Abstract
Background
Of the three lethal coronaviruses, in addition to the ongoing pandemic-causing SARS-CoV 2, Middle East Respiratory Syndrome Coronavirus (MERS-CoV) remains in circulation. Information on MERS-CoV has relied on small sample of patients. We updated the epidemiology, laboratory and clinical characteristics, and survival patterns of MERS-CoV retrospectively with the largest sample of followed patients.
Methods
We conducted a retrospective review of line-listed records of non-random, continuously admitted patients who were suspected (6,873) or confirmed with MERS-CoV (501) admitted to one of the four MERS-CoV referral hospitals in Saudi Arabia, 2014-2019.
Findings
Of the 6,873 MERS-CoV suspected persons, the majority were male (56%) and Saudi nationals (83%) and 95% had no known history that increased their risk of exposure to MERS-CoV patients or vectors (95%). More confirmed cases reported history that increased their risk of MERS-CoV infection (41%). Among the suspected, MERS-CoV confirmation (7.4% overall) was independently associated with being male, known transmission link to MERS-CoV patients or vectors, fever, symptoms for 7 days, admission through intensive care unit, and diabetes. Among persons with confirmed MERS-CoV, single symptoms were reported by 20%, 3-symptom combinations (fever, cough and dyspnea) reported by 21% and 2-symptom combinations (fever, cough) reported by 16%. Of the two-thirds (62%) of MERS-CoV confirmed patients who presented with co-morbidity, 32% had 2-"comorbidities (diabetes, hypertension). More than half of the MERS-CoV patents showed abnormal chest X-ray, elevated aspartate aminotransferase, and creatinine kinase. About a quarter of MERS-CoV patients had positive cultures on blood, urine, or respiratory secretions. During an average hospital stay of 18 days (range 11 to 30), 64% developed complications involving liver, lungs, or kidneys. Ventilation requirement (29% of MERS-CoV cases) was independently associated with abnormal chest X-ray, viremia (Ct value <30), elevated creatinine, and prothrombin time. Death (21% overall) was independently associated with older age, dyspnea and abnormal chest X-ray on admission, and low hemoglobulin levels.
Interpretations
With two-thirds of the symptomatic persons developing multiorgan complications MERS-CoV remains the coronavirus with the highest severity (29%) and case fatality rate (21%) among the three lethal coronaviruses. Metabolic abnormalities appear to be an independent risk factor for sustained MERS-CoV transmission. The poorly understood transmission dynamics and non-specific clinical and laboratory features call for high index of suspicion among respiratory disease experts to help early detection of outbreaks. We reiterate the need for case control studies on transmission.
Funding
No special funding to declare.
Keywords: MERS-CoV, Saudi Arabia, Epidemiology
Research in context.
Evidence before this study
MERS-CoV, a lethal zoonotic novel β-coronaviruses emerged in 2012, expanded to 27 countries and remains in circulation with various transmission dynamics. Intermittent sporadic cases, community clusters, nosocomial outbreaks, and human-to-human transmissions of MERS-CoV resulted in a total 2,574 (June 2020) laboratory confirmed cases of MERS-CoV. About 80% of reported MERS-CoV cases were reported from Saudi Arabia. Of the three lethal coronavirus, MERS-CoV is unique in that it has the highest reported case fatality, and, due to the relatively low number of cases, remains the least well-defined in terms of its epidemiology and natural history.
Added value of this study
We used the line-listed hospital care records of all suspected (6,873) MERS-CoV cases admitted to one of the four Mers-CoV referral hospitals in Saudi Arabia, 2014 -2019. In this cohort, we found a Mers-CoV positivity rate averaging at 7.3% (512 cases) during 2014 through 2019. Nearly two thirds of Mers-CoV patients had comorbidities, diabetes being the most common, and more than half of the patients had two or more concurrent comorbidities. Mers-CoV patients were hospitalized for an average of 18 days, two-thirds (64%) developed organ complications, more than a quarter (29%) developed severity requiring ventilation, and 21% died at hospital.
Implications of all the available evidence
Our findings on key characteristics of MERS-CoV (percent with comorbidity, days of hospitalization, percent who require ventilation, case fatality rate) are in the lowest range of values reported to date. Our report updates information on the most common concurrent infections that are likely to be observed in settings that have implemented most advanced infection control measures. It appears, the confluence of three factors- presence of highly susceptible population notably with metabolic disorders, high MERS-CoV burden among the host, and sufficient interaction between the host and susceptible population.- should be met for efficient and extensive transmission of MERS-CoV.
Alt-text: Unlabelled box
1. Introduction
The vulnerability of human populations to lethal zoonotic novel β-coronaviruses increased recently with three such novel coronaviruses identified in the past 17 years. The three most lethal coronaviruses identified in 2002, 2012, and 2019 respectively are the severe acute respiratory syndrome coronavirus (SARS-CoV) 2002, the Middle East respiratory syndrome coronavirus (MERS-CoV), and the SARS-CoV-2, the cause of the ongoing COVID-19 pandemic [1,2]. All the three lethal β- coronaviruses, which primarily affect the respiratory system, are listed in the World Health Organization (WHO) Blueprint list of priority pathogens because of their pandemic potential and the limitations of pharmaceutical countermeasures [3,4].
The first coronavirus outbreak caused by SARS-CoV in 2002 lasted for nine months and abruptly ended in July 2003 after causing 8,098 cases in 29 countries [5], [6], [7], [8]. The second coronavirus outbreak, MERS-CoV, started in 2012, expanded to 27 countries and remains in circulation with various transmission dynamics [7,8]. Intermittent sporadic cases, community clusters, nosocomial outbreaks, and human-to-human transmissions of MERS-CoV resulted in a total 2,574 (June 2020) laboratory confirmed cases of MERS-CoV [9], [10], [11]. About 80% of reported MERS-CoV cases were reported from Saudi Arabia, with the largest outbreak outside the Middle Eastern region occurring in South Korea in 2015 [7,8,12,13]. Of the three lethal coronavirus, MERS-CoV is unique that it has the highest reported case fatality [14,15], and, due to the relatively low number of cases, remains the least well-defined in terms of its epidemiology and natural history [2,11,16]. Other than surveillance, summary reports or sub analysis of specific variables, comprehensive data on MERS-CoV epidemiology, exposure history, clinical characteristics, disease progression, and sequelae are limited to reports of family clusters and hospital outbreaks [11, 12,13, 14,15]. Also, those reports predated changes in case definitions by the World Health Organization (WHO) and the Saudi Arabian Ministry of Health. COVID-19 has heightened the interest in coronavirus infections [19,20]. Given the sparsity of information on previous lethal coronavirus, there is an urgent need to continually update the epidemiology and clinical features of MERS-CoV with the largest data sets available [16,21].
Since the first reported case of MERS-CoV, the Saudi Arabian Ministry of Health (MoH) mandated that all patients with respiratory illnesses needing admission to intensive care should be tested for the virus [17,18]. In this report we used data from the largest continuous cohort of suspected (6,873) and confirmed (501) MERS-CoV cases ever reported from one of the four MERS-CoV referral centers in Saudi Arabia from April 2014 up to November 12, 2019. We present a comprehensive description of demographic, epidemiological, clinical, laboratory findings and survival patterns to address some of the knowledge gaps outlined earlier [1] and in the WHO R&D blueprint initiative consultation on MERS [4,22].
2. Methods
We conducted a retrospective review of line-listed records of non-random, continuously admitted patients who were suspected or confirmed with MERS-CoV at the Prince Mohammed Bin Abdulaziz Hospital (PMAH) [15,23,24], and followed through discharge, transfer or death from the hospital, during April 2014 through November, 2019.
PMAH is a 500-bed teaching and referral hospital under the Ministry of Health located in the eastern part of Riyadh city with a catchment area of 2,500,000 inhabitants. The hospital covers all medical and surgical services with an annual admission of about 11,000.
PMAH is a center of excellence for MERS, and one of the four MERS-CoV regional referral hospitals in Saudi Arabia where all MERS-CoV suspected persons are referred for further investigation by all hospitals and clinics in the catchment region. Since 2015, all referring hospitals conform to the MERS-CoV patient data collection system required by the MoH and the WHO and report summary data containing select variables to the Ministry of Health which maintains a public use version of all MERS-CoV cases in the country [25]. The Saudi Arabia national MERS data collection system does not include detailed line-listed patient information on demographics, full list of risk factors, clinical course and outcome to the extent that is available in the referral hospital data systems.
2.1. Case definitions
All patients presenting with compatible symptoms defined in the Saudi Arabia Ministry of Health case definitions [19,26] during the study period with >14 days separating illness episodes for persons in whom MERS-CoV infection is suspected are admitted to the hospital [25]. Asymptomatic patients with an epidemiological link to MERS-CoV confirmed patients are also subjected to MERS-CoV evaluation.
The data used here were collected after the release of the World Health Organization MERS-CoV case definitions, July 2013. https://www.who.int/csr/disease/coronavirus_infections/MERS_CoV_investigation_guideline_Jul13.pdf As with all novel pathogens, case definitions for suspicion of MERS in Saudi Arabia changed over time. Following the initial World Health Organization case definition, the MOH of Saudi Arabia updated the case definitions in 2014, 2015, 2017 and 2018 (supplementary appendices 1-5). In Saudi Arabia, persons meeting the MoH case definition [26] for suspected MERS-CoV infection are hospitalized and undergo detailed investigations until they are tested negative for the virus. Patients with severe illness are admitted directly to intensive care unit (ICU) [23,27]. Patients with non-severe illness are admitted to a special airborne isolation ward [28,29]. Case definitions have evolved over time. In July 2018, the MoH implemented a 4-category revision to the 2015 case definition, with the goal of making the definition more specific. In April 2018, the MoH revised the case definition for suspected MERS-CoV [26].
2.2. Laboratory confirmation of MERS-CoV
Nasopharyngeal swabs were tested for MERS-CoV by using real time reverse transcriptase polymerase chain reaction (RT-PCR) as described previously [5,30,31]. The PCR test the upstream E protein (upE gene) and ORF1 of MERS-CoV. A positive test was considered when both assays were positive, and the samples were considered negative when the MERS-CoV Rt-PCR was negative [5,30]. High viremia was used interchangeably with low cycle threshold (CT) value of respiratory sample or high viral load in nasopharyngeal swab.
2.3. Hospital infection control procedures
Infection control protocols and measures include prevention of crowding in any part of the hospital, allocation of special areas in the hospital to triage and mange suspected and or confirmed cases of MERS-CoV [1,10,23,24]. Separate buildings housed a dedicated respiratory zone emergency department (R-ED) to receive patients with respiratory symptoms [24]. Patients from the main emergency room were triaged by receiving medical staff who ascertained a history of respiratory symptoms, fever, history of contact with MERS-CoV patients or visiting the emergency room of any health care facility in the past two weeks for respiratory diseases [28,32,33]. Patients who responded affirmatively to these questions and their attendants were required to wear a surgical mask during the transfer to the respiratory emergency department. The R-ED implements, surface hygiene, physical distancing in waiting rooms and registration area, and consists of negative pressure isolation rooms and high efficiency particulate air filters throughout. Infection control nurse monitors staff compliance with personal protective equipment use and isolation precautions [1,10,24].
2.4. Contact investigation
In general, contact investigations of community acquired MERS-CoV infections are conducted by the MOH Ministry of Health staff. However, contact investigations of PMAH health care workers were done by the PMAH infection control staff.
2.5. Data
PMAH maintains a database of line listed, routinely collected epidemiological, demographic, clinical, laboratory, and clinical procedures and outcome during the period of hospitalization on each patient in a standardized Microsoft Excel database. The source data system includes unique patient identification numbers with patients’ national identification numbers, name, age, and addresses. Repeat admissions for the same patient are documented and can be distinguished from previous admissions by dates. Patients were followed until they were transferred to another hospital, discharged from the hospital, or died. Events that occurred after discharge from the hospital were not recorded in the PMAH data.
2.6. Statistical analysis
We used STATA® software, version 15 (StataCorp, College Station, TX, USA) for all analyses. We summarized the baseline characteristics of patients at the time of admission for categorical variables as counts and percentages. Continuous variables (mainly laboratory values) were summarized as medians because they tended to have non-normal distributions, an assumption confirmed using the Shapiro-Wilk test. Among all suspected cases (N=6,873), the primary outcome was laboratory confirmation of MERS diagnosis. We used logistic regression to assess potential associations with MERS confirmation, including demographic characteristics of patients (age, gender, employment as a healthcare worker, time and place of admission, symptom history (any symptoms, gastrointestinal symptoms, dyspnea, cough, sore throat, fever, duration of symptoms prior to admission), and comorbidities (any comorbidity, diabetes, asthma, hypertension, chronic obstructive pulmonary disease, cancer, liver disease, and congestive heart failure).Variables that were significant (P <0.05) in bivariate models were included in a first multivariable model. If significant in the first model, they were included in the second model. If significant in the second model, they were included in a final model. Variables with P<0.05 in the final model were interpreted as significantly associated with MERS confirmation.
Among the 501 confirmed cases, we chose two primary outcome measures: receipt of mechanical ventilation and death. We used Kaplan-Meier curves to describe the probability of survival after admission to the hospital. Exit time was set as the primary outcome date (of mechanical ventilation or death) or the hospital discharge or transfer date, whichever occurred first. We assessed the statistical significance of variables associated with mechanical ventilation and death using used multivariable Cox-Proportional hazard models to assess the statistical significance of variables associated with mechanical ventilation and death. We assessed the same demographic and clinical variables listed above for the “MERS confirmation” outcome and results of laboratory investigations performed after admission, including, brain magnetic resonance imaging, microbiology, blood culture, urine culture, respiratory culture, MERS CoV PCR CT < 30, white blood cell count, hemoglobin, platelet count, neutrophil creatinine kinase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, albumin, bilirubin, prothrombin time (PT), partial thromboplastin time (PTT), and lactate dehydrogenase (LDG). We modeled the two outcomes separately. We expressed the exponentiated regression coefficients in terms of a hazard ratio (HR) in bivariate models and adjusted HR (aHR) in multivariable models. Variables that were significant (P <0.05) in bivariate models were included in a first multivariable model. If significant in the first model, they were included in second model. If significant in the second model, they were included in a final model. Variables with P<0.05 in the final model were interpreted as significantly associated with death or mechanical ventilation.
We refrained from statistical analysis of trends over time in diagnosis or outcomes due to potential bias introduced by the frequent updates in case definitions and availability of various palliative treatments available and modified over the study period. Data were missing for several variables relating to laboratory and clinical investigations among some confirmed cases. Due to the variability in timing of admission, severity and course of disease, comorbid conditions, and other clinical variables, some variability in laboratory and clinical investigations among medical staff can be expected, as can some missingness of data for different patients. We therefore assumed that these data were missing not completely at random. Because we were not able to test this assumption based on the available data, we opted to not impute data for some independent variables, rather than exclude cases with incomplete data. Counts of missing data are presented in the results tables.
Ethical Approval: As per the ethical standards and by laws, retrospective analysis of routinely collected data from patients admitted to service delivery points such as primary, secondary, tertiary and specialty hospitals are not required to undergo ethical review. As such the proposed analysis is exempt from a review, Central Ministry of Health IRB Log number 21-110M.
Role of funding: No special funding to declare.
3. Results
3.1. MERS-CoV suspected persons
Overall, of the 6,873 persons who met the criteria for a suspected case of MERS-CoV and were admitted to PMAH the majority were Saudi men (55.9%) aged less than 60 years (53.7%) with no known exposure information (95.3%) (Table 1). Most were admitted to the non-emergency wards (77.6%). Only a small percent of the suspected persons (4.7%) had a known exposure link or were health care workers (2%). The majority of suspected cases (71%) reported symptom onset within seven days prior to hospitalization. Over half had symptoms of cough (64.0%), fever (59%), or dyspnea (51.8%). Two or more concurrent symptoms were reported by more than half of patients, with concurrent cough, fever and dyspnea being the most reported combination. Two-thirds of the MERS-CoV suspected persons had comorbidities with hypertension or diabetes (both 44%) as the most common.
Table 1.
Characteristics of 6,873 MERS-CoV suspected and 501 confirmed persons, Saudi Arabia, 2014-2019
| Number and % distribution of suspected cases | Number and % of suspected cases that were confirmed | P-value | |||
|---|---|---|---|---|---|
| Total | 6,873 | 100 | 501 | 7.4 | |
| Gender | |||||
| Female | 3,028 | 44.1 | 154 | 5.1 | <0.001 |
| Male | 3,845 | 55.9 | 346 | 9 | |
| Age in years | |||||
| 0-20 | 316 | 4.6 | 14 | 4.4 | <0.001 |
| 21-40 | 1,542 | 22.4 | 165 | 10.7 | |
| 41-60 | 1,835 | 26.7 | 209 | 11.4 | |
| >60 | 3,180 | 46.3 | 114 | 3.6 | |
| Saudi national | |||||
| No | 1,206 | 17.5 | 180 | 14.9 | <0.001 |
| Yes | 5,667 | 82.5 | 323 | 5.7 | |
| Year of admission | |||||
| 2014 | 164 | 2.4 | 58 | 35.4 | <0.001 |
| 2015 | 1,091 | 15.9 | 189 | 17.3 | |
| 2016 | 1,132 | 16.5 | 58 | 5.1 | |
| 2017 | 1,696 | 24.7 | 76 | 4.5 | |
| 2018 | 1,813 | 26.4 | 47 | 2.6 | |
| 2019 | 975 | 14.2 | 70 | 7.2 | |
| Healthcare worker | |||||
| No | 6,734 | 98 | 431 | 6.4 | <0.001 |
| Yes | 139 | 2 | 72 | 51.8 | |
| History of travel or exposure to MERS CoV cases or vectors | |||||
| Unknown | 6,548 | 95.3 | 295 | 4.5 | <0.001 |
| Positive case | 243 | 3.5 | 142 | 58.4 | |
| Related of MERS CoV patient | 54 | 0.8 | 47 | 87 | |
| Animal | 23 | 0.3 | 15 | 65.2 | |
| Travel | 2 | 0 | 1 | 50 | |
| Admitted through Intensive Care Unit (ICU) | |||||
| No | 5,331 | 77.6 | 331 | 6.2 | <0.001 |
| Yes | 1,542 | 22.4 | 170 | 11 | |
| Days symptomatic before admissiona | |||||
| <=7 days | 3,272 | 71.2 | 213 | 6.5 | 0.002 |
| >7 days | 1,321 | 28.8 | 124 | 9.4 | |
| Any symptoms | |||||
| No | 136 | 2 | 51 | 37.5 | <0.001 |
| Yes | 6,737 | 98 | 451 | 6.7 | |
| Gastrointestinal symptoms | |||||
| No | 5,879 | 85.5 | 406 | 6.9 | 0.010 |
| Yes | 994 | 14.5 | 96 | 9.7 | |
| Shortness of breath | |||||
| No | 3,310 | 48.2 | 305 | 9.2 | <0.001 |
| Yes | 3,563 | 51.8 | 196 | 5.5 | |
| Cough | |||||
| No | 2,475 | 36 | 200 | 8.1 | 0.140 |
| Yes | 4,398 | 64 | 299 | 6.8 | |
| Sore throat | |||||
| No | 6,626 | 96.4 | 477 | 7.2 | 0.199 |
| Yes | 247 | 3.6 | 26 | 10.5 | |
| Fever | |||||
| No | 2,821 | 41 | 150 | 5.3 | 0.001 |
| Yes | 4,052 | 59 | 353 | 8.7 | |
| Any comorbidity | |||||
| No | 1,663 | 24.2 | 190 | 11.4 | <0.001 |
| Yes | 5,210 | 75.8 | 313 | 6 | |
| Total number of comorbidities | |||||
| 0 | 1,663 | 24.2 | 190 | 11.4 | <0.001 |
| 1 | 2,673 | 38.9 | 179 | 6.7 | |
| 2 | 2,004 | 29.2 | 110 | 5.5 | |
| 3 | 493 | 7.2 | 20 | 4.1 | |
| 4 | 40 | 0.6 | 1 | 2.5 | |
| Diabetes | |||||
| No | 3,854 | 56.1 | 304 | 7.9 | 0.007 |
| Yes | 3,019 | 43.9 | 196 | 6.5 | |
| Asthma | |||||
| No | 6,191 | 90.1 | 464 | 7.5 | 0.115 |
| Yes | 682 | 9.9 | 39 | 5.7 | |
| Hypertension | |||||
| No | 3,865 | 56.2 | 340 | 8.8 | <0.001 |
| Yes | 3,008 | 43.8 | 162 | 5.4 | |
| Chronic Obstructive pulmonary disease (COPD) | |||||
| No | 6,593 | 95.9 | 501 | 7.6 | <0.001 |
| Yes | 280 | 4.1 | 4 | 1.4 | |
| Cancer | |||||
| No | 6,811 | 99.1 | 497 | 7.3 | 0.547 |
| Yes | 62 | 0.9 | 6 | 9.7 | |
| Liver disease | |||||
| No | 6,818 | 99.2 | 498 | 7.3 | 0.695 |
| Yes | 55 | 0.8 | 3 | 5.5 | |
| Congestive heart failure | |||||
| No | 6,514 | 94.8 | 489 | 7.5 | <0.001 |
| Yes | 359 | 5.2 | 12 | 3.3 | |
Among 6,737 patients with any symptoms. Data were missing from 2,166 (32.2%) of these patients.
3.2. MERS-CoV confirmation among suspected persons
Overall, 7.4% of the 6,873 persons suspected of MERS-COV were confirmed positive. MERS-CoV seropositivity decreased from 2014 through 2016 and did not vary significantly thereafter (Table 1), which likely reflects changes in case definitions in 2015 and 2018. Within the socio-demographics subgroups of suspected persons with MERS-CoV, the percentages of confirmed positive were highest among men, those aged 21-60 years, non-Saudi nationals, health care workers, and those who had contact with animals or positive cases (Table 1).
Analysis of the 501 MERS-CoV confirmed cases by the source of origin of the patients, 75.5% of the MERS-CoV diagnosis was confirmed among persons referred to PMAH for MERS-CoV evaluation, and the remainder of the patients were admitted directly to PMAH.
Of note, though exposure link was not known for 59% (295/501) of the MERS-CoV confirmed patients, the fact that 15 of the MERS-CoV patients were diagnosed from 23 suspected persons (65.2%), followed by 47 cases who had link to a positive case (58.4%) underscores potential missing link in transmission dynamics.
Confirmed diagnoses were greater among patients who were admitted through the ICU, reported symptoms for more than seven days, and reported dyspnea, fever, or gastrointestinal symptoms (Table 1). More males with confirmed MERS-CoV were admitted to the ICU than females (37.6% vs 25.5%).
Results of the final multivariate logistic regression (Table 2) indicate that male gender [AOR = 2.55 (95% CI: 1.85-3.51), P<0.001], being a non-Saudi national [AOR=1.50 (95%CI: 1.07-2.09 P=0.02], having a known transmission link [AOR=132.54 (95% CI: 74.52-235.76), P<0.001], admission through the ICU [AOR: 2.05 (95% CI, 1.50-2.82), P<0.001], reported symptoms >7 days before admission [AOR= 2.04 (95% CI: 1.51-2.76), P<0.001, fever [AOR=5.77 (95% CI: 3.95-8.44), P<0.001], and diabetes [AOR=1.52 (95% CI: 1.13-2.05), P=0.01] were significantly associated with a confirmed MERS-CoV diagnosis. (Table 2). Other symptoms and comorbidities did not independently increase the likelihood of a confirmed MERS-CoV diagnosis.
Table 2.
MERS CoV, Saudi Arabia, 2014-2019: Final multivariate logistic regression model for MERS CoV confirmation among 6,873 suspected cases.a
| Independent variables | Adjusted odds ratio | 95% CI | P-value |
|---|---|---|---|
| Male gender | 2.55 | (1.85-3.51) | <0.001 |
| Age 41 to 60 years | 2.22 | (1.65-2.99) | <0.001 |
| Non-Saudi national | 1.50 | (1.07-2.09) | 0.02 |
| Known transmission link | 132.54 | (74.52-235.76) | <0.001 |
| Admitted through intensive care unit. | 2.05 | (1.50-2.82) | <0.001 |
| Symptomatic > 7 days before admission | 2.04 | (1.51-2.76) | <0.001 |
| Fever | 5.77 | (3.95-8.44) | <0.001 |
| Diabetes | 1.52 | (1.13-2.05) | 0.01 |
The first multivariate model (not shown) included all variables with p<=0.05 in the single variable models in Table 1. After removing non-significant variables, the independent variables in the final model shown here were adjusted for each other.
3.3. Symptoms among MERS-CoV confirmed patients
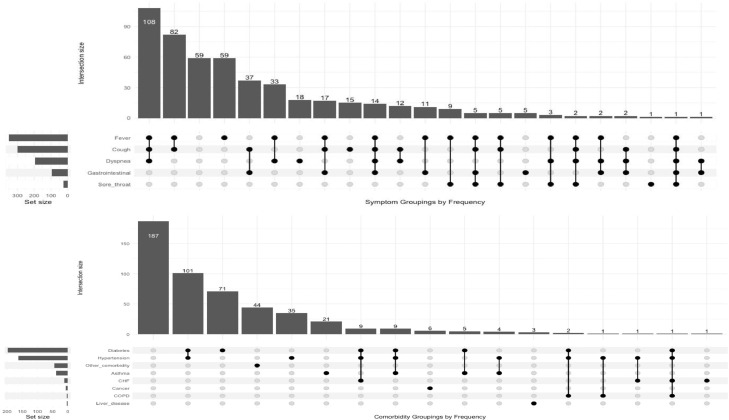
Few (10.2%) MERS-CoV confirmed patients presented as asymptomatic (Table 3), nearly all of whom were contacts of MERS-CoV positive cases identified though contact tracing. Fever (70.3%), cough (60.1%) and dyspnea (39.1%) were the most commonly reported symptoms among confirmed cases Table 3. One in five confirmed cases reported only one symptom (20%), including fever (10%), cough (3%), dyspnea (4%), gastrointestinal (1%), and sore throat (0.2%) (Figure 1). The most frequently reported concurrent symptoms among confirmed cases were concurrent fever, cough, and dyspnea (21%) and concurrent fever and cough (16%) (Figure 1).
Table 3.
Demographic, clinical, laboratory and radiographic findings among MERS-CoV patients overall and by severity, Saudi Arabia, 2014-2019
| All MERS-CoV cases: number and % distribution | MERS CoV cases who required ventilation (n, %) | P-value | MERS CoV cases who died at the hospital (n, %) | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Total | 501 | 100 | 146 | 29.1 | 106 | 21.2 | ||
| Gender | ||||||||
| Female | 155 | 30.9 | 36 | 23.2 | 0.062 | 24 | 15.5 | 0.025 |
| Male | 346 | 69.1 | 110 | 31.8 | 82 | 23.7 | ||
| Age in years | ||||||||
| 0-20 | 14 | 2.8 | 5 | 35.7 | 0.019 | 1 | 7.1 | 0.001 |
| 21-40 | 165 | 32.9 | 34 | 20.6 | 21 | 12.7 | ||
| 41-60 | 208 | 41.5 | 61 | 29.3 | 43 | 20.7 | ||
| >60 | 114 | 22.8 | 46 | 40.4 | 41 | 36.0 | ||
| Saudi national | ||||||||
| no | 180 | 35.9 | 44 | 24.4 | 0.045 | 32 | 17.8 | 0.107 |
| yes | 321 | 64.1 | 102 | 31.8 | 74 | 23.1 | ||
| Year of admission | ||||||||
| 2014 | 58 | 11.6 | 16 | 27.6 | 0.150 | 9 | 15.5 | 0.554 |
| 2015 | 189 | 37.9 | 42 | 22.2 | 43 | 22.8 | ||
| 2016 | 58 | 11.6 | 19 | 32.8 | 14 | 24.1 | ||
| 2017 | 76 | 15.2 | 31 | 40.8 | 19 | 25.0 | ||
| 2018 | 48 | 9.6 | 17 | 35.4 | 12 | 25.0 | ||
| 2019 | 70 | 14 | 21 | 30.0 | 9 | 12.9 | ||
| Healthcare worker | ||||||||
| no | 429 | 85.6 | 143 | 33.3 | 0.000 | 105 | 24.5 | 0.000 |
| yes | 72 | 14.4 | 3 | 4.2 | 1 | 1.4 | ||
| History of travel or exposure to MERS -CoV cases or vectors | ||||||||
| Unknown | 293 | 58.8 | 114 | 38.9 | 0.000 | 82 | 28.0 | 0.001 |
| Patient | 142 | 28.5 | 24 | 16.9 | 21 | 14.8 | ||
| Relative of patient | 47 | 9.4 | 2 | 4.3 | 1 | 2.1 | ||
| Animal | 15 | 3 | 4 | 26.7 | 2 | 13.3 | ||
| Travel | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | ||
| Admitted through Intensive Care Unit | ||||||||
| no | 332 | 66.3 | 30 | 9.0 | 0.000 | 31 | 9.3 | 0.000 |
| yes | 169 | 33.7 | 116 | 68.6 | 75 | 44.4 | ||
| Days symptomatic before admission | ||||||||
| <=7 days | 212 | 63.1 | 67 | 31.5 | 0.219 | 45 | 21.2 | 0.242 |
| >7 days | 124 | 36.9 | 32 | 26.0 | 20 | 16.1 | ||
| Any symptoms | ||||||||
| no | 51 | 10.2 | 5 | 9.8 | 0.001 | 6 | 11.8 | 0.071 |
| yes | 450 | 89.8 | 141 | 31.3 | 100 | 22.2 | ||
| Gastrointestinal symptoms | ||||||||
| No | 405 | 80.8 | 124 | 30.5 | 0.163 | 92 | 22.7 | 0.119 |
| Yes | 96 | 19.2 | 22 | 23.2 | 14 | 14.6 | ||
| Dyspnea | ||||||||
| No | 305 | 60.9 | 54 | 17.7 | 0.000 | 41 | 13.4 | 0.000 |
| Yes | 196 | 39.1 | 92 | 46.9 | 65 | 33.2 | ||
| Cough | ||||||||
| No | 200 | 39.9 | 59 | 29.4 | 0.927 | 41 | 20.5 | 0.803 |
| Yes | 301 | 60.1 | 87 | 29.0 | 65 | 21.6 | ||
| Sore throat | ||||||||
| No | 475 | 94.8 | 145 | 30.5 | 0.000 | 105 | 22.1 | 0.002 |
| Yes | 26 | 5.2 | 1 | 3.8 | 1 | 3.8 | ||
| Fever | ||||||||
| No | 149 | 29.7 | 44 | 29.3 | 0.948 | 36 | 24.2 | 0.223 |
| Yes | 352 | 70.3 | 102 | 29.1 | 70 | 19.9 | ||
| Any comorbidity | ||||||||
| No | 190 | 37.9 | 29 | 15.3 | 0.000 | 16 | 8.4 | 0.000 |
| Yes | 311 | 62.1 | 117 | 37.6 | 90 | 28.9 | ||
| Diabetes | ||||||||
| No | 305 | 60.9 | 71 | 23.4 | 0.000 | 50 | 16.4 | 0.000 |
| Yes | 196 | 39.1 | 75 | 38.1 | 56 | 28.6 | ||
| Asthma | ||||||||
| No | 462 | 92.2 | 134 | 29.1 | 0.888 | 99 | 21.4 | 0.607 |
| Yes | 39 | 7.8 | 12 | 30.0 | 7 | 17.9 | ||
| Hypertension | ||||||||
| No | 338 | 67.5 | 75 | 22.2 | 0.000 | 50 | 14.8 | 0.000 |
| Yes | 163 | 32.5 | 71 | 43.6 | 56 | 34.4 | ||
| Chronic Obstructive Pulmonary disease | ||||||||
| No | 497 | 99.2 | 143 | 28.8 | 0.057 | 102 | 20.5 | 0.000 |
| Yes | 4 | 0.8 | 3 | 75.0 | 4 | 100.0 | ||
| Cancer | ||||||||
| No | 495 | 98.8 | 144 | 29.1 | 0.811 | 103 | 20.8 | 0.063 |
| Yes | 6 | 1.2 | 2 | 33.3 | 3 | 50.0 | ||
| Liver disease | ||||||||
| No | 498 | 99.4 | 144 | 28.9 | 0.020 | 104 | 20.9 | 0.002 |
| Yes | 3 | 0.6 | 2 | 66.7 | 2 | 66.7 | ||
| Congestive heart failure | ||||||||
| No | 489 | 97.6 | 141 | 28.8 | 0.293 | 101 | 20.7 | 0.022 |
| Yes | 12 | 2.4 | 5 | 45.5 | 5 | 41.7 | ||
| Chest X-ray | ||||||||
| Negative | 155 | 36.3 | 10 | 6.5 | 0.000 | 9 | 5.8 | 0.000 |
| Positive | 272 | 63.7 | 114 | 41.9 | 86 | 31.6 | ||
| missing | 74 | 22 | 11 | |||||
| Brain Magnetic Resonance Imaging | ||||||||
| Negative | 370 | 73.9 | 99 | 26.8 | 0.140 | 75 | 20.3 | 0.522 |
| Positive | 131 | 26.1 | 47 | 35.9 | 31 | 23.7 | ||
| Microbiology | ||||||||
| Negative | 238 | 64 | 34 | 14.3 | 0.000 | 25 | 10.5 | 0.000 |
| Positive | 134 | 36 | 75 | 56.0 | 59 | 44.0 | ||
| Missing | 129 | 37 | 22 | |||||
| Blood culture | ||||||||
| Negative | 332 | 74.1 | 77 | 23.2 | 0.010 | 60 | 18.1 | 0.113 |
| Positive | 116 | 25.9 | 55 | 47.4 | 34 | 29.3 | ||
| Missing | 53 | 14 | 12 | |||||
| Urine culture | ||||||||
| Negative | 341 | 76.5 | 91 | 26.7 | 0.146 | 65 | 19.1 | 0.419 |
| Positive | 105 | 23.5 | 40 | 38.1 | 27 | 25.7 | ||
| Missing | 55 | 15 | 13 | |||||
| Respiratory culture | ||||||||
| Negative | 337 | 75.6 | 65 | 19.3 | 0.000 | 46 | 13.6 | 0.000 |
| Positive | 109 | 24.4 | 66 | 60.6 | 47 | 43.1 | ||
| Missing | 55 | 155 | 13 | |||||
| MERS CoV PCR CT < 303 | ||||||||
| No | 141 | 39.5 | 15 | 10.6 | 0.000 | 13 | 9.2 | 0.000 |
| Yes | 216 | 60.5 | 92 | 42.6 | 69 | 31.9 | ||
| Missing | 144 | 39 | 24 | |||||
| White blood cell count | ||||||||
| Below normal | 93 | 21.7 | 17 | 18.3 | 0.000 | 12 | 12.9 | 0.000 |
| Normal | 270 | 63.1 | 72 | 26.7 | 54 | 20.0 | ||
| Above normal | 65 | 15.2 | 36 | 55.4 | 31 | 47.7 | ||
| Missing | 73 | 21 | 9 | |||||
| Hemoglobin | ||||||||
| Below normal | 211 | 49.3 | 94 | 44.5 | 0.000 | 76 | 36.0 | 0.000 |
| Normal | 211 | 49.3 | 29 | 13.7 | 20 | 9.5 | ||
| Missing | 73 | 21 | 9 | |||||
| Platelet count | ||||||||
| Below normal | 113 | 26.4 | 41 | 36.3 | 0.032 | 31 | 27.4 | 0.353 |
| Normal | 299 | 69.9 | 78 | 26.1 | 62 | 20.7 | ||
| Missing | 73 | 21 | 9 | |||||
| Neutrophil | ||||||||
| Below normal | 36 | 8.7 | 2 | 5.6 | 0.000 | 2. | 5.6 | 0.000 |
| Normal | 295 | 71.4 | 72 | 24.4 | 50 | 16.9 | ||
| Above normal | 82 | 19.9 | 48 | 58.5 | 43 | 52.4 | ||
| Missing | 88 | 24 | 11 | |||||
| Creatinine kinase | ||||||||
| Below normal | 49 | 24.1 | 15 | 30.6 | 0.171 | 12 | 24.5 | 0.426 |
| Normal | 45 | 22.2 | 13 | 28.9 | 11 | 24.4 | ||
| Above normal | 109 | 53.7 | 44 | 40.0 | 35 | 32.1 | ||
| Missing | 298 | 75 | 48 | |||||
| Alanine aminotransferase (ALT) | ||||||||
| Normal | 253 | 66.6 | 76 | 30.0 | 0.768 | 59 | 23.3 | 0.858 |
| Above normal | 126 | 33.2 | 37 | 29.4 | 29 | 23.0 | ||
| Missing | 121 | 33 | 18 | |||||
| Aspartate aminotransferase (AST) | ||||||||
| Normal | 122 | 32.9 | 13 | 10.7 | 0.000 | 9 | 7.4 | 0.000 |
| Above normal | 249 | 67.1 | 91 | 36.5 | 73 | 29.3 | ||
| Missing | 130 | 42 | 24 | |||||
| Creatinine | ||||||||
| Below normal | 95 | 26 | 19 | 20.0 | 0.000 | 15 | 15.8 | 0.000 |
| Normal | 203 | 55.6 | 39 | 19.2 | 27 | 13.3 | ||
| Above normal | 67 | 18.4 | 41 | 61.2 | 35 | 52.2 | ||
| Missing | 136 | 47 | 29 | |||||
| Albumin | ||||||||
| Below normal | 216 | 49.3 | 106 | 49.1 | 0.000 | 75 | 34.7 | 0.000 |
| Normal | 215 | 49.1 | 29 | 13.5 | 24 | 11.2 | ||
| Missing | 63 | 10 | 7 | |||||
| Bilirubin | ||||||||
| Normal | 338 | 85.6 | 87 | 25.8 | 0.000 | 66 | 19.5 | 0.000 |
| Above normal | 49 | 12.4 | 29 | 59.2 | 24 | 49.0 | ||
| Missing | 106 | 27 | 15 | |||||
| Prothrombin Time (PT) | ||||||||
| Normal | 231 | 69.6 | 52 | 22.6 | 0.000 | 42 | 18.2 | 0.000 |
| Above normal | 88 | 26.5 | 56 | 64.0 | 41 | 46.6 | ||
| Missing | 169 | 34 | 22 | |||||
| Partial Thromboplastin Time (PTT) | ||||||||
| Normal | 202 | 62.9 | 51 | 25.2 | 0.000 | 39 | 19.3 | 0.001 |
| Above normal | 73 | 22.7 | 45 | 61.6 | 33 | 45.2 | ||
| Missing | 180 | 41 | 26 | |||||
| Lactate dehydrogenase (LDH) | ||||||||
| Normal | 11 | 10.7 | 3 | 27.3 | 0.130 | 1 | 9.1 | 0.070 |
| Above normal | 91 | 88.4 | 50 | 55.0 | 39 | 42.9 | ||
| Missing | 398 | 93 | 66 | |||||
1Patients admitted to one of the four MERS-CoV referral hospitals in Saudi Arabia. 2Denominator for laboratory and other investigations vary. 3CT (Cycle Threshold) value is the number of cycles necessary to spot the virus, lower threshold indicates high viral count; PCR, polymerase chain reaction. The number of missing values are reported when missing in >1% of subjects.
Figure 1.
MERS CoV, Saudi Arabia, 2014-2019: Prevalence and single and combination symptoms and comorbidities among 501 confirmed cases admitted to one of the four MERS-CoV referral hospitals
3.4. Comorbidities among MERS-CoV confirmed patients
A majority of MERS-CoV confirmed patients had at least one comorbidity (62%), among which diabetes (39.1%) and hypertension (32.5%) were the most common (Table 3). Almost half of confirmed cases had a single comorbidity (7.8%), including diabetes (14%), hypertension (9%), and asthma (4%) (Figure 1). The most frequently reported concurrent comorbidities were concurrent diabetes and hypertension (32%) and concurrent diabetes, hypertension, and congestive heart failure or (3%). (Figure 1).
To assess whether the differences in co-morbidity may underscore the gender difference in MERS-CoV confirmation, we conducted a sub analysis of comorbidities by gender among suspected vs confirmed cases. Asthma and cancer occurred with similar frequency among men and women in both groups (data not shown in tables). MERS-CoV suspected but unconfirmed women had a greater diabetes prevalence than MERS-CoV confirmed (49% vs 31%), but among men diabetes was more prevalent among MERS-CoV confirmed patients (40% vs 43%).
3.5. Laboratory and diagnostic findings among MERS-CoV confirmed cases
Among MERS-CoV confirmed persons, the three most common laboratory and diagnostic findings observed among more than half of the patients were abnormal chest X-ray (63.7%), elevated levels of aspartate aminotransferase (AST) (67.1%), and elevated creatinine kinase (53.7%) (Table 3). The next three frequent abnormal findings were decreased albumin and hemoglobin levels noted among half of the patients (49.3% each), and elevated alanine aminotransferase (ALT) among one-third of the patients (33.2%). About two-thirds of patients (60.5%) had significant viremia measured by Cycle Threshold (CT) values <30 at the time of diagnosis.
About a quarter of MERS-CoV confirmed persons had above normal prothrombin (26.5%) and partial thromboplastin time (22.7%). Other hematological abnormalities were observed among fewer patients and included: leukocytosis (21.7%), leukopenia (15.2%), neutrophilia (19.9%), neutropenia (8.7%), and thrombocytopenia (26.4%). Elevated lactate dehydrogenase was observed among 88% of the 113 patients who were tested. Though multiple diagnostic or laboratory abnormalities were common, we did not observe any notable patterns. Given that high burden of lung pathology shown by chest X-ray, most of the patients with abnormal chest findings also had other concurrent laboratory anomalies.
Microbiology showed positive findings among one third of patients (36%), and one fourth had positive culture findings on all three types of specimens: blood (25.9%), urine (23.5%), respiratory (24.4%). (Table 3). The three most pathogens identified by blood culture were staphylococcus species (38%) klebsiella species (13%), and candida (13%) (data not shown in tables). In urine culture, the three most common findings were enterococcus (33%), candida (28%), and klebsiella pneumoniae species (15%). In respiratory culture, the three most common pathogens were pseudomonas (23%), Acinetobacter baumanii complex (MDRO) (17%) and klebsiella species (15%) (data not shown in table).
Among MERS-CoV confirmed cases, we conducted sub analysis by gender to assess the significance of variations in the above-mentioned laboratory findings (Table 4). Among MERS-CoV confirmed cases, abnormal findings on chest X-ray were more prevalent among men than women (69.1% vs 52.8%). Gender differences in hematology, blood chemistry, and cultures were also statistically significant.
Table 4.
Clinical and laboratory features and clinical outcome of 501 MERS-CoV patients by gender, Saudi Arabia, 2014-20191
| Female | Male | P-value | |
|---|---|---|---|
| Hematology (reference value), median (IQR) | |||
| WBC (4.0-11.0) | 6.9 (4.6-9.4) | 5.7 (3.9-8.8) | 0.0143 |
| Hgb (13.5-175) | 12.5 (9.9-14.1) | 13.9 (11.3-15.3) | <0.001 |
| Neutrophil (1.50-7.8) | 4.3 (2.7-6.4) | 4.0 (2.4 - 6.7) | 0.29 |
| Platelets (150-450) | 251 (181 - 306) | 195 (133-249) | <0.001 |
| Blood Chemistry, median (IQR) | |||
| Creatinine (62-115) | 56 (64-78.8) | 77 (67.8 -129.7) | <0.001 |
| Albumin (32-46) | 33(28-40) | 31 (25-37) | 0.003 |
| AST(5-34) | 39 (24-90) | 72.5 (35-118) | <0.001 |
| ALT (5-55) | 24 (14-53) | 43 (24-84) | <0.001 |
| Bilirubin (3.4-20.5) | 7.7 (5.6-11.3) | 10.1 (7-15.6) | <0.001 |
| LDH (125-243) | 477 (216-603) | 546 (375-821) | 0.04 |
| Microbiology and culture positive, number of patients (%) | |||
| Microbiology test, | 44 (35.2) | 90 (36.4) | 0.81 |
| Blood Culture | 26 (18.7) | 90 (29.1) | 0.02 |
| Urine Culture | 25 (18.1) | 80 (26.0) | 0.07 |
| Respiratory culture | 25 (18.0) | 84(27.4) | 0.03 |
| Other culture | 22 (16.1) | 88 (29.0) | 0.004 |
| Complications, number of patents (%) | |||
| Chest x-ray, positive | 75 (52.8) | 197 (69.1) | 0.001 |
| Acute lung Injury | 7 (16.7) | 24 (16.11) | 0.93 |
| Acute renal injury | 1 (2.4) | 5 (3.4) | 0.74 |
| Pneumonia | 12 (27.9) | 39 (26.2) | 0.82 |
| Pleural effusion | 2 (4.7) | 8 (5.4) | 0.88 |
| Cardiac arrest | 5 (11.9) | 18 (12.2) | 0.96 |
| Seizure, stroke, or shock | 3 (7.1) | 6 (4.1) | 0.41 |
| Other | 14 (32.3) | 14 (29.9) | 0.74 |
| No complications | 12 (27.9) | 59 (38.6) | 0.20 |
| Admitted to, number of patients (%) | |||
| Intensive care unit | 39 (25.2) | 130 (37.6) | 0.01 |
| Ward | 116 (74.8) | 216 (62.4) | 0.01 |
| Duration of hospital stay, median (IQR) | |||
| 16 (11-28) | 19 (14-30) | 0.16 | |
| Clinical outcome, number of patients (%) | |||
| Died | 24 (15.5) | 82 (23.7) | 0.04 |
| Discharged | 124 (80.0) | 248 (71.7) | 0.05 |
| Transferred | 3 (1.9) | 8 (2.3) | 0.79 |
1Patients admitted to one of the four MERS-CoV referral hospitals in Saudi Arabia.2Denominator for laboratory and other investigations vary. AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; LDH, lactate dehydrogenase
3.6. Clinical outcome
Overall, during an average hospital stay of 18 days (range 11 to 30), over one-third (36%) of MERS-CoV confirmed patients did not develop complications. The 64% of patients who developed complications confirms earlier observations of multiorgan involvement in MERS-CoV infection. Pneumonia (26%), acute lung injury (16%), and cardiac arrest (12%) were the leading complications. Cerebrovascular events were noted among 5% and acute renal injury among 3%. Contrary to significant gender differences in diagnostic and laboratory findings, there was no statistically significant differences by gender in development of complications during hospital stay or in the duration of hospital stay (IQR, male 19 days, females 16 days, p=0.16) among MERS confirmed case, except for the likelihood of being admitted to the ICU. Men were significantly more likely to be admitted to the ICU (37.6% vs 25.2%) (Table 4).
3.7. Severe outcomes (ventilation requirement, death)
Overall, 29.1% (146/501) of MERS-CoV patients required ventilation, an index of disease severity (Table 3). In the unadjusted analysis, the factors that increased the risk of ventilation included older age, unknown transmission link, dyspnea, admission through intensive care unit, comorbid diabetes, hypertension or liver disease and various laboratory findings, including positive findings on chest X-ray, microbiology positive, respiratory culture positive, and high viremia (Table 3). However, only six of these factors showed significance in the multivariate model adjusting for other variables, including Positive chest X-ray, [AHR=2.58 (95% CI: 1.11-5.99), P=0.03], positive microbiology [AHR=2.18 (95% CI: 1.07-4.42), P=0.03], positive blood culture [AHR=3.21 (1.57-6.57), P=0.001], high MERS CoV viremia [AHR=2.85 (95% CI: 1.45-5.60), P=0.002], elevated creatinine [AHR=1.87 (95% CI: 1.02-3.42), P=0.04], and elevated prothrombin time [AHR=2.85 (95% CI: 1.56-5.22), P=0.001] (Table 5).
Table 5.
MERS CoV, Saudi Arabia, 2014-2019: Final multivariate cox proportional hazard models for ventilation and death among 501 confirmed cases.a
| Outcome | Independent variables | Adjusted hazard ratio | 95% CI | P-value |
|---|---|---|---|---|
| Ventilation | ||||
| Unknown transmission link | 2.09 | (0.91-4.77) | 0.08 | |
| Chest X-ray positive | 2.58 | (1.11-5.99) | 0.03 | |
| Microbiology positive | 2.18 | (1.07-4.42) | 0.03 | |
| Blood culture positive | 3.21 | (1.57-6.57) | 0.001 | |
| MERS CoV PCR cycle threshold value < 30 b | 2.85 | (1.45-5.60) | 0.002 | |
| Elevated creatinine | 1.87 | (1.02-3.42) | 0.04 | |
| Elevated prothrombin time | 2.85 | (1.56-5.22) | 0.001 | |
| Death | ||||
| Age >60 years | 2.37 | (1.23-4.59) | 0.01 | |
| Dyspnea | 2.48 | (1.26-4.91) | 0.009 | |
| Hemoglobin below normal | 2.74 | (1.39-5.42) | 0.004 | |
| Chest X-ray positive | 3.74 | (1.44-9.69) | 0.01 | |
| Microbiology positive | 4.47 | (2.16-9.27) | <0.001 | |
| Elevated alanine aminotransferase | 2.85 | (1.09-7.44) | 0.03 |
The first multivariate models for each outcome included all variables with p<=0.05 in the single variable models in Table 3. After removing non-significant variables from the first and second multivariable models (not shown), the independent variables in the final models here were adjusted for each other.
The PCR cycle threshold (CT) value is the number of cycles necessary to spot the virus. A lower threshold indicates high viral count.
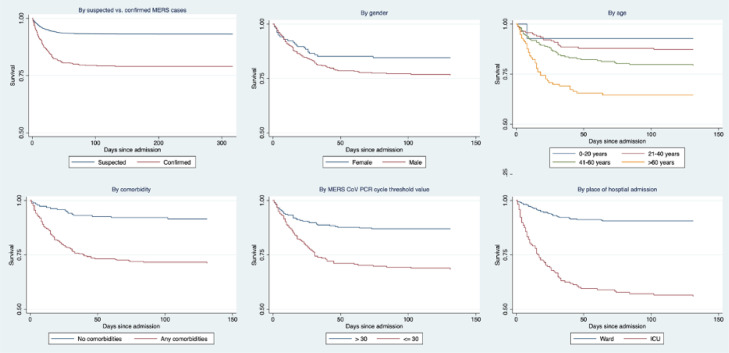
Overall, 21% of MERS-CoV patients died, with a greater percentage of men dying than women (23.7% vs. 15.5%). Kaplan Meier survival analysis indicated lower survival among MERS-CoV confirmed cases compared to suspected unconfirmed cases (Figure 2). Among MERS-CoV confirmed patients, survival decreased with increasing age, and was the lowest among male, those with co-morbidities, those admitted through the intensive care unit, and those with high viremia (PCR Ct <30) (Figure 2).
Figure 2.
MERS-CoV in Saudi Arabia, 2014-2019: Kaplan Meier survival analysis of 6873 suspected (501 confirmed) cases admitted to one of the four MERS-CoV referral hospitals. Curves shown for survival among all suspected laboratory negative and laboratory confirmed cases, and among laboratory confirmed cases by gender, age, comorbidity, place of first admission, and PCR Cycle threshold (CT) values
In the final multivariate model, independent predictors of death varied slightly from that of factors predictive of ventilation requirement. Factors significantly associated with death included age >60 years [AOR=2.37 (95% CI: 1.23-4.59), P=0.01], dyspnea [AOR=2.48 (95% CI: 1.26-4.91), P=0.009], hemoglobin below normal [AOR=2.74 (95% CI: 1.39-5.42), P=0.004], chest X-ray positive [AOR=3.74 (95% CI: 1.44-9.69), P=0.01], microbiology positive [AOR=4.47 (95% CI: 2.16 9.27), P<0.001], and elevated ALT [AOR=2.85 (95% CI: 1.09-7.44), P=0.03]. Over time, the variation in the percentage of MERS-CoV patients who required ventilation or died was not significant in univariate or multivariate analysis.
4. Discussion
In this cohort of 6,873 persons suspected of MERS-CoV infection, we found a MERS-CoV positivity rate averaging at 7.4% during 2014 through 2019. Nearly two thirds of MERS-CoV patients had comorbidities, diabetes being the most common, and more than half of the patients had two or more concurrent comorbidities. MERS-CoV patients were hospitalized for an average of 18 days, two-thirds (64%) developed organ complications, more than a quarter (29%) developed severity requiring ventilation, and 21% died at hospital. Our findings on key characteristics of MERS-CoV (percent with comorbidity, days of hospitalization, percent who require ventilation, case fatality rate) are in the lowest range of values reported to date [7,15,34,35]. Our report updates information on the most common concurrent infections that are likely to be observed in settings that have implemented most advanced infection control measures.
The most important contribution of this report is the updating of earlier epidemiologic and clinical observations of MERS-CoV based on small samples of patients, pooled data, or time-limited analyses providing more accurate mean values. Our findings emanate from the largest ever reported single case series on persons with MERS-CoV and followed through the entire course of illness in the only remaining MERS-CoV endemic country, Saudi Arabia MERS-CoV. Data in this report were obtained after the revision in 2013 of the WHO interim surveillance guidelines that allowed screening over a wider geographic area.
Our data is subject to some limitations. In this cohort, while the larger sample size of patients gives more accurate mean values, the high index of suspicion among health care providers and more streamlined clinical case definitions may have resulted in early detection of the MERS-CoV infections than in the early stages of the outbreak when MERS-CoV diagnosis may have been delayed [2]. Another limitation is that all patients in our report are hospitalized and have benefited from a range of potential new or repurposed pharmaceutical treatment options on trial influencing the clinical progression and survival [15,26]. The MERS-CoV case definition is symptom-based and therefore clinical patient-based reports may not capture the true population prevalence of MERS-CoV or accurately describe the characteristics of human MERS-CoV infections in the population [25,26]. In addition, our analysis is based on a non-random sample of patients receiving care at a large referral hospital. Although we assume that the demographic and risk profiles of these patients are comparable to non-sampled patients seeking diagnoses and treatment for MERS elsewhere, we were not able to confirm this assumption and its effect on the generalizability of our results to the broader Saudi population. However, given the severity of MERS-CoV, characterization of MERS-CoV infection may be sufficient to inform clinical practice guidelines. Another limitation of this report that BMI data were not routinely reported in a standardized way during the period of analysis. In the analysis we could not maintain covariates in the models as potential confounders, even if they demonstrated non-significance in bivariate analyses/single models. And Lastly, we did not test for interactions in the multivariable models.
The characteristics that are independent predicters of the diagnosis of MERS-CoV among the suspected cases are non-specific and were observed earlier at least in univariate analyses—male gender, 40-60 years of age, known transmission link, fever, symptoms for >7 days, admission through the ICU, and co-morbid diabetes. As observed with other lethal coronaviruses SARS and SARS-COV-2, the non-specificity of epidemiologic and clinical symptoms highlights the limitation of their utility in differentiating MERS-CoV infection form other and respiratory infections including that caused by the other two lethal coronaviruses.
The reasons for men being disproportionately affected by MERS-CoV is not well understood [2,15,32,36,37]. Based on the large sample of patients in this cohort, we reflect on the transmission dynamics of MERS-CoV with reference to metabolic syndrome related conditions, male gender, age, the geography of MERS-CoV prevalence among camels, and per-capita camel population [2,11,38,39]. The fact that over 95% of the suspected persons and 59% of MERS-CoV confirmed patients had no known exposure to potential source of infection, coupled with high correlation of infection among those with animal and case contact underscores the poorly understood transmission dynamics of MERS-CoV [11].
As indicated by earlier observations on the relationship between MERS-CoV and diabetes, the higher prevalence of diabetes among men in KSA (34%) relative to women (27%) and as high as 50% among persons over 50 years, and the fact that about 40% of MERS-CoV patients had diabetes helps to strengthen the diabetes-male MERS risk explanations [34,[40], [41], [42], [43], [44]]. Population prevalence of overweight and obesity (2019, overall, 38% and 28% respectively) has been on the increase in KSA [34,40]. In KSA, men are more likely to be overweight than women (41% vs 35%) whereas their obesity prevalence is lower than that among women (26% vs 29%) [40,45]. The cellular receptor for MERS-CoV is the surface molecule DPP4. Studies that induced DPP4 in mice using a high fat diet led to the diabetic mice exhibiting a more prolonged and severe course of disease following MERS-CoV infection [41,46]. Metabolic disorders are known to down-regulate key mediators of the host innate immune response to pathogenesis [46,47]. In our cohort, MERS-CoV suspected but unconfirmed women had a greater diabetes prevalence than MERS-CoV infected indicating that women with diabetes are more likely to present with community acquired non-MERS-CoV respiratory diseases leading to their inclusion among the suspected cases [15]. However, among men, diabetes was more prevalent among MERS-CoV confirmed patients [34,45]. This would mean that men aged 40-60 years in Saudi Arabia have greater exposure to the source of infection than women. Further, the high prevalence of metabolic diseases -related pathophysiology in men [14,34,35,40,41] predisposes them to the expression of MERS-CoV disease. KSA has the 4th largest camel to population ratio (1 to 40 persons) after Somalia, Mauritania (1 to 1.4) and Sudan (1 to 8.6) [39,48]. The prevalence of diabetes in these countries with high per-capita camel population (Mauritania 6.7%, Somalia 5.1%, Sudan 7.7%) is however substantially lower than that of Saudi Arabia and may not provide sufficient susceptible population to cause large outbreaks [48,49]. The most recent seroprevalence of MERS-CoV among camels in Saudi Arabia was 92.7% and RNA detection rate was 17.2%, with some variation between imported and resident herds, but RNA detection was as high as 35.5% among resident camel herds than imported [11,29,48,49]. Therefore, it appears the confluence of three factors- presence of a highly susceptible population notably with metabolic syndrome conditions, high MERS-CoV burden among the host, and sufficient interaction between with the host and susceptible population —should be met for efficient and extensive transmission of MERS-CoV [38,50]. Case-control studies among camel herders and controlling for diabetes and phylogenetic analyses of MERS-CoV would help clarify the diabetes-camel-MERS-CoV interactions.
Our report helps to update the prevalence of clinical outcomes with more generalizable data. We note also that clinical symptoms, laboratory and diagnostic imaging findings of MERS-CoV are non-specific and similar to that of other community-acquired respiratory tract infections [15,51,52]. Though imaging and chest X-ray features resemble the findings of community acquired pneumonia, the high rates of progression to acute respiratory distress syndrome and death distinguishes MERS-CoV from other common respiratory infections and coronaviruses [16,41,53,54]. Pathological changes in the lungs include evidence of focal hemorrhagic necrotizing pneumonia with exudative diffuse alveolar damage, indistinguishable from findings detected in severe pneumonia caused by other viral agents. Patients with MERS can typically present with fever, chills, rigors, headache, a non-productive cough, sore throat, arthralgia, and myalgia followed by dyspnea [15,51,55]. Other associated symptoms include coryza, nausea, vomiting, dizziness, sputum production, diarrhea, and abdominal pain [8]. Our patients did not show high burden of kidney anomalies as reported earlier based on 70 patients in a center with high concentration of patients with renal pathologies [27]. This may be due to the difference in underlying comorbidities of patients between the two groups.
Co-infection with MERS-CoV and SARS CoV-2 have been reported in Saudi Arabia yet. Of the 8 patients reported, 4 (50%) were discharged home and 3 (37.5%) died [21]. Co-infection of MERS-CoV with other respiratory viruses (such as parainfluenza virus, rhinovirus, influenza A or B virus, respiratory syncytial virus, enteroviruses, and human metapneumovirus) and nosocomial bacterial infections has been reported in patients receiving intensive care [2,34,56]. Given that hospital infection control procedures have been updated and strengthened for MERS-CoV in KSA, the continued role of concurrent infections affecting over one-third of MERS-CoV patients may be reflective of the multi-system immunopathology of MERS-CoV infection [28,56,57].
Independent predictors of severity and death varied slightly in this cohort and probably relates to advance care and management received by MERS-CoV patients. The independent risk for both ventilation requirement and death were lung pathology (indicated by positive chest X-ray as an independent risk) and susceptibility to concurrent infections (indicated by microbiology positively as an independent risk) underscores the significance of lung involvement in coronavirus infections [15]. Our multivariate regression models did not identify comorbidity or gender as a determinant of MERS-CoV disease progression as has been reported earlier [15,27]. Pooling of data small sample sizes, and shorter timeframe of evaluation may all have contributed to such observations in earlier reports [5,10,27,37].
Of note, the odds of viremia were higher among those required ventilation predisposing them to more concurrent infections as shown by blood culture and microbiology results among patients requiring ventilation. Access to repurposed therapeutic options may have averted death among some patients. Therefore, not all the independent risk factors for severity may be relevant for death.
In summary, this report serves as the most comprehensive and large sample-based epidemiological and clinical outcome data gleaned from following over six thousand MERS-CoV suspected cases through confirmation and the entire duration of clinical course. Given the rarity of MERS-CoV, continued collection, storage, and analysis of MERS-CoV data remains a strategic priority to inform global emerging disease research.
Data sharing statement
All the data used for the study are included in the manuscript and supplementary material.
Funding source
No special funding to declare.
Contributions
SHA, ZAM, AMA and NAA provided clinical care to patients whose data are used in this report, supervised and the data collection and storage. SHE developed the analytical framework, and AM performed all statistical analyses, developed graphics and tables and wrote the relevant methods section. UK conducted preliminary data analysis and formatted the tables and conducted literature search. SHE wrote the drafts of the full paper with input from all co-authors. SHE, SAA and ZAM, AMA reviewed country specific risk factors and epidemiology. All authors approved the final draft.
Declaration of Competing Interest
Nothing to declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101191.
Contributor Information
Shahul H. Ebrahim, Email: ebrahimsh2@gmail.com.
Andrew D. Maher, Email: Andrew.maher@ucsf.edu.
Udhayashankar Kanagasabai, Email: k.udhayashankarmd@gmail.com.
Sarah H. Alfaraj, Email: alfarajsa@pmah.med.sa.
Nojom A. Alzahrani, Email: alzahranin@pmah.med.sa.
Saleh A. Alqahtani, Email: Salqaht1@jhmi.edu.
Abdullah M. Assiri, Email: abasiri@me.com.
Ziad A. Memish, Email: zmemish@yahoo.com.
Appendix. Supplementary materials
References
- 1.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DAT, et al. Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N Engl J Med [Internet] 2013 Jun 19;369(5):407–416. doi: 10.1056/NEJMoa1306742. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet [Internet] 2015 Sep 5;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. A research and development Blueprint for action to prevent epidemics - Plan Of Action May 2016. 2016;(May):44. Available from: https://www.who.int/blueprint/about/r_d_blueprint_plan_of_action.pdf
- 4.Modjarrad K, Moorthy VS, Ben Embarek P, Van Kerkhove M, Kim J, Kieny M-P. A roadmap for MERS-CoV research and product development: report from a World Health Organization consultation. Nat Med. 2016 Jul;22(7):701–705. doi: 10.1038/nm.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assiri A, Al-tawfi JA, Al-rabeeah AA, Al-rabiah FA, Al-hajjar S, Al-barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia : a descriptive study. Lancet. 2013;13(September) doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Clinical management of severe acute respiratory infection when Middle East respiratory syndrome coronavirus (MERS-CoV) infection is suspected. Interim guidance Updated January 2019 WHO/MERS/Clinical/15.1 Revision 1. 2019;1–12.
- 7.Perlman S, Azhar EI, Memish ZA, Hui DS, Zumla A. Confronting the persisting threat of the Middle East respiratory syndrome to global health security. Lancet Infect Dis [Internet] 2020 Feb;20(2):158–160. doi: 10.1016/S1473-3099(19)30347-0. https://pubmed.ncbi.nlm.nih.gov/31279728 2019/07/03Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memish ZA, Perlman S, Van Kerkhove MD, Zumla A. Middle East respiratory syndrome. Lancet [Internet] 2020;395(10229):1063–1077. doi: 10.1016/S0140-6736(19)33221-0. http://www.sciencedirect.com/science/article/pii/S0140673619332210 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health organization (WHO). Middle East respirtaory syndrome coronavirus (MERS-CoV) - The United Arab Emirates [Internet]. Emergencies preparedness, response. 2020 [cited 2020 Nov 22]. Available from: https://www.who.int/csr/don/08-january-2020-mers-uae/en/
- 10.Alfaraj SH, Al-taw JA, Gautret P, Alenazi MG, Asiri AY, Memish ZA. Evaluation of visual triage for screening of Middle East respiratory syndrome coronavirus patients. 2018;49–52. [DOI] [PMC free article] [PubMed]
- 11.Killerby M, Biggs H, Midgley C, Gerber S, Watson J. Middle East Respiratory Syndrome Coronavirus Transmission. Emerg Infect Dis J [Internet] 2020;26(2):191. doi: 10.3201/eid2602.190697. https://wwwnc.cdc.gov/eid/article/26/2/19-0697_article Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.{WHO}. Middle East respiratory syndrome coronavirus (MERS-CoV): Summary and Risk Assessment of Current Situation in the Republic of Korea and China [Internet]. 2015. Available from: https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov )
- 13.Kim T, Jung J, Kim S, Seo D, Lee YS. Transmission among healthcare worker contacts with a Middle East respiratory syndrome patient in a single Korean centre Appendix A . Supplementary data. Clin Microbiol Infect [Internet] 2016;22(2):e11–e13. doi: 10.1016/j.cmi.2015.09.007. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinedo-Torres I, Flores-Fernández M, Yovera-Aldana M, Gutierrez-Ortiz C, Zegarra-Lizana P, Intimayta-Escalante C, et al. Prevalence of Diabetes Mellitus and Its Associated Unfavorable Outcomes in Patients With Acute Respiratory Syndromes Due to Coronaviruses Infection: A Systematic Review and Meta-Analysis. Clin Med Insights Endocrinol Diabetes [Internet] 2020 Oct 19;13 doi: 10.1177/1179551420962495. https://pubmed.ncbi.nlm.nih.gov/33177910 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfaraj SH, Al-taw A, Assiri AY, Alzahrani NA, Alanazi AA, Memish ZA. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection : A cohort study. Travel Med Infect Dis. 2019;29(October 2018):48–50. doi: 10.1016/j.tmaid.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen B, Tian EK, He B, Tian L, Han R, Wang S, et al. Overview of lethal human coronaviruses. Signal Transduct Target Ther. 2020;5(1) doi: 10.1038/s41392-020-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haagmans BL, Al Dhahiry SHS, Reusken CBEM, Raj VS, Galiano M, Myers R, et al. Middle East respiratory syndrome coronavirus in dromedary camels : an outbreak investigation. Lancet Infect Dis [Internet] 2014;14(2):140–145. doi: 10.1016/S1473-3099(13)70690-X. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfaraj SH, Al-Tawfiq JA, Alzahrani NA, Altwaijri TA, Memish ZA. The impact of co-infection of influenza A virus on the severity of Middle East Respiratory Syndrome Coronavirus. J Infect [Internet] 2017;74(5):521–523. doi: 10.1016/j.jinf.2017.02.001. http://www.sciencedirect.com/science/article/pii/S0163445317300488 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madani TA. Case definition and management of patients with MERS coronavirus in Saudi Arabia. Lancet Infect Dis [Internet] 2014 Oct;14(10):911–913. doi: 10.1016/S1473-3099(14)70918-1. https://pubmed.ncbi.nlm.nih.gov/25253396 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bialek SR, Allen D, Alvarado-Ramy F, Arthur R, Balajee A, Bell D, et al. First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities - May 20. Am J Transplant. 2014;14(7):1693–1699. [PMC free article] [PubMed] [Google Scholar]
- 21.Elhazmi A, Al-Tawfiq JA, Sallam H, Al-Omari A, Alhumaid S, Mady A, Al Mutair A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) coinfection: A unique case series. Travel Med Infect Dis. 2021;(3) doi: 10.1016/j.tmaid.2021.102026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization WHO Research and Development Blueprint 2018 Annual review of diseases prioritized under the Research and Development Blueprint. World Heal Organ [Internet] 2018;(February):6–7. http://www.who.int/emergencies/diseases/2018prioritization-report.pdf?ua=1 February 2018, Geneva, Switzerland. Available from: [Google Scholar]
- 23.Ghazal H, Ghazal S, Alharbi T, Al Nujaidi M, Memish Z. Middle-East respiratory syndrome-coronavirus: Putting emergency departments in the spotlight. J Heal Spec [Internet] 2017 Apr 1;5(2):51–54. https://www.thejhs.org/article.asp?issn=2468-6360 Available from: [Google Scholar]
- 24.Alfaraj SH, Al-taw JA, Memish ZA. Middle East respiratory syndrome coronavirus intermittent positive cases : Implications for infection control. Am J Epidemiol. 2019;47:290–293. doi: 10.1016/j.ajic.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim C, Choi WS, Jung Y, Kiem S, Seol HY, Woo HJ, et al. Surveillance of the Middle East respiratory syndrome (MERS) coronavirus (CoV) infection in healthcare workers after contact with confirmed MERS patients : incidence and risk factors of MERS-CoV seropositivity. Clin Microbiol Infect. 2016;22:880–886. doi: 10.1016/j.cmi.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gautret P, Mccloskey B, Memish ZA. Case definition and management of patients with MERS coronavirus in Saudi Arabia. Lancet. 2014;14(October):911–913. doi: 10.1016/S1473-3099(14)70918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Abdul M, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection : a single-center experience in Saudi Arabia. Int J Infect Dis [Internet] 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libby P. Middle East Respiratory Syndrome Coronavirus Infections in Health Care Workers. N Engl J Med. 2013:9–11. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 29.Azhar EI, El-kafrawy SA, Farraj SA, Hassan AM, Al-saeed MS, Hashem AM, et al. Evidence for Camel-to-Human Transmission of MERS Coronavirus. N Engl J Med. 2014:1–7. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 30.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, Assiri A, Alhakeem RF, Albarrak A, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014 Nov;210(10):1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance [Internet] 2020;25(3):1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohd HA, Memish ZA, Alfaraj SH, Mcclish D, Altuwaijri T, Alanazi MS, et al. Predictors of MERS-CoV infection : A large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia. Travel Med Infect Dis [Internet] 2016;14(5):464–470. doi: 10.1016/j.tmaid.2016.09.008. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfaraj SH, Al-tawfiq JA, Edin F, London F. Middle East respiratory syndrome coronavirus transmission among health care workers : Implication for infection control. AJIC Am J Infect Control [Internet] 2018;46(2):165–168. doi: 10.1016/j.ajic.2017.08.010. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis [Internet] 2016;49(January):129–133. doi: 10.1016/j.ijid.2016.06.015. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan S, El Morabet R, Khan RA, Bindajam A, Alqadhi S, Alsubih M, et al. Where we missed? Middle East Respiratory Syndrome (MERS-CoV) epidemiology in Saudi Arabia; 2012-2019. Sci Total Environ [Internet] 2020 Dec 10;747 doi: 10.1016/j.scitotenv.2020.141369. https://pubmed.ncbi.nlm.nih.gov/32791417 2020/08/03Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perlman S, Azhar EI, Memish ZA, Hui DS, Zumla A. Confronting the persisting threat of the Middle East respiratory syndrome to global health security. Lancet Infect Dis. 2020;20(2):158–160. doi: 10.1016/S1473-3099(19)30347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol [Internet] 2016 Aug;14(8):523–534. doi: 10.1038/nrmicro.2016.81. https://pubmed.ncbi.nlm.nih.gov/27344959 2016/06/27Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alshukairi AN, Zheng J, Zhao J, Nehdi A, Baharoon SA, Layqah L, et al. High Prevalence of MERS-CoV Infection in Camel Workers in Saudi Arabia. MBio. 2018;9(5):1–10. doi: 10.1128/mBio.01985-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gossner C, Danielson N, Gervelmeyer A, Berthe F, Faye B, Kaasik Aaslav K, et al. Human–Dromedary Camel Interactions and the Risk of Acquiring Zoonotic Middle East Respiratory Syndrome Coronavirus Infection. Zoonoses Public Health [Internet] 2016 Feb 1;63(1):1–9. doi: 10.1111/zph.12171. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Raddadi R, Bahijri SM, Jambi HA, Ferns G, Tuomilehto J. The prevalence of obesity and overweight, associated demographic and lifestyle factors, and health status in the adult population of Jeddah, Saudi Arabia. Ther Adv Chronic Dis [Internet] 2019 Sep 30;10 doi: 10.1177/2040622319878997. https://pubmed.ncbi.nlm.nih.gov/31632623 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazucanti CH, Egan JM. SARS-CoV-2 disease severity and diabetes: why the connection and what is to be done? Immun Ageing [Internet] 2020;17(1):21. doi: 10.1186/s12979-020-00192-y. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20) doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alanazi K, Abedi G, Midgley C, Alkhamis A, Alsaqer T, Almoaddi A, et al. Diabetes Mellitus, Hypertension, and Death among 32 Patients with MERS-CoV Infection, Saudi Arabia. Emerg Infect Dis J [Internet] 2020;26(1):166. doi: 10.3201/eid2601.190952. https://wwwnc.cdc.gov/eid/article/26/1/19-0952_article Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benskin LL. A Basic Review of the Preliminary Evidence That COVID-19 Risk and Severity Is Increased in Vitamin D Deficiency. Front Public Heal. 2020;8(September):1–25. doi: 10.3389/fpubh.2020.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinedo-Torres I, Flores-Fernández M, Yovera-Aldana M, Gutierrez-Ortiz C, Zegarra-Lizana P, Intimayta-Escalante C, et al. Prevalence of Diabetes Mellitus and Its Associated Unfavorable Outcomes in Patients With Acute Respiratory Syndromes Due to Coronaviruses Infection: A Systematic Review and Meta-Analysis. Clin Med Insights Endocrinol Diabetes [Internet] 2020 Oct 19;13 doi: 10.1177/1179551420962495. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7592335/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI insight. 2019 Oct;4(20) doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamel KS, Schreiber M, Carlotti APCP, Halperin ML. Approach to the Treatment of Diabetic Ketoacidosis. Am J Kidney Dis [Internet] 2016;68(6):967–972. doi: 10.1053/j.ajkd.2016.05.034. Available from: [DOI] [PubMed] [Google Scholar]
- 48.Tolah AM, AL Masaudi SB, El-Kafrawy SA, Mirza AA, Harakeh SM, Hassan AM, et al. Cross-sectional prevalence study of MERS-CoV in local and imported dromedary camels in Saudi Arabia, 2016-2018. PLoS One [Internet] 2020 May 26;15(5) doi: 10.1371/journal.pone.0232790. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farag E, Sikkema R, Mohamedani A, de Bruin E, Munnink BO, Chandler F, et al. MERS-CoV in Camels but Not Camel Handlers, Sudan, 2015 and 2017. Emerg Infect Dis J [Internet] 2019;25(12):2333. doi: 10.3201/eid2512.190882. https://wwwnc.cdc.gov/eid/article/25/12/19-0882_article Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adney DR, Letko M, Ragan IK, Scott D, van Doremalen N, Bowen RA, et al. Bactrian camels shed large quantities of Middle East respiratory syndrome coronavirus (MERS-CoV) after experimental infection. Emerg Microbes Infect. 2019;8(1):717–723. doi: 10.1080/22221751.2019.1618687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drosten C, Seilmaier M, Corman VM, Hartmann W, Scheible G, Sack S, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet. 2013:745–751. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Omrani AS, Matin MA, Haddad Q, Al-Nakhli D, Memish ZA. Albarrak AM. A family cluster of middle east respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis [Internet] 2013;17(9):e668–e672. doi: 10.1016/j.ijid.2013.07.001. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinzerling A, Stuckey MJ, Scheuer T, Xu K, Perkins KM, Resseger H, et al. Transmission of COVID-19 to Health Care Personnel During Exposures to a Hospitalized Patient — Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, et al. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses. 2019 Jan;11(1) doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omrani AS, Matin MA, Haddad Q, Al-Nakhli D, Memish ZA. Albarrak AM. A family cluster of Middle East Respiratory Syndrome Coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis [Internet] 2013;17(9):e668–e672. doi: 10.1016/j.ijid.2013.07.001. http://www.sciencedirect.com/science/article/pii/S1201971213002257 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans C, Hospital ST, Bernal JL, England PH. Respiratory viral infections, their interactions with SARS-CoV-2 and implications for a winter resurgence of COVID-19 [Internet]. London; Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/905056/S0627_NERVTAG_Respiratory_viral_infections__their_interactions_with_SARS-CoV-2_and_implications_for_a_winter_resurgence.pdf
- 57.Amer H, Alqahtani AS, Alaklobi F, Altayeb J, Memish ZA. Healthcare worker exposure to Middle East respiratory syndrome coronavirus (MERS-CoV): Revision of screening strategies urgently needed. Int J Infect Dis [Internet] 2018;71:113–116. doi: 10.1016/j.ijid.2018.04.001. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.