Abstract
Objective:
Default frequency filters of cochlear implant (CI) devices assign frequency information irrespective of intracochlear position, resulting in varying degrees of frequency-to-place mismatch. Substantial mismatch negatively influences speech recognition in postlingually deafened CI recipients, and acclimatization may be particularly challenging for older adults due to effects of aging on the auditory pathway. The present report investigated the influence of mismatch and age at implantation on speech recognition within the initial 6 months of CI use.
Study Design:
Retrospective review.
Setting:
Tertiary referral center.
Subjects and Methods:
Forty-eight postlingually deafened adult CI recipients of lateral wall electrode arrays underwent postoperative computed tomography to determine angular insertion depth of each electrode contact. Frequency-to-place mismatch was determined by comparing spiral ganglion place frequencies to default frequency filters. Consonant-nucleus-consonant (CNC) scores in the CI-alone condition at 1, 3, and 6 months post-activation were compared to the degree of mismatch at 1500 Hz and age at implantation.
Results:
Younger adult CI recipients experienced more rapid growth in speech recognition during the initial 6 months post-activation. Greater degrees of frequency-to-place mismatch were associated with poorer performance, yet older listeners were not particularly susceptible to this effect.
Conclusions:
While older adults are not necessarily more sensitive to detrimental effects of frequency-to-place mismatch, other factors appear to limit early benefit with a CI in this population. These results suggest that minimizing mismatch could optimize outcomes in adult CI recipients across the lifespan, which may be particularly beneficial in the elderly considering auditory processing deficits associated with advanced age.
Keywords: Cochlear implant, Frequency-to-place mismatch, Age, Speech recognition
Introduction
Cochlear implant (CI) devices are designed to take advantage of the natural tonotopicity of the cochlea, such that low- to high-frequency information is logarithmically distributed along electrode contacts from the apex to base. Individual differences in cochlear duct length1–3 and differences in electrode array length4 contribute to substantial variability in the angular insertion depth (AID) of each electrode contact. Nonetheless, current default mapping procedures assign electric frequency filters regardless of intracochlear location. The resulting discrepancy between electric frequency information and natural tonotopic organization of the cochlea leads to variable degrees of frequency-to-place mismatch, which is perceived as a spectral degradation in the speech signal through the CI. This is a particularly relevant consideration for postlingually deafened CI recipients who learned speech with a normal frequency-to-place function along the basilar membrane prior to hearing loss and must subsequently learn to use spectrally shifted information.
The human auditory system displays a remarkable degree of plasticity in adapting to tonotopic mismatch over time; nevertheless, there is a large body of evidence to suggest that this spectral shift may prolong the acclimatization period with a CI, and in some cases adaptation remains incomplete even after extensive listening experience5–9. One metric for characterizing deviations from the natural tonotopicity associated with acoustic hearing is the frequency-to-place mismatch at 1500 Hz, the approximate spectral center of important speech information10. The mismatch at 1500 Hz has been shown to negatively correlate with speech recognition during the initial 6 months of CI device use11.
Advanced age at implantation may further compromise the ability to acclimatize to frequency-to-place mismatch. While cochlear implantation is clearly a viable and effective treatment option for sensorineural hearing loss in the elderly12,13, older CI recipients take longer to reach asymptotic performance than young adults14, which may be related to higher rates of cognitive impairment in the older cohort. Interpreting degraded input provided by the CI requires top-down processing15, a cognitive function that declines with advancing age16. This decline can adversely impact postoperative speech recognition17–19. Additionally, it is possible that CI recipients rely more on temporal cues compared to those with normal hearing when listening to spectrally degraded speech20. Temporal processing declines with advanced age21, and temporal processing deficits could degrade older adults’ ability to recognize speech presented with a large frequency-to-place mismatch22.
Despite the above findings highlighting the effects of aging on auditory processing abilities, controversy exists regarding the impact of age on speech recognition in postlingually deafened CI recipients. While several studies have consistently demonstrated poorer performance in older adults compared to young adults23–27, others indicate no age effect28,29. In light of conflicting results in prior literature, it is possible that differences in frequency-to-place mismatch across devices and populations of CI users could play a role in the age effects that are sometimes observed. The primary aim of the present study was to investigate whether older CI recipients have more difficulty adapting to spectrally shifted information provided with frequency-to-place mismatch than younger adult CI recipients.
Methods
Subjects
The Biomedical Institutional Review Board at the University of North Carolina approved the retrospective assessment of eligible subjects from a prospectively collected database (protocol 19–2328). The database was queried for postlingually deafened adults listening in the CI-alone condition who: 1) received a MED-EL GmbH (Innsbruck, Austria) Flex24 (24 mm), Flex28 (28 mm), or FlexSOFT/Standard (31.5 mm) electrode array, 2) underwent postoperative high-resolution temporal bone cone-beam computed tomography (CT), and 3) completed speech recognition assessment at 1, 3, and 6 months post-activation. To minimize confounding variables, patients with cochlear malformations, partial electrode array insertions, revision surgery, mapping deviations from the default frequency filters, or incomplete speech recognition data were excluded from the study. A partial insertion was defined as having at least one extracochlear electrode contact on review of imaging.
Measurement of Angular Insertion Depth
The postoperative CT was analyzed with OTOPLAN®, an otologic imaging analysis tool developed by CAScination AG (Bern, Switzerland) in collaboration with MED-EL, as previously described30. In short, a user-defined cochlear coordinate system is used to identify the location of the modiolus, round window, and individual electrode contacts in the cochlear view, which are subsequently used to determine the AID of each electrode contact. These values support derivation of the spiral ganglion (SG) place frequency for each contact, as described by Stakhovskaya et al. (2007)31.
Degree of Frequency-to-Place Mismatch at 1500 Hz
To quantify the extent of frequency-to-place mismatch, we first determined the difference between the center frequency associated with each electrode contact and the associated SG place frequency. The center frequencies for the default frequency filters were obtained for each subject from the clinical mapping software (MED-EL, Maestro, version 7), with all CI recipients mapped with a default frequency range of either 70- or 100–8500 Hz. Second, a fourth-order polynomial function was fit to the semitone deviation from the SG map as a function of AID for each subject. Lastly, the absolute value of the frequency deviation in semitones was estimated at 1500 Hz (approximately 267° on the SG map) based on these fits.
Postoperative Speech Recognition
Speech recognition was assessed with the consonant-nucleus-consonant (CNC) word test32 at 1, 3, and 6 months in the CI-alone condition, which was the familiar listening condition for all subjects. Recorded materials were presented at 60 dB SPL in a soundproof booth with the patient seated 1 meter away from the sound source. Percent correct scores were transformed into rationalized arcsine units (RAUs) to normalize error variance33. Speech recognition was compared to frequency-to-place mismatch at 1500 Hz and age at implantation.
Statistical Analysis
Pearson correlations were used to assess the relationship between speech recognition, degree of mismatch, and age. A linear mixed model evaluated trends in speech recognition over time, using the R statistical software34. A one-way analysis of variance (ANOVA) followed by a Tukey test for honestly significant differences (HSD) was performed for analyses of categorical variables. Statistical analyses were performed with SPSS version 25 for Windows (IBM Corp, Armonk, New York) and significance was defined as P < .05.
Results
Subject Demographics
A summary of subject demographics for the 48 CI recipients listening with a CI-alone device is shown in Table 1. Fifty-two percent of the subjects were male. Age at implantation ranged from 42 to 95 years, with a mean of 67.4 years (SD: 13.4 years). Of the 48 subjects, 2 were implanted with a Flex24 (4.2%), 21 with a Flex28 (43.7%) and 25 with a FlexSOFT/Standard (52.1%) electrode array.
Table 1.
Subject demographics.
| Variable | All Patients (n = 48) |
|---|---|
| Sex | |
| Female | 23 (47.9%) |
| Male | 25 (52.1%) |
| Age, mean (range), years | 67.4 (42–95) |
| Device | |
| Flex24 | 2 (4.2%) |
| Flex28 | 21 (43.7%) |
| FlexSOFT/Standard | 25 (52.1%) |
| Absolute frequency-to-place mismatcha, mean (range), semitones | 4.7 (0.22–11.56) |
Quantified at 1500 Hz (approximately 267° on the spiral ganglion map)
Angular Insertion Depth
Based on postoperative CT, the mean AID of the most apical electrode contact for the entire cohort was 597° ± 72.0° (range, 407° to 751°), with electrode-specific values of 464° ± 20.5° for Flex24, 570° ± 71.3° for Flex28, and 630° ± 51.2° for FlexSOFT/Standard arrays. A one-way ANOVA demonstrated a statistically significant difference in AID of the most apical electrode contact across the three array types (P = .001), and a post hoc Tukey HSD analysis revealed significant differences for all three pairwise comparisons between arrays (P < .05).
Frequency-to-Place Mismatch
The absolute semitone deviation from the SG map at 1500 Hz was quantified for each subject (one octave is equal to 12 semitones). The mean deviation for all subjects was 4.7 ± 2.7 semitones. As expected, shorter arrays were associated with a greater frequency-to-place mismatch, with electrode-specific values of 9.9 ± 0.9 for Flex24, of 5.0 ± 3.0 for Flex28 and 4.0 ± 2.0 for FlexSOFT/Standard arrays. Absolute frequency deviation in semitones differed significantly across arrays on ANOVA (P = .007). A post hoc Tukey HSD analysis demonstrated significant differences between Flex24 and Flex28 arrays (P = .028) and between Flex24 and FlexSOFT/Standard arrays (P = .006). No significant difference was noted when comparing Flex28 to FlexSOFT/Standard arrays (P = .368).
Postoperative Speech Recognition
The mean percent correct for CNC word recognition was 32.1 ± 18.0% at 1 month, 46.9 ± 18.6% at 3 months, and 50.8 ± 18.7% at 6 months. Electrode-specific mean CNC scores at 6 months were 19.0 ± 9.9% for Flex24, 46.5 ± 20.3% for Flex28, and 56.3 for ± 13.3% FlexSOFT/Standard arrays. Word scores were transformed to RAUs for subsequent statistical analyses. The top row of Figure 1 shows speech recognition scores at 1, 3, and 6 months post-activation as a function of age at implantation. While there was no correlation between age and CNC scores at 1 month (r = .032, P = .828), a significant negative correlation was noted at 3 months (r = −.339, P = .019) and 6 months (r = −.423, P = .003). The bottom row of Figure 1 shows speech recognition scores at each test interval as a function of the absolute frequency-to-place mismatch at 1500 Hz. The mismatch at 1500 Hz negatively correlated with CNC scores at 1 month (r = −.338, P = .019), 3 months (r = −.304, P = .036), and 6 months (r = −.394, P = .006). Correlations between CNC scores and frequency-to-place mismatch appear to be driven by the 5 subjects with the greatest mismatch. Recalculating correlations without these subjects indicated no significant effects, which could indicate that listeners are tolerant of mismatches < 7 semitones.
Figure 1.
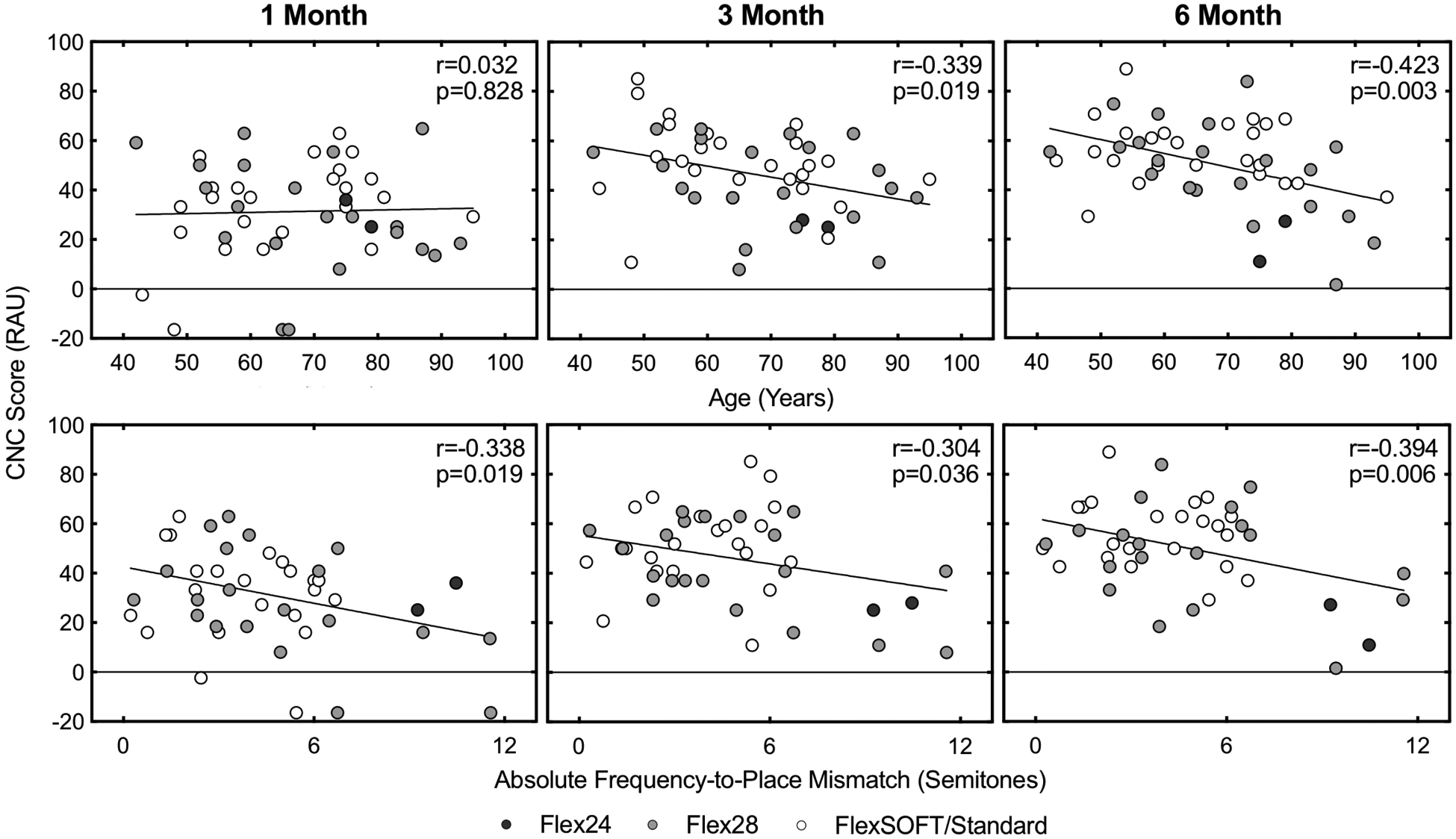
CNC word scores as a function of age at implantation (top row), and absolute frequency-to-place mismatch at 1500 Hz on the SG map (bottom row). Abbreviations: SG, spiral ganglion; CNC, consonant-nucleus-consonant.
Table 2 reports a linear mixed model predicting CNC word scores with the independent variables of frequency-to-place mismatch, interval, age, and the interaction between age and interval; subject was included in this model as a random factor to accommodate repeated measures. Speech recognition improved over test intervals (P < .001), and frequency-to-place mismatch negatively affected performance (P = .007). There was a significant interaction between age at implantation and test interval (P = .002), reflecting the observation of more marked improvement across test intervals in younger listeners than older listeners. Including an interaction between mismatch and age at implantation into the model indicated no significant change in susceptibility to mismatch with age (P = .617). Restricting the analysis to the 6-month test interval also failed to reveal a significant interaction between mismatch and age (P = .147). These results suggest that both older age at implantation and frequency-to-place mismatch negatively influence early speech recognition abilities, but there is no indication that older adults are more susceptible to the detrimental effects of mismatch.
Table 2.
Linear mixed model evaluating the effects of frequency-to-place mismatch, test interval (1, 3, 6 months), and age at implantation (in years). Subject was included as a random effect.
| Coef. | SE | t | P value | |
|---|---|---|---|---|
| (Intercept) | 32.61 | 13.64 | 2.39 | 0.019 |
| Mismatcha | −2.15 | 0.76 | −2.84 | 0.007 |
| Interval | 11.41 | 2.47 | 4.61 | <0.001 |
| Age | 0.12 | 0.20 | 0.59 | 0.556 |
| Age:Interval | −0.11 | 0.04 | −3.19 | 0.002 |
Quantified at 1500 Hz (approximately 267° on the spiral ganglion map)
Discussion
Despite substantial advances in the field of cochlear implantation, predicting postoperative speech recognition with a CI remains an elusive task35–39. Although there are numerous factors that correlate with performance (see Holden et al., 201340), the focus here was on the trend for better outcomes for patients with a younger age at implantation23–27 and smaller frequency-to-place mismatch11. In general, older listeners tend to be more detrimentally affected by signal degradation, perhaps due to poorer temporal processing abilities and reduced cognitive function. Results from the present study support the idea that both advanced age at cochlear implantation and greater degrees of frequency-to-place mismatch negatively influence early speech recognition, yet older listeners are not necessarily more susceptible to detrimental effects associated with larger mismatches.
These findings are generally consistent with prior studies demonstrating that older CI recipients perform worse than younger peers with respect to speech recognition24. However, the finding that age did not affect the ability to adapt to mismatch was somewhat unexpected. Previous vocoder simulation experiments have shown that normal-hearing listeners rely on temporal cues when listening to spectrally degraded speech20. Furthermore, aging has been shown to reduce the ability to utilize spectral information and temporal cues in speech segments22. As such, we initially hypothesized that a reduction in mismatch – providing improved spectral cues – would benefit older CI recipients more than younger recipients.
The present study demonstrated that speech recognition abilities amongst younger listeners improved rapidly, while older CI recipients displayed a slower rate of growth, a finding that could be related to their reduced temporal processing abilities and/or cognitive capacity. The trend observed for younger listeners to improve more quickly in the initial 6 months of CI use supports the role of plasticity in the acclimatization to spectrally degraded speech signals. While the present study did not demonstrate a significant difference between the acclimatization to frequency-to-place mismatch in younger and older CI recipients, there are reasons not to exclude this possibility. Speech recognition was quantified in the present study using CNC words. These stimuli are simple and relatively short. In contrast, sentence recognition, particularly masked sentence recognition, would be expected to place greater demands on working memory capacity41, and could reveal deficits related to diminished cognitive processing capacity. Given the marked heterogeneity of the CI population, clinically relevant effects may not be evident even for a study sample of 48 patients. Ultimately, future work is still warranted to fully understand the detrimental effects of frequency-to-place mismatch as a function of listener age.
While age is a non-modifiable factor, frequency-to-place mismatch can be controlled to some degree when selecting the electrode array to be implanted and mapping the CI device. Frequency-to-place mismatch is typically small with a fully inserted long lateral wall array and default frequency filters; this is evident in the trend for less mismatch with longer arrays in the present study. For traditional CI candidates with severe-to-profound sensorineural hearing loss destined for the CI-alone condition with a lateral wall electrode array, the authors generally advocate for use of an individualized approach in determining cochlear duct length to select the longest array necessary to achieve maximal cochlear coverage. In the case of an individual with a small cochlea, a 28 mm array may be preferred over a 31.5 mm array to avoid extracochlear electrodes. This approach is supported by prior work demonstrating better speech recognition with deeply inserted lateral wall arrays in the CI-alone condition27,42–46; one potential mechanism for this benefit may be closer tonotopic alignment with the default frequency filters11. Although this evidence supports the use of long lateral wall arrays to reduce frequency-to-place mismatch for traditional CI candidates, the optimal array for the growing population of candidates with residual hearing in the implanted ear is more challenging as these patients may gain substantial benefit from electric-acoustic stimulation (EAS) devices47–55. Shorter arrays generally maximize hearing preservation with less trauma to the apical region of the cochlea with residual low-frequency hearing56–58; however, for cases in which hearing is lost with a short array, recipients must adapt to substantial frequency-to-place mismatch with a CI-alone device. In this scenario, a possible recourse to more closely align frequency information with the natural tonotopic organization of the cochlea would be to shift frequency filters of individual electrode contacts.
Advances in post-implantation imaging with CT have allowed for an accurate assessment of AID, which can be used to derive an estimated cochlear place frequency for each electrode contact based on the distribution of SG cells31. Preliminary investigations of place-based mapping procedures differ in approach, but generally aim to align electric frequency filters with the tonotopic organization of the cochlea59,60. In theory, place-based mapping may facilitate growth in speech recognition by limiting the need to acclimate to a spectrally-shifted signal. The individualized approach to programming could be particularly important in the elderly population, to counteract age-related deficits in auditory processing. Studies are ongoing to assess this strategy.
Limitations of this study include its retrospective nature, lack of data assessing cognitive function, and the focus on lateral wall arrays. Cognitive abilities have been shown to mediate the effects of aging on speech recognition19, and future studies will additionally determine the influence of these factors. Future studies should address the ability to adapt to frequency-to-place mismatch as a function of array design (i.e., pre-curved versus lateral wall). Another consideration is the fact that speech recognition was only evaluated through 6 months post-activation; while this is the interval associated with asymptotic speech recognition in young adults61, older adults may require several years to reach asymptotic performance14. Long-term studies are required to determine if older CI recipients overcome the observed deficit in speech recognition ability with extended listening experience.
Conclusions
Both advanced age at cochlear implantation and frequency-to-place mismatch negatively influence early speech recognition, as assessed by CNC words in quiet, yet older adults are not necessarily more sensitive to the detrimental effects of mismatch. Older CI recipients generally perform worse in the initial 6 months of device use, and when combined with frequency-to-place mismatch these individuals may be challenged by poor performance. Reducing mismatch may optimize speech recognition outcomes for CI recipients across the lifespan, yet this consideration may be particularly relevant for older listeners who also experience auditory processing deficits.
Acknowledgements
This work was funded in part by the NIH through NIDCD (T32 DC005360). A portion of this dataset was presented at the 2019 Conference on Implantable Auditory Prostheses in Lake Tahoe, CA, July 14-19, 2019 and is under consideration by Ear and Hearing in “Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients.”
Footnotes
This article was presented at the 2019 AAO-HNSF Annual Meeting and OTO Experience; September 15–18, 2019; New Orleans, Louisiana.
References
- 1.Hardy M. The length of the organ of corti in man. Am J Anat. 1938;63:291–311. [Google Scholar]
- 2.Wurfel W, Lanfermann H, Lenarz T, Majdani O. Cochlear length determination using Cone Beam Computed Tomography in a clinical setting. Hear Res. 2014;316:65–72. [DOI] [PubMed] [Google Scholar]
- 3.Meng J, Li S, Zhang F, Li Q, Qin Z. Cochlear Size and Shape Variability and Implications in Cochlear Implantation Surgery. Otol Neurotol. 2016;37(9):1307–1313. [DOI] [PubMed] [Google Scholar]
- 4.Dhanasingh A, Jolly C. An overview of cochlear implant electrode array designs. Hear Res. 2017;356:93–103. [DOI] [PubMed] [Google Scholar]
- 5.Svirsky MA, Fitzgerald MB, Sagi E, Glassman EK. Bilateral cochlear implants with large asymmetries in electrode insertion depth: implications for the study of auditory plasticity. Acta Otolaryngol. 2015;135(4):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svirsky MA, Silveira A, Neuburger H, Teoh SW, Suarez H. Long-term auditory adaptation to a modified peripheral frequency map. Acta Otolaryngol. 2004;124(4):381–386. [PubMed] [Google Scholar]
- 7.Sagi E, Fu QJ, Galvin JJ 3rd, Svirsky MA. A model of incomplete adaptation to a severely shifted frequency-to-electrode mapping by cochlear implant users. J Assoc Res Otolaryngol. 2010;11(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiss LA, Turner CW, Karsten SA, Gantz BJ. Plasticity in human pitch perception induced by tonotopically mismatched electro-acoustic stimulation. Neuroscience. 2014;256:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan CT, Martin B, Svirsky MA. Pitch Matching between Electrical Stimulation of a Cochlear Implant and Acoustic Stimuli Presented to a Contralateral Ear with Residual Hearing. J Am Acad Audiol. 2017;28(3):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ANSI/ASA. American National Standard Methods for Calculation of the Speech Intelligibility Index. 1997;American National Standards Institute, New York:S3.5. [Google Scholar]
- 11.Canfarotta MW, Dillon MT, Buss E, Pillsbury HC, Brown KD, O’Connell BP. Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear Hear. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labadie RF, Carrasco VN, Gilmer CH, Pillsbury HC 3rd. Cochlear implant performance in senior citizens. Otolaryngol Head Neck Surg. 2000;123(4):419–424. [DOI] [PubMed] [Google Scholar]
- 13.Lundin K, Nasvall A, Kobler S, Linde G, Rask-Andersen H. Cochlear implantation in the elderly. Cochlear Implants Int. 2013;14(2):92–97. [DOI] [PubMed] [Google Scholar]
- 14.Dillon MT, Buss E, Adunka MC, et al. Long-term speech perception in elderly cochlear implant users. JAMA Otolaryngol Head Neck Surg. 2013;139(3):279–283. [DOI] [PubMed] [Google Scholar]
- 15.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24(3):761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54(1–3):35–54. [DOI] [PubMed] [Google Scholar]
- 17.Lyxell B, Andersson J, Arlinger S, Bredberg G, Harder H, Ronnberg J. Verbal information-processing capabilities and cochlear implants: implications for preoperative predictors of speech understanding. J Deaf Stud Deaf Educ. 1996;1(3):190–201. [DOI] [PubMed] [Google Scholar]
- 18.Heydebrand G, Hale S, Potts L, Gotter B, Skinner M. Cognitive predictors of improvements in adults’ spoken word recognition six months after cochlear implant activation. Audiol Neurootol. 2007;12(4):254–264. [DOI] [PubMed] [Google Scholar]
- 19.Moberly AC, Vasil KJ, Wucinich TL, et al. How does aging affect recognition of spectrally degraded speech? Laryngoscope. 2018;128 Suppl 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winn MB, Chatterjee M, Idsardi WJ. The use of acoustic cues for phonetic identification: effects of spectral degradation and electric hearing. J Acoust Soc Am. 2012;131(2):1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grose JH, Hall JW 3rd, Buss E. Temporal processing deficits in the pre-senescent auditory system. J Acoust Soc Am. 2006;119(4):2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goupell MJ, Gaskins CR, Shader MJ, Walter EP, Anderson S, Gordon-Salant S. Age-Related Differences in the Processing of Temporal Envelope and Spectral Cues in a Speech Segment. Ear Hear. 2017;38(6):e335–e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatelin V, Kim EJ, Driscoll C, et al. Cochlear implant outcomes in the elderly. Otol Neurotol. 2004;25(3):298–301. [DOI] [PubMed] [Google Scholar]
- 24.Beyea JA, McMullen KP, Harris MS, et al. Cochlear Implants in Adults: Effects of Age and Duration of Deafness on Speech Recognition. Otol Neurotol. 2016;37(9):1238–1245. [DOI] [PubMed] [Google Scholar]
- 25.Williamson RA, Pytynia K, Oghalai JS, Vrabec JT. Auditory performance after cochlear implantation in late septuagenarians and octogenarians. Otol Neurotol. 2009;30(7):916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin FR, Chien WW, Li L, Clarrett DM, Niparko JK, Francis HW. Cochlear implantation in older adults. Medicine (Baltimore). 2012;91(5):229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connell BP, Cakir A, Hunter JB, et al. Electrode Location and Angular Insertion Depth Are Predictors of Audiologic Outcomes in Cochlear Implantation. Otol Neurotol. 2016;37(8):1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson ML, Breen JT, Gifford RH, et al. Cochlear implantation in the octogenarian and nonagenarian. Otol Neurotol. 2010;31(8):1343–1349. [DOI] [PubMed] [Google Scholar]
- 29.Park E, Shipp DB, Chen JM, Nedzelski JM, Lin VY. Postlingually deaf adults of all ages derive equal benefits from unilateral multichannel cochlear implant. J Am Acad Audiol. 2011;22(10):637–643. [DOI] [PubMed] [Google Scholar]
- 30.Canfarotta MW, Dillon MT, Buss E, Pillsbury HC, Brown KD, O’Connell BP. Validating a New Tablet-Based Tool in the Determination of Cochlear Implant Angular Insertion Depth. Otol Neurotol. 2019;40(8):1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stakhovskaya O, Sridhar D, Bonham BH, Leake PA. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol. 2007;8(2):220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. [DOI] [PubMed] [Google Scholar]
- 33.Studebaker GA. A “rationalized” arcsine transform. J Speech Hear Res. 1985;28(3):455–462. [DOI] [PubMed] [Google Scholar]
- 34.R, Core, Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. [Google Scholar]
- 35.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol. 1996;1(5):293–306. [DOI] [PubMed] [Google Scholar]
- 36.Blamey P, Artieres F, Baskent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol. 2013;18(1):36–47. [DOI] [PubMed] [Google Scholar]
- 37.Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102(12):909–916. [DOI] [PubMed] [Google Scholar]
- 38.Green KM, Bhatt Y, Mawman DJ, et al. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants Int. 2007;8(1):1–11. [DOI] [PubMed] [Google Scholar]
- 39.Lazard DS, Vincent C, Venail F, et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One. 2012;7(11):e48739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34(3):342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winn M. Rapid Release From Listening Effort Resulting From Semantic Context, and Effects of Spectral Degradation and Cochlear Implants. Trends Hear. 2016;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hochmair I, Arnold W, Nopp P, Jolly C, Muller J, Roland P. Deep electrode insertion in cochlear implants: apical morphology, electrodes and speech perception results. Acta Otolaryngol. 2003;123(5):612–617. [PubMed] [Google Scholar]
- 43.Yukawa K, Cohen L, Blamey P, Pyman B, Tungvachirakul V, O’Leary S. Effects of insertion depth of cochlear implant electrodes upon speech perception. Audiol Neurootol. 2004;9(3):163–172. [DOI] [PubMed] [Google Scholar]
- 44.Buchman CA, Dillon MT, King ER, Adunka MC, Adunka OF, Pillsbury HC. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35(10):1773–1779. [DOI] [PubMed] [Google Scholar]
- 45.Chakravorti S, Noble JH, Gifford RH, et al. Further Evidence of the Relationship Between Cochlear Implant Electrode Positioning and Hearing Outcomes. Otol Neurotol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchner A, Illg A, Majdani O, Lenarz T. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One. 2017;12(5):e0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14 Suppl 1:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn CC, Perreau A, Gantz B, Tyler RS. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol. 2010;21(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helbig S, Van de Heyning P, Kiefer J, et al. Combined electric acoustic stimulation with the PULSARCI(100) implant system using the FLEX(EAS) electrode array. Acta Otolaryngol. 2011;131(6):585–595. [DOI] [PubMed] [Google Scholar]
- 50.Gifford RH, Dorman MF. The Psychophysics of Low-Frequency Acoustic Hearing in Electric and Acoustic Stimulation (EAS) and Bimodal Patients. J Hear Sci. 2012;2(2):33–44. [PMC free article] [PubMed] [Google Scholar]
- 51.Gifford RH, Dorman MF, Sheffield SW, Teece K, Olund AP. Availability of binaural cues for bilateral implant recipients and bimodal listeners with and without preserved hearing in the implanted ear. Audiol Neurootol. 2014;19(1):57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gifford RH, Dorman MF, Skarzynski H, et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear. 2013;34(4):413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rader T, Fastl H, Baumann U. Speech perception with combined electric-acoustic stimulation and bilateral cochlear implants in a multisource noise field. Ear Hear. 2013;34(3):324–332. [DOI] [PubMed] [Google Scholar]
- 54.Dillon MT, Buss E, Adunka OF, Buchman CA, Pillsbury HC. Influence of Test Condition on Speech Perception With Electric-Acoustic Stimulation. Am J Audiol. 2015;24(4):520–528. [DOI] [PubMed] [Google Scholar]
- 55.Pillsbury HC 3rd, Dillon MT, Buchman CA, et al. Multicenter US Clinical Trial With an Electric-Acoustic Stimulation (EAS) System in Adults: Final Outcomes. Otol Neurotol. 2018;39(3):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. Laryngoscope. 2016;126(4):962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suhling MC, Majdani O, Salcher R, et al. The Impact of Electrode Array Length on Hearing Preservation in Cochlear Implantation. Otol Neurotol. 2016;37(8):1006–1015. [DOI] [PubMed] [Google Scholar]
- 58.O’Connell BP, Hunter JB, Haynes DS, et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. 2017;127(10):2352–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiam NT, Gilbert M, Cooke D, et al. Association Between Flat-Panel Computed Tomographic Imaging-Guided Place-Pitch Mapping and Speech and Pitch Perception in Cochlear Implant Users. JAMA Otolaryngol Head Neck Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiam NT, Pearl MS, Carver C, Limb CJ. Flat-Panel CT Imaging for Individualized Pitch Mapping in Cochlear Implant Users. Otol Neurotol. 2016;37(6):672–679. [DOI] [PubMed] [Google Scholar]
- 61.Lenarz M, Sonmez H, Joseph G, Buchner A, Lenarz T. Long-term performance of cochlear implants in postlingually deafened adults. Otolaryngol Head Neck Surg. 2012;147(1):112–118. [DOI] [PubMed] [Google Scholar]


