimensions, respectively. Although both the databases showed promising benefits in covering a wide range of topics, Scopus provided a more comprehensive coverage possibly due to its recognition among various researchers compared to Dimensions (Stahlschmidt and Stephen 2021). Figure 2 illustrates the summary of country-wise analysis for the research contribution according to bibliometric data extracted from (a) Scopus and, (b) Dimensions. The pie charts show the top 5 countries have contributed to more than 56% and 71% in Scopus and Dimensions, respectively, as compared to the rest of the world. It can also be inferred that China contributes to one of the highest number of articles along with the USA and Denmark. A significant contribution was also seen by scientists in countries like Germany, Korea, and France providing promising research outputs to the topic.
Fig. 2.
Summary of country-wise analysis for the research contribution according to bibliometric data extracted from a Scopus and b Dimensions. The input data showed significant contributions made by the USA, China, and Korea for both the databases
Keywords play an important role in evaluating the scope of the work as well as bringing out emerging and popular themes for the topic. The keywords “foods,” “proteins,” “beverages,” “agriculture,” “food safety,” “food pathogens” or “food processing” were given in addition to “lab-on-a-chip devices,” “lab-on-chip devices,” “lab on a chip” or “microfluidics” were used for the present study based on authors linguistic abilities. The minimum number of occurrences for a keyword was set to 10 words aiming for a robust keyword clustering analysis. Since a greater number of articles were retrieved using the Scopus database, it was used for further analysis and interpretations.
VOSviewer software was used to map the data from the Scopus database and presented using keyword cluster analysis (van Eck and Waltman 2010, 2017). Figure 3 illustrates the future themes formed along with the number of items (keywords) present based on data extracted from Scopus. In the network view, the color-coding depicts the number of similar areas and their links that collectively account for a theme. A total of 5 clusters were formed through keywords connecting to the topic of this study. For instance, Cluster I (red color) constituting 378 items highlight the themes protein synthesis, biosensing, and nanotechnology using keywords like “protein,” “nanofabrication,” “bioassay” and “metal nanoparticles.” Similarly, the keywords “cell migration,” “in-vitro study” or “stem cells” link specific areas in drug delivery and health care (Cluster II—Green color). The words “food safety,” “food analysis,” “food control” and “microbiology” clearly stated the trends in food technology occupying 115 items (Cluster III—blue color). In addition to these themes, importance was also given to genetic engineering (Cluster IV—yellow color) and the pathology (Cluster V—purple color).
Fig. 3.
Possible future themes using keyword cluster analysis of the bibliometric data extracted from Scopus using VOSviewer software. Keyword cluster analysis is conducted to visualize the research hotspots and transitions in the last two decades
The bibliometric mapping and analysis revealed rapid advancements in lab-on-a-chip technologies for food systems. Considering the above points, this review is structured to focus on the application of LOC technologies in various aspects of the food industry. Several fabrication techniques like photolithography, plotting, inkjet printing, laser printing, wax screen printing, and plasma oxidation used in the food industry have been highlighted. In addition, their food-related applications are discussed. Different fabricating materials and patterning agents with a focus on biopolymers have also been reviewed. The applications of LOC devices in practically relevant areas like food safety, processing, and packaging have been critically examined. The detection of pathogens and microorganisms in the area of food safety has been possible by using technologies like electrochemical, optical, colorimetric, chemiluminescence, and biological methods like polymerase chain reaction (PCR), which have been presented here. Further, the growing potential of such miniaturized devices in emulsion, food formulation, nutraceutical developments, radio frequency detection systems (RFID) scanning, and indicator techniques have been elucidated.
Several interesting review articles have been published with a major focus on the analysis of pathogens, mycotoxins, pesticide residues, and other contaminants for food safety applications (Choi et al. 2019; Nelis et al. 2020; Xu et al. 2020; Saravanan et al. 2021). The need of the hour lies in exploring deeper into the value of LOC devices in the food industry. The present study encompasses a broader scope in the food industry giving importance to advancements in lab-on-a-chip technology in the area of safety, processing, and packaging. The inferences from literature findings reported in the work could open opportunities for LOC devices in the food industry addressing key issues like food quality, adaptability, fabrication, and cost.
Fabrication and detection of lab-on-a-chip devices
Lab-on-a-chip devices hold promising platforms for on-site sample testing as they are easily portable. Figure 4 shows the characteristics of conventional techniques and lab-on-a-chip technology. They serve as efficient tools in rapid analysis for pathogen testing in laboratories. Additionally, these devices require only small reagent and sample volumes and generate minimal byproducts thus making them safe and cost-effective (Sri Sruthi et al. 2021). These devices could also be useful in minimizing quality-related issues before the material reaches the consumer (Moschou and Tserepi 2017).
Fig. 4.
Characteristics of traditionally used techniques and lab-on-a-chip technology. Lab-on-a-chip technology comprises of miniature devices incorporating multiple laboratory functions on a single platform. These devices find applications in diverse areas such as precise fluid delivery, intensified processing, and analytical studies
Various LOC device fabrication methods have been explored for creating different flow patterns for controlled mixing and detection. The commonly used fabrication technologies in LOC development include photolithography (Phiphatanaphiphop et al. 2020), inkjet printing (Papamatthaiou et al. 2020), screen printing (Sitanurak et al. 2019), plasma oxidation (Wong et al. 2020), and laser treatment (Grist et al. 2012). Table 1 summarizes the chief features of various LOC device fabrication techniques and their food-related applications. The technologies work on the principle of fluid dynamics and can be categorized into active mixing (fluid flow by application of external force) and passive mixing (use of capillarity and geometrical features to drive the fluid flow) (Lee et al. 2016). Fluid flow parameters, fluid composition, mixing patterns, and device design features determine the overall efficiency of the microfluidic device (Calado et al. 2016).
Table 1.
Lab-on-a-chip device fabrication techniques and food-related applications
| Fabrication technique | Features | Process limitation | Food-related applications | Reference |
|---|---|---|---|---|
| Photolithography | Utilizes a photoresist to fabricate the microfluidic device. In the presence of an ultraviolet lamp, the mask is prepared using an inkjet printer or can even be hand-drawn. Post-exposure, the photoresist is removed using the solvent, leaving a photoresist barrier pattern. Detection is then done by adding appropriate reagents | Laborious. High precision instruments. Cleanroom facilities. Time-consuming. Costly | Agarose-biodegradable and compatible substrate which can be used for dual applications (in energy storage as a flexible biodegradable battery) | Verma et al. (2018) |
| Plotting | Used to create flow channels and sensing areas on substrates. Polydimethylsiloxane is generally used as a substrate for plotting. After curing, bending or folding can be done without destruction. The plotting is done in a continuous fashion (line drawing method) | Suitable mainly in research laboratories. Less resolution. Difficult to dispense the liquid in some cases | Proteins-detection and sensing | Li et al. (2012), Nie et al. (2012) |
| Inkjet printing | Printing method for fabrication of microfluidic devices requiring low temperature and minimal resources for its operation. A unique feature of this technology is that the whole process gets completed within one cycle and relies completely on the inkjet printing equipment. High volumes of final product can be manufactured | Sensing material cannot be inkjet printed. Other techniques like casting need to be used for completion. Rheological conditions of inks like viscosity and surface tension need to be carefully followed | Water and oxygen sensing through additive inkjet printing-food packaging, pharmaceutical, and biomedical applications | Moya et al. (2017), Maddipatla et al. (2019) |
| Volatile compounds, glucose, protein-analytical chemistry | Yamada et al. (2015) | |||
| Laser printing | This type of printing is generally a combination of laser printing and laminating process. The laser deposits the material layer by layer on transparent sheets with white regions representing microfluidic channels. Printing is followed by laminating process which seals the microfluidic channels | Distance between the two channels could sometimes be an issue | Proteins, glucose-healthcare industry (testing kits for people living in rural regions who have limited access to hospitals and clinics) | Oliverira (2015) |
| Food dyes | Gharaghani et al. (2020) | |||
| Finely ground pork-food safety and consumer health | Xia et al. (2016), Trofimchuk et al. (2020) | |||
| Wax screen printing | The wax printing process can be explained in the following steps: first, the wax is printed on the surface of the material using the paper screen-printing method. This is followed by the melting of wax onto the paper to form hydrophobic barriers. The process is feasible as the wax is of low cost and can be purchased easily | The patterned mesh is necessary for prototyping. This technique can sometimes require a heating step bringing questions about energy efficiency | Emulsions in food processing | Sher et al. (2017) |
| Plasma oxidation | Fabricating material is generally baked in an oven for about 100 °C for 45 min to cure the solution. The flexible patterns are then made on the baked fabricated material. The technology is generally used for making functional components like control switches, microreactors, or microfilters | Over-etching may take place sometimes due to excess baking | Ara h1 allergen detection in peanut and wheat | Weng et al. (2016) |
In recent years, significant developments have been carried out to understand the mixing performance and improvements in device design. For instance, researchers created a low-cost 3D printed microfluidic device using a solvent bonding method of polymethyl methacrylate and acrylonitrile butadiene styrene (Duong and Chen 2019). The overall mixing efficiency achieved post optical transmission was 89% with a bonding strength of 8 bars. Similar studies were also investigated to understand the design parameters to fabricate a hybrid microfluidic mixer using finite element analysis. Mixing efficiency of 48–53% was achieved showing its potential for future iterations in additive manufacturing (Agarwal et al. 2020). Thus, the effective construction of devices based on samples acts as a pivot for determining the success of these microdevices.
Apart from fabrication methods, different patterning agents also play a critical role in device development. A variety of hydrophobic agents have been extensively studied. These range from expensive agents like photoresists to cheaper options like wax (Martinez et al. 2008). Table 2 gives a list of substrates used for performing LOC study in various foods samples. LOC studies commonly use polymer-based patterning agents due to their strong binding power and versatile characteristics. They are rigid, durable, and can generate high-resolution patterns. However, the fabrication of polymer-based LOCs is complicated and costly. Further, these devices require external energy using pumps for efficient transport of liquid and mixing. In contrast, paper-based devices have gained more interest recently due to the simplicity of the fabrication process, cost-effectiveness, and elimination of the need for pumps or any external devices for fluid transport (Hu and Lu 2020).
Table 2.
Substrates used for performing lab-on-a-chip studies in food samples
| Substrate | Concept used | Food material | Results | Reference |
|---|---|---|---|---|
| Polydimethylsiloxane | Gel electrophoresis | Ovalbumin-Texas red dye protein | The analyte enrichment gave detection limits of 250 pM with an increase in temperature to 30 °C. The involvement of temperature showed an increase of 30% in a single run | Peli Thanthri et al. (2020) |
| Glass fiber paper | Surface-enhanced Raman scattering and ELISA method | Fish samples | Concentration range of Malachite green residue in fish: 1 × 10–7 mol/L to 1 × 10–5 mol/L. Limit of detection: 5 × 10–10 mol/L. The technique proved better than the ELISA method showing responses within 1 h | Deng et al. (2019) |
| Polyethersulfone and glass fiber | Recombinase polymerase amplification method | Milk | Detection of Escherichia coli and Staphylococcus aureus = 102 CFU/mL. The device had the potential to be integrated with simple nucleic acid extraction for food pathogen detection using limited resources | Ahn et al. (2018) |
| Paper layer containing carbon ink | Microfluidic sensing tongue | Orange juice and Cola beverage | One-step sampling design with a linear chip range measuring concentrations between 0.5 and 15 mM provided excellent precision for noting glucose levels | Amor-Gutiérrez et al. (2019) |
| Paper | Real-time Polymerase chain reaction test | Drinking water and milk | Three-dimensional book-shaped paper device for pathogen identification done based on sensitivity and specificity. DNA recovery rate: 25 ng/µL. Paper extraction recovery rate: 60–70% | He et al. (2020c) |
| Polystyrene | Different T-junction geometries | Water–oil–water emulsion with an oil phase containing polystyrene | The value of flowrate at the second junction provides the most effective parameter for controlling the inner diameter, outer diameter, and thickness of the shell | Viza and Harding (2018) |
| Regenerated silk | Transfer printing process | Banana-film packaging | 30% water permeability reduction observed after applying on food sample post-fabrication. The shelf life of perishable food increased by 7 days | Valentini et al. (2018) |
| Chitosan | Electrochemical deposition | Active bacteria (E. coli) resistance using a hydrogel film | The fabricated device showed its potential for the on-site detection of viable food pathogens. Can be used for detecting pathogens during the fermentation process | Li et al. (2018) |
| Chitosan reinforced with cellulose | ELISA | Listeria monocytogenes in p60 protein | Using this special biopolymer membrane, the technique improved by: 17%—when kept in Tryptic soy broth and 24%—when kept in 0.5% dextrose broth. No cross-reaction was observed | Etty et al. (2019) |
DNA: Deoxyribonucleic acid
ELISA: Enzyme-linked immunosorbent assay
CFU: Colony forming unit
In recent years, a significant number of biopolymers like cellulose, chitin, and collagen have shown promising results as flexible substrates. Biodegradable fabricating materials have found applications in flexible edible food biosensors (Tao et al. 2012). Besides these, proteins and carbohydrates have also been tried for fabricating LOCs through pattern transfer from silicon substrates. For instance, collagen was taken to fabricate a strain temperature sensor using thermal techniques through a shadow mask (Moreno et al. 2015). However, key aspects of safety and regulatory measures should be considered for a potential scale-up.
The increased interest in designing and patterning techniques has led to advancements in detection, optimization, control, and efficiency improvements. For instance, a tree-shaped microfluidic device was designed and fabricated using paper and demonstrated for multiple analyte detection (Wang et al. 2010). The design gave a promising approach for colorimetric detection of protein to measure the concentration of analyte and color intensity with self-calibration. Another study showed the fabrication of a 3D printed acrylate-based polymer microchip using magnetic zinc–imidazole framework as an adsorbent. The portable device showed a dual approach in the simultaneous determination of total phenolic acids and flavonoids present in tea and honey samples with detection limits in the range of 0.04–0.10 µg/mL along with bioactive recovery rate 95.4–104.1% (Bagheri et al. 2021). Similar work has been done to develop a one-step approach with 3D wax printed paper-based device for glucose determination. The conclusions gave a strong linear relationship of R2 = 0.985 and resolution of 468 ± 72 µm with effective barrier properties, thus giving a better manufacturing approach for paper-based devices (Chiang et al. 2019). Although several iterations and feature additions are being done aiming to improve the microscale device efficiency, aspects on cost, convenience, pattern resolution, and scale-up are to be considered.
Application of lab-on-a-chip devices in the food industry
Food safety
With the increasing demand for nutritious meals, the safety of food plays a vital role in achieving balanced human health. Food safety refers to proper food preparation, management, handling, storage, and treatment to prevent any sort of contamination (Zhang et al. 2019). In recent years, there have been many cases of microorganisms, food poisoning, and adulteration causing harm to consumer health (Ellis et al. 2012; Bansal et al. 2017; Kong et al. 2017; El Sheikha 2019). External factors like allergens, physical contaminants, toxins, pesticides, and heavy metals are equal contributors and need to be removed for safe consumption.
Numerous analytical methods like high-performance liquid chromatography and gas chromatography have been evaluated to ensure the quality and safety of food. These technologies are well-established and provide high precision and sensitivity. Various technological approaches have been explored for food quality testing. Table 3 gives a detailed list of detection methods for improving the sensing capabilities in food samples. However, it is equally important for consumers to be aware of the quality and safety of the foods they consume.
Table 3.
Detection methods for improved sensing capabilities in food samples
| Detection type | Type of LOC | Unique characteristics | Food sample | Conditions | Conclusions | References |
|---|---|---|---|---|---|---|
| Electrochemical detection | Impedance LOC | The cell membrane of the sample prevents the electric field lines from penetrating the cell. When a signal frequency is applied, the total impedance decreases due to short-circuiting. The properties of the sample can then be evaluated | Cow milk | Amplitude: 100 mV Frequency range: 100 Hz–1 MHz | Analysis of impedance and conventional measurements indicate that the former can be used to sense the presence of soap adulteration of 0.9% toxicity in milk | Brazey et al. (2018), Das et al. (2018) |
| Carbon nanotube LOC | Such LOCs can be used to minimize the contact surface between microfluidic device channels giving higher precision during fluid control. The unique characteristic of this type of LOC is its electronic properties, higher sensitivities, and lower voltages | Vegetable extracts | Solution concentration: 4.75 × 10–5 mol/L Current: 4 A pH: 5 | Composite carbon paste (60:40 w/w) and paraffin binder gave the best results. The device proved effective in detecting pesticides, vitamins, and pro-vitamins thus showing high activity and catalytic properties | Oliveira et al. (2013), Ghasemi et al. (2017) | |
| Optical detection | Lateral flow assay (or dipstick assay) | Simplest form of sensing technology. First applied during pregnancy tests. The user either applies the drop of sample onto the strip or dips the strip into the liquid sample. This causes a reaction between the strip and sample showing color changes, or line | Corn | Dissolving solution: tris-HCl (10 mL) pH: 8.5 Concentration of corn sample: 3,10, 30 ng/mL | Improvement in the recovery of mycotoxins in corn samples from 96.4 to 104.67% | Zhang et al. (2018) |
| Drinking water | Pesticide samples were diluted with 1 mg/mL acetonitrile, 5 mM phosphate buffer. pH: 7 Storage temperature: 4ºC | Lateral flow sensors showed the rate of inhibition reaction. Rate was determined by sensor output system. The calibration curve obtained for chlorpyrifos was 2–45 µg/L and for carbaryl, 0.24–2 µg/L. Reproducibility obtained was 4.2–5%. No specific sample preparation was required | Fernández-Ramos et al. (2020) | |||
| ELISA LOC | Generally performed on a microplate. The target solution continuously flows into the microchannel. This continuous flow makes the rinsing very effective and detects pathogens | Potato and corn chips | Amount of sample taken: 1 g Incubation temperature and time: 50 °C for 60 min | Analytical recovery for detection of acrylamide in potato and corn chips: 91.8% to 96%. Limit of detection: 5 ng/mL. High specificity with the technique having the potential for quick, simple, and reliable screening analysis | Franek et al. (2014) | |
| Gluten-free pieces of bread, sandwich spreads, and fried foods | Sample size: 0.25 g to 1 g | An evaluation of gluten cross-contact was done. 93.6% of results showed no significant cross contacts | Parsons et al. (2020) | |||
| Surface plasmon resonance LOC | Unique technology involving no labeling step with multiple rinsing as compared to lateral flow assay and ELISA. Surface plasmon resonance uses the total internal reflection principle causing variation in the refractive index of metal and liquid. The sample detection is done using concepts of polarization and oscillations | Milk | Limit of detection: 0.164 µg/mL | Antibody capturing using the sensor. This method proved better as compared to the ELISA-based technique for food allergen risk management in food manufacturing | Ashley et al. (2018) | |
| Peanut | Limit of detection: 5.54 ng/mL | Polyclonal antibodies enhanced the sensitivities using surface plasmon resonance and proved as a better method of detection as compared to ELISA | Wu et al. (2016), Zhou et al. (2019) | |||
| PCR detection | Stationary chamber PCR | The technology uses heat transfer principles. The sample is heated at different temperatures to achieve thermocycling. The volume taken is the least (in microliters) thus yielding a faster heat transfer. Generally used for detecting pathogens or adulterants in foods | Meat mixtures | Initial activation: 95 °C for 5 min Denaturation: 95 °C for 15 s Annealing: 60 °C at 15 s Extension: 72 °C for 10 s | Pork DNA detection in binary meat mixtures was conducted using conventional and real-time PCR. Real-time PCR detected pork DNA at 0.0001 ng/µL or less. Detection limits of pork DNA in meat mixtures were 0.22, 0.047, 0.048, 0.015 ng/µL | Al-Kahtani et al. (2017) |
| Cashew | Limit of detection: 10 ppm Initial boiling: 121 °C for 15 min and 135 °C for 30 min | Real-time PCR detection proved better as compared to ELISA. Boiling did not affect cashew detectability | Sanchiz et al. (2018) | |||
| Isothermal PCR LOCs | The principle of isothermal PCR is based on the amplification of a target sample under a specific temperature. The technique is fast, ultrasensitive, and takes place within a single step. Monitoring is then generally done using a fluorescent reader. Much easier to fabricate and operate as compared to real-time PCR | Salmonella in Chicken | Sample usage: 1 mL Time: 5 h Temperature for enrichment: 42 °C | Isothermal PCR required less expertise, was economical and reliable. It proved a rapid testing tool for interpretation. Detection time was obtained in less than 8 h including enrichment and DNA extraction steps | Tsen et al. (2013), Kumar (2021) | |
| Colorimetric detection | Tree-shaped self-calibrating device | Widely applied for the analysis of proteins, chemicals, and metals. This tree-shaped device integrates self-calibration on the test strip due to which effects of environmental conditions can be minimized | Proteins | Bovine serum assay usage: 0–5 mg/mL | The device showed its potential to be coupled with digital transmission of images for remote sensing systems in food control and environmental analysis | Wang et al. (2010) |
| Chemiluminescence | Lab-on-paper device | Type of detection requiring less instrumentation and providing high sensitivity. Paper was chosen as a fabricating material considering cost efficiency for sample analysis | Cabbage leaves and tomato skin | Skins and leaves were washed using 20 mL distilled water for 40 s and then filtered before adding into analytical devices | Limit of detection of dichlorvos in the vegetables 0.8 ng/mL Sensitivity determination of dichlorvos in vegetables ranged between 3 ng/mL and 1 µg/mL making the technology a promising approach for environmental monitoring and food analysis | Liu et al. (2015), Al Mughairy and Al-Lawati (2020) |
LOC: Lab-on-a-chip
ELISA: Enzyme-linked immunosorbent assay
PCR: Polymerase chain reaction
DNA: Deoxyribonucleic acid
Developments in different biosensing technologies play a fundamental role in implementing new sensors to ensure the safety and quality of foods. Various detection techniques have been employed aiming to combine complex technical advances onto a single platform. The most commonly used detection techniques involve enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) effective for detecting different kinds of pathogens and mainly used in agriculture and food industries. For instance, a study done in Manila, Philippines, showed the detection of Salmonella species in the meat where 57.64% was found to be contaminated post PCR testing (Santos et al. 2020). Similar studies were investigated for rapid detection of E. coli in beef using the ELISA method where the whole operation time for detection was less than 3 h (Zhao et al. 2020). In PCR, the target pathogen and genetic material are analyzed. The primers and enzymes are then added followed by parameter optimization by varying the temperatures, agitation, or operating cycles. The potential of target material is later detected using gel electrophoresis (Yoon and Kim 2012).
Table 4 shows a list of target groups that need to be considered for food safety. In recent years, non-conventional fabrication materials have been evaluated which showed improved characteristics and safety at a reduced cost. The selection of efficient materials plays an important role as they could aid in saving costs, environmental impact as well as provide better performance. However, these materials should deliver precise results within a shorter period as well as be portable (Dincer et al. 2019). Jokerst et al. developed a paper-based device for colorimetric detection of E. coli O157:H7, L. monocytogenes, and Salmonella in ready-to-eat meat products (Jokerst et al. 2012). The fabricated analytical device could detect concentrations as low as 101 colony forming unit (CFU)/mL in meat within 12 h or less in comparison with the standard detection methods. Chitosan reinforced with cellulose nanocrystals was also used for the detection of listeria monocytogenes in proteins using ELISA. After biopolymer optimization, the detection and safety characteristics improved by 17 to 24% (Etty et al. 2019). Thus, such techniques and fabrication materials give more accurate results overcoming the drawbacks.
Table 4.
Target groups for food safety applications
| Category | Target group | Technique used | Food sample | Limit of detection | Reference |
|---|---|---|---|---|---|
| Pathogen | Salmonella | Loop-mediated isothermal amplification | Tomato | 5 × 10–3 ng/µL | Sayad et al. (2016) |
| Salmonella Typhimurium | Photolithography | Chicken | 103 CFU/mL | Kim et al. (2015) | |
| E. coli | Nanofiber light sensor | Orange juice | 102 CFU/mL | Shaibani et al. (2018) | |
| Salmonella enterica | Surface plasmon resonance | Cheese | 103–105 CFU/mL | Bouguelia et al. (2013) | |
| Salmonella spp. | Polymerase chain reaction detection | Meat products | 102 CFU/ 40 mL | Poltronieri et al. (2016) | |
| Biotoxins | Aflatoxins | Platinum nanoparticle-based microfluidic chip | Beer | 0.55 ppb | Ma et al. (2016) |
| Aflatoxins | Enzyme-linked immunosorbent assay | Wheat | 0.33–0.4 ppb | Uludag et al. (2016) | |
| Aflatoxin M1 | Colorimetric detection | Milk | 3 pM to 10 pM | Kasoju et al. (2020) | |
| Ricin | Edge embossing (cell-free protein synthesis) | Orange juice, diet soda | Orange juice: 2 pM Diet soda: 170 pM | Khnouf et al. (2015) | |
| Heavy metal | Copper ions | Colorimetric detection | Tomato, rice, water | 1 ng/mL | Chaiyo et al. (2015) |
| Pesticides and other compounds | Carbaryl paraoxon parathion, malathion, diazinon, chlorpyrifos (Pesticides) | Ink-based printing | Tap water, apple juice, rice | Carbaryl 29 ng/mL Paraoxon: 22 ng/mL Parathion: 32 ng/mL Malathion: 17 ng/mL Diazinon: 45 ng/mL Chlorpyrifos: 36 ng/mL | Bordbar et al. (2020) |
| Chlorpyrifos | Screen printing | Tomato juice | 3 ng/L | Nagabooshanam et al. (2020) |
CFU: Colony forming unit
The integration of nanotechnology has developed sensors in providing a significant enhancement to overall performance. For instance, techniques involving nano-biosensors using magnetic beads and quantum dots have gained a lot of interest in recent years (Mekkaoui et al. 2018; Moerland et al. 2019; Saadat et al. 2020). The portable device primarily includes two inlets, one outlet, and a detection source. The antibodies conjugated to the magnetic beads capture the analytes present in the sample and concentrate the target analytes. In the case of quantum dot labeling separation, the detection method takes place in three steps (reaction, washing, and isolation). Such technologies have shown promising approaches for selective detection of Salmonella in various foods (Kim et al. 2015). Magnetic-based sample separation using loop-mediated isothermal amplification for detection of Salmonella species in chicken breast has also provided merits (Kim et al. 2015). The quantum dot magnetic nanoparticles were water-soluble with high fluorescence properties effectively detecting S. Typhimurium and E. coli with the limit of detection of 103 CFU/mL for both. Similar magnetic bead-based integrated lab-on-chip systems were made for the separation of Salmonella species in food samples (Sun et al. 2015).
Another novel LOC technology was implemented by integrating electrokinetic magnetic bead-based assay which can moderate and inhibit the permitted mycotoxin level in infant foods. The technology was designed using a T-junction layout. An enzymatic reaction takes place on the application of an electric field driving the inner mixing of fluids for performing reactions. Simple low-cost magnetic actuators trap and move the beads within the microfluidic chamber providing more than 2.7-fold sensitivity enhancements compared to conventional systems (Berenguel-Alonso et al. 2014). The LOC approach has been shown to give more reliability in terms of ease of sample preparation, cost, and monitoring safety (Escarpa 2014).
The application of microfluidic devices has also kindled interest in digitalization mainly due to the ease of testing in a short duration (Balasubramanian et al. 2021). For instance, the use of smartphone-assisted LOC devices primarily involves performing the test using spot or lateral flow assay and positioning the detection zone for digital imaging (Ghosh et al. 2019). The strip is scanned using a smartphone and it captures the data accordingly. Colorimetric smartphone-based sensors deal with the complementary metal–oxide–semiconductor filters of phones to obtain red, green, and blue components of light. Thereby, changes in optical density are detected by giving light signals to the user for various experimental conditions (Ross et al. 2018). Smartphone-based sensing has shown promising potential for various foods like yogurt, egg, and milk (Zeinhom et al. 2018; Costa et al. 2020). When it comes to cost-effectiveness, different fabrication materials like glass, acrylic, polydimethylsiloxane, polysulfone, and hydrogels were investigated. However, paper-based analytical devices have rapidly progressed over the recent years as it poses a viable method for fast detection (Govindarajalu et al. 2019). These paper-based chips have been implemented for quantitative analysis in various food safety and quality applications (Chanu et al. 2020; Muthukumar et al. 2020).
Food production and processing
The food production industry is vital for human health. The global food production industry revolves around the following few major factors like economic growth, technological advancements, integration with electrical and communication technology as well as transportation (Sridhar et al. 2021a, b, c). Microfluidics and LOC devices represent a reliable technological system integrating multiple technologies like sensing and nanotechnology. These systems rely on the concept of a micrototal analysis system, which combines multiple analytical sensing systems into a single device converting chemical information to digital information (Guijt and Manz 2018). These systems give an edge over other conventional technologies in terms of rapid processing, least raw material requirement, and minimal space. Few developing food-related applications of these devices include emulsion processing, sensory analysis, and formulation as well as nutraceuticals.
Emulsion processing
The most simplified categorization of an emulsion is primarily oil in water (for example, mayonnaise) and water in oil (for example, butter) (Galus and Kadzińska 2015; He et al. 2020a). Generation of emulsion using conventional methods requires a tremendous amount of energy, mixing equipment, time, and raw material. Figure 5 shows the mechanism of conventional emulsion processing for different compounds and lab-on-a-chip-based approach in emulsion technology. An increased amount of energy is normally used up for the processing and denaturing of compounds during conventional emulsion processing. Non-thermal techniques like high-pressure homogenization and ultrasonic homogenization have been considered suitable processes for producing emulsions due to their stability, oil droplet dispersion, and high speed in obtaining results (Gharibzahedi et al. 2013; Wang et al. 2015). For instance, a study was done to investigate the stability of emulsions at different pressures (40, 80, and 120 MPa) using high-pressure homogenization. Pressure treatments showed an increase in emulsifying activity index from 56.93 to 87.68 m2/g and the stability index rise from 59.33 to 154.62 min. The process parameters were shown to improve the solubility from 16.5% to 75.1% producing a stable emulsion at 80 MPa (Cha et al. 2019).
Fig. 5.
Mechanism of conventional emulsion process and lab-on-a-chip-based approach. Droplet size, droplet size distribution, and composition of the phases are critical factors that affect the stability of emulsions
Another study showed that both ultrasound and high-pressure treatment had a stable emulsion for more than 30 days post-processing making it feasible in improving the performance and estimating shelf life in the food and beverage industry (Li and Xiang 2019). In another study, a co-flowing step emulsification strategy was adopted for in-line control of microdroplets (Lian et al. 2019). The study considered a mathematical approach using computational fluid dynamics for analyzing droplet size, generation, and initial pressure as well as providing predictive equations. This allowed the devices to dispense liquid in a controlled manner consuming less energy during droplet mixing. Additionally, greater control over particle size distribution of emulsified particles with faster response time was achieved compared to conventional emulsion processing (Clegg et al. 2016).
Food formulation
LOC devices have paved the way for their usage in understanding food formulation and commercial food processing. The technology offers promise to overcome the challenges faced by various sensory experts and participants for analysis and study for different foods. For instance, a sensory evaluation technique involved 12 sensory panelists for the effective assessment of wine (Guld et al. 2020). Similar sensory and consumer studies were done for different foods namely soy sauce, olive oils, and soups (Yang and Lee 2019). These studies allow better prediction of descriptors that influence consumer acceptability, leading to product modifications. In contrast, rapid testing technologies require the least manpower and limited resources for functioning. In addition to these aspects, the tests are portable with easy operation and multiple reusability options (Romao et al. 2017). Further, it can be easily cleaned for better performance with precise results in a short duration. Although such techniques are in their early stages, they have a promising potential for a possible scale-up in the future.
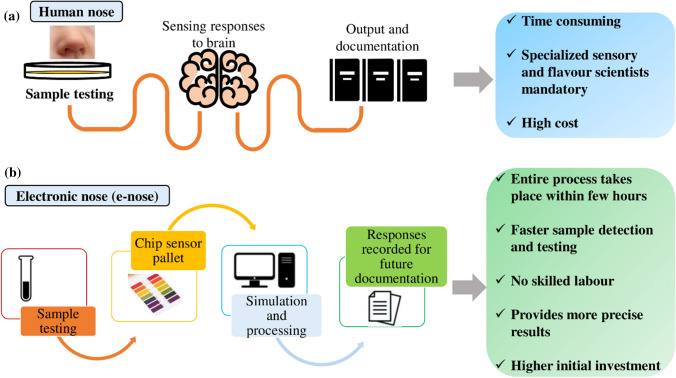
A novel technology that extensively uses the concept of microfluidics and sensors is electric nose and electronic tongue testing. Figure 6 shows the comparison of the functioning of the human nose with an e-nose device. The former has the potential to measure different tastes using a pattern recognition system while the latter can detect different odors, flavors, and quality of foods simultaneously in the form of electrical or optical signals. The e-nose instrument has completely replaced the need for human interaction with foods thereby minimizing any microbiological risks. In addition, these instruments are reproducible, reliable, inexpensive, simple, fast, and can operate at very low levels (parts per billion) (Chilo 2016; Huang et al. 2019). Further, the integration of bioelectronics with olfactory research aid in significant improvements in rapid, sensitive quantitative assessments of odorant molecules (Lee and Park 2010).
Fig. 6.
Comparison of the human nose with an electronic nose based on mimicking human olfaction. The e-nose devices are powerful analytical instruments for evaluating the quality and freshness of drinks, foods, volatile compounds, aromatic oils at a shorter time with precise results
The technology has been successfully applied to different foods like apple fruit and meat for determining the aroma profiles and freshness. Studies conducted on apples showed great variation in sourness, saltiness, and umami with the significant amount of esters and the presence of volatile compounds like hexyl butyrate and toluene (Zhu et al. 2020). Similar studies were evaluated to analyze the meat freshness (Weng et al. 2020). It is noteworthy to mention the most recent advancement of 3D printed chip technology. The technology can be used to print microchannels based on specific demands within few hours enabling rapid prototyping (Jamaledin et al. 2020).
Microneedles or patches then act as a pathway to transport the viscous materials or proteins through the nozzle onto the preparation tray. 3D printing has provided increasing interest among scientists and the commercial arena to manufacture food of different shapes and structures as per convenience by monitoring different printing variables. For instance, Derossi et al. investigated the effect of two printing factors, print speed, and flow level to manufacture a fruit-based formulation for young children (Derossi et al. 2017). The studies concluded a flow rate less than 70% and a print speed of 50 m/s would be ideal for preparing the formulation and restrict irregular structures and oversized porosities. Similar experiments were conducted to understand the textural properties and the variation in the structure of 3D printed processed cheese (Le et al. 2018). Thus, such research provides an insight on the customization of foods, personalized nutrition catering to consumer needs as well as in faster food packaging applications.
Recently, studies have also been undertaken on integrating artificial neural networks, machine learning, and computer vision with e-nose testing. Such e-nose technology coupled with artificial intelligence concept offers practical implementation in field conditions using sensor networks. These technologies have also been used in food samples like wines by modeling different parameters like amount of phenol, the concentration of berries, and consumer sensory tests as a function of time. The models showed a promising regression value ranging between 0.98 and 0.99 (Fuentes et al. 2020). Similar e-nose systems equipped with machine learning and neural network models have been used for aroma testing of different gas releases through sensors as a function of time in beer. The total sensor testing time was between 50 and 400 s producing regression values between 0.93 and 0.97 (Gonzalez Viejo et al. 2020). Thus, such technologies could form a path toward effective data recognition and analysis for understanding food formulation.
Nutraceutical development
With changing lifestyles, there is a growing need for diet-rich foods and supplements for maintaining body immunity and health. Developments are being carried in the application of LOCs for understanding the protein–polysaccharide interactions in the nutraceutical industry. The isolation of the food matrix, the interaction between bioactive compounds, and the determination of antioxidants and carotenoids have been widely explored (Bealer et al. 2020). As these compounds are difficult to isolate, innovative techniques through LOC-based technology by encapsulation or entrapment are being investigated to determine efficient nutrient deliveries (Sridhar et al. 2021b). During encapsulation, the particle size and wall material must be carefully considered. Vortex fluidic device (VFD) has been gaining a lot of interest in recent years due to its scalable, thin-film microfluidic flow process involving efficient mass transfer technique (He et al. 2019). The rotation of the tube kept at an angle provides efficient mixing between the two products. Figure 7 shows a schematic of a VFD device with two sample inlets with one outlet. The application of this concept has been shown to reduce the processing time of raw milk pasteurization from 30 to 10 min. In recent years, the technique has also been widely applied in fish oil encapsulation and for the rapid processing of biologically active proteins (Luo et al. 2016; He et al. 2020b).
Fig. 7.
Vortex fluid device (VFD) with two sample inlets and one sample outlet. The device is a simple, low-cost platform that operates based on the principles of different shear regimes and organic reactions resulting in the formation of a suitable emulsion
Although studies using VFD for food production and processing are scarce, a lot of scope lies in the effective scale-up of this technique due to low cost and easy operation. The cost of LOC devices ranges between $ 15,000–$ 17,000 compared to $ 150,000 for a high-pressure microfluidic device (He et al. 2020a). The VFD technology has also been successfully implemented for enzymatic hydrolysis of milk protein from 3 h to 20 min and pasteurization time from 30 to 10 min thereby suggesting its high potential (He et al. 2019).
Food packaging
Product packaging development primarily runs on four basic functions: protection, communication, convenience, and containment (Yam 2005). Food packaging is primarily done to protect food from any sort of contamination and maintain the desired quality. Excessive water through condensation, oxidation, and microbial growth are the major causes of deterioration of food quality leading to foodborne illnesses. Although traditional packaging has contributed massively to early development, it is no longer sufficient to satisfy the complex and evolving consumer needs. Consumers demand food that is safe, diet rich, attractive, and healthy. Rapid advancements have been carried out in packaging technology introducing the concept of smart packaging, time–temperature-based indicators, gas sensors, and absorbers as well as RFID systems.
Smart packaging system
Smart packaging devices are small inexpensive labels or tags that are attached to the main package (pouches, trays, labs, bottles, and caps). They work on the principle of collecting, storing, and transmitting data. These devices are generally connected to a host computer where data recording is done for traceability purposes. For instance, miniature stick packaging integrated with a pressure-controlled seal serves as an effective technology for foods containing high moisture as well as aids in determining shelf life (Van Oordt et al. 2011). The study revealed an adjustment of pressure between 20 and 100 kPa showed promising results for a low-cost mass production system. Similar food packaging wraps were developed by printing DNAzyme probe to a cyclo-olefin polymer film (Yousefi et al. 2018). The flexible fabricated wrap has the potential to generate a fluorescence signal when a target bacterium like E. coli comes in contact with the wrapping film. The biosensor was successfully tested for E. coli in meat and apple juice for concentrations as low as 103 CFU/mL at pH 3–9 up to 14 days.
Radio frequency identification tags
Radio frequency identification (RFID) is an advanced form of carrying data for product identification and traceability. The tags are generally put for easier tracking of items. In recent years, they have broadened their application in providing nutrient information of the specific packaged product. Figure 8 shows the application of RFID technology for product identification. In a typical RFID system, the reader emits radio frequency waves on the food commodity to capture the data. This data is transferred to the tag which is connected to the main server. The user then checks the server for analysis and decision-making (Zheng et al. 2020). An ideal RFID tag works on a small microchip with a tiny antenna for transferring signals to the user (Reyes et al. 2013; Gillenson 2019).
Fig. 8.
Application of radio frequency identification (RFID) technology for product identification. These tags are attached to almost all food products to aid in reducing shoplifting and counterfeiting in addition to improvements in the supply chain
The technology has proven advantageous as compared to traditional barcode scanners. For instance, it can store larger amounts of data (more than 1 megabyte), provides real-time information, and does not require a specific site area for scanning as compared to barcodes (Chen and Tu 2009). In recent years, a displacement and tilt detection method has been found using a passive ultra-high-frequency RFID reader. The novel method uses high-frequency waves to measure the phase variation and tag response using the polarized reader. The results showed displacement less than 2 mm and a tilt angle of 2.5° and 500 mm working range would be the ideal conditions for detection (Lai et al. 2018). Similar fabrication studies were done combining RFID with microfluidics and inkjet printing technology for sensing contaminants in the fluid. The low-cost and rapid microfluidic RFID device showed promising results as it could detect less than 1% water adulteration in alcohol samples (Cook et al. 2013). Thus, the concept has gained a growing interest in retail centers, food industries, supply chains (Wang et al. 2019; Alfian et al. 2020), health care (Hariraj and Selvarajah 2020), and security controls (Khalil et al. 2019).
In recent years, technology with the concept of the Internet of Things (IoT) has introduced the idea of “smart shopping” where an RFID chip is attached to smart carts and shelves. The technology involves an artificial intelligence approach where the cart saves all the selected goods and produces the bill by updating it on the LCD screen of the shopping cart thus removing any human intervention (Hussien et al. 2020). Similar RFID-based systems compiled with machine learning models have been developed for better sensing of tagged products in perishable foods, which move through the gates thus improving traceability and efficiency (Alfian et al. 2020).
Indicators
Indicators are small miniaturized self-adhesive labels attached to food packages and containers. These special labels show visual indications using sensors for maintaining the required temperature or giving a warning through a color change (freshness indicators). There are three major classes of indicators; gas indicators, freshness indicators, and time–temperature indicators (Vanderroost et al. 2014). Table 5 shows the characteristics of different types of indicators used in the food industry. These technologies have gained continuous up-gradation in the information and communication technology sector. Systems like wireless networks, mobile phones, touch screens, and global positioning systems play a key role in making up the IoT. The concept of “making products smart” has increased the use of temperature/freshness or gas-based indicators for ensuring efficient delivery as well as food safety across the supply chain to meet the consumer standards (Maksimović et al. 2015).
Table 5.
Characteristics of indicators used in the food industry
| Indicator name | Characteristics | Target to be detected | Application in foods | References |
|---|---|---|---|---|
| Gas indicators | To determine the scavenging activities in the food commodity. They offer an alternative approach for identifying any leakages or sealing issues in the pack. The chip detects the leak and converts the signals into visual colorimetric change | Gases like CO2, O2 | Oxygen inhibitor films using an alginate polymer coating | Vu and Won (2013) |
| Freshness indicators | Provide immediate product quality data regarding microbial growth or any chemical changes or modifications in the packaged food. Generally used for maintaining shelf life | Monitoring of nitrogen, amines, ammonia, CO2 pH sensing in food packages | Meat and fish | Kuswandi (2017) |
| Time–temperature indicators | Used for measuring food deterioration. Lab-on-a-chip device provides visual information concerning food quality, distribution, spoilage issues, and storage | Bacterial adulteration | Milk and other perishable foods | Zhang et al. (2013), Vanderroost et al. (2014) |
There has been a growing interest in recent times in developing irreversible spoilage sensors for foods. For instance, Liu et al. developed an irreversible food sensor that shows halochromic behavior toward amines and volatile compounds in seafood, meat products, and protein-based foods (Liu et al. 2020). Similar rapid indicator systems with single-cell detection methods were developed to analyze foodborne pathogens (Salmonella contamination) in milk samples. The results showed a detection limit of 50 CFU/mL with fluorescence-based pathogen identification within 5 h (An et al. 2020). Experiments on polymer-based flexible strain sensors with visual LED indicators were also fabricated for providing an effective packaging system. The light intensity (maximum brightness = 67 lx) decreased as spoilage was observed in the food package (Escobedo et al. 2020). Thus, such technologies can form an initial path for detection ensuring greater food safety and public health.
Challenges
Significant advancements have taken place in the area of LOC devices and their application in the food industry. A bibliometric analysis is conducted to evaluate the scope and progress of lab-on-a-chip devices in different areas. The review emphasizes the recent developments in the potential of LOCs in the food industry in specific areas of food safety, production, processing, and packaging. Food fraud along with microbial contamination has instilled the need for better safety standards and improved practices. However, as of today, the system still lacks traceability and transparency. Although continuous improvements through revised policies are being implemented by regulatory authorities such as Food and Drug Administration, more intensive monitoring across the entire supply chain is required. The research findings in our study point toward the integration of detection techniques with information and communication technology.
Smartphone-based sensors pose an excellent option for portable analysis as they can instantly acquire data, analyze and store results. Such sensors, if installed along the production line, could help detect contaminants at specific stages. Nanotechnology could offer a possible path for development of precise and accurate sensors by facilitating design of novel materials and enabling selective surface modification. In recent years, materials like graphite, graphene (Shahdeo et al. 2020; Bauer et al. 2021), carbon nanotubes (Sobhan et al. 2020), and chitosan (Lin et al. 2020) have shown promising results for the development of lab-on-a-chip devices and their scale-up. Further, scale-out strategies must also be explored to achieve higher throughput in production.
However, challenges like mass-scale production and user acceptability still need to be considered before they arrive in the market. A systematic investigation needs to be done in choosing analytes, dosages, material as well as fabrication approaches. Improvements in developing re-usable sensors for production and safety testing could be one of the possible ways forward. More importance should be given to device performance, its integration in food processing lines as well as testing standards for it to become commercially available. Lab-on-a-chip devices should also be combined with strategies for valorization and agricultural and food waste for integrated technological solutions in environmental protection (Ponnuchamy et al. 2020). Lab-on-a-chip technology has potential to play a major role in facilitating end-to-end traceability throughout the food production chain.
Conclusion
The promising potential of the lab-on-a-chip sensing technique has given an attractive option for rapid detection with minimal sample and reagent volumes. The review discusses the few most promising applications of chips in the food industry. The innovative fabrication techniques along with advancements in materials and design aspects could be beneficial for the development of advanced micrototal analysis systems. Other prospective uses of lab-on-a-chip systems in foods, which were not discussed but are not limited to, include nano-encapsulated nutrient delivery, security inks, or nano-barcodes to protect against counterfeiting as well as analyzing gut microbiota profiles using chips. In addition to continuous technology advancements, equal importance on marketing, public satisfaction and adequate support from the government would help in the successful implementation of these applications. It is needless to mention that these microscale devices could drastically save time, as well as bring high efficiency to the current systems if a constant dialogue is maintained between the scientists and companies who purchase them. These benefits could ultimately make an efficient food supply chain, reduce wastage, and ensure the safety of food products for consumption.
Abbreviations
- CFU
Colony forming unit
- DNA
Deoxyribonucleic acid
- ELISA
Enzyme-linked immunosorbent assay
- IoT
Internet of Things
- LOC
Lab-on-a-chip
- PCR
Polymerase chain reaction
- RFID
Radio frequency identification
- VFD
Vortex fluidic device
Funding
This research did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ashish Kapoor, Email: ashishko@srmist.edu.in.
Ponnusamy Senthil Kumar, Email: senthilkumarp@ssn.edu.in.
References
- Agarwal A, Salahuddin A, Wang H, Ahamed MJ. Design and development of an efficient fluid mixing for 3D printed lab-on-a-chip. Microsyst Technol. 2020;26:2465–2477. doi: 10.1007/s00542-020-04787-9. [DOI] [Google Scholar]
- Ahn H, Batule BS, Seok Y, Kim MG. Single-Step recombinase polymerase amplification assay based on a paper chip for simultaneous detection of multiple foodborne pathogens. Anal Chem. 2018;90:10211–10216. doi: 10.1021/acs.analchem.8b01309. [DOI] [PubMed] [Google Scholar]
- Al Mughairy B, Al-Lawati HAJ. Recent analytical advancements in microfluidics using chemiluminescence detection systems for food analysis. TrAC - Trends Anal Chem. 2020;124:115–802. doi: 10.1016/j.trac.2019.115802. [DOI] [Google Scholar]
- Alfian G, Syafrudin M, Farooq U, et al. Improving efficiency of RFID-based traceability system for perishable food by utilizing IoT sensors and machine learning model. Food Control. 2020;110:1–30. doi: 10.1016/j.foodcont.2019.107016. [DOI] [Google Scholar]
- Al-Kahtani HA, Ismail EA, Asif Ahmed M. Pork detection in binary meat mixtures and some commercial food products using conventional and real-time PCR techniques. Food Chem. 2017;219:54–60. doi: 10.1016/j.foodchem.2016.09.108. [DOI] [PubMed] [Google Scholar]
- Amicarelli V, Bux C, Lagioia G. How to measure food loss and waste? A material flow analysis application. Br Food J. 2020;123:67–85. doi: 10.1108/BFJ-03-2020-0241. [DOI] [Google Scholar]
- Amor-Gutiérrez O, Costa-Rama E, Fernández-Abedul MT. Sampling and multiplexing in lab-on-paper bioelectroanalytical devices for glucose determination. Biosens Bioelectron. 2019;135:64–70. doi: 10.1016/j.bios.2019.04.006. [DOI] [PubMed] [Google Scholar]
- An X, Zuo P, Ye BC. A single cell droplet microfluidic system for quantitative determination of food-borne pathogens. Talanta. 2020;209:120–571. doi: 10.1016/j.talanta.2019.120571. [DOI] [PubMed] [Google Scholar]
- Ashley J, D’Aurelio R, Piekarska M, et al. Development of a β-Lactoglobulin sensor based on SPR for milk allergens detection. Biosensors. 2018;8:1–11. doi: 10.3390/bios8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri N, Al Lawati HAJ, Hassanzadeh J. Simultaneous determination of total phenolic acids and total flavonoids in tea and honey samples using an integrated lab on a chip device. Food Chem. 2021;342:128–338. doi: 10.1016/j.foodchem.2020.128338. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Udayabhanu A, Kumar PS, et al. Digital colorimetric analysis for estimation of iron in water with smartphone-assisted microfluidic paper-based analytical devices. Int J Environ Anal Chem. 2021 doi: 10.1080/03067319.2021.1893711. [DOI] [Google Scholar]
- Bansal S, Singh A, Mangal M, et al. Food adulteration: sources, health risks, and detection methods. Crit Rev Food Sci Nutr. 2017;57:1174–1189. doi: 10.1080/10408398.2014.967834. [DOI] [PubMed] [Google Scholar]
- Bauer M, Wunderlich L, Weinzierl F, et al. Electrochemical multi-analyte point-of-care perspiration sensors using on-chip three-dimensional graphene electrodes. Anal Bioanal Chem. 2021;413:763–777. doi: 10.1007/s00216-020-02939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bealer EJ, Onissema-karimu S, Rivera-galletti A, et al. Protein polysaccharide composite materials. Polym (Basel) 2020;12:1–28. doi: 10.3390/polym12020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguel-Alonso M, Granados X, Faraudo J, et al. Magnetic actuator for the control and mixing of magnetic bead-based reactions on-chip. Anal Bioanal Chem. 2014;406:6607–6616. doi: 10.1007/s00216-014-8100-5. [DOI] [PubMed] [Google Scholar]
- Bhalla N, Pan Y, Yang Z, Payam AF. Opportunities and challenges for biosensors and nanoscale analytical tools for pandemics: COVID-19. ACS Nano. 2020;14:7783–7807. doi: 10.1021/acsnano.0c04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordbar MM, Nguyen TA, Arduini F, Bagheri H. A paper-based colorimetric sensor array for discrimination and simultaneous determination of organophosphate and carbamate pesticides in tap water, apple juice, and rice. Microchim Acta. 2020;187:1–13. doi: 10.1007/s00604-020-04596-x. [DOI] [PubMed] [Google Scholar]
- Bouguelia S, Roupioz Y, Slimani S, et al. On-chip microbial culture for the specific detection of very low levels of bacteria. Lab Chip. 2013;13:4024–4032. doi: 10.1039/c3lc50473e. [DOI] [PubMed] [Google Scholar]
- Brazey B, Cottet J, Bolopion A, et al. Impedance-based real-time position sensor for lab-on-a-chip devices. Lab Chip. 2018;18:818–831. doi: 10.1039/c7lc01344b. [DOI] [PubMed] [Google Scholar]
- Calado B, dos Santos A, Semiao V. Characterization of the mixing regimes of Newtonian fluid flows in asymmetrical T-shaped micromixers. Exp Therm Fluid Sci. 2016;72:218–227. doi: 10.1016/j.expthermflusci.2015.11.010. [DOI] [Google Scholar]
- Cha Y, Shi X, Wu F, et al. Improving the stability of oil-in-water emulsions by using mussel myofibrillar proteins and lecithin as emulsifiers and high-pressure homogenization. J Food Eng. 2019;258:1–8. doi: 10.1016/j.jfoodeng.2019.04.009. [DOI] [Google Scholar]
- Chaiyo S, Siangproh W, Apilux A, Chailapakul O. Highly selective and sensitive paper-based colorimetric sensor using thiosulfate catalytic etching of silver nanoplates for trace determination of copper ions. Anal Chim Acta. 2015;866:75–83. doi: 10.1016/j.aca.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Chanu OR, Kapoor A, Karthik V. Digital image analysis for microfluidic paper based pH sensor platform. Mater Today Proc. 2020;40:S64–S68. doi: 10.1016/j.matpr.2020.03.503. [DOI] [Google Scholar]
- Chen RS, Tu M. Development of an agent-based system for manufacturing control and coordination with ontology and RFID technology. Expert Syst Appl. 2009;36:7581–7593. doi: 10.1016/j.eswa.2008.09.068. [DOI] [Google Scholar]
- Chen S, Zhang Y, Li H, et al. Differentiation of fish species in Taiwan Strait by PCR-RFLP and lab-on-a-chip system. Food Control. 2014;44:26–34. doi: 10.1016/j.foodcont.2014.03.019. [DOI] [Google Scholar]
- Chiang CK, Kurniawan A, Kao CY, Wang MJ. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta. 2019;194:837–845. doi: 10.1016/j.talanta.2018.10.104. [DOI] [PubMed] [Google Scholar]
- Chilo J. E-nose application to food industry production. IEE Instrum Meas. 2016;19:27–33. doi: 10.1109/MIM.2016.7384957. [DOI] [Google Scholar]
- Choi JR, Yong KW, Choi JY, Cowie AC. Emerging point-of-care technologies for food safety analysis. Sens (Switzerland) 2019;19:1–31. doi: 10.3390/s19040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg PS, Tavacoli JW, Wilde PJ. One-step production of multiple emulsions: microfluidic, polymer-stabilized and particle-stabilized approaches. Soft Matter. 2016;12:998–1008. doi: 10.1039/c5sm01663k. [DOI] [PubMed] [Google Scholar]
- Cook BS, Cooper JR, Tentzeris MM. An inkjet-printed microfluidic rfid-enabled platform for wireless lab-on-chip applications. IEEE Trans Microw Theory Tech. 2013;61:4714–4723. doi: 10.1109/TMTT.2013.2287478. [DOI] [Google Scholar]
- Costa RA, Morais CLM, Rosa TR, et al. Quantification of milk adulterants (starch, H2O2, and NaClO) using colorimetric assays coupled to smartphone image analysis. Microchem J. 2020;156:104968. doi: 10.1016/j.microc.2020.104968. [DOI] [Google Scholar]
- Das C, Chakraborty S, Karmakar A, Chattopadhyay S. On-chip detection and quantification of soap as an adulterant in milk employing electrical impedance spectroscopy. 2018 Int Symp Devices. Circuits Syst ISDCS. 2018;2018:1–4. doi: 10.1109/ISDCS.2018.8379634. [DOI] [Google Scholar]
- Deng D, Lin Q, Li H, et al. Rapid detection of malachite green residues in fish using a surface-enhanced Raman scattering-active glass fiber paper prepared by in situ reduction method. Talanta. 2019;200:272–278. doi: 10.1016/j.talanta.2019.03.021. [DOI] [PubMed] [Google Scholar]
- Derossi A, Caporizzi R, Azzollini D, Severini C. Application of 3D printing for customized food. A case on the development of a fruit-based snack for children. J Food Eng. 2017;220:65–75. doi: 10.1016/j.jfoodeng.2017.05.015. [DOI] [Google Scholar]
- Dincer C, Bruch R, Costa-Rama E, et al. Disposable sensors in diagnostics, food, and environmental monitoring. Adv Mater. 2019;31:1–28. doi: 10.1002/adma.201806739. [DOI] [PubMed] [Google Scholar]
- Dooley JJ, Sage HD, Brown HM, Garrett SD. Improved fish species identification by use of lab-on-a-chip technology. Food Control. 2005;16:601–607. doi: 10.1016/j.foodcont.2004.06.022. [DOI] [Google Scholar]
- Duong LH, Chen PC. Simple and low-cost production of hybrid 3D-printed microfluidic devices. Biomicrofluidics. 2019;13:1–11. doi: 10.1063/1.5092529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sheikha AF. DNAFoil: novel technology for the rapid detection of food adulteration. Trends Food Sci Technol. 2019;86:544–552. doi: 10.1016/j.tifs.2018.11.012. [DOI] [Google Scholar]
- Ellis DI, Brewster VL, Dunn WB, et al. Fingerprinting food: current technologies for the detection of food adulteration and contamination. Chem Soc Rev. 2012;41:5706–5727. doi: 10.1039/c2cs35138b. [DOI] [PubMed] [Google Scholar]
- Escarpa A. Lights and shadows on Food Microfluidics. Lab Chip. 2014;14:3213–3224. doi: 10.1039/c4lc00172a. [DOI] [PubMed] [Google Scholar]
- Escobedo P, Bhattacharjee M, Nikbakhtnasrabadi F, Dahiya R (2020) Flexible Strain Sensor with NFC Tag for Food Packaging FLEPS 2020 - IEEE Int Conf Flex Printable Sensors Syst, 9781728152:16–19. Doi: 10.1109/FLEPS49123.2020.9239568
- Etty MC, D’Auria S, Shankar S, et al. New immobilization method of anti-PepD monoclonal antibodies for the detection of Listeria monocytogenes p60 protein – Part B: Rapid and specific sandwich ELISA using antibodies immobilized on a chitosan/CNC film support. React Funct Polym. 2019;143:104317. doi: 10.1016/j.reactfunctpolym.2019.104317. [DOI] [Google Scholar]
- Fernández-Abedul MT. Paper based sensors. Anal Bioanal Chem. 2021;413:3143–3144. doi: 10.1007/s00216-021-03277-9. [DOI] [Google Scholar]
- Fernández-Ramos MD, Ogunneye AL, Barbarinde NAA, et al. Bioactive microfluidic paper device for pesticide determination in waters. Talanta. 2020;218:121108. doi: 10.1016/j.talanta.2020.121108. [DOI] [PubMed] [Google Scholar]
- Franek M, Rubio D, Diblikova I, Rubio F. Analytical evaluation of a high-throughput enzyme-linked immunosorbent assay for acrylamide determination in fried foods. Talanta. 2014;123:146–150. doi: 10.1016/j.talanta.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Fuentes S, Summerson V, Viejo CG, et al. Assessment of smoke contamination in grapevine berries and taint in wines due to bushfires using a low-cost e-nose and an artificial intelligence approach. Sens (Switzerland) 2020;20:1–15. doi: 10.3390/s20185108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galus S, Kadzińska J. Food applications of emulsion-based edible films and coatings. Trends Food Sci Technol. 2015;45:273–283. doi: 10.1016/j.tifs.2015.07.011. [DOI] [Google Scholar]
- Gharaghani FM, Akhond M, Hemmateenejad B. A three-dimensional origami microfluidic device for paper chromatography: Application to quantification of Tartrazine and Indigo carmine in food samples. J Chromatogr A. 2020;1621:461049. doi: 10.1016/j.chroma.2020.461049. [DOI] [PubMed] [Google Scholar]
- Gharibzahedi SMT, Razavi SH, Mousavi SM. Ultrasound-assisted formation of the canthaxanthin emulsions stabilized by arabic and xanthan gums. Carbohydr Polym. 2013;96:21–30. doi: 10.1016/j.carbpol.2013.03.085. [DOI] [PubMed] [Google Scholar]
- Ghasemi A, Amiri H, Zare H, et al. Carbon nanotubes in microfluidic lab-on-a-chip technology: current trends and future perspectives. Microfluid Nanofluidics. 2017;21:1–19. doi: 10.1007/s10404-017-1989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Vaishampayan V, Mahapatra A, et al. Enhancement of limit of detection by inducing coffee-ring effect in water quality monitoring microfluidic paper-based devices. Desalin Water Treat. 2019;156:316–322. doi: 10.5004/dwt.2019.23715. [DOI] [Google Scholar]
- Ghosh S, Aggarwal K, Ahn CH (2020) A new mobile healthcare system using smartphone and lab-on-a-chip for on-site diagnostics of malaria. 21st Int Conf Miniaturized Syst Chem Life Sci MicroTAS 2017 1:233–234
- Gillenson ML. I’ve got you under my skin: The past, present and future use of RFID technology in people and animals. J Inf Technol Manag. 2019;30:19–29. [Google Scholar]
- Gonzalez Viejo C, Fuentes S, Godbole A, et al. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens Actuat, B Chem. 2020;308:127688. doi: 10.1016/j.snb.2020.127688. [DOI] [Google Scholar]
- Govindarajalu AK, Ponnuchamy M, Sivasamy B, et al. A cellulosic paper-based sensor for detection of starch contamination in milk. Bull Mater Sci. 2019;42:1–6. doi: 10.1007/s12034-019-1958-2. [DOI] [Google Scholar]
- Grist S, Oyunerdene N, Flueckiger J, et al. Fabrication and laser patterning of polystyrene optical oxygen sensor films for lab-on-a-chip applications. RSC Adv. 2012;139:5718–5727. doi: 10.1039/C4AN00765D. [DOI] [PubMed] [Google Scholar]
- Guijt RM, Manz A. Miniaturised total chemical-analysis systems (ΜTAS) that periodically convert chemical into electronic information. Sens Actuat, B Chem. 2018;273:1334–1345. doi: 10.1016/j.snb.2018.06.054. [DOI] [Google Scholar]
- Guld Z, Nyitrainé Sárdy D, Gere A, Rácz A. Comparison of sensory evaluation techniques for Hungarian wines. J Chemom. 2020;34:1–15. doi: 10.1002/cem.3219. [DOI] [Google Scholar]
- Guo L, Feng J, Fang Z, et al. Application of microfluidic “lab-on-a-chip” for the detection of mycotoxins in foods. Trends Food Sci Technol. 2015;46:252–263. doi: 10.1016/j.tifs.2015.09.005. [DOI] [Google Scholar]
- Hariraj S, Selvarajah V. Implementation of rfid technology in managing health information in a hospital. Int J Curr Res Rev. 2020;12:177–182. doi: 10.31782/IJCRR.2020.122029. [DOI] [Google Scholar]
- He S, Joseph N, Luo X, Raston CL. Vortex fluidic mediated food processing. PLoS ONE. 2019;14:1–12. doi: 10.1371/journal.pone.0216816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Joseph N, Feng S, et al. Application of microfluidic technology in food processing. Food Funct. 2020;11:5726–5737. doi: 10.1039/d0fo01278e. [DOI] [PubMed] [Google Scholar]
- He S, Joseph N, Mirzamani M, et al. Vortex fluidic mediated encapsulation of functional fish oil featuring in situ probed small angle neutron scattering. Npj Sci Food. 2020;4:1–11. doi: 10.1038/s41538-020-00072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Li J, Liu L, et al. Origami-based “book” shaped three-dimensional electrochemical paper microdevice for sample-to-answer detection of pathogens. RSC Adv. 2020;10:25808–25816. doi: 10.1039/d0ra03833d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Jeong SG, Shim G, et al. Improvement in the reproducibility of a paper-based analytical device (PAD) using stable covalent binding between proteins and cellulose paper. Biotechnol Bioprocess Eng. 2018;23:686–692. doi: 10.1007/s12257-018-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Cai G, Zheng L, Lin J. A microfluidic signal-off biosensor for rapid and sensitive detection of Salmonella using magnetic separation and enzymatic catalysis. Food Control. 2019;103:186–193. doi: 10.1016/j.foodcont.2019.04.008. [DOI] [Google Scholar]
- Hu Y, Lu X. Rapid pomegranate juice authentication using a simple sample-to-answer hybrid paper/polymer-based lab-on-a-chip device. ACS Sens. 2020;5:2168–2176. doi: 10.1021/acssensors.0c00786. [DOI] [PubMed] [Google Scholar]
- Huang X, Yu S, Xu H, et al. Rapid and nondestructive detection of freshness quality of postharvest spinaches based on machine vision and electronic nose. J Food Saf. 2019;39:1–8. doi: 10.1111/jfs.12708. [DOI] [Google Scholar]
- Hussien NA, Alsaidi SAAA, Ajlan IK, et al. Smart shopping system with RFID technology based on internet of things. Int J Interact Mob Technol. 2020;14:17–29. doi: 10.3991/ijim.v14i04.13511. [DOI] [Google Scholar]
- Jamaledin R, Di Natale C, Onesto V, et al. Progress in microneedle-mediated protein delivery. J Clin Med. 2020;9:542. doi: 10.3390/jcm9020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokerst JC, Adkins JA, Bisha B, et al. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal Chem. 2012;84:2900–2907. doi: 10.1021/ac203466y. [DOI] [PubMed] [Google Scholar]
- Kasoju A, Shahdeo D, Khan AA, et al. Fabrication of microfluidic device for Aflatoxin M1 detection in milk samples with specific aptamers. Sci Rep. 2020;10:1–8. doi: 10.1038/s41598-020-60926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil G, Doss R, Chowdhury M. A comparison survey study on RFID based anti-counterfeiting systems. J Sens Actuator Netw. 2019;8:1–15. doi: 10.3390/jsan8030037. [DOI] [Google Scholar]
- Khnouf R, Chapman B, Jin S, et al. Detection of ricin in beverages using cell-free protein synthesis in a microfluidic device. Sens Actuat B Chem. 2015;221:723–729. doi: 10.1016/j.snb.2015.06.077. [DOI] [Google Scholar]
- Kim G, Moon J, Moh C, Lim J. A micro fluidic nano-biosensor for the detection of pathogenic Salmonella. Biosens Bioelectron. 2015;67:243–247. doi: 10.1016/j.bios.2014.08.023. [DOI] [PubMed] [Google Scholar]
- Kim J, Campbell AS, de Ávila BEF, Wang J. Wearable biosensors for healthcare monitoring. Nat Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Squire K, Chong X, Wang AX. Ultra-sensitive lab-on-a-chip detection of Sudan I in food using plasmonics-enhanced diatomaceous thin film. Food Control. 2017;79:258–265. doi: 10.1016/j.foodcont.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Y. Isothermal amplification-based methods for assessment of microbiological safety and authenticity of meat and meat products. Food Control. 2021;121:107679. doi: 10.1016/j.foodcont.2020.107679. [DOI] [Google Scholar]
- Kuswandi B (2017) Freshness Sensors for Food Packaging. 10.1016/B978-0-08-100596-5.21876-3
- Lai X, Cai Z, Xie Z, Zhu H. A novel displacement and tilt detection method using passive UHF RFID technology. Sens (Switzerland) 2018;18:1–14. doi: 10.3390/s18051644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le C, Sullivan JJO, Drapala KP, et al. Effect of 3D printing on the structure and textural properties of processed cheese. J Food Eng. 2018;220:56–64. doi: 10.1016/j.jfoodeng.2017.02.003. [DOI] [Google Scholar]
- Lee SH, Park TH. Recent advances in the development of bioelectronic nose. Biotechnol Bioprocess Eng. 2010;15:22–29. doi: 10.1007/s12257-009-3077-1. [DOI] [Google Scholar]
- Lee CY, Wang WT, Liu CC, Fu LM. Passive mixers in microfluidic systems: a review. Chem Eng J. 2016;288:146–160. doi: 10.1016/j.cej.2015.10.122. [DOI] [Google Scholar]
- Li Y, Xiang D. Stability of oil-in-water emulsions performed by ultrasound power or high-pressure homogenization. PLoS ONE. 2019;14:1–14. doi: 10.1371/journal.pone.0213189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ballerini DR, Shen W. A perspective on paper-based microfluidics: current status and future trends. Biomicrofluidics. 2012;6:1–14. doi: 10.1063/1.3687398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Y, Kim E, et al. Electrodeposition of a magnetic and redox-active chitosan film for capturing and sensing metabolic active bacteria. Carbohydr Polym. 2018;195:505–514. doi: 10.1016/j.carbpol.2018.04.096. [DOI] [PubMed] [Google Scholar]
- Lian J, Zheng S, Liu C, et al. Investigation of microfluidic co-flow effects on step emulsification: wall contact angle and critical dimensions. Coll Surf A Physicochem Eng Asp. 2019;580:123733. doi: 10.1016/j.colsurfa.2019.123733. [DOI] [Google Scholar]
- Lin CT, Kuo SH, Lin PH, et al. Hand-powered centrifugal microfluidic disc with magnetic chitosan bead-based ELISA for antibody quantitation. Sens Actuat, B Chem. 2020;316:1–10. doi: 10.1016/j.snb.2020.128003. [DOI] [Google Scholar]
- Liu W, Guo Y, Luo J, et al. A molecularly imprinted polymer based a lab-on-paper chemiluminescence device for the detection of dichlorvos. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2015;141:51–57. doi: 10.1016/j.saa.2015.01.020. [DOI] [PubMed] [Google Scholar]
- Liu B, Gurr PA, Qiao GG. Irreversible spoilage sensors for protein-based food. ACS Sens. 2020;5:2903–2908. doi: 10.1021/acssensors.0c01211. [DOI] [PubMed] [Google Scholar]
- Luo X, Smith P, Raston CL, Zhang W. Vortex fluidic device-intensified aqueous two phase extraction of C-phycocyanin from spirulina maxima. ACS Sustain Chem Eng. 2016;4:3905–3911. doi: 10.1021/acssuschemeng.6b00756. [DOI] [Google Scholar]
- Ma Y, Mao Y, Huang D, et al. Portable visual quantitative detection of aflatoxin B1 using a target-responsive hydrogel and a distance-readout microfluidic chip. Lab Chip. 2016;16:3097–3104. doi: 10.1039/c6lc00474a. [DOI] [PubMed] [Google Scholar]
- Maddipatla D, Narakathu BB, Ochoa M, et al. Rapid prototyping of a novel and flexible paper based oxygen sensing patch via additive inkjet printing process. RSC Adv. 2019;9:22695–22704. doi: 10.1039/c9ra02883h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimović M, Vujović V, Omanović-Mikličanin E. Application of internet of things in food packaging and transportation. Int J Sustain Agric Manag Inform. 2015;1:333–350. doi: 10.1504/IJSAMI.2015.075053. [DOI] [Google Scholar]
- Mark D, Haeberle S, Roth G, et al. Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev. 2010;39:1153–1182. doi: 10.1039/b820557b. [DOI] [PubMed] [Google Scholar]
- Marquez S, Liu J, Morales-Narváez E. Paper-based analytical devices in environmental applications and their integration with portable technologies. Curr Opin Environ Sci Heal. 2019;10:1–8. doi: 10.1016/j.coesh.2019.08.002. [DOI] [Google Scholar]
- Martinez AW, Phillips ST, Wiley BJ, et al. FLASH: a rapid method for prototyping paper-based microfluidic devices. Lab Chip. 2008;8:2146–2150. doi: 10.1039/b811135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekkaoui S, Le Roy D, Audry MC, et al. Arrays of high aspect ratio magnetic microstructures for large trapping throughput in lab-on-chip systems. Microfluid Nanofluidics. 2018;22:1–10. doi: 10.1007/s10404-018-2141-6. [DOI] [Google Scholar]
- Min J, Kim J-H, Kim S. Microfluidic device for bio analytical systems. Biotechnol Bioprocess Eng. 2004;9:100–106. doi: 10.1007/BF02932991. [DOI] [Google Scholar]
- Moerland CP, Van Ijzendoorn LJ, Prins MWJ. Rotating magnetic particles for lab-on-chip applications-a comprehensive review. Lab Chip. 2019;19:919–933. doi: 10.1039/C8LC01323C. [DOI] [PubMed] [Google Scholar]
- Moreno S, Baniasadi M, Mohammed S, et al. Biocompatible collagen films as substrates for flexible implantable electronics. Adv Electron Mater. 2015;1:1–8. doi: 10.1002/aelm.201500154. [DOI] [Google Scholar]
- Moschou D, Tserepi A. The lab-on-PCB approach: tackling the μTAS commercial upscaling bottleneck. Lab Chip. 2017;17:1388–1405. doi: 10.1039/c7lc00121e. [DOI] [PubMed] [Google Scholar]
- Moya A, Gabriel G, Villa R, Javier del Campo F. Inkjet-printed electrochemical sensors. Curr Opin Electrochem. 2017;3:29–39. doi: 10.1016/j.coelec.2017.05.003. [DOI] [Google Scholar]
- Muthukumar R, Kapoor A, Balasubramanian S, et al. Detection of adulteration in sunflower oil using paper-based microfluidic lab-on-a-chip devices. Mater Today Proc. 2020;34:496–501. doi: 10.1016/j.matpr.2020.03.099. [DOI] [Google Scholar]
- Nagabooshanam S, Sharma S, Roy S, et al. Development of field deployable sensor for detection of pesticide from food chain. IEEE Sens J XX. 2020;21:4129–4134. doi: 10.1109/jsen.2020.3030034. [DOI] [Google Scholar]
- Nelis JLD, Tsagkaris AS, Dillon MJ, et al. Smartphone-based optical assays in the food safety field. TrAC - Trends Anal Chem. 2020;129:1–13. doi: 10.1016/j.trac.2020.115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Zhang Y, Lin L, et al. Low-cost fabrication of paper-based microfluidic devices by one-step plotting. Anal Chem. 2012;84:6331–6335. doi: 10.1021/ac203496c. [DOI] [PubMed] [Google Scholar]
- Oliveira TMBF, Fátima Barroso M, Morais S, et al. Biosensor based on multi-walled carbon nanotubes paste electrode modified with laccase for pirimicarb pesticide quantification. Talanta. 2013;106:137–143. doi: 10.1016/j.talanta.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Oliverira (2015) Mobile health technologies: Methods and protocols. Mob Heal Technol Methods Protoc 1256:1–496. 10.1007/978-1-4939-2172-0
- Van Oordt T, Barb Y, Zengerle R, Von Stetten F (2011) Miniature stick-packaging - An industrial technology for pre-storage and release of reagents in lab-on-a-chip systems. 15th Int Conf Miniaturized Syst Chem Life Sci 2011, MicroTAS 2011 1:437–439 10.1039/C3LC50404B [DOI] [PubMed]
- Orduña-Malea E, López-Cózar ED. Dimensions: re-discovering the ecosystem of scientific information. Prof La Inf. 2018;27:420–431. doi: 10.3145/epi.2018.mar.21. [DOI] [Google Scholar]
- Papamatthaiou S, Zupancic U, Kalha C, et al. Ultra stable, inkjet-printed pseudo reference electrodes for lab-on-chip integrated electrochemical biosensors. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-74340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons K, Brown L, Clark H, et al. Gluten cross-contact from common food practices and preparations. Clin Nutr. 2020;40:3279–3287. doi: 10.1016/j.clnu.2020.10.053. [DOI] [PubMed] [Google Scholar]
- Peli Thanthri SH, Ward CL, Cornejo MA, Linz TH. Simultaneous preconcentration and separation of native protein variants using thermal gel electrophoresis. Anal Chem. 2020;92:6741–6747. doi: 10.1021/acs.analchem.0c00876. [DOI] [PubMed] [Google Scholar]
- Phiphatanaphiphop C, Leksakul K, Phatthanakun R, et al. Multiwalled carbon nanotubes in microfluidic chip for the separation of X- And Y-sperm based on a photolithography technique. J Microelectromech Syst. 2020;29:1264–1277. doi: 10.1109/JMEMS.2020.3020130. [DOI] [Google Scholar]
- Poenar DP. Microfluidic and micromachined/MEMS devices for separation, discrimination and detection of airborne particles for pollution monitoring. Micromachines. 2019;10:1–34. doi: 10.3390/mi10070483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltronieri P, Cimaglia F, De Lorenzis E, et al. Protein chips for detection of Salmonella spp. from enrichment culture. Sens (Switzerland) 2016;16:1–13. doi: 10.3390/s16040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnuchamy M, Kapoor A, Pakkirisamy B, et al. Optimization, equilibrium, kinetic and thermodynamic studies on adsorptive remediation of phenol onto natural guava leaf powder. Environ Sci Pollut Res. 2020;27:20576–20597. doi: 10.1007/s11356-019-07145-z. [DOI] [PubMed] [Google Scholar]
- Ponnuchamy M, Kapoor A, Senthil P, et al. Sustainable adsorbents for the removal of pesticides from water : a review. Environ Chem Lett. 2021;19:2425–2463. doi: 10.1007/s10311-021-01183-1. [DOI] [Google Scholar]
- Puangbanlang C, Sirivibulkovit K, Nacapricha D, Sameenoi Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta. 2019;198:542–549. doi: 10.1016/j.talanta.2019.02.048. [DOI] [PubMed] [Google Scholar]
- Rasmi Y, Li X, Khan J, et al. Emerging point-of-care biosensors for rapid diagnosis of COVID-19: current progress, challenges, and future prospects. Anal Bioanal Chem. 2021;413:4137–4159. doi: 10.1007/s00216-021-03377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi R, Prakash M, Bhat KK. Characterization of aroma active compounds of cumin (Cuminum cyminum L.) by GC-MS, E-Nose, and sensory techniques. Int J Food Prop. 2013;16:1048–1058. doi: 10.1080/10942912.2011.576356. [DOI] [Google Scholar]
- Reyes PI, Li J, Duan Z, et al. ZnO surface acoustic wave sensors built on zein-coated flexible food packages. Sens Lett. 2013;11:539–544. doi: 10.1166/sl.2013.2822. [DOI] [Google Scholar]
- Rios A, Zougagh M, Avila M. Miniaturization through lab-on-a- chip: Utopia or reality for routine laboratories ? A review. Anal Chim Acta. 2018;740:1–10. doi: 10.1016/j.aca.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Romao VC, Martins SAM, Germano J, et al. Lab-on-Chip devices: gaining ground losing size. ACS Nano. 2017;11:10659–10664. doi: 10.1021/acsnano.7b06703. [DOI] [PubMed] [Google Scholar]
- Ross GMS, Bremer MGEG, Nielen MWF. Consumer-friendly food allergen detection: moving towards smartphone-based immunoassays. Anal Bioanal Chem. 2018;410:5353–5371. doi: 10.1007/s00216-018-0989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat M, Shafii MB, Ghassemi M. Numerical investigation on mixing intensification of ferrofluid and deionized water inside a microchannel using magnetic actuation generated by embedded microcoils for lab-on-chip systems. Chem Eng Process - Process Intensif. 2020;147:107727. doi: 10.1016/j.cep.2019.107727. [DOI] [Google Scholar]
- Sanchiz Á, Ballesteros I, Marqués E, et al. Evaluation of locked nucleic acid and TaqMan probes for specific detection of cashew nut in processed food by real time PCR. Food Control. 2018;89:227–234. doi: 10.1016/j.foodcont.2018.02.021. [DOI] [Google Scholar]
- Santos PDM, Widmer KW, Rivera WL. PCR-based detection and serovar identification of Salmonella in retail meat collected from wet markets in Metro Manila, Philippines. PLoS ONE. 2020;15:1–17. doi: 10.1371/journal.pone.0239457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan A, Kumar PS, Hemavathy RV, et al. Methods of detection of food-borne pathogens: a review. Environ Chem Lett. 2021;19:189–207. doi: 10.1007/s10311-020-01072-z. [DOI] [Google Scholar]
- Sayad AA, Ibrahim F, Uddin SM, et al. A microfluidic lab-on-a-disc integrated loop mediated isothermal amplification for foodborne pathogen detection. Sens Actuat, B Chem. 2016;227:600–609. doi: 10.1016/j.snb.2015.10.116. [DOI] [Google Scholar]
- Shahdeo D, Roberts A, Abbineni N, Gandhi S. Graphene based sensors. Compr Anal Chem. 2020;91:175–199. doi: 10.1016/bs.coac.2020.08.007. [DOI] [Google Scholar]
- Shaibani PM, Etayash H, Jiang K, et al. Portable nanofiber-light addressable potentiometric sensor for rapid escherichia coli detection in orange juice. ACS Sens. 2018;3:815–822. doi: 10.1021/acssensors.8b00063. [DOI] [PubMed] [Google Scholar]
- Sher M, Zhuang R, Demirci U, Asghar W. Paper-based analytical devices for clinical diagnosis: recent advances in the fabrication techniques and sensing mechanisms. Expert Rev Mol Diagn. 2017;17:351–366. doi: 10.1080/14737159.2017.1285228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Singh P, Karmakar M, et al. The journal coverage of web of science, Scopus and dimensions: a comparative analysis. Scientometrics. 2021;126:5113–5142. doi: 10.1007/s11192-021-03948-5. [DOI] [Google Scholar]
- Sitanurak J, Fukana N, Wongpakdee T, Thepchuay Y. Talanta T-shirt ink for one-step screen-printing of hydrophobic barriers for 2D- and 3D-microfluidic paper-based analytical devices. Talanta. 2019;205:120113. doi: 10.1016/j.talanta.2019.120113. [DOI] [PubMed] [Google Scholar]
- Sobhan A, Oh JH, Park MK, Lee J. Reusability of a single-walled carbon nanotube-based biosensor for detecting peanut allergens and Y enterocolitica. Microelectron Eng. 2020;225:111281. doi: 10.1016/j.mee.2020.111281. [DOI] [Google Scholar]
- Sri Sruthi P, Balasubramanian S, Senthil Kumar P, et al. Eco-friendly pH detecting paper-based analytical device: towards process intensification. Anal Chim Acta. 2021;1182:338953. doi: 10.1016/j.aca.2021.338953. [DOI] [PubMed] [Google Scholar]
- Sridhar A, Kapoor A, Kumar PS, et al. Conversion of food waste to energy : a focus on sustainability and life cycle assessment. Fuel. 2021;302:121069. doi: 10.1016/j.fuel.2021.121069. [DOI] [Google Scholar]
- Sridhar A, Ponnuchamy M, Kumar PS, et al. Techniques and modeling of polyphenol extraction from food: a review. Environ Chem Lett. 2021;19:3409–3443. doi: 10.1007/s10311-021-01217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar A, Ponnuchamy M, Kumar PS, Kapoor A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: a review. Environ Chem Lett. 2021;19:1715–1735. doi: 10.1007/s10311-020-01126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar A, Kannan D, Kapoor A, Prabhakar S. Extraction and detection methods of microplastics in food and marine systems: a critical review. Chemosphere. 2022;286:131–653. doi: 10.1016/j.chemosphere.2021.131653. [DOI] [PubMed] [Google Scholar]
- Stahlschmidt S, Stephen D (2021) From indexation policies through citation networks to normalized citation impacts : Web of Science , Scopus , and Dimensions as varying resonance chambers. Cornell arXiv 1–17
- Sun Y, Quyen TL, Hung TQ, et al. A lab-on-a-chip system with integrated sample preparation and loop-mediated isothermal amplification for rapid and quantitative detection of Salmonella spp. in food samples. Lab Chip. 2015;15:1898–1904. doi: 10.1039/c4lc01459f. [DOI] [PubMed] [Google Scholar]
- Tao H, Brenckle MA, Yang M, et al. Silk-based conformal, adhesive, edible food sensors. Adv Mater. 2012;24:1067–1072. doi: 10.1002/adma.201103814. [DOI] [PubMed] [Google Scholar]
- Teymourian H, Barfidokht A, Wang J. Electrochemical glucose sensors in diabetes management: an updated review (2010–2020) Chem Soc Rev. 2020;49:7671–7709. doi: 10.1039/d0cs00304b. [DOI] [PubMed] [Google Scholar]
- Trofimchuk E, Hu Y, Nilghaz A, et al. Development of paper-based microfluidic device for the determination of nitrite in meat. Food Chem. 2020;316:126396. doi: 10.1016/j.foodchem.2020.126396. [DOI] [PubMed] [Google Scholar]
- Tsen HY, Shih CM, Teng PH, et al. Detection of Salmonella in chicken meat by insulated isothermal PCR. J Food Prot. 2013;76:1322–1329. doi: 10.4315/0362-028X.JFP-12-553. [DOI] [PubMed] [Google Scholar]
- Ude C, Hentrop T, Lindner P, et al. New perspectives in shake flask pH control using a 3D-printed control unit based on pH online measurement. Sens Actuat, B Chem. 2015;221:1035–1043. doi: 10.1016/j.snb.2015.07.017. [DOI] [Google Scholar]
- Uludag Y, Esen E, Kokturk G, et al. Lab-on-a-chip based biosensor for the real-time detection of aflatoxin. Talanta. 2016;160:381–388. doi: 10.1016/j.talanta.2016.07.060. [DOI] [PubMed] [Google Scholar]
- Valentini L, Bittolo Bon S, Pugno NM. Combining living microorganisms with regenerated silk provides nanofibril-based thin films with heat-responsive wrinkled states for smart food packaging. Nanomaterials. 2018;8:518. doi: 10.3390/nano8070518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck NJ, Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. 2017;111:1053–1070. doi: 10.1007/s11192-017-2300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderroost M, Ragaert P, Devlieghere F, De Meulenaer B. Intelligent food packaging: the next generation. Trends Food Sci Technol. 2014;39:47–62. doi: 10.1016/j.tifs.2014.06.009. [DOI] [Google Scholar]
- Verma S, Gaganjot TJ, et al. Biodegradable photolithography compatible substrate for transparent transient electronics and flexible energy storage devices. Appl Mater Today. 2018;13:83–90. doi: 10.1016/j.apmt.2018.08.010. [DOI] [Google Scholar]
- Viza ND, Harding DR. Performance of different “Lab-On-Chip” geometries for making double emulsions to form polystyrene shells. Fusion Sci Technol. 2018;72:248–257. doi: 10.1080/15361055.2017.1391662. [DOI] [Google Scholar]
- Vu CHT, Won K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chem. 2013;140:52–56. doi: 10.1016/j.foodchem.2013.02.056. [DOI] [PubMed] [Google Scholar]
- Wahyuni H, Vanany I, Ciptomulyono U. Food safety and halal food in the supply chain: review and bibliometric analysis. J Ind Eng Manag. 2019;12:373–391. doi: 10.3926/jiem.2803. [DOI] [Google Scholar]
- Wang W, Wu WY, Wang W, Zhu JJ. Tree-shaped paper strip for semiquantitative colorimetric detection of protein with self-calibration. J Chromatogr A. 2010;1217:3896–3899. doi: 10.1016/j.chroma.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wei X, Li J, et al. Study on nanocellulose by high pressure homogenization in homogeneous isolation. Fibers Polym. 2015;16:572–578. doi: 10.1007/s12221-015-0572-1. [DOI] [Google Scholar]
- Wang R, Tsai W-T, He J, et al. Logistics management system based on permissioned blockchains and RFID technology. Int Conf Comput Netw, Commun Inf Syst. 2019;88:426–432. doi: 10.2991/cnci-19.2019.58. [DOI] [Google Scholar]
- Weng X, Gaur G, Neethirajan S. Rapid detection of food allergens by microfluidics ELISA-Based optical sensor. Biosensors. 2016;6:24. doi: 10.3390/bios6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng X, Luan X, Kong C, et al. A comprehensive method for assessing meat freshness using fusing electronic nose, computer vision, and artificial tactile technologies. J Sens. 2020;2020:1–14. doi: 10.1155/2020/8838535. [DOI] [Google Scholar]
- Wong B, Kasparek E, Robillard A, et al. Improving the longevity of passive microfluidic systems through plasma polymer films with a vertical chemical gradient. Microfluid Nanofluidics. 2020;24:1–8. doi: 10.1007/s10404-020-2324-9. [DOI] [Google Scholar]
- Wongsrichanalai C, Barcus MJ, Muth S, et al. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT) Am J Trop Med Hyg. 2007;77:119–127. doi: 10.4269/ajtmh.2007.77.119. [DOI] [PubMed] [Google Scholar]
- Wu X, Li Y, Liu B, et al. Two-Site antibody immunoanalytical detection of food allergens by surface plasmon resonance. Food Anal Methods. 2016;9:582–588. doi: 10.1007/s12161-015-0232-5. [DOI] [Google Scholar]
- Xia Y, Si J, Li Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: a review. Biosens Bioelectron. 2016;77:774–789. doi: 10.1016/j.bios.2015.10.032. [DOI] [PubMed] [Google Scholar]
- Xu B, Guo J, Fu Y, et al. A review on microfluidics in the detection of food pesticide residues. Electrophoresis. 2020;41:821–832. doi: 10.1002/elps.201900209. [DOI] [PubMed] [Google Scholar]
- Yam P. Intelligent packaging: concepts and applications. J Food Sci. 2005;66:379–379. doi: 10.1111/j.1365-2621.2001.tb16112.x. [DOI] [Google Scholar]
- Yamada K, Henares TG, Suzuki K, Citterio D. Paper-based inkjet-printed microfluidic analytical devices. Angew Chemie - Int Ed. 2015;54:5294–5310. doi: 10.1002/anie.201411508. [DOI] [PubMed] [Google Scholar]
- Yang J, Lee J. Application of sensory descriptive analysis and consumer studies to investigate traditional and authentic foods: a review. Foods. 2019;8:1–17. doi: 10.3390/foods8020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Kim KR, Kim CS. Biosensor for rapid and sensitive detection of influenza virus. Biotechnol Bioprocess Eng. 2018;23:371–382. doi: 10.1007/s12257-018-0220-x. [DOI] [Google Scholar]
- Yoon JY, Kim B. Lab-on-a-chip pathogen sensors for food safety. Sens (Switzerland) 2012;12:10713–10741. doi: 10.3390/s120810713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi H, Ali MM, Su HM, et al. Sentinel wraps: real-time monitoring of food contamination by printing DNAzyme probes on food packaging. ACS Nano. 2018;12:3287–3294. doi: 10.1021/acsnano.7b08010. [DOI] [PubMed] [Google Scholar]
- Zeinhom MMA, Wang Y, Song Y, et al. A portable smart-phone device for rapid and sensitive detection of E. coli O157:H7 in Yoghurt and Egg. Biosens Bioelectron. 2018;99:479–485. doi: 10.1016/j.bios.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yin AX, Jiang R, et al. Time-temperature indicator for perishable products based on kinetically programmable Ag overgrowth on Au nanorods. ACS Nano. 2013;7:4561–4568. doi: 10.1021/nn401266u. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zhu C, Huang Y, et al. A lateral flow strip based aptasensor for detection of Ochratoxin a in corn samples. Molecules. 2018;23:1–12. doi: 10.3390/molecules23020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li G, Wu D, et al. Recent advances in emerging nanomaterials based food sample pretreatment methods for food safety screening. TrAC - Trends Anal Chem. 2019;121:1–71. doi: 10.1016/j.trac.2019.115669. [DOI] [Google Scholar]
- Zhao Y, Zeng D, Yan C, et al. Rapid and accurate detection of: escherichia coli O157:H7 in beef using microfluidic wax-printed paper-based ELISA. Analyst. 2020;145:3106–3115. doi: 10.1039/d0an00224k. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zou M, Hu G. Implementation of a lightweight RFID security authentication protocol system. J Phys Conf Ser. 2020;1549:1–6. doi: 10.1088/1742-6596/1549/4/042038. [DOI] [Google Scholar]
- Zhou J, Qi Q, Wang C, et al. Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens Bioelectron. 2019;142:111449. doi: 10.1016/j.bios.2019.111449. [DOI] [PubMed] [Google Scholar]
- Zhu D, Ren X, Wei L, et al. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci Hortic (Amsterdam) 2020;260:108879. doi: 10.1016/j.scienta.2019.108879. [DOI] [Google Scholar]