Abstract
Vascular basement membrane (BM) thickening has been hailed over half a century as the most prominent histological lesion in diabetic microangiopathy, and represents an early ultrastructural change in diabetic retinopathy (DR). Although vascular complications of DR have been clinically well established, specific cellular and molecular mechanisms underlying dysfunction of small vessels are not well understood. In DR, small vessels develop insidiously as BM thickening occurs. Studies examining high resolution imaging data have established BM thickening as one of the foremost structural abnormalities of retinal capillaries. This fundamental structural change develops, at least in part, from excess accumulation of BM components. Although BM thickening is closely associated with the development of DR, its contributory role in the pathogenesis of DR is coming to light recently. DR develops over several years before clinical manifestations appear, and it is during this clinically silent period that hyperglycemia induces excess synthesis of BM components, contributes to vascular BM thickening, and promotes structural and functional lesions including cell death and vascular leakage in the diabetic retina. Studies using animal models show promising results in preventing BM thickening with subsequent beneficial effects. Several gene regulatory approaches are being developed to prevent excess synthesis of vascular BM components in an effort to reduce BM thickening. This review highlights current understanding of capillary BM thickening development, role of BM thickening in retinal vascular lesions, and strategies for preventing vascular BM thickening as a potential therapeutic strategy in alleviating characteristic lesions associated with DR.
Keywords: BM thickening, Hyperglycemia, Apoptosis, Angiogenesis, DR
2. Introduction
2.1. Diabetic Retinopathy
Diabetic retinopathy (DR), a prevalent microvascular complication of diabetes, is the leading cause of blindness and vision loss in the working age population (Duh et al., 2017). DR develops in two stages: background DR (BDR) or non-proliferative DR (NPDR) seen in the early stage, and the late stage represented by proliferative DR (PDR) (Cunha-Vaz et al., 2014; Duh et al., 2017; Simo and Hernandez, 2015; Stitt et al., 2016; Tang and Kern, 2011). BDR is clinically characterized by microaneurysms, intraretinal microvascular abnormalities (IRMA), and vascular permeability. The advanced stage PDR is marked by hypoxia, neovascularization, and angiogenesis. PDR develops as a long-term complication of diabetes generally emerging after 15 years of hyperglycemia (Fong et al., 2004). Importantly, hyperglycemia-driven vascular BM thickening is not only the most common histological hallmark in DR but is also present among the two other diabetic microangiopathies, diabetic nephropathy and diabetic neuropathy (Richner et al., 2018; Williamson and Kilo, 1983). The universal presence of vascular BM thickening in diabetic tissues highlights its importance in DR.
2.2. BM overview
BM is a ubiquitous, multicomponent, ultrastructural layer that provides structural integrity to small blood vessels, and displays diverse functions. The composition of vascular BM has been widely studied and the collective findings indicate presence of several key components including collagen type IV (collagen IV), fibronectin, laminin, and perlecan, a BM specific heparan sulfate proteoglycan (HSPG) (Das et al., 1990b; Jerdan and Glaser, 1986) (Table 1). BM components assemble in a highly organized manner several of those through crosslinking to form a continuous layer that serves as a substratum for attachment of endothelial cells on the luminal surface, and pericytes on the abluminal surface of capillaries. Additionally, BM plays a critical role in cell spreading, migration, growth, repair and differentiation (Ffrench-Constant et al., 1989; Furcht et al., 1984; Morrissey and Sherwood, 2015; Podesta et al., 1997; Yurchenco, 2015). At the ultrastructural level, BM exhibits morphologically distinct zones (Carlson and Bjork, 1990), the lamina densa representing the more electron-dense central region with lamina rara interna facing the cellular side and lamina rara externa facing the stromal side. In individuals with long-term diabetes, the BM becomes significantly thickened in microvasculature of all tissues (Kilo et al., 1972; Siperstein et al., 1968; Williamson et al., 1969) (Table 2). It is important to note that the extent to which vascular BM thickness develops varies in different tissues (Cherian 2009).
Table 1.
Diabetes-induced changes in ECM component levels associated with BM thickening
| ECM Component | Level | Species | References |
|---|---|---|---|
| Fibronectin | Increased | Human | (Ljubimov et al., 1996; Ljubimov et al., 1998; Monnier et al., 1999; Monnier et al., 1986; Nwomeh et al., 1998; Risteli et al., 1987; Roux et al., 1977; Roy et al., 1996; Salmela et al., 1989) |
| Laminin-111 (α1, β1, γ1) | Increased | Human, Rat | (Bek and Ledet, 1996; Das et al., 1990) |
| Vitronectin | Increased | Human | (Bek and Ledet, 1996; Weller et al., 1991) |
| Collagen type I | Increased | Human | (Salmela et al., 1989) |
| Collagen type III | Increased | Human | (Ljubimov et al., 1998; Salmela et al., 1989) |
| α1 Collagen type IV | Increased | Human, Mouse, Rat | (Cooper et al., 1998; Das et al., 1990; Ljubimov et al., 1996; Rasmussen and Ledet, 1993; Roy et al., 1994; Rumble et al., 1998) |
| α2 Collagen type IV | Increased | Human, Rat | (Ljubimov et al., 1996) |
| Collagen type V | Increased /Unchanged | Human | (Labat-Robert and Robert, 1988; Rasmussen and Ledet, 1993; Salmela et al., 1989) |
| Collagen type VI | Increased | Human, Rat | (Bek and Ledet, 1996; Shinoda, 1992; Spiro and Crowley, 1993) |
| Tenascin | Increased | Human | (Monnier et al., 1999; Roux et al., 1977; Salmela et al., 1989) |
| Chondroitin sulfate | Decreased/ Increased | Human | (Anderson, 1993; Hagedorn et al., 1993) |
| Hyaluronic acid | Increased | Human | (Brenner, 1994) |
| Heparan sulfate | Increased/Unchanged/Decreased | Human, Rat | (Anderson, 1993; Chowdhury et al., 1995; Das et al., 1990; Huijberts et al., 1994; Monnier et al., 1999; Nakamura et al., 1993) |
Table 2.
Vascular basement membrane thickening in human diabetic microangiopathies
| Tissue | Normal BM Thickness (nm) | Diabetic BM Thickness (nm) | References |
|---|---|---|---|
| Retina | 292 ± 24 | 583.1 ± 38.52 | (Bianchi et al., 2016) |
| Glomerulus | 321 ± 21 | 482 ± 151 | (Rayat et al., 2005) |
| Skeletal Muscles | 108 ± 2.7 | 240.3 ± 11.9 | (Longhurst et al., 1975) |
| Quadricep Muscles | 118 ± 2.7 | 203.1 ± 18.7 | (Lindsay et al., 1994; Quabbe et al., 1983; Siperstein et al., 1968) |
| Skin | 250 ± 34 | 353.3 ± 38 | (Bae et al., 1987) |
| Lungs | 164 ± 14 | 223 ± 27 | (Weynand et al., 1999) |
| Perineurium | 405.86 ± 4.2 | 524.55 ± 5.9 | (Hill and Williams, 2004) |
2.3. BM thickening as a pathogenetic factor – a historical perspective
The term “basement membrane” was first introduced by the great ophthalmologist, Sir William Bowman (Bowman and Todd, 1858). As early as 1921, Henry Wagener and Russel Wilder from Mayo Clinic observed that DR, then referred to as “retinitis of diabetes”, is almost always accompanied by vascular complications of the retina (Wagener and Wilder, 1921). Long before electron microscopy came into vogue, Norman Ashton using light microscopy observed that the BM of retinal capillaries in diabetic subjects were intensely stained for PAS (Ashton, 1949), offering early insights that abnormal vascular BM thickening is closely associated with DR. One of the first indications that capillary BM thickening plays a critical role in the development of microvascular complications in diabetes came to light when studies using electron microscopy revealed a close association between BM thickening in glomerular capillaries and diabetic patients with glomerulosclerosis (Bergstrand and Bucht, 1957; Farquhar et al., 1959). Subsequently, a study using electron microscopy investigating ultrastructural changes in the diabetic retina revealed significant BM thickening in retinal capillaries of individuals with DR compared to those of non-diabetic subjects, providing further evidence that the thickened retinal capillary BM is a key histological feature of DR (Toussaint and Dustin, 1963). Furthermore, studies investigating microangiopathy in diabetic dogs revealed significant thickening of capillary BMs in the retina, glomerulus, and muscle suggesting that vascular BM thickening is a prevalent characteristic in diabetic microangiopathy (Bloodworth et al., 1969; Bloodworth and Molitor, 1965). Similarly, BM thickening in retinal capillaries of diabetic rodents (Fig. 1), and in other animal models of diabetes has been reported. Importantly, capillary BM thickening was noted prior to the development of microaneurysms, which are the earliest clinical manifestations in diabetic retinas (Bloodworth, 1963), thus highlighting thickened vascular BMs as an initial change in the pathogenesis of DR. Shortly after, Norman Ashton, a well-known pathologist, suggested that the thickened vascular BM is a likely contributor to the development and progression of DR (Ashton, 1974). During this period, diabetes was considered a “basement membrane disease” (Hayden et al., 2005; Williamson and Kilo, 1977). Although, our current understanding of the role of BM thickening in the pathogenesis of DR has advanced significantly, further studies are needed to gain insights into mechanisms underlying thickened BM-driven cellular abnormalities in the pathogenesis of DR.
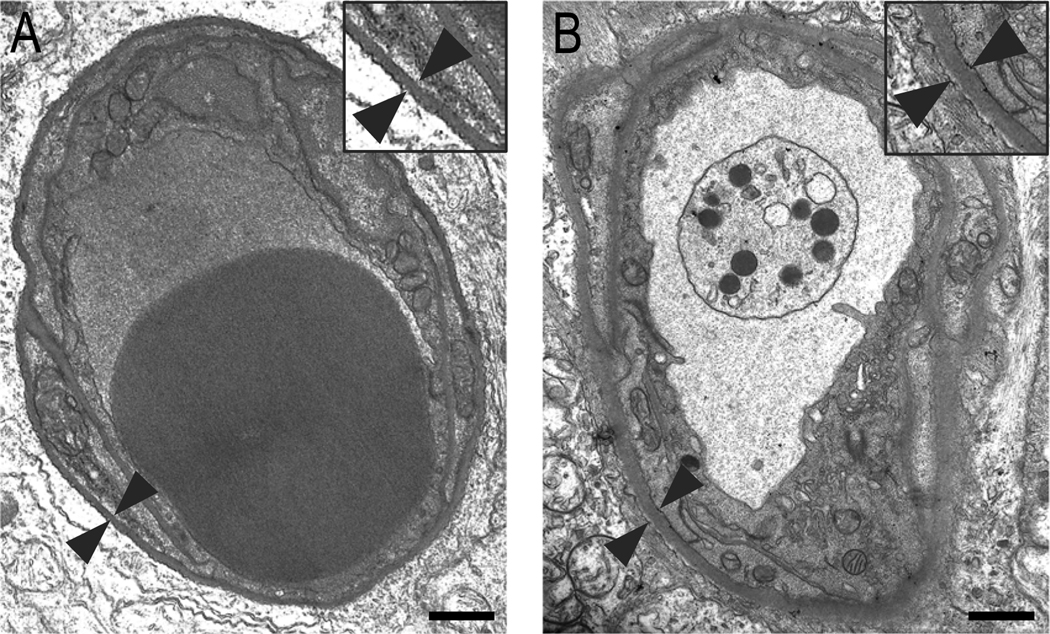
Figure 1. Transverse section of a retinal capillary from a normal rat and a diabetic rat.
Compared to retinal capillary BM of the (A) normal rat, note significant thickening of vascular BM in the (B) diabetic rat retina. Arrowheads indicate a representative area of the retinal capillary BM from outer plexiform layer. Insets represent enlarged view of corresponding fields. Scale bar: 1.0 μm.
3. BM components
Vascular BMs are composed of various components including collagen IV, fibronectin, laminin, and perlecan (Gay et al., 1981; Martinez-Hernandez and Amenta, 1983; Martinez-Hernandez et al., 1982) (Fig. 2). According to some investigators these components are present in specific areas of the BM; however, some disagreement exists in their ultrastructural distribution in the BM.
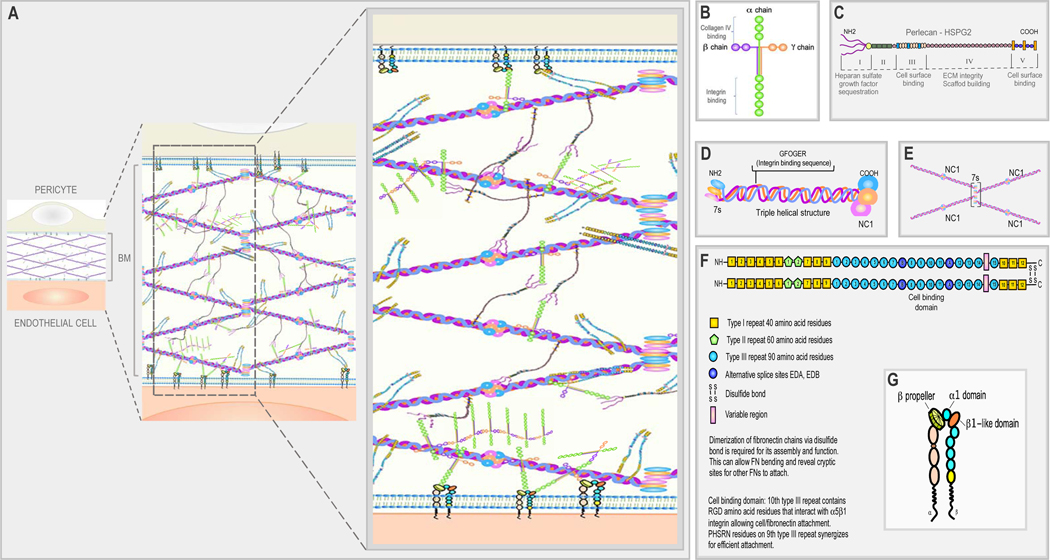
Figure 2. Schematic illustration of retinal vascular BM structure and its components.
(A) BM is a highly organized structure composed of various components including collagen IV, laminin, fibronectin, and HSPG (perlecan). (B) General structure of laminin and its domain-binding sites. The NH2 end of laminin is represented by three “arms” of α chain, β chain, and γ chain, followed by a coiled-coil structure and five large globular domains at the COOH end representing the integrin binding region. (C) Structure of perlecan, a BM-specific HSPG. Perlecan has five globular domains, which are connected by a protein core of a single polypeptide chain. The NH2 end of the first globule (domain I) is attached to three HSPG side chains responsible for sequestration of growth factors. Domains III and V represent cell surface binding regions while domain IV facilitates ECM scaffold formation and maintenance of ECM integrity. (D) Triple helical structure of collagen IV. A protomer of collagen IV is made up of three α-helical polypeptide chains with different combinations; most commonly two α1, and one α2 chains, each with a 7s domain at the NH2 end and a NC1 domain at the COOH end. (E) Formation of collagen IV tetramer: four triple-helical collagen IV molecules undergo cross-linking at the 7s domains, while the NC1 domains dock to another set of NC1 domains, forming a NC1 hexamer and the formation of a “chicken wire” network. (F) Fibronectin exists as a dimer consisting of two similar subunits, which are bound together by disulfide bonds located at the COOH end of each subunit. Dimerization of fibronectin chains is required for its assembly, function and bending that allows cryptic site for other fibronectin polypeptides to attach. Each subunit has 12 type I repeats, two type II repeats, and 15–17 type III repeats. These domains represent specialized areas for cell binding including RGD sites for integrin binding. (G) Schematic diagram of integrin structure: integrins are transmembrane receptors that consist of one α and one β chain forming heterodimers. The “coil” structure represents the transmembrane domain while the “tail” represents the cytoplasmic domain. The specificity of integrins binding to their ligand is primarily conferred through the α chain.
Collagen IV
Collagen IV are exclusively found in the BM, where each collagen molecule is composed of three α-helical polypeptide chains, that are rich in hydroxyproline and hydroxylysine (Marchand et al., 2019; Martinez-Hernandez and Amenta, 1983). In vertebrates, six distinct chains of collagen IV have been identified, referred to as α1(IV) up to α6(IV) chains (Khoshnoodi et al., 2008; Komori et al., 2018). Traditionally, each triple helical collagen IV molecule is composed of two identical α1 chains and one α2 chain (Timpl et al., 1978). More recently, analyses through advancements in ultrastructural assays, different combinations of α chains have been reported indicating three distinct collagen IV heterotrimers, α1α1α2, α3α4α5, or α5α5α6 (Jayadev et al., 2019; Jayadev and Sherwood, 2017).
Collagen IV chains are incorporated into the BM where the collagen network is connected to laminin polymers via linker proteins, nidogen. Collagen IV undergo crosslinking among four triple-helical type IV chains at unique 7S regions at the NH2 ends (Jayadev et al., 2019; Jayadev and Sherwood, 2017; Martinez-Hernandez and Amenta, 1983; Risteli et al., 1980; Timpl et al., 1982; Timpl et al., 1981). At the opposite ends of the four collagen IV molecules, each -COOH end joins to a -COOH end of another collagen IV tetramer. This arrangement of four collagen IV molecules to one another leads to the formation of a polygonal network representing the framework of a BM. Collagen IV networks also serve as scaffolds, which can bind to other BM components, enzymes, and growth factors (Brown et al., 2017; Parkin et al., 2011; Wang et al., 2008). Compared to other interstitial collagen molecules, collagen IV molecules have fundamental distinctions. Apart from having glycine in every third position in the primary protein sequence, it has no homology with other collagens (Babel and Glanville, 1984; Khoshnoodi et al., 2008; Killen et al., 1988). In addition, both α1 and α2 chains contain several interruptions of the Glycine-X-Y motif (Brazel et al., 1988; Schuppan et al., 1980; Schwarz et al., 1986), which provides flexibility to the macromolecular structure. Notably, mutations in COL4A3, COL4A4, and COL4A5 genes leading to dysfunctional collagen IV synthesis and assembly have been reported to promote Alport’s syndrome, characterized by hematuria, nephritis, hearing loss, and even ocular complications (Khoshnoodi et al., 2008; Savige et al., 2010)
Laminin
Laminins represent one of the most prevalent components of BM. In higher organisms, 16 trimeric laminin isoforms have been identified to date with different extents of cell/tissue specificity (Domogatskaya et al., 2012; Hamill et al., 2009; Hohenester and Yurchenco, 2013). Laminins represent a large family of heterotrimeric multidomain proteins that are composed of one of five α chains, one of four β chains and one of three γ chains (Domogatskaya et al., 2012; Hohenester and Yurchenco, 2013; Yurchenco, 2015). The nomenclature for laminins has undergone recent revision where laminin trimers are referred to by their subunit composition with the exclusion of Greek letters, such that α1β1γ1 laminin, then known as laminin-1, is now referred to as laminin-111 (Hamill et al., 2009), whereas α2β1γ3, then known as laminin-12, is now referred to as laminin-213. Further details are well presented in this review by (Hamill et al., 2009). Importantly, laminins exhibit multiple cell-specific functions including cell adhesion, cell differentiation, cell migration, and cell phenotype maintenance (Domogatskaya et al., 2012). At the C-terminal end of the long arm of laminin, α, β, and γ chains assemble via a coiled coil structure, followed by five large globular domains named LG1-LG5, which represent the cellular binding region (Domogatskaya et al., 2012; Hamill et al., 2009; Yurchenco, 2015). The three chains, α, β, and γ, vary in their sizes: 200–440 kD, ~120–200 kD, and ~120–200 kD, respectively. The resultant trimer formed by these three chains (400–800 kD) can link to form a network, which is connected via nidogen to the collagen IV network. While the homology between the tandem repeats of the three chains are similar, a significant difference exists between α chains on one hand and the β and γ chains on the other in terms of their functionality (Domogatskaya et al., 2012).
Perlecan
Perlecan represents a specific heparan sulfate proteoglycan (HSPG) which is present in the vascular BMs, referred to as HSPG2 (Gubbiotti et al., 2017). Perlecan molecules are composed of three long glycosaminoglycan side chains (100–170 nm in length, 65-kD each) that are covalently linked to a protein core of a single polypeptide chain (80 nm in length, 400-kD) comprised of five globular domains (I-V) (Charonis and Tsilibary, 1990; Farach-Carson and Carson, 2007; Gubbiotti et al., 2017; Martinez-Hernandez et al., 1981b; Yamashita et al., 2018). Notably, domain I is responsible for sequestration of growth factors, while domain III and V represent cell surface binding regions (Gubbiotti et al., 2017). Domain IV of perlecan facilitates ECM scaffold formation and maintenance of ECM integrity (Gubbiotti et al., 2017). The glycosaminoglycans are attached to the protein core through a region containing glucuronic acid, galactose, and xylose (Martinez-Hernandez and Amenta, 1983). In the BM, perlecans are most dense in the lamina rara (Ha et al., 2004; Mynderse et al., 1983), and are known to mediate cell attachment (Martinez-Hernandez and Amenta, 1983) and even influence angiogenesis and autophagy (Gubbiotti et al., 2017).
Fibronectin
Fibronectin represents a prominent primary constituent of BM components. Fibronectin is a multidomain glycoprotein that is contiguous to the connective tissue stroma of the BM (Boselli et al., 1981; Martinez-Hernandez and Amenta, 1983; Martinez-Hernandez et al., 1981a). Fibronectin participates in various intra and extra-cellular activities, and exists as two different forms: a soluble form in the plasma and an insoluble form in the ECM of the cell. Soluble fibronectin is produced by hepatocytes, and exists in the blood, saliva, and other body fluids, and participates during blood clotting (Vaca et al., 2020). In parallel, the insoluble form of fibronectin is primarily synthesized by endothelial cells and fibroblasts which ultimately incorporates into the ECM (Vaca et al., 2020). Furthermore, there are three distinct subunits within each monomer; type 1, type II, and type III subunits. Structurally, fibronectin is a dimer consisting of two identical subunits, which are bound together by disulfide bonds located at the C-terminus of each subunit (Miller et al., 2017; Pankov and Yamada, 2002; Vaca et al., 2020). Dimerization of fibronectin chains via disulfide bond is required for its assembly and function, allowing bending of fibronectin to reveal cryptic sites for other fibronectin molecules to attach (Klotzsch et al., 2009). There are 12 type I repeats, two type II repeats, and 15–17 type III repeats, which accounts for approximately 90% of the fibronectin sequence (Pankov and Yamada, 2002). Notably, the 10th type III repeat contains RGD amino acid residues that interact with α5β1 integrin, allowing cell-fibronectin attachment. In addition, the PHSRN residues on the 9th type III repeat synergizes for efficient attachment. Interestingly, fibronectin also undergoes alternative splicing, which are tissue-specific, producing at least 20 distinct isoforms in humans (Chauhan et al., 2004; Schwarzbauer, 1991; White et al., 2008). Primary functions of fibronectin include cellular adhesion, migration, growth and differentiation (Pankov and Yamada, 2002). In order to maintain a highly organized BM structure, fibronectin facilitates specific binding to collagen, heparan sulfate, and other proteoglycans, and is referred to as the “molecular glue” (Hynes et al., 1984).
Nidogen
Nidogen is a 150 kD sulfated monomeric glycoprotein, a component of the BM, which is also commonly referred to as entactin. The “dumb-bell” structure of nidogen consists of an N-terminal globule, which is linked to a C-terminal globule by a stalk. Vertebrates have two distinct nidogens, a shorter 30 nm nidogen-1, and a longer 40 nm nidogen-2 isoform (Ho et al., 2008; Timpl et al., 1983). Notably, the C-terminal globular domain of nidogen has been reported to bind to the center of the “cross-shaped” laminin as well as the triple-helical domain of collagen IV (Aumailley et al., 1989); therefore, nidogen-1 effectively facilitates the noncovalent molecular connections between collagen IV and laminin (Breitkreutz et al., 2013; Dai et al., 2018; Lakshmanan et al., 2020; Wolfstetter et al., 2019).
3.1. BM assembly
BM components are capable of self-assembly as each of the components harbors information needed for binding to specific sites on other macromolecules. The assembly of BM is a multistep process and is facilitated by the binding of laminin to the cell surface via integrins (Li et al., 2005; Li et al., 2002; McKee et al., 2009; McKee et al., 2007; Smyth et al., 1998). The binding of laminins and polymerization of type IV collagen fibrils produce a scaffold of matrix on which other ECM components are assembled in a supramolecular architecture (Kalb and Engel, 1991; Li et al., 2005; McKee et al., 2007). The primary interaction is initiated by the binding of laminin LG domain to integrins, such as α3β1, α6β1, α7β1 and α6β4 integrins (Hohenester and Yurchenco, 2013), as well as sulfated glycolipids, dystroglycan, and heparan sulfate. The assembly of BM is a carefully regulated process, which involves the local degradation of existing BM by matrix metalloproteinases (MMPs) followed by synthesis and accumulation of new BM components. Assembly of the BM is also achieved through interactions between different BM components as well as ECM interactions with cell surface molecules (Yurchenco et al., 2004). Additionally, cell polarity and tissue shape are established during the assembly of BM (Morrissey and Sherwood, 2015).
3.2. BM function
The primary role of the BM is to provide a substratum for cell attachment and act as a physical barrier between different cell types and tissues, thereby maintaining cell shape or size (Timpl et al., 1981). The assembly of the components into their supramolecular architecture involves collagen IV, laminin, fibronectin, and nidogen and other components (Welling and Grantham, 1972). Alterations in these BM components may influence cell behavior including decreased cell attachment (Chakravarti et al., 1990).
The BM also plays a significant role in the selective permeability of molecules to maintain a homeostatic balance between the inner and outer layers of the retinal vasculature. In the retinal capillaries, the inner layer is lined by endothelial cells, which overlay a thin layer of BM. The BM, thus, serves as a substratum for cell attachment, prevents leakage of growth factors, hormones, and polysaccharides out of the blood stream into the connective tissue. In the capillary endothelial BM, the tight junctions that are formed between the endothelial cells in the paracellular region regulate permeability. Collagen IV and laminin have been shown to contribute to the proper assembly of tight junctions, suggesting that the BM contributes to preservation of selective permeability in the retinal capillaries (Jayadev and Sherwood, 2017).
In addition, the BM has been shown to regulate retinal blood flow. Retinal blood flow is regulated, at least in part, by pericyte contractility and relaxation (Trost et al., 2016). When pericytes relax, the pericyte processes spiraling along the longitudinal axis of the retinal microvessels loosen allowing increase in vessel patency. Inversely, when the pericytes contract, the diameter of retinal capillaries is reduced. Through this relaxation and contraction activity mediated by pericytes, retinal blood flow is regulated. Moreover, pericytes as well as endothelial cells contribute to the synthesis of BM components, and loss of these two cell types as seen in DR can promote hyperdilation of the retinal capillaries which could negatively impact retinal blood flow (Trost et al., 2016). Furthermore, the BM has been found to stiffen in high glucose (HG) conditions (Yang et al., 2016), which could alter the elasticity of the vessels, thereby compromising the ability of the pericytes to regulate retinal blood flow (Yang et al., 2016). Taken together, the BM plays an important role in the maintenance of retinal homeostasis, and HG- or diabetes-induced structural and functional alterations in the BM that can fundamentally lead to retinal dysfunction seen in DR.
4. Vascular BM thickening in diabetes
BM thickening is the most prominent histological hallmark of diabetic microangiopathy. BM thickening as measured by the electron microscopy morphometric techniques have shown not only that there is extra accumulation of BM components, but also that morphological changes in diabetes exist as “Swiss cheese-like” vacuolization and deposition of fibrillar collagen within the homogenous structure of the BM. In retinal capillaries of galactosemic rats, not only is the retinal capillary BM thickness increased (Roy et al., 2003), but also “Swiss cheese-like” changes in the vascular BM have been reported (Frank et al., 1983).
In the context of diabetic nephropathy, there is evidence of glomerular capillary BM thickening, at least in part, due to increased synthesis of ECM components, such as collagen IV by podocytes, as well as expansion of the mesangial matrix and thickening of the tubular BM (Tsilibary, 2003). Additionally, a significant increase in ECM levels was noted in glomerular BMs of diabetic rats (Seon et al., 1999). Interestingly, BM thickening in glomerular capillaries is characterised by an atypical form of thickening, forming nodular deposits called Kimmelstiel-Wilson bodies (Tervaert et al., 2010). Of note, glomerular capillaries are also morphologically different than retinal capillaries in that they consist of fenestrated endothelium underlying the glomerular BM, which participates in the regulation of glomerular filtration barrier. While diabetes-induced changes in retinal pericytes primarily affect retinal blood flow, diabetes-induced insult to podocytes can lead to local renal damage leading to microalbuminuria. Retinal vascular leakage mainly results from the failure of the blood-retinal barrier integrity. Tight junctions involving ZO-1, occludins, and claudins are compromised in retinal vascular cells, which leads to excess permeability. Importantly, BM acts as another layer of barrier that participates in the regulation of retinal capillary leakage. Although the BM itself is thickened, the ultrastructural integrity is compromised, in part due to excess crosslinking of ECM components by lysyl oxidase, resulting in increased interfibrillar space between collagen IV tetramers, ultimately promoting excess permeability. In contrast, the glomerular BM of glomerular capillaries becomes leaky, in part, due to at least two structural changes: (1) the BM integrity is compromised, tilting in favor of leakage, and 2) loss of podocytes can render the slit diaphragm complex between foot processes of podocytes non-functional, contributing to compromised filtration and ultimately microalbuminuria. While changes in the vascular BM in DR and diabetic nephropathy are similar in many aspects, these findings indicate that vascular BM thickening can compromise tissue functionality in different ways.
Diabetic neuropathy, another well-established diabetic microangiopathy, is also characterized by BM thickening surrounding the endoneurial microvessels, which can lead to degradation of tight junctions and endothelial cell hypertrophy, ultimately resulting in the breakdown of the blood-neural-barrier (Richner et al., 2018). Interestingly, capillary BM thickening has also been reported in the stria vascularis of the inner ear in diabetic rats, demonstrating that the thickened BM in the vasculature contributes, at least in part, to hearing loss in diabetic patients (Smith et al., 1995). Diabetic hepatosclerosis, which is a recently reported form of diabetic microangiopathy, is also characterized by hepatic sinusoidal fibrosis and excess BM deposition with notable increase in laminin and collagen IV (Hudacko et al., 2009). Additionally, a study reported that glomerular, muscular, and retinal capillary BM thickening was evident in diabetic dogs (Bloodworth et al., 1969), supporting the notion that BM thickening is associated with diabetic microangiopathy. Vascular BM thickening also develops in various diseases aside from diabetic microangiopathy, such as congestive heart failure (Longhurst et al., 1975), cystic fibrosis (Rodman et al., 1986), collagen diseases, and atherosclerotic vascular disease (Williamson and Kilo, 1983). In macrovessels, in identical twins, a study demonstrated that quadricep muscle BM thickening developed in the diabetic twin compared to the non-diabetic counterpart (Williamson and Kilo, 1983), clearly suggesting that diabetes is a key contributor in promoting vascular BM thickening. Interestingly, several studies attempted to identify a relationship between muscle capillary BM thickening and clinical manifestations of DR; however, these studies failed to reliably use muscle capillary BM thickening for predicting clinically significant DR (Klein et al., 1987; Williamson et al., 1988). Taken together, vascular BM thickening acts differently in different microenvironments, and requires further investigation to elucidate its role in diabetic disease processes.
5. Vascular BM thickening in DR
5.1. Development of BM thickening in diabetes
To investigate the mechanisms by which BM thickening develops, in vitro studies were initially carried out. A pivotal study showed for the first time that collagen IV and fibronectin mRNA expression levels were significantly upregulated in human umbilical vein endothelial cells by HG, showing that HG-induced insult alone is sufficient to mimic the effects of diabetes on overexpression of BM components (Cagliero et al., 1988). A follow-up study then demonstrated that HG promotes gene transcription of BM components in a coordinated manner, which requires several days of HG exposure, through effects exerted intracellularly or at the cell-matrix boundary (Cagliero et al., 1991). Furthermore, relative contributions of retinal endothelial cells and pericytes to the synthesis of ECM components were investigated, which showed that pericytes produce ten-fold more fibronectin than that of endothelial cells but approximately equal laminin production in both cell types under physiological conditions (Mandarino et al., 1993). However, under HG condition, endothelial cells were reported to show a three-fold increase in fibronectin expression while pericytes showed a generalized but insignificant fibronectin upregulation (Mandarino et al., 1993). Interestingly, an experiment using [35S] methionine radiolabelling immunoprecipitation also demonstrated that endothelial cells and pericytes synthesize different forms of fibronectin, with endothelial cells producing a dimer of two subunits each weighing 220 kD whereas pericytes producing fibronectin consisting of multiple subunits, with one predominant form at 225 kD (Mandarino et al., 1993). Both pericytes and endothelial cells synthesize an α- and two β-chains of laminin of similar size but were reported to have differential post-translational modification in each cell type. It is therefore postulated that due to preferential loss of pericytes under diabetic conditions, and since pericytes and endothelial cells contribute different forms and amounts of fibronectin and laminin to the retinal capillary BM, the net outcome results in BM thickening leading to endothelial cell dysfunction seen in DR. Table 3 shows a list of ECM components known to be upregulated in HG or diabetic conditions. Our previous study has also shown that retinal capillaries of diabetic individuals exhibit fibronectin overexpression (Roy et al., 1996). While vascular BM thickening develops in diabetes, it should also be noted that aging promotes BM thickening as well (Bianchi et al., 2016; Nagata et al., 1986). A recent study showed that retinal Müller cells may deposit BM material and contribute to retinal BM thickening (Bianchi et al., 2016).
Table 3.
Increased synthesis of BM components in HG or diabetic conditions
| BM Components | Cell Type / Tissue | References |
|---|---|---|
| FN, Coll IV | Rat Retinal Endothelial Cells | (Nguyen et al., 2020) |
| FN, Coll IV | Rat Retinal Endothelial Cells | (Roy et al., 2015) |
| FN | Human Retinas | (To et al., 2013) |
| FN | Rat Retinas | (Roy et al., 2011) |
| FN, Coll IV | Rat Retinal Endothelial Cells | (Chronopoulos et al., 2011) |
| FN, Coll IV, LM | Human Retinal Pigment Epithelial Cells | (Trudeau et al., 2011) |
| FN | Rat Retinas | (Cherian et al., 2009) |
| FN, Coll IV, LM | Rat Microvascular Endothelial Cells | (Oshitari et al., 2006) |
| FN | Rat Retinas | (Roy et al., 2003) |
| FN | Rat Mesangial Cells | (Noh et al., 2002) |
| FN, Coll IV | Rat Retinas | (Roy and Lorenzi, 1996) |
| FN | Human Retinas | (Roy et al., 1996) |
| Coll IV | Human Retinas | (Roy et al., 1994) |
| FN | Bovine Retinal Endothelial Cells and Pericytes | (Mandarino et al., 1993) |
| FN receptor | Human Umbilical Vein Endothelial Cells & Human Retinal Capillaries | (Roth et al., 1993) |
| FN | Human Umbilical Vein Endothelial Cells & Rat Retinas | (Roy et al., 1990) |
| Coll IV | Rat Retinas | (Das et al., 1990) |
| FN, Coll IV | Human Umbilical Vein Endothelial Cells | (Cagliero et al., 1988) |
| Coll IV | Bovine Retinal Pericytes | (Li et al., 1984) |
| FN | Human Glomeruli | (Parthasarathy and Spiro, 1982) |
In addition to increased synthesis of BM components, studies revealed that BM thickening can be attributed to decreased degradation of the BM components under HG conditions. MMPs, or matrix metalloproteinases, are enzymes responsible for degrading the ECM to allow for ECM remodelling in normal conditions (Nagase et al., 2006). MMPs are also regulated by TIMPs, or tissue inhibitors of metalloproteinases, which are endogenous inhibitors of MMPs (Nagase et al., 2006). In physiological conditions, a critical balance exists between the MMPs and TIMPs to promote ECM remodelling necessary for normal maintenance of the BM. In the context of DR, type IV collagenases, MMP-2 and MMP-9, were detected in the vitreous of diabetic patients with PDR (Brown et al., 1994). A later study demonstrated that MMP-2 and MMP-9 are produced by human retinal microvascular endothelial cells (Grant et al., 1998). In vitro studies also demonstrated that MMP-2 activity could be elevated in HG conditions in retinal pericytes, and MMP-9 activity could be elevated in human umbilical vein endothelial cells (Tarallo et al., 2010). Taken together, these findings indicate increased activity of MMP-2 and MMP-9 during late stage development of DR. Therefore, it remains plausible that in early stages of DR, degradation of ECM components is decreased ultimately leading to thickening of the BM, while in the later stages of DR, increased local activity of MMP-2 and MMP-9 allows endothelial cell sprouting during neovascularization leading to angiogenesis (Abreu and de Brito Vieira, 2016; Mohammad and Siddiquei, 2012; Naduk-Kik and Hrabec, 2008). In parallel, several studies suggest that levels of plasminogen activator inhibitor-1 (PAI-1), a serine protease, which can lower matrix degradation of BM components such as laminin and fibronectin (Krag et al., 2005; Levin and Santell, 1987), are elevated in retinal microvessels of diabetic subjects (Lorenzi et al., 1998) and in the vitreous of non-proliferative DR patients (Grant et al., 1996). These findings support the suggestion that in early stages of DR, excess synthesis of ECM components may also be accompanied by decreased degradation, contributing to the overall thickening of the retinal vascular BM.
In contrast to increased MMP activity in late stage DR, a reverse phenomenon occurs in the pathogenesis of diabetic nephropathy where MMP activity is decreased. Both MMP-1 and MMP-3 mRNA levels were significantly reduced in diabetic rat glomeruli concomitant with TIMP-1 upregulation (Nakamura et al., 1994). MMP-7 and MMP-9 activity were also reduced in human mesangial cells grown in HG conditions with corresponding increase in TIMP-1 activity (Abdel Wahab and Mason, 1996). MMP-2 and MMP-9 activity were also significantly reduced in diabetic rat glomeruli (Song et al., 1999). Interestingly, mesangial cells grown in HG medium exhibited reduced expression of membrane type MMP (MT1-MMP), which is responsible for activating MMP-2, with a corresponding decrease in MMP-2 activity despite a two-fold increase in MMP-2 expression (Boucher et al., 2006; McLennan et al., 2000), suggesting that this decrease in MMP-mediated ECM degradation could contribute to mesangial matrix accumulation even in the presence of MMP gene overexpression associated with diabetic nephropathy. These data suggest that there is decreased degradation of ECM in the process of BM thickening, at least in the context of diabetic nephropathy (Garcia-Fernandez et al., 2020).
While BM thickening develops during early DR, a recent study reported that the thickened BM is also characterized by increased stiffening (Yang et al., 2016). This can promote endothelial cell activation and play a major role in retinal inflammation by producing intercellular adhesion molecule (ICAM)-1 and promote leukostasis (Yang et al., 2016). In addition, connective tissue growth factor (CTGF), a regulatory protein known to modulate several growth factors and ECM proteins, plays a role in contributing to capillary BM thickening and pericyte loss in both early and late stages of DR (Klaassen et al., 2015). In PDR, CTGF participates in switching from neovascularization to a fibrotic phase (Klaassen et al., 2015). These studies suggest a possibility that reducing diabetes-induced CTGF overexpression may have beneficial effects in PDR (Klaassen et al., 2015; Yang et al., 2010).
Advanced glycation and oxidative stress are known to contribute to retinal vascular BM thickening in diabetes. Under hyperglycemic conditions, ECM components, such as collagen IV, are subjected to increased levels of advanced glycation, which can promote stiffening and irreversible thickening of the BM (Beltramo et al., 2003; Hayden et al., 2005). A study showed that administration of anti-glycated albumin reduced diabetes-induced retinal capillary BM thickening despite severe hyperglycemia (Clements et al., 1998), suggesting that glycation alone can contribute to the BM thickening process. In diabetic rats, treatment with aminoguanidine, a non-specific inhibitor of advanced glycation end-products (AGEs), led to significant protection against retinal vascular BM thickening (Gardiner et al., 2003b), suggesting that AGE may contribute to BM thickening associated with DR. Oxidative stress has also been implicated as a contributory factor in promoting retinal capillary BM thickening in diabetes (Obrosova and Kador, 2011). Reactive oxygen species (ROS), known to promote oxidative stress, has been shown to induce collagen IV accumulation and ECM fibrosis (Hayden et al., 2005). Taken together, these studies suggest that glycation and oxidative stress are important factors that can contribute to the process of retinal vascular BM thickening associated with DR.
Pro-inflammatory cytokines may also influence BM thickening, at least in part, by promoting increased synthesis of ECM components. Studies have indicated that diabetes enhances TNF-α production in retinal microvascular cells in diabetic retinopathy (Behl et al., 2008; Huang et al., 2011). In addition, in the presence of TNF-α, fibronectin deposition is increased in endothelial cells, which results in substantial accumulation of fibronectin in the ECM (Lee et al., 2019). Furthermore, TNF-α has been found to be localized in ECM under HG condition (Bianchi et al., 2015; Bianchi et al., 2016; To et al., 2013). Taken together, these findings suggest that pro-inflammatory cytokines can participate promoting ECM synthesis and ultimately contributing to the development of BM thickening.
5.2. Hyperglycemia – a key contributor to BM thickening
Diabetes is primarily characterized by chronic hyperglycemia, which has been identified as the principal contributing factor leading to the thickening of BM seen in DR among other diabetic complications (Frank, 1984). In animal models of diabetes, it was reported for the first time that experimentally induced hyperglycemia mediated by STZ can induce or enhance capillary BM thickening in mouse retinas (Beauchemin et al., 1975). Shortly after, Sima et al. demonstrated that STZ-induced diabetic rats exhibited retinal capillary BM thickening, which was completely prevented in animals that received successful pancreatic islet allografts, clearly indicating that hyperglycemic conditions play a causal role in the development of BM thickening in retinal capillaries (Sima et al., 1988). In humans, a novel study demonstrated that collagen IV expression was upregulated in retinas of patients with background DR compared to that in retinas of non-diabetic subjects (Roy et al., 1994), suggesting that BM thickening can be attributed to excess synthesis of specialized components of the ECM induced by hyperglycemic conditions. Another study demonstrated that retinal capillary BM of patients with DR exhibited accumulations of fibronectin and collagen type I, III, IV, and V compared to that of non-diabetic subjects and diabetic patients without DR (Ljubimov et al., 1996). Interestingly, a porcine model of diabetes demonstrated that hyperglycemic condition, but not high fat diet, promotes retinal capillary BM thickness (Hainsworth et al., 2002) supporting previous reports. In addition, retinal capillary BM thickening was also observed in diabetic dogs compared to non-diabetic controls (Gardiner et al., 2003a). Importantly, a key study demonstrated that there was a strong correlation between retinal and glomerular capillary BM thickening in diabetic rats with elevated HbA1c level, as well as fibronectin overexpression in both tissues (Cherian et al., 2009). Moreover, diabetic rats with tight glycemic control showed protective effects against hyperglycemia-induced fibronectin upregulation as well as retinal capillary BM thickening (Cherian et al., 2009). Interestingly, in a primate model of DR, hyperhexosemia-induced marmosets exhibited retinal capillary BM thickening concomitant with the development of acellular capillaries, pericytes loss, macular edema, and microaneurysms, similar to retinal vascular changes seen in human DR (Chronopoulos et al., 2015). Collectively, these findings clearly indicate that hyperglycemia promotes excess synthesis of ECM components and that it is a key contributor to retinal vascular BM thickening seen in DR.
A significant thickening of the retinal vascular BM was observed using light and transmission electron microscopy in diabetic human eyes compared to those of non-diabetic human eyes (To et al., 2013). Moreover, this thickening was also accompanied by accumulation of fibronectin and tenascin, an ECM glycoprotein, in the retinal vascular BM of diabetic human eyes compared to that of non-diabetic controls using immunocytochemistry and Western blot analysis (To et al., 2013), suggesting that an increase in BM thickness results not only from excess synthesis of BM components but also from induction of diabetes-specific ECM proteins which are not normally found in the retinal vascular BM. Interestingly, a study using electron microscopy reported that thickening of the retinal capillary BM was prominent at the glial aspect of the retina obtained from diabetic human eyes (Bianchi et al., 2016), suggesting that hyperglycemic conditions affect both inner and outer layers of the BM. It was also shown that retinal vascular BM of diabetic patients exhibited collagen accumulation as well as higher abundance of seventeen other ECM-associated proteins, of which most were implicated in complement-mediated inflammatory process in the diabetic retinal vasculature (Halfter et al., 2017). Specifically, the vascular BM of diabetic patients showed presence of C4 and C9, two members of the complement family involved in inflammation, which were interestingly not detectable in that of non-diabetic donors (Halfter et al., 2017). It is also theorized that this increase in complements localized in the retinal vascular BM could contribute to pericyte loss, a characteristic hallmark of diabetic microangiopathy. In addition, the investigators also reported that there was more than a two-fold increase in the thickness of ocular BMs in both type 1 and type II diabetic patients (Halfter et al., 2017), lending further support that hyperglycemia plays a causal role in the BM thickening process.
Vascular BM thickening depends largely on the dynamic interactions of synthetic and degradative enzymes regulating BM components. It is of interest that in smaller animals, the extent of BM thickening in diabetes is less compared to that of larger animals; however, when compared to the baseline BM thickness, the percent change in thickness is similar. For example, in diabetic mice, 3–4 months of hyperglycemic condition promotes significant retinal capillary BM thickening, whereas in rats, it would take a longer duration for the same to occur. Similarly, in cats and dogs, it takes longer about few years for vascular BM thickening to develop, and in non-human primates, such as rhesus macaques and baboons, about 5–7 years (Gibbs et al., 1966; Stout et al., 1986; Thomson et al., 2008). Generally, in humans, vascular BM thickening is evident after greater than 10 years of hyperglycemia (To et al., 2013). Our studies have shown that the extent to which BM thickening develops, even within the same species, is highly regulated by the level of hyperglycemia. We have previously shown that even within the same diabetic animal, vascular BM thickening can develop at different rates in different tissues, such as in glomerular versus retinal capillaries (Cherian et al., 2009). Table 4 presents retinal vascular BM thickening in diabetic animal models.
Table 4.
Retinal vascular basement membrane thickening in diabetic animal models
| Species | Mode of diabetes Induction | BMT in Non-Diabetic (nm) | BMT in Diabetic (nm) | References |
|---|---|---|---|---|
| Porcine | Alloxan-induced diabetes with normal diet | 97.7±11.2 | 152.2 ± 16.3 | (Hainsworth et al., 2002) |
| Porcine | Alloxan-induced diabetes with high fat diet | 97.7 ± 11.2 | 120.2 ± 13 | (Hainsworth et al., 2002) |
| Wistar Kyoto (WKY) rat | Galactose | 160.4 ± 31.2 | 203.3 ± 376 | (Frank et al., 1983) |
| Spontaneously hypertensive (SHR) rat | Galactose | 159.4 ± 36.1 | 230.7 ± 623 | (Frank et al., 1983) |
| Marmosets | Galactose | 147 ± 34 | 244 ± 30 | (Chronopoulos et al., 2015) |
| Dog | Alloxan-induced diabetes | 141 + 25 | 236 ± 46 | (Engerman and Kern, 1984; Engerman et al., 1993; Gardiner et al., 2003) |
| Dog | Galactose | 141 ± 25 | 258 ± 88 | (Engerman and Kern, 1984; Engerman et al., 1993) |
| Human | Diabetic subject | 292.4 ± 24.3 | 583.1 ± 38.52 | (Bianchi et al., 2016) |
| Yorkshire swine | Streptozotocin | 2.6 ± 15.0 | 121.5 ± 21.8 | (Lee et al., 2010) |
| Spiny mice | Streptozotocin | 124.2 ± 1.7 | 150.8 ± 1.4 | (Beauchemin et al., 1975) |
| Cat | Partial Pancreatectomy | 72 ± 12 | 114 ± 15 | (Mansour et al., 1990) |
| Sprague-Dawley rats | 30% Galactose | 159 ± 23 | 227 ± 37 | (Kern and Engerman, 1994; Robison et al., 1986) |
| Sprague-Dawley rats | 50% Galactose | 159 ± 23 | 276 ± 226 | (Kern and Engerman, 1994) |
| Sprague-Dawley rats | 50% Galactose | 159 ± 23 | 216 ± 36 | (Kern and Engerman, 1994) |
| BALB/c mice | Streptozotocin | 149 ± 45 | 295 ± 72 | (Cuthbertson et al., 1989) |
5.3. Direct effects of BM thickening
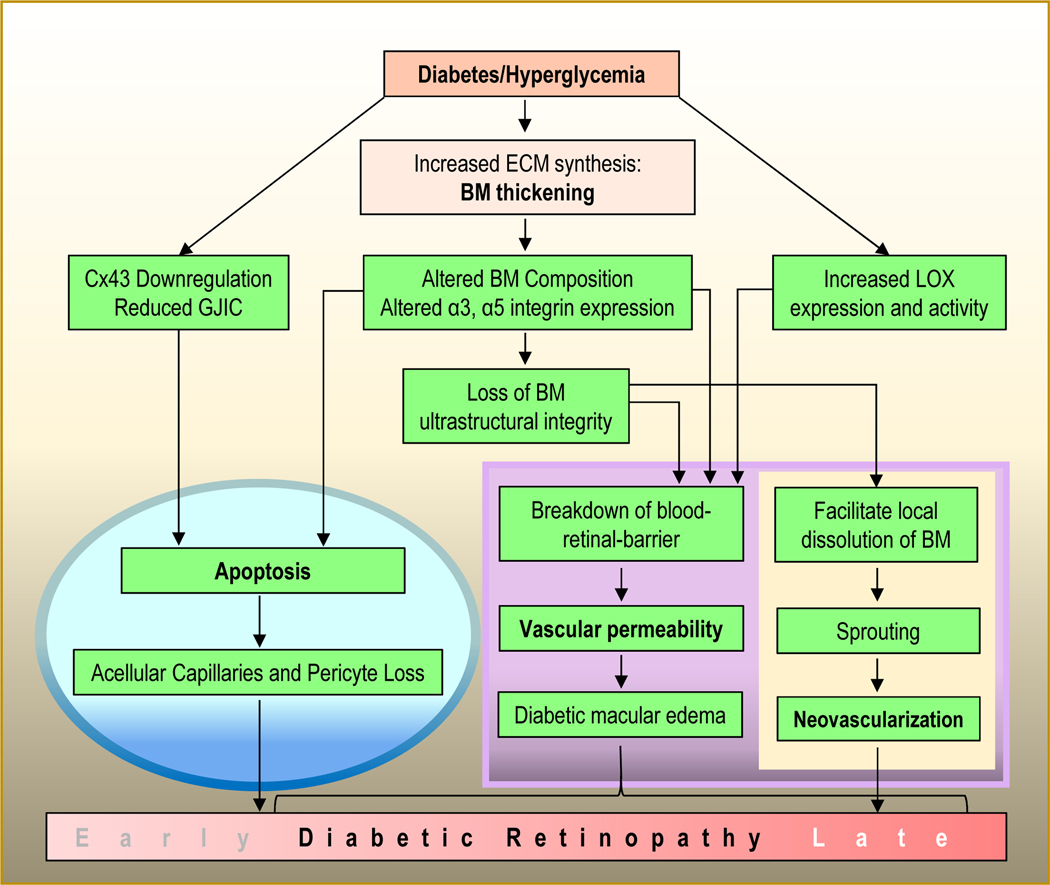
BM is involved in regulating multiple cellular functions (Fig. 3), which can be profoundly affected from its change in morphology, in particular, the thickening of the BM. Specifically, in the context of DR pathogenesis, BM thickening can have significant effects on maintenance of BRB characteristics and cell survival. An emerging role of BM in DR that has gained attention is its participation in retinal neovascularization associated with DR. Furthermore, recent studies suggest that vascular BM thickening can impact cell-cell communication, and thereby modulate cell metabolism and exchange of small molecules, which, in turn, can impact cell survival.
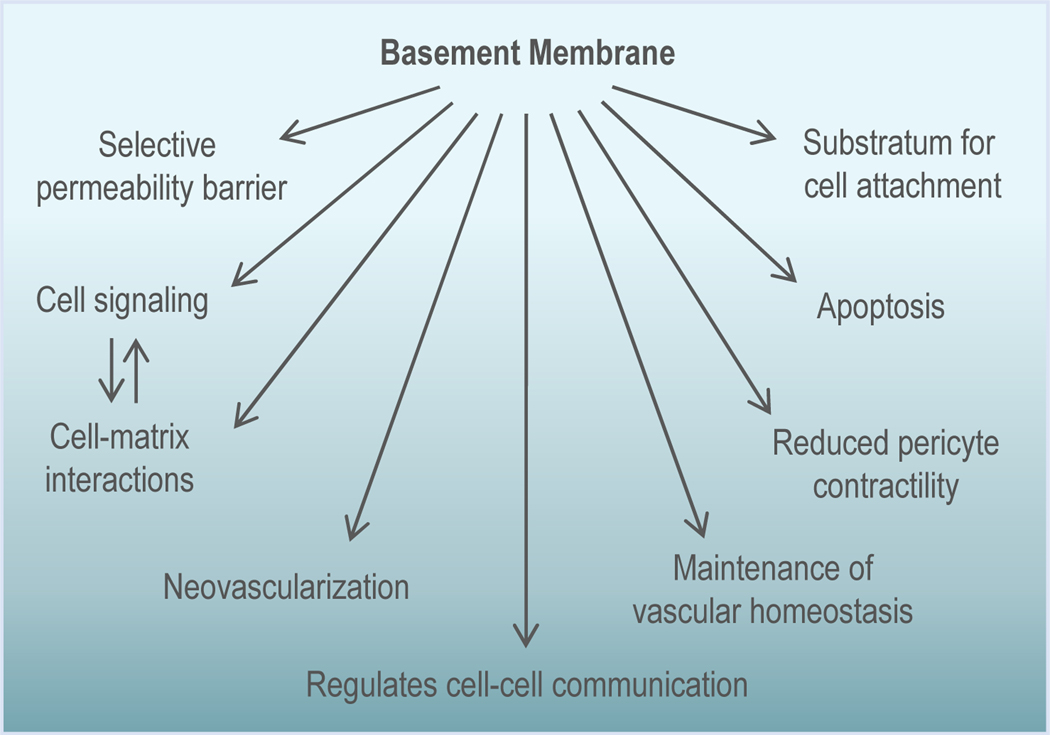
Figure 3. Multi-faceted functions of the BM.
The vascular BM is a multifunctional unit that serves as a selective permeability barrier, mediates cell-matrix interactions, acts as a substratum for cell attachment, and is also involved in regulating apoptosis, pericyte contractility, vascular homeostasis, neovascularization, and cell-cell communication.
5.3.1. Thickened BM promotes increased permeability and vascular leakage
Besides its functional role as a substratum for vascular cells in the retinal capillaries, the vascular BM also serves as a selective barrier to regulate capillary permeability (Roy et al., 2010). When vascular BM thickening develops in diabetes, the ultrastructural integrity of the BM is compromised, and can interfere with the exchange of substances between the inner and outer capillary environments resulting in loss of BRB characteristics (Bianchi et al., 2016). This phenomenon may seem paradoxical in that these remodelled BMs appear thickened on close examination but results in leakage. However, this may be due to the thickened BM’s loss of its filtering capacity, or “sieving” function (Hayden et al., 2005).
Conventionally, it was thought that compromised tight junctions and increased vesicular transport were the only factors contributing to excess capillary leakage in DR. While compromised tight junctions, or paracellular pathway, (Antonetti et al., 1998; Barber and Antonetti, 2003; Harhaj and Antonetti, 2004) and transcellular pathway mediated by vesicles (Klaassen et al., 2009; Vinores et al., 1998; Vinores et al., 1993) have been identified in promoting vascular permeability in DR, these changes do not fully account for the excess permeability in totality as they only pertain to the cellular layer of the retinal vasculature. To better understand how the thickened BM may promote excess permeability, a study using cells grown in HG for short-term in which no changes in tight junctions or vesicle formation was observed, showed ECM upregulation concomitant with increased cell monolayer permeability in these cells (Chronopoulos et al., 2011). Thus, this finding demonstrates that excess ECM accumulation can contribute to increased permeability. This observation was further supported by studies in which reducing collagen IV, fibronectin, and laminin resulted in preventing HG-induced cell monolayer permeability and diabetes-induced vascular leakage in rat retinas (Oshitari et al., 2005; Oshitari et al., 2006). These findings indicate that the thickened BM contributes to the development of excess permeability seen in DR.
To better understand how the thickened BM promotes leakiness and excess permeability, the relationship between BM components and tight junctions is of high interest. It has been reported that vascular BM components laminin and collagen IV are critical for proper formation of tight junctions between endothelial cells and for the maintenance of transendotheilal electrical resistance in the blood-brain barrier (Jayadev and Sherwood, 2017). Therefore, it is plausible that excess synthesis of BM components could fundamentally disrupt the natural formation of tight junctions and thus compromise the blood-retinal barrier leading to increased permeability. In addition, it has been reported that alteration in collagen IV and fibronectin can modulate levels of gap junction protein Cx43 (Moon et al., 2011), which is closely associated with the functionality of tight junction proteins ZO-1 and occludin (Tien et al., 2013). Specifically, siRNA-mediated Cx43 downregulation resulted in reduced expression of ZO-1 and occludin in retinal endothelial cells. These findings suggest that excess collagen IV and fibronectin, and the resultant thickened BM may disrupt tight junctions and compromise barrier characteristics, at least in part, through decrease in Cx43 expression (Sato et al., 2002).
The breakdown of the BM’s ultrastructural integrity has also been implicated as a key factor in contributing to excess permeability. For proper development and maturation of the BM, collagen fibrils undergo post-translational modification during assembly process and integration into the ECM mediated by lysyl oxidase (LOX), a cross-linking enzyme that aids in the formation of covalent cross-links in the ECM (Rucker et al., 1998). While LOX-mediated cross-linking activity is normal in physiological states, studies have suggested that excess cross-linking in pathological states could lead to formation of overly compact collagen fibrils (Grant et al., 1997; Ortolan et al., 2008), resulting in increased interfibrillar space and ultimately promoting excess permeability. This was further confirmed in retinal endothelial cells grown in HG condition in which elevated LOX expression and activity resulted in excess cell monolayer permeability (Chronopoulos et al., 2010). Similarly, LOX upregulation was noted in retinal microvessels of diabetic rats with corresponding increase in retinal vascular leakage (Chronopoulos et al., 2010; Song et al., 2018). Interestingly, when cells grown in HG medium were transfected with LOX siRNA or treated with β-aminopropionitrile to block LOX expression or activity, respectively, HG-induced cell monolayer permeability was reduced. In addition, diabetic rats that received monthly intravitreal injections of LOX siRNA spanning three months displayed reduced retinal vascular leakage (Song et al., 2018). In an animal model of diabetes using LOX heterozygous knockout mice, we observed that diabetic LOX heterozygous knockout mice exhibited reduced LOX expression as expected but also these animals showed decreased retinal vascular leakage compared to that of diabetic wild type mice (Kim et al., 2019b). Taken together, these findings suggest that abnormal LOX overexpression and excess cross-linking activity could compromise cell barrier functional integrity. Importantly, a recent study provided evidence for abnormal LOX upregulation and its involvement in PDR that shed light to its pathological role in human DR. Specifically, upregulation of LOX expression was found in vitreous of diabetic subjects with advanced DR (Subramanian et al., 2019). This finding showed for the first time that increased LOX levels in the diabetic retina may promote PDR and raises the prospect of targeting LOX overexpression for treatment of PDR.
Studies have also investigated perlecans as a potential contributor to increased permeability seen in DR. Perlecan, an integral component of the BM, is responsible for regulating charge-selective properties pertaining to filtration of polyanions. Therefore, a proposition dubbed the Steno hypothesis stipulated that changes in HSPGs can negatively impact the barrier properties of the BM and compromise charge-specific permeability of molecules (Deckert et al., 1989). A study showed that changes in HSPGs localized to the rabbit glomerular BM are correlated with loss of charge selectivity, and hence increased glomerular permeability, in an animal model of nephropathy (Groggel et al., 1988). In the retina, thickening of the capillary BM was linearly correlated to reduction in anionic site density in diabetic rat retinas, suggesting that diabetes leads to loss of perlecan in the retinal vascular BM and that this may play a role in compromising vascular permeability (Chakrabarti et al., 1991). Moreover, diabetic rat retinas exhibited decreased protein synthesis and mRNA expression of perlecan (Bollineni et al., 1997), suggesting that reduction in perlecan synthesis could potentially account for the apparent loss of anionic sites in retinal vascular BM and contribute to increased capillary BM seen in DR. However, in a study using human donor samples, it was ultimately determined that increased microvascular permeability seen in DR was not attributed to changes in the expression of perlecan, but rather due to elevated levels of VEGF that promoted overexpression of a perlecan side chain (Witmer et al., 2001). Collectively, these findings suggest that increased retinal vascular permeability in DR is a complex process involving the abnormally thickened BM, where disruption of the BM composition impacts interactions among the BM components. Further studies are needed for better understanding of BM component interactions and their role in the vascular leakage associated with DR.
5.3.2. BM produced under HG condition promotes apoptosis
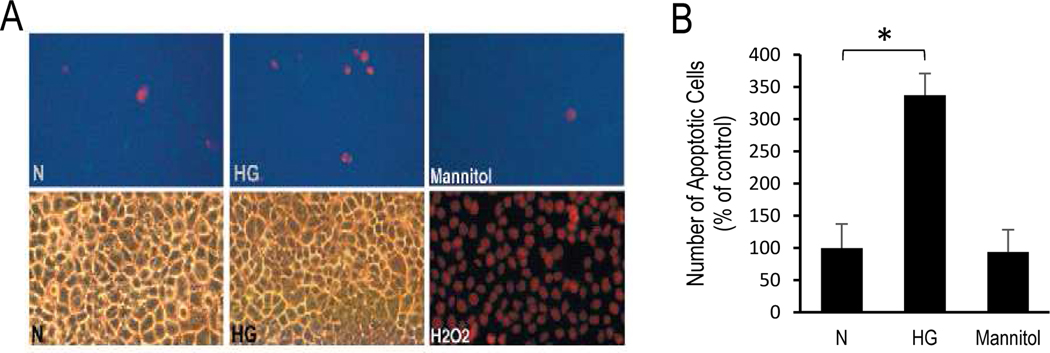
Studies show that ECM accumulation produced by endothelial cells in HG condition or the thickened BM in diabetes can have detrimental effects on cell survival and barrier characteristics (Chronopoulos et al., 2011; Roy et al., 2011; Roy et al., 2003; Trudeau et al., 2011). To determine whether HG-induced excess ECM triggers apoptosis, matrix laid down by RRECs grown in normal or HG medium were tested. Cells plated on ECM laid down by HG cells (HG-matrix) showed a significantly higher number of apoptotic cells compared to cells grown on N-matrix (337.5±33% of control, P<0.05, Fig. 4). These findings suggest that ECM laid down by cells in HG media is abnormal and is capable of promoting apoptosis. Additionally, while LOX is conventionally responsible for crosslinking ECM components for the development and maturation of the BM, studies suggest that excess LOX can also promote apoptosis (Kim et al., 2019a; Kim et al., 2019b; Kim et al., 2017; Song et al., 2018). However, further studies are needed to better understand how the thickened BM and abnormal LOX expression are involved in pro-apoptotic signalling associated with the development and progression of DR.
Figure 4. HG-induced excess ECM promotes apoptosis.
(A) Representative images show RRECs plated on HG-matrix and grown in N condition exhibit increased number of apoptotic cells (red) compared to those plated on N-matrix and grown in N condition. Cells exposed to hydrogen peroxide (H2O2) served as positive control. (B) Bar graph shows cumulative data indicating that ECM produced by cells grown in HG condition (HG-matrix) is capable of promoting apoptosis in cells grown in N medium. Cells exposed to mannitol exhibited no difference in apoptosis compared to cells grown in N medium. *p<0.05; n=6.
5.4. Indirect effects of BM thickening on cellular functions
5.4.1. BM alterations and its effects on cell-cell communication
The effects of BM thickening on structural changes are accompanied by functional changes. While BM serves as a substratum for endothelial cell and pericyte attachment and regulates blood-retinal-barrier, it can have a profound regulatory role on cell survival. In DR, HG- or diabetes-induced downregulation of Cx43 has been shown to contribute to the development of vascular lesions in the diabetic retina (Bobbie et al., 2010; Roy et al., 2003; Tien et al., 2014).
BM components, fibronectin, laminin, and collagen IV, have been shown to regulate Cx43 expression and gap junction intercellular communication (GJIC) activity in various cell types. In granulosa cells, when Cx43 was downregulated, a reciprocal effect was seen wherein ECM components, fibronectin, collagen IV, and laminin expression were increased within granulosa cell layers in late stages of follicular atresia (Huet et al., 1998). In alveolar epithelial cells, laminin-332 has been shown to alter Ca2+ exchange via gap junction channels, which resulted in Cx43 downregulation and cell morphology change (Isakson et al., 2006). A study using Cx43 knockout mice showed increased collagen expression in late phases of granuloma organization (Oloris et al., 2007), indicating that accumulation of BM components is associated with reduced Cx43 expression. A similar finding reported that increased collagen secretion reduces Cx43 expression in tenocytes (Waggett et al., 2006). Importantly, in keratinocytes, Cx43 downregulation using antisense oligonucleotides led to a significant increase in collagen type I mRNA and protein expression, further supporting a clear association between Cx43 and BM components (Mori et al., 2006). Cell-cell communication is essential for cell survival and cell growth (Li and Roy, 2009; Roy et al., 2017). Cx43-mediated GJIC is one of the processes through which cell-cell communication is facilitated in the retinal vasculature. A study from our lab showed that HG-induced upregulation of laminin and collagen IV decreases Cx43 expression in microvascular endothelial cells (Pinheiro and Roy, 2007), suggesting that ECM accumulation can impact Cx43 expression. Importantly, reducing HG-induced fibronectin and collagen IV overexpression in retinal endothelial cells positively impacted Cx43 expression, upregulating Cx43 to near normal levels and thereby restoring GJIC (Moon et al., 2011). Such improved intercellular communication was shown to prevent HG-induced apoptosis (Moon et al., 2011). Overall, these reports indicate that HG-induced overexpression of fibronectin, laminin, and collagen IV may influence Cx43 expression and GJIC activity, and thereby compromise vascular BM function towards vascular cell loss seen in DR.
There is a growing realization that in DR pathogenesis, the breakdown of neuronal components along with the vasculature are involved, and that the inter-dependence of endothelium, pericytes, Müller glia, neurons, microglia, perivascular immune cells, and the vascular BM are disturbed as part of the neurovascular unit (NVU) (Simo et al., 2018). In the retinal environment where the vascular BM serves as a common barrier between Müller cells and pericytes, the exchange of critical cell survival factors can be hampered (Muto et al., 2014). Importantly, in the context of cell-cell interactions, HG-driven altered expression of BM components is known to influence CX43 expression (Roy et al., 2017), which can compromise intercellular communication. In addition, aquaporin 4 and Kir4.1 are critically altered in the diabetic retina (Daruich et al., 2018; Zhang et al., 2011), which could negatively impact cell-cell exchange across a modified BM within the neurovascular unit in the diabetic retina. While gap junctions have been observed between Müller cells (Nishizono et al., 1993), currently, it is unclear if BM thickening between the Müller glial end feet and vascular BM plays a role in the pathogenesis of DR. Growing evidence also suggests that neuroprotective drugs may prevent retinal vascular leakage in experimental diabetes (Hernandez et al., 2016; Hernandez et al., 2017; Hernandez et al., 2013); however, whether this effect is mediated by changes in the vascular BM remains to be elucidated. Recent studies indicate that retinal glial cells may be involved in promoting retinal capillary BM thickening (Bianchi et al., 2016; Coorey et al., 2012; Feher et al., 2018; Lee et al., 2010). Notably, a study using electron microscopy demonstrated that BM thickening develops primarily at the glial aspect in the human diabetic retina, and that retinal Müller cells also contribute to excess deposition of ECM components, leading to thickened BM in diabetic human retinal capillaries (Bianchi et al., 2016). These findings highlight vascular BM thickening as a product of pathogenetic cellular processes including those of the NVU in the diabetic retina and underscore the need for further investigation into contributory factors influencing BM thickening.
The vascular BM is also known to sequester growth factors. It has been reported that the vascular BM, namely HSPGs, can sequester growth factors, such as basic fibroblast growth factor (bFGF), a heparin-binding angiogenic protein, outside endothelial cells (Vlodavsky et al., 1987). A follow-up study further demonstrated that the vascular BM can deposit up to 30% of cellular bFGF into the ECM network, which can then be released in the presence of heparin or upon proteolytic degradation, suggesting that the BM serves as a storage for growth factors which can be released upon BM dissolution (Folkman et al., 1988). In addition, fibronectin and laminin, key components of the BM, have been shown to sequester angiogenic growth factors into the ECM (Bhatwadekar et al., 2008; Ishihara et al., 2018). These findings support the possibility that in later stage DR, as the thickened BM undergoes local dissolution, angiogenic growth factors originally sequestered in the BM become released, initiating the process of neovascularization (Forrester et al., 1993). Interestingly, a study showed that nitritemodified ECM, a model of Bruch’s membrane thickening in age-related macular degeneration, led to accelerated release of growth factors such as VEGF and PEDF in the overlying retinal pigmented epithelial cells (Fields et al., 2017), suggesting that the altered BM can affect cellular exchange of trophic and vasoactive growth factors. Whether similar mechanisms occur in the diabetes-modified thickened retinal vascular BM is currently unclear and requires further investigation.
5.4.2. Effects of BM thickening on cell signalling
There is no longer any doubt that interactions between cells and the ECM initiate a flow of information that acts to regulate many fundamental processes throughout development, including cell migration, organ formation, and growth and differentiation of various cell types (Adams and Watt, 1993; Hay, 2013). The BM also relays critical signals to the cell that affects cell viability, cell migration, and adhesiveness. Integrins, a family of transmembrane receptors consisting of α and β subunits that form heterodimers (Hynes, 1992), are the main conduits mediating cell-matrix interactions but other non-integrin receptors such as dystroglycan, syndecans and discoidin domain receptors have also been reported (Short et al., 1998). However, in the context of DR, integrins are of particular interest.
Crosstalk between integrins and ECM allows cells to respond to biochemical and mechanical cues from ECM to the cell and from the cell to the ECM. The α subunits of integrins are specific and can bind to the Arg-Gly-Asp (RGD) motif of fibronectin. This motif was later reported in several other ECM proteins (Ruoslahti, 1996). The presence of RGD motif regulates specific spatial distribution of integrins necessary for cell response (Chen et al. 1997). Similarly, the α3 subunit of integrin is involved in communicating signals specifically from collagen IV to and from the cells. When BM composition is altered in HG condition, the communication between ECM components and the actin cytoskeleton via integrins is compromised. It has also been reported that excess HG-induced upregulation of integrins can have detrimental effects including firmer cell-matrix adhesion and compromised cell survival.
BM components have also been reported to influence cell shape as reported recently (Randles et al., 2020). Cells plated on Collagen IV may promote a round morphology, with large adhesion complexes rich in actin-binding proteins, whereas, cells plated on laminin can assume a polygonal shape with small adhesion complexes. To what extent these shapes are dictated by BM ligands and how they influence cell signaling and functionality are currently under investigation.
Cooperation between integrins and soluble mitogens is essential to allow efficient propagation of signals to downstream kinases (Short et al., 1998). Specifically, α5β1 integrin-dependent adhesion of endothelial cells has been reported to promote tyrosine phosphorylation of important cellular targets including focal adhesion kinase and paxillin (Short et al., 1998). Anchorage to the ECM through α2, α3, and α5 integrins has also been shown to be necessary for efficient MAPK activation by cytokines in endothelial cells (Davis and Senger, 2005). It has been reported that β1 integrins communicate cell-matrix signaling through focal adhesion kinase and integrin-linked kinase, which then modulate MAPK and PI3K/AKT signaling pathways (Davis and Senger, 2005). Of particular note, it has been indicated that integrins α1β1 and α2β1 play a role in the regulation of collagen and MMP synthesis and are thus of special importance for ECM turnover or BM degradation (Niland and Eble, 2012). Taken together, these findings highlight the importance of integrins in relaying cellular signaling between the BM and the cell and more studies will be necessary to further elucidate how vascular BM thickening impacts signaling pathways leading to the pathogenesis of DR.
Currently, several clinical trials are ongoing assessing the use of integrin antagonists for treatment of diabetic retinal diseases. In a Phase 2 trial, intravitreal injection of ALG-1001 (Risuteganib), a novel peptide targeting α5β3, α5β5, α5β1, and α5β2 integrins, has shown greater efficacy over Avastin therapy against diabetic macular edema (DME) (ClinicalTrials.gov Identifier: NCT02348918) (Bhatwadekar et al., 2020). Topical administration of SF-0166, an inhibitor of α5β3 integrin, was shown to reduce VEGF-induced retinal vascular leakage in animal studies (Askew et al., 2018), and led to a reduction in retinal thickness with concomitant improvements in visual acuity in 53% of DME patients conducted in a Phase 1/2 trial (ClinicalTrials.gov Identifier: NCT02914613 (Bhatwadekar et al., 2020). Use of another agent called THR-687, an inhibitor of α5β3, α5β5 and α5β1 integrins, led to reduced retinal vascular permeability in animal studies while attaining safety endpoints and showing promising improvements in visual acuity in DME patients in a Phase 1 trial (ClinicalTrials.gov Identifier: NCT03666923) (Bhatwadekar et al., 2020; Hu et al., 2019). The common denominator of these aforementioned clinical trials is the targeting of α5β3 integrin, which has been implicated in activation of VEGF receptor-2 associated with the initiation of neovascularization and angiogenesis (Soldi et al., 1999). Therefore, further studies are necessary to determine whether these integrin antagonists provide beneficial effects against retinal vascular leakage through interactions with the thickened BM associated with DR.
5.4.3. Downregulation of HG-induced overexpression of BM components modulates integrin expression in retinal endothelial cells
Impaired cell-matrix interactions under HG condition have been shown to promote apoptosis (Beltramo et al., 2002). Studies suggest that HG altered cell-matrix interactions may compromise pericyte adhesion to the matrix, and thereby contribute to pericyte loss (Beltramo et al., 2003; Beltramo et al., 2002), and that overexpression of ECM components may play a critical role in HG or diabetes-induced apoptosis (Roy et al., 2015; Roy et al., 2011). Currently, it is unknown whether excess ECM synthesized by cells grown in HG mediates apoptotic cell death through modulation of integrin expression.
Integrins act as the key signalling conduits between ECM and the internal components of the cell. Integrins are transmembrane receptors present as heterodimers and consist of an α and a β subunit (Hynes, 2002). They are widely distributed in various tissues and mediate diverse biological functions, including interactions between cells and matrix via induction of signal transduction cascades regulating among other functions the recruitment of structural and signalling molecules to adhesion sites (Schoenwaelder and Burridge, 1999). Such interactions are mediated through integrin-signalling, which has been shown to regulate ECM synthesis in a feedback dependent manner (Beekman et al., 1997). Addition of fibronectin to chondrocytes increased expression of α5 integrin subunit (Beekman et al., 1997), and synthesis of the α2β1 integrin was selectively upregulated when fibroblasts were seeded on type I collagen gels (Klein et al., 1991). Beekman et al reported that through integrin-mediated mechanism, extracellular collagen accumulation downregulated collagen synthesis in a negative feedback manner (Beekman et al., 1997). These studies suggest that feedback mechanisms are operative between cells and ECM via integrin in various cell types. However, currently it is unknown whether feedback mechanisms are affected by HG-induced modulation of ECM component expression and its corresponding integrin subunit expression in retinal endothelial cells.
Despite the knowledge in aberrant ECM synthesis in diabetes and its contribution in the pathophysiology of diabetic complications, its role in the control of specific integrin subunit content related to retinal vascular cell loss remains to be determined. Having downregulated specific fibronectin and Collagen IV overexpression with antisense oligos (Oshitari et al., 2006; Roy et al., 1999) and siRNAs (Oshitari et al., 2005; Roy et al., 2011), we investigated whether modulation of fibronectin or Collagen IV expression alters expression of corresponding integrin subunits associated with fibronectin or Collagen IV in RRECs, and whether the abnormal ECM produced by cells grown in HG mediates apoptosis by modulating the expression of specific integrin subunits.
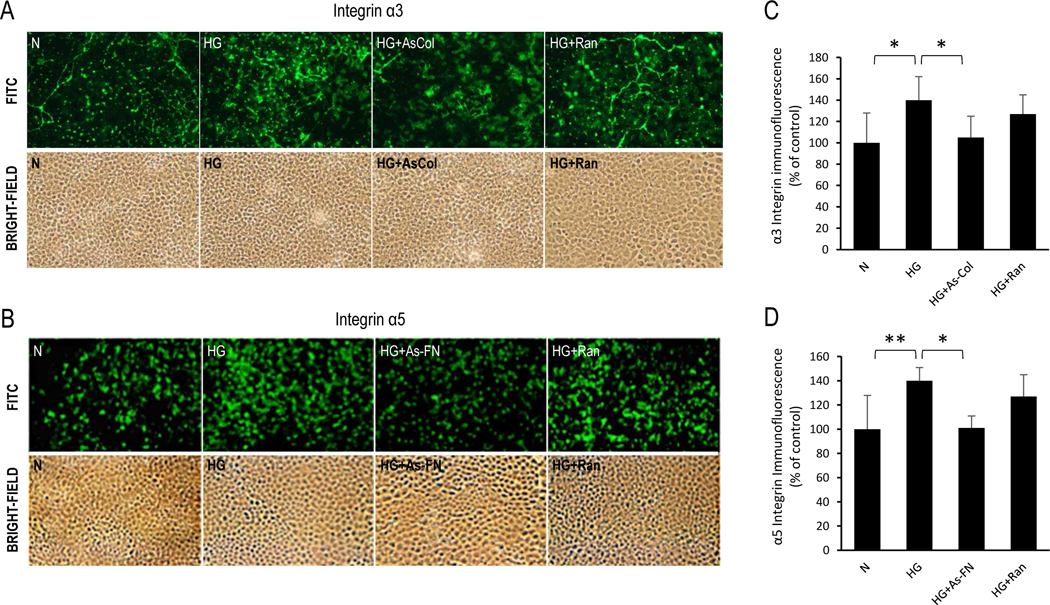
Western blot data revealed that cells grown in HG medium exhibited increased fibronectin (218±65% of control, P<0.05, Fig. 5) and integrin α5 expression (233±23% of control, P<0.01, Fig. 5) compared to those of cells grown in N medium. In cells grown in HG medium and transfected with AS-fibronectin (As-FN) oligos, expression levels of fibronectin (107±42% of control, P<0.05, Fig. 5) and integrin α5 subunit were significantly reduced (161±30% of control, P<0.01, Fig. 5) compared to that of cells grown in HG condition. Cells grown in HG medium and transfected with random oligos showed no effect in either integrin α5 subunit or fibronectin expression. Results investigating the effects of Collagen IV levels on integrin α3 protein expression through Western Blot analysis revealed that cells grown in HG medium exhibited a significant increase in ColI IV (133.0±17.0% of control, P<0.01, Fig. 5) and integrin α3 expression (142.5±13.9% of control, P<0.01, Fig. 5) compared to cells grown in N medium. In cells grown in HG medium and transfected with AS-Col IV oligos, Col IV expression (101.6±20.7% of control, P<0.05, Fig. 5) and integrin α3 expression (116.5±9.1% of control, P<0.05, Fig. 5) were significantly reduced compared to cells grown in HG medium. Cells grown in HG medium and transfected with random oligos showed no effect in either integrin α3 subunit or Collagen IV expression.
Figure 5. Effect of FN and Col IV antisense oligos on integrin α5 and α3 expression in RRECs.
Representative western blot image demonstrates that expression levels of (A) FN and α5 integrin, as well as those of (B) Coll IV and α3 integrin, are increased in cells grown in HG medium compared to that of cells grown in normal medium. Bar graphs representing cumulative data indicate that reducing (C) FN and (E) Col IV overexpression using antisense oligos decrease (D) α5 and (F) α3 integrin levels, respectively. Data is presented as mean ± standard deviation; *p<0.05. **p<0.01. N: normal media; HG: high glucose media; As-FN: HG media + antisense Col IV oligos; As-Col: HG media + antisense Col IV oligos; HG+Ran: HG medium + random oligos.
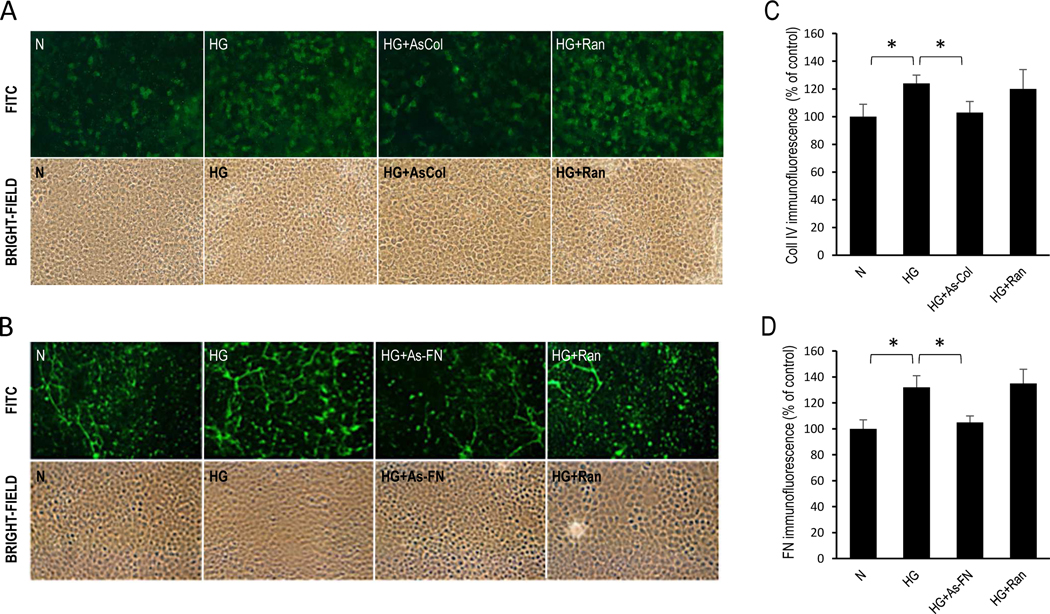
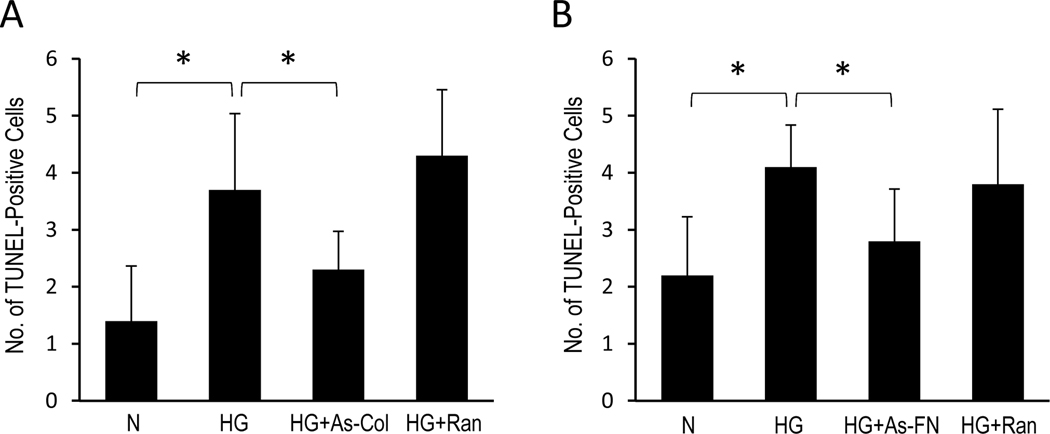
Immunostaining data revealed increased fibronectin (132±9% of control, P<0.05, Fig. 6) and integrin α5 in RRECs grown in HG medium (140±11% of control, P<0.01, Fig. 7) compared to cells grown in N medium. α5 integrin immunostaining was markedly reduced (101±10% of control, P<0.05, Fig. 7) in cells transfected with As-FN oligos compared to cells grown in HG condition. Cells grown in HG medium and transfected with random oligos showed no effect. Additionally, increased immunostaining of Collagen IV (124±6% of control, P<0.05, Fig. 6) and integrin α3 (140±22% of control, P<0.05, Fig. 7) was observed in RRECs grown in HG medium compared to cells grown in N medium. In cells grown in HG medium and transfected with AS-Col IV oligos, immunostaining of integrin α3 (105±20% of control, P<0.05, Fig. 7) was significantly reduced compared to cells grown in HG medium. Importantly, reduction in collagen IV overexpression using As-Collagen IV oligos resulted in a significant decrease in the number of TUNEL-positive cells (2.3±0.7) compared to that of cells grown in HG medium (3.7±1.3, P<0.05, Fig. 8) or cells grown in HG medium transfected with random oligos (4.3±0.9, P<0.05, Fig. 8). Similarly, cells grown in HG medium and transfected with As-FN oligos exhibited a significant decrease in the number of TUNEL-positive cells (2.8±0.8, Fig. 8) compared to that of cells grown in HG medium (4.1±0.7, P<0.05, Fig. 8) or cells grown in HG medium transfected with random oligos (3.8±1.3, P<0.05, Fig. 8).
Figure 6. Effect of As-Coll IV and As-FN oligos on Coll IV and FN immunostaining in RRECs.
Immunohistochemical analysis show efficacy of (A) AS-Col oligos and (B) AS-FN oligos in reducing Col IV and FN levels, respectively, in RRECs grown in HG condition. Corresponding bright-field image is shown below each FITC-labeled photomicrograph. Bar graphs of cumulative data show AS-Col-oligos and AS-FN-oligos downregulate (C) Coll IV and (D) FN, respectively. Data is presented as mean ± standard deviation; *p<0.05. N: normal media; HG: high glucose media; As-FN: HG media + antisense FN oligos; As-Col: HG media + antisense Col IV oligos; HG+Ran: HG medium + random oligos; n=6.
Figure 7. Effect of Coll IV and FN antisense oligos on α3 and α5 integrin immunostaining in RRECs.
Immunohistochemical analysis show (A) α3 integrin and (B) α5 integrin immunoreactivity in RRECs. Corresponding bright-field image is shown below each FITC-labeled photomicrograph. An increase in integrin α5 and integrin α3 immunoreactivity were observed in cells grown in HG medium compared to that of cells grown in normal medium. Bar graphs of cumulative data show that Coll IV and FN downregulation using antisense oligos decreases immunostaining of integrin α3 and integrin α5, respectively. Data is presented as mean ± standard deviation; *p<0.05, **p<0.01. N: normal media; HG: high glucose media; As-FN: HG media + antisense FN oligos; As-Col: HG media + antisense Col IV oligos; HG+Ran: HG medium + random oligos; n=6.
Figure 8. Effect of Coll IV and FN antisense oligos on apoptosis.
Bar graphs of cumulative data indicate that cells grown in HG medium exhibit increased number of TUNEL-positive cells. Interestingly, cells grown in HG medium and transfected with antisense Coll IV oligos or antisense FN oligos exhibit a decrease in the number of TUNEL-positive cells compared to that of cells grown in HG medium or cells grown in HG medium transfected with random oligo. Data is presented as mean ± standard deviation; *p<0.05. N: normal media; HG: high glucose media; As-FN: HG media + antisense FN oligos; As-Col: HG media + antisense Col IV oligos; HG+Ran: HG medium + random oligos; n=6.
Findings from this study indicate that the ECM participates in cell-matrix interactions via integrins. Since HG or diabetes upregulates fibronectin and Collagen IV with concomitant increase in fibronectin-specific α5β1, and Collagen IV specific α3β1 receptors, a strategy that simultaneously reduces both ECM components and integrin subunits could be useful in thwarting DR progression. As reported in the above-mentioned study, reducing ECM component expression decreases the corresponding integrin, whereas another study reported inhibiting α5 integrin reduces fibronectin accumulation in lung BMs (Lu et al., 2020), suggesting that targeting integrin subunits can modulate ECM levels and thus ameliorate BM thickening. Also, integrin-mediated interactions between the cells and the ECM are critical for cell survival, and abnormal overexpression of ECM components and integrins may compromise such interactions with profound consequences including cell death. Thus, reduction of integrin levels may be beneficial for maintenance of normal interaction between cell and BM.
5.5. Neovascularization involves the local dissolution of BM – a critical step in PDR
BM thickening likely occurs not only due to excess synthesis of ECM components but also due to decreased degradation. Studies were conducted to follow-up on in vitro findings on MMPs and their inhibitors, TIMPs, in diabetic human patients. It was first revealed that MMP-1, MMP-2, MMP-3, MMP-9, TIMP-1, TIMP-2, and TIMP-3 were present in human vitreous samples (De La Paz et al., 1998; Plantner et al., 1998), suggesting that normal remodelling of the BM occurs regularly even in non-diabetic subjects. In retinas of 12-week STZ-induced diabetic rats, MMP-2, MMP-9, and MMP-14 mRNA expression was found to be upregulated concomitant with increased retinal vascular leakage (Giebel et al., 2005). MMP-9 expression was also significantly elevated in retinal endothelial cells grown in HG condition (Giebel et al., 2005). In addition, cells treated with purified MMP-2 or MMP-9 led to degradation of occludin, a tight junction protein, suggesting that MMPs disrupt tight junctions and thus facilitate the breakdown of the blood-retinal barrier, leading to vascular leakage associated with DR. Interestingly, in diabetic patients with retinopathy, an elevated circulating level of MMP-9 was observed as well as increased MMP-9/TIMP-1 ratio compared to those of diabetic patients without retinopathy (Abu El-Asrar et al., 2017; Jacqueminet et al., 2006; Jayashree et al., 2018), suggesting that serum levels of MMP-9 could potentially serve as a biomarker of retinopathy in diabetic patients. Furthermore, significant elevation in the plasma level of MMP-2 was noted in type I diabetic patients with proliferative retinopathy compared to those without retinopathy (Peeters et al., 2015). In epiretinal neovascular membranes obtained from human diabetic patients, both pro- and active forms of MMP-2 and MMP-9 as well as high- and low-molecular weight forms of urokinase, a serine proteinase involved in the degradation of ECM, were significantly increased (Das et al., 1999), indicating that degradation of the ECM mediated by these molecules promotes neovascularization clinically seen in late stages of DR. Taken together, these data suggest that plasma levels of MMP-2 and MMP-9 are associated with the severity and progression of DR.
For angiogenesis to occur, local dissolution of BM is essential for new tip cells to sprout and undergo endothelial cell invasion. Participation of MMP-2 and MMP-9, in part mediated by PI3K/Akt and ERK1/2 pathways (Mohammad and Siddiquei, 2012), orchestrates local dissolution of BM allowing the initiation of controlled neovascularization. Studies have shown that diabetes-induced VEGF production correlates with MMP-2 and MMP-9 upregulation indicating that MMPs are closely associated with VEGF-driven angiogenesis. Furthermore, in hypoxic condition, Müller cells produce VEGF, which in turn induces MMP-2 expression and activity in retinal endothelial cells (Rodrigues et al., 2013). Presence of MMP-2 on invading endothelial cell surface has also been reported (Brooks et al., 1998; Kowluru and Mishra, 2017), suggesting that MMP-2 is associated with neovascularization. The involvement of other MMPs in neovascularization has recently been reported showing MMP-10 and MMP-14 to have significant involvement in patients with PDR (Drankowska et al., 2019). While further studies are necessary to better understand the role of different MMPs, findings from these recent reports provide substantial evidence that implicate MMPs as critical players in the angiogenic process seen in late stages of DR.
Uncontrolled neovascularization is the hallmark of PDR. Initiation of neovascularization involves complex interactions of several factors, enzymes, and local dissolution of vascular BM (Fig. 9). In response to hypoxia, VEGF produced by Muller cells activate endothelial cells to release MMP-2, MMP-9, and urokinase, which orchestrate local degradation of the BM, allowing endothelial cells to breakout from vessel lumen, and exhibit sprouting. Sprouting angiogenesis is led by endothelial tip cells with their filopodia, which possess highly enriched actin filaments capable of responding to cues from growth factors. This process is dictated, at least in part, by the binding of growth factors to endothelial cell receptors on pre-existing vessels, and ultimately results in stromal invasion. As cells proliferate, migrate, and lay collagen IV scaffolds, other ECM molecules assemble on the collagen framework in a highly organized manner, eventually leading to the development of a matured BM. Simultaneously, cells keep migrating to the site of angiogenesis, and facilitate the extension and formation of the neovessel. During PDR, fibronectin plays a major role in the assembly of the neovessel BM, which is facilitated in part by PDGF and TGF-β. However, the neovessel architecture is characterized as aberrant due to altered matrix assembly. Furthermore, PDGF-driven pericyte recruitment (Caporarello et al., 2019) may result in reduced pericyte coverage in the neovessels. Taken together, the aberrant neovessel lacks functional and blood-retinal barrier characteristics contributing to leakiness. Intense research is underway to identify mechanisms underlying regulation of sprouting angiogenesis in diabetes. Specifically, studies have focused on identifying distinct responses by specific endothelial cells involving sprouting. For example, tip cells were found to be capable of sensing and responding to guidance cues from growth factors, such as VEGF (Ruhrberg et al., 2002), exhibiting spatially restricted patterning cues in blood vessel branching morphogenesis. Other studies indicate filopodial extension to be mediated by VEGFR2 expressed on endothelial tip cells (Fruttiger, 2002; Gerhardt et al., 2003). Taken together, neovessels that develop from pre-existing vessels are abnormal and contribute to vascular leakage, retinal detachment, and other retinal complications in DR.
Figure 9. A schematic illustration showing major steps in retinal neovascularization.
This multi-step process is hypoxia-driven and involves endothelial cell participation, local BM dissolution, endothelial cell sprouting, ECM synthesis, ECM assembly, BM maturation, and neovessel formation.
6. BM remodelling – a biomarker of DR
As biomarkers become more available for the diagnosis and identification of DR development and progression, not much is known about the role of abnormal vascular BM and its components as biomarkers in DR.
Effective diagnosis and treatment of DR depends on reliable biomarkers. Hyperglycemia-driven vascular BM thickening is known to play a key role in the pathogenesis of DR (Fig. 10). However, the abnormal levels of BM components have not yet been fully studied as potential biomarkers of DR. A study suggests that collagen IV, a major component of the BM, could serve as a potential biomarker of DR, in that plasma levels of collagen IV was found to be significantly elevated in patients with DR compared to those of diabetic patients without DR (Simo-Servat et al., 2016). Additionally, in retinas of patients with DR, there was evidence indicating excess accumulation of fibronectin and collagen types I, II, IV (α1- α 2), and V in the inner limiting membrane (Ljubimov et al., 1996).
Figure 10. Hyperglycemia-induced retinal vascular BM thickening contributes to the development and progression of DR.
Schematic diagram highlights major steps in the development of vascular lesions characteristic of DR. GJIC: gap junction intercellular communication.
Moreover, age- and gender-adjusted plasma level of fibronectin was found to be increased in diabetic patients with DR compared to that of non-diabetic subjects or diabetic subjects without DR (De Giorgio et al., 1984). Another study demonstrated that collagen IV levels were significantly elevated in serum and in the vitreous samples of patients with DR compared to those of non-diabetic subjects with non-inflammatory retinopathy such as those with macular holes or retinal detachment (Kotajima et al., 2001). Studies have also suggested that serum laminin-P1 levels may serve as a useful biomarker of DR but not in the early stages of DR pathogenesis (Masmiquel et al., 2000; Masmiquel et al., 1999). Furthermore, serum laminin-P1 levels were correlated with an increase in retinal thickness in subjects with type 2 diabetes, suggesting that circulating levels of laminin-P1 may be a useful tool in monitoring early stages of DR (Hernandez et al., 2020). A recent report also found that elevated levels of circulating oxidized low-density lipoprotein immune complexes could serve as a potential biomarker to predict risk for severe non-PDR and PDR (Simo et al., 2013). Furthermore, another study showed that MMPs, zinc-dependent proteinases responsible for degrading ECM proteins, specifically, MMP-2 and MMP-9, were elevated in plasma samples of patients with PDR (Beranek et al., 2008; Maxwell et al., 2001). As discussed previously, plasma levels of MMP-2 and MMP-9 (Peeters et al., 2015), and urokinase levels in epiretinal neovascular membranes (Das et al., 1999), were found to be significantly elevated in DR patients. However, further studies are needed to definitively establish reliable biomarkers that are clinically indicative of DR.
7. BM thickening – a potential therapeutic target for treatment of DR
Given that current treatments for DR are limited in scope, and that BM thickening is a characteristic hallmark of this disease process, there have been attempts to ameliorate the effects of thickened BM for inhibiting retinal vascular lesions. A study by Gardiner et al. showed that oral administration of Sulindac, a non-steroidal anti-inflammatory drug, prevented retinal capillary BM thickening in diabetic dogs, suggesting that thickening of the BM can be halted in mechanisms independent of the polyol pathway activity, advanced glycation, or oxidative stress (Gardiner et al., 2003a). Another study reported that tight glycemic control with insulin therapy resulted in complete prevention of BM thickening in both deep and superficial capillary beds of the diabetic rat retina, whereas treatment with ponelrestat, an aldose reductase inhibitor, reduced BM thickening in the deep capillary bed but not in the superficial capillary bed of the diabetic retina (Chakrabarti and Sima, 1989). Similarly, tight glycemic control was found to normalize diabetes-induced fibronectin overexpression and also reduce vascular BM thickening in retinal and glomerular capillaries of diabetic rats (Cherian et al., 2009). Interestingly, treatment with another aldose reductase inhibitor, Sorbinil, was found to prevent collagen IV and laminin overexpression and thereby reduce capillary BM thickening in galactose-fed rat retinas (Das et al., 1990a). A different aldose reductase inhibitor, Tolrestat, was found to attenuate glomerular capillary BM thickening (Donnelly et al., 1996) and retinal capillary BM thickening in diabetic rats (McCaleb et al., 1991; Robison et al., 1986; Robison et al., 1983; Robison et al., 1988). While Tolrestat showed beneficial effects in animal models of diabetes, it was deemed ineffective in ameliorating microaneurysms, soft exudates, capillary closures, and neovascularization in patients with DR as part of a clinical trial (van Gerven et al., 1994).
One of the pioneering efforts to modulate abnormal vascular BM thickening in the diabetic retina was the use of antisense oligonucleotides via intravitreal delivery targeting fibronectin overexpression (Roy et al., 1999). This led to downregulation of fibronectin expression, reduced retinal capillary BM thickness, and also led to a decrease in the number of pericyte ghosts and acellular capillaries (Roy et al., 2003). This suggests that targeting BM components, which are overexpressed in the diabetic retina, is at least in part, beneficial towards preventing retinal vascular lesions associated with DR. The antisense oligonucleotides administered via intravitreal injection were shown to localize in the vascular cells of retinal capillaries.
Once it was determined that fibronectin antisense oligonucleotides were effective in reducing fibronectin overexpression and ultimately BM thickness, a more powerful approach to fully reduce BM thickness towards normal level was attempted. To achieve this, a combined antisense oligonucleotide approach was strategized using an antisense oligonucleotide cocktail targeting fibronectin, collagen IV, and laminin, three prominent BM components which are overexpressed in diabetes (Oshitari et al., 2006). This combined antisense oligonucleotide approach was more effective than a single antisense oligonucleotide approach targeting one component of the BM. Ultimately, the proof of this approach proved effective in reducing BM thickening and preventing retinal vascular permeability (Oshitari et al., 2006). Overall, findings from this study indicate that BM thickening is a potential therapeutic target in DR.
Since DR is a long-term complication, administering intravitreal injections on a regular basis is undesirable. A long-term strategy that will allow longer intervals between intravitreal injections is therefore important. To design such an approach that can have the same type of effect in reducing BM thickening, short interfering RNAs (siRNAs) were developed. siRNAs were considered for this purpose of long-term duration of efficacy because it works on the principle of targeting the RISC complex of the mRNA, disrupting the mRNA, and importantly the siRNA is “re-usable”, as opposed to antisense oligonucleotides that are of “one-time” use. In contrast to antisense oligonucleotides, siRNAs were tested as an alternative modality to reduce BM thickening as they do not require a vector such as that used for antisense oligonucleotide delivery. Importantly, a long-term regimen involving intravitreal injections of siRNA targeting fibronectin was found to be effective in reducing diabetes-induced retinal capillary BM thickening, as well as preventing vascular cell loss (Roy et al., 2011) with no untoward effects. Of note, antisense oligonucleotide administration required monthly intravitreal injections (Roy et al., 2003; Roy et al., 1999) while siRNA administration was efficacious for up to 6 weeks (Roy et al., 2011). As such, the siRNA approach for gene modulation is robust and has recently been preferred over antisense oligonucleotides as a potential treatment modality due to its long duration of efficacy and ease of use.
Interestingly, data obtained from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial has shown that administration of fenofibrate, a drug designed to treat hyperlipidemia and hypercholesterolemia, markedly blocked the development of PDR and alleviated macular edema in diabetic patients by 30% (Keech et al., 2005; Keech et al., 2007). Although it is well documented that fenofibrate lowers cholesterol by reducing the levels of low-density lipoprotein, very low-density lipoprotein, and triglycerides while increasing levels of high-density lipoprotein, how this drug protects against the progression of non-PDR to PDR is not well known. PDR is characterized by uncontrolled neovascularization accompanied by vascular leakage, at least in part, due to abnormally thickened BMs which contribute to excess leakiness. Studies conducted from our lab have demonstrated that fenofibric acid can reduce HG-induced fibronectin and collagen overexpression (Roy et al., 2015; Trudeau et al., 2011) concomitant with a decrease in cyclooxygenase-2, a pro-inflammatory molecule, as well as improving tight junction functionality by upregulating zonula occludens-1 (ZO-1) expression (Roy et al., 2015). Additionally, retinal endothelial cells grown in HG condition exhibited increased cell monolayer permeability whereas cells grown in HG and treated with fenofibric acid showed reduced cell monolayer permeability, indicating fenofibric acid’s beneficial effects against vascular permeability (Roy et al., 2015). Moreover, in an in vivo environment, oral administration of fenofibrate halted retinal capillary BM thickening, downregulated VEGF and ROS expression, and prevented retinal vascular permeability in diabetic rats (Li et al., 2018), suggesting that fenofibrate can preserve BM functionality and prevent retinal vascular leakage associated with DR. Taken together, while further studies are necessary to better characterize fenofibrate efficacy and other gene modulatory strategies in human DR, targeting abnormal vascular BM thickening holds promise amidst the growing need for therapeutic strategies to treat DR.
8. Conclusions
Novel targets associated with BM thickening are being assessed for better understanding of the pathogenesis of DR. In particular, LOX has gained attention for its abnormal levels and activity in hyperglycemic condition and for its unusually high level in vitreous samples of patients with PDR. Findings support the possibility that altered LOX levels promote BRB breakdown and retinal vascular cell loss associated with DR. Based on current studies LOX may be useful in accurately classifying disease stage of DR patients and designing therapeutic strategies accordingly, and this raises the prospect of targeting abnormal LOX overexpression as a therapeutic target for PDR treatment.
A balance between ECM formation and the level of expression of the LOX isoforms is critical, as factors that disrupt this balance could lead to BM thickening and compromise BM function. Intense research is currently underway attempting to establish if a link exists between LOX isoforms and the early stage of DR. Further studies are needed to determine if pharmacological inhibition of LOXs is feasible in clinical practice to slow the progression of DR.
The role of integrins in mediating signals from ECM to intracellular action and vice versa are critical in understanding how abnormal BM mediates changes to its microenvironment and affects cell function and survival. Collagen is known to activate α2β1 integrin-regulated signal transduction pathway through focal adhesion kinases, and signals that sustain cell survival and initiate cell proliferation are elicited by a synergistic action of growth factors and ECM components. Anchorage of cells with their surrounding ECM through integrins is necessary for MEK/MAPK and PI3K/Akt driven pathways and cell survival. Thus, maintenance of proper ECM structure and function could prove to be a therapeutic target for supporting growth factor action and importantly, cell survival.
Our current findings also highlight an association between altered expression of ECM components and its influence on Cx43 expression in mediating apoptosis in retinal vascular cells under HG condition. Thus, identifying mechanisms underlying HG-induced changes in ECM expression and its effects on cell-cell communication may provide novel insights into the deleterious role of abnormal BM thickening in DR.
Vascular BM thickening may not be the only contributory factor to the pathogenesis of the vasculature in tissues targeted by diabetes (Bae et al., 1987; Weynand et al., 1999). Hyperglycemia-driven changes could be quite complex as it is still unknown why some individuals with 50 years of diabetes have not developed any pathological microvascular changes (Sun et al., 2011). Whether tissues have genetic protection against certain pathological changes are not well understood. Some studies indicate that, for example, retina, kidney, and nerves succumb to pathological changes from hyperglycemia, while other tissues are spared against hyperglycemia-induced insult. It is unclear why no lung complications are associated with diabetes despite pulmonary capillaries exhibiting BM thickening. While BM thickening clearly plays an important role in the development and progression of DR, other pathological factors are at play in contributing to the development of characteristic lesions associated with DR (Duh et al., 2017; Stitt et al., 2016). Biochemical mechanisms underlying the development of BM thickening are now better defined and the detrimental effects of the thickened vascular BMs are better understood during the development and progression of DR - the challenge now is to test whether preventing vascular BM thickening has potential merit in treating DR.
Supplementary Material
Acknowledgments
Research was supported by NIH grants R01 EY014702 (SR) and EY025528 (SR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel Wahab N, Mason RM, 1996. Modulation of neutral protease expression in human mesangial cells by hyperglycaemic culture. Biochem J 320 ( Pt 3), 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu BJ, de Brito Vieira WH, 2016. Metalloproteinase Changes in Diabetes. Adv Exp Med Biol 920, 185–190. [DOI] [PubMed] [Google Scholar]
- Abu El-Asrar AM, Ahmad A, Alam K, Siddiquei MM, Mohammad G, Hertogh G, Mousa A, Opdenakker G, 2017. Extracellular matrix metalloproteinase inducer (EMMPRIN) is a potential biomarker of angiogenesis in proliferative diabetic retinopathy. Acta Ophthalmol 95, 697–704. [DOI] [PubMed] [Google Scholar]
- Adams JC, Watt FM, 1993. Regulation of development and differentiation by the extracellular matrix. Development 117, 1183–1198. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW, 1998. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 47, 1953–1959. [DOI] [PubMed] [Google Scholar]
- Ashton N, 1949. Vascular changes in diabetes with particular reference to the retinal vessels; preliminary report. Br J Ophthalmol 33, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N, 1974. Vascular basement membrane changes in diabetic retinopathy. Montgomery lecture, 1973. Br J Ophthalmol 58, 344–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew BC, Furuya T, Edwards DS, 2018. Ocular Distribution and Pharmacodynamics of SF0166, a Topically Administered alphavbeta3 Integrin Antagonist, for the Treatment of Retinal Diseases. J Pharmacol Exp Ther 366, 244–250. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Wiedemann H, Mann K, Timpl R, 1989. Binding of nidogen and the laminin-nidogen complex to basement membrane collagen type IV. Eur J Biochem 184, 241–248. [DOI] [PubMed] [Google Scholar]
- Babel W, Glanville RW, 1984. Structure of human-basement-membrane (type IV) collagen. Complete amino-acid sequence of a 914-residue-long pepsin fragment from the alpha 1(IV) chain. Eur J Biochem 143, 545–556. [DOI] [PubMed] [Google Scholar]
- Bae HY, Oh KT, Chae JK, Chung CH, Hong SP, Cho KK, 1987. Subepidermal capillary basement membrane thickness of the skin obtained by punch biopsy in patients with non insulin dependent diabetes mellitus. Korean J Intern Med 2, 234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, 2003. Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci 44, 5410–5416. [DOI] [PubMed] [Google Scholar]
- Beauchemin ML, Leuenberger PM, Babel J, 1975. Retinal capillary basement membrane thickness in spiny mice (Acomys cahirinus) with induced and spontaneous diabetes. Invest Ophthalmol 14, 560–562. [PubMed] [Google Scholar]
- Beekman B, Verzijl N, Bank RA, von der Mark K, TeKoppele JM, 1997. Synthesis of collagen by bovine chondrocytes cultured in alginate; posttranslational modifications and cell-matrix interaction. Exp Cell Res 237, 135–141. [DOI] [PubMed] [Google Scholar]
- Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT, 2008. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol 172, 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo E, Buttiglieri S, Pomero F, Allione A, D’Alu F, Ponte E, Porta M, 2003. A study of capillary pericyte viability on extracellular matrix produced by endothelial cells in high glucose. Diabetologia 46, 409–415. [DOI] [PubMed] [Google Scholar]
- Beltramo E, Pomero F, Allione A, D’Alu F, Ponte E, Porta M, 2002. Pericyte adhesion is impaired on extracellular matrix produced by endothelial cells in high hexose concentrations. Diabetologia 45, 416–419. [DOI] [PubMed] [Google Scholar]
- Beranek M, Kolar P, Tschoplova S, Kankova K, Vasku A, 2008. Genetic variations and plasma levels of gelatinase A (matrix metalloproteinase-2) and gelatinase B (matrix metalloproteinase-9) in proliferative diabetic retinopathy. Mol Vis 14, 1114–1121. [PMC free article] [PubMed] [Google Scholar]
- Bergstrand A, Bucht H, 1957. Electron microscopic investigations on the glomerular lesions in diabetes mellitus (diabetic glomerulosclerosis). Lab Invest 6, 293–300. [PubMed] [Google Scholar]
- Bhatwadekar AD, Glenn JV, Li G, Curtis TM, Gardiner TA, Stitt AW, 2008. Advanced glycation of fibronectin impairs vascular repair by endothelial progenitor cells: implications for vasodegeneration in diabetic retinopathy. Invest Ophthalmol Vis Sci 49, 1232–1241. [DOI] [PubMed] [Google Scholar]
- Bhatwadekar AD, Kansara V, Luo Q, Ciulla T, 2020. Anti-Integrin therapy for retinovascular diseases. Expert Opin Investig Drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Ripandelli G, Feher J, Plateroti AM, Plateroti R, Kovacs I, Plateroti P, Taurone S, Artico M, 2015. Occlusion of retinal capillaries caused by glial cell proliferation in chronic ocular inflammation. Folia Morphol (Warsz) 74, 33–41. [DOI] [PubMed] [Google Scholar]
- Bianchi E, Ripandelli G, Taurone S, Feher J, Plateroti R, Kovacs I, Magliulo G, Orlando MP, Micera A, Battaglione E, Artico M, 2016. Age and diabetes related changes of the retinal capillaries: An ultrastructural and immunohistochemical study. Int J Immunopathol Pharmacol 29, 40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodworth JM Jr., 1963. Diabetic microangiopathy. Diabetes 12, 99–114. [DOI] [PubMed] [Google Scholar]
- Bloodworth JM Jr., Engerman RL, Powers KL, 1969. Experimental diabetic microangiopathy. I. Basement membrane statistics in the dog. Diabetes 18, 455–458. [DOI] [PubMed] [Google Scholar]
- Bloodworth JM Jr., Molitor DL, 1965. Ultrastructural aspects of human and canine diabetic retinopathy. Invest Ophthalmol 4, 1037–1048. [PubMed] [Google Scholar]
- Bobbie MW, Roy S, Trudeau K, Munger SJ, Simon AM, Roy S, 2010. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest Ophthalmol Vis Sci 51, 3758–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollineni JS, Alluru I, Reddi AS, 1997. Heparan sulfate proteoglycan synthesis and its expression are decreased in the retina of diabetic rats. Curr Eye Res 16, 127–130. [DOI] [PubMed] [Google Scholar]
- Boselli JM, Macarak EJ, Clark CC, Brownell AG, Martinez-Hernandez A, 1981. Fibronectin: its relationship to basement membranes. I. Light microscopic studies. Coll Relat Res 1, 391–404. [DOI] [PubMed] [Google Scholar]
- Boucher E, Mayer G, Londono I, Bendayan M, 2006. Expression and localization of MT1-MMP and furin in the glomerular wall of short- and long-term diabetic rats. Kidney Int 69, 1570–1577. [DOI] [PubMed] [Google Scholar]
- Bowman W, Todd RB, 1858. The Physiological Anatomy and Physiology of Man. Br Foreign Med Chir Rev 21, 1–28. [PMC free article] [PubMed] [Google Scholar]
- Brazel D, Pollner R, Oberbaumer I, Kuhn K, 1988. Human basement membrane collagen (type IV). The amino acid sequence of the alpha 2(IV) chain and its comparison with the alpha 1(IV) chain reveals deletions in the alpha 1(IV) chain. Eur J Biochem 172, 35–42. [DOI] [PubMed] [Google Scholar]
- Breitkreutz D, Koxholt I, Thiemann K, Nischt R, 2013. Skin basement membrane: the foundation of epidermal integrity--BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res Int 2013, 179784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA, 1998. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell 92, 391–400. [DOI] [PubMed] [Google Scholar]
- Brown D, Hamdi H, Bahri S, Kenney MC, 1994. Characterization of an endogenous metalloproteinase in human vitreous. Curr Eye Res 13, 639–647. [DOI] [PubMed] [Google Scholar]
- Brown KL, Cummings CF, Vanacore RM, Hudson BG, 2017. Building collagen IV smart scaffolds on the outside of cells. Protein Sci 26, 2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliero E, Maiello M, Boeri D, Roy S, Lorenzi M, 1988. Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest 82, 735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliero E, Roth T, Roy S, Lorenzi M, 1991. Characteristics and mechanisms of high-glucose-induced overexpression of basement membrane components in cultured human endothelial cells. Diabetes 40, 102–110. [DOI] [PubMed] [Google Scholar]
- Caporarello N, D’Angeli F, Cambria MT, Candido S, Giallongo C, Salmeri M, Lombardo C, Longo A, Giurdanella G, Anfuso CD, Lupo G, 2019. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EC, Bjork NJ, 1990. SEM and TEM analyses of isolated human retinal microvessel basement membranes in diabetic retinopathy. Anat Rec 226, 295–306. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Ma N, Sima AA, 1991. Anionic sites in diabetic basement membranes and their possible role in diffusion barrier abnormalities in the BB-rat. Diabetologia 34, 301–306. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Sima AA, 1989. Effect of aldose reductase inhibition and insulin treatment on retinal capillary basement membrane thickening in BB rats. Diabetes 38, 1181–1186. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Tam MF, Chung AE, 1990. The basement membrane glycoprotein entactin promotes cell attachment and binds calcium ions. J Biol Chem 265, 10597–10603. [PubMed] [Google Scholar]
- Charonis AS, Tsilibary EC, 1990. Assembly of Basement Membrane Protein, in: Adair SW, Mecham RP (Eds.), Organization and Assembly of Plant and Animal Extracellular Matrix. Academic Press, Inc., pp. 85–117. [Google Scholar]
- Chauhan AK, Iaconcig A, Baralle FE, Muro AF, 2004. Alternative splicing of fibronectin: a mouse model demonstrates the identity of in vitro and in vivo systems and the processing autonomy of regulated exons in adult mice. Gene 324, 55–63. [DOI] [PubMed] [Google Scholar]
- Cherian S, Roy S, Pinheiro A, Roy S, 2009. Tight glycemic control regulates fibronectin expression and basement membrane thickening in retinal and glomerular capillaries of diabetic rats. Invest Ophthalmol Vis Sci 50, 943–949. [DOI] [PubMed] [Google Scholar]
- Chronopoulos A, Roy S, Beglova E, Mansfield K, Wachtman L, Roy S, 2015. Hyperhexosemia-Induced Retinal Vascular Pathology in a Novel Primate Model of Diabetic Retinopathy. Diabetes 64, 2603–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulos A, Tang A, Beglova E, Trackman PC, Roy S, 2010. High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes 59, 3159–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulos A, Trudeau K, Roy S, Huang H, Vinores SA, Roy S, 2011. High glucose-induced altered basement membrane composition and structure increases trans-endothelial permeability: implications for diabetic retinopathy. Curr Eye Res 36, 747–753. [DOI] [PubMed] [Google Scholar]
- Clements RS Jr., Robison WG Jr., Cohen MP, 1998. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J Diabetes Complications 12, 28–33. [DOI] [PubMed] [Google Scholar]
- Coorey NJ, Shen W, Chung SH, Zhu L, Gillies MC, 2012. The role of glia in retinal vascular disease. Clin Exp Optom 95, 266–281. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, Ribeiro L, Lobo C, 2014. Phenotypes and biomarkers of diabetic retinopathy. Prog Retin Eye Res 41, 90–111. [DOI] [PubMed] [Google Scholar]
- Dai J, Estrada B, Jacobs S, Sanchez-Sanchez BJ, Tang J, Ma M, Magadan-Corpas P, Pastor-Pareja JC, Martin-Bermudo MD, 2018. Dissection of Nidogen function in Drosophila reveals tissue-specific mechanisms of basement membrane assembly. PLoS Genet 14, e1007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild PR, Omri S, Gelize E, Jonet L, Delaunay K, De Kozak Y, Berdugo M, Zhao M, Crisanti P, Behar-Cohen F, 2018. Mechanisms of macular edema: Beyond the surface. Prog Retin Eye Res 63, 20–68. [DOI] [PubMed] [Google Scholar]
- Das A, Frank RN, Zhang NL, Samadani E, 1990a. Increases in collagen type IV and laminin in galactose-induced retinal capillary basement membrane thickening--prevention by an aldose reductase inhibitor. Exp Eye Res 50, 269–280. [DOI] [PubMed] [Google Scholar]
- Das A, Frank RN, Zhang NL, Turczyn TJ, 1990b. Ultrastructural localization of extracellular matrix components in human retinal vessels and Bruch’s membrane. Arch Ophthalmol 108, 421–429. [DOI] [PubMed] [Google Scholar]
- Das A, McGuire PG, Eriqat C, Ober RR, DeJuan E Jr., Williams GA, McLamore A, Biswas J, Johnson DW, 1999. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci 40, 809–813. [PubMed] [Google Scholar]
- Davis GE, Senger DR, 2005. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97, 1093–1107. [DOI] [PubMed] [Google Scholar]
- De Giorgio LA, Seghieri G, Gironi A, Mammini P, Bartoli U, Bartolomei G, 1984. Raised plasma fibronectin concentration is related to the presence of diabetic retinopathy. Acta Diabetol Lat 21, 251–256. [DOI] [PubMed] [Google Scholar]
- De La Paz MA, Itoh Y, Toth CA, Nagase H, 1998. Matrix metalloproteinases and their inhibitors in human vitreous. Invest Ophthalmol Vis Sci 39, 1256–1260. [PubMed] [Google Scholar]
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A, 1989. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32, 219–226. [DOI] [PubMed] [Google Scholar]
- Domogatskaya A, Rodin S, Tryggvason K, 2012. Functional diversity of laminins. Annu Rev Cell Dev Biol 28, 523–553. [DOI] [PubMed] [Google Scholar]
- Donnelly SM, Zhou XP, Huang JT, Whiteside CI, 1996. Prevention of early glomerulopathy with tolrestat in the streptozotocin-induced diabetic rat. Biochem Cell Biol 74, 355–362. [DOI] [PubMed] [Google Scholar]
- Drankowska J, Kos M, Kosciuk A, Marzeda P, Boguszewska-Czubara A, Tylus M, Swiech-Zubilewicz A, 2019. MMP targeting in the battle for vision: Recent developments and future prospects in the treatment of diabetic retinopathy. Life Sci 229, 149–156. [DOI] [PubMed] [Google Scholar]
- Duh EJ, Sun JK, Stitt AW, 2017. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farach-Carson MC, Carson DD, 2007. Perlecan--a multifunctional extracellular proteoglycan scaffold. Glycobiology 17, 897–905. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Hopper J Jr., Moon HD, 1959. Diabetic glomerulosclerosis: electron and light microscopic studies. Am J Pathol 35, 721–753. [PMC free article] [PubMed] [Google Scholar]
- Feher J, Taurone S, Spoletini M, Biro Z, Varsanyi B, Scuderi G, Orlando MP, Turchetta R, Micera A, Artico M, 2018. Ultrastructure of neurovascular changes in human diabetic retinopathy. Int J Immunopathol Pharmacol 31, 394632017748841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO, 1989. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 109, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields MA, Bowrey HE, Gong J, Moreira EF, Cai H, Del Priore LV, 2017. Extracellular matrix nitration alters growth factor release and activates bioactive complement in human retinal pigment epithelial cells. PLoS One 12, e0177763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I, 1988. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am J Pathol 130, 393–400. [PMC free article] [PubMed] [Google Scholar]
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R, American Diabetes A, 2004. Retinopathy in diabetes. Diabetes Care 27 Suppl 1, S84–87. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Shafiee A, Schroder S, Knott R, McIntosh L, 1993. The role of growth factors in proliferative diabetic retinopathy. Eye (Lond) 7 ( Pt 2), 276–287. [DOI] [PubMed] [Google Scholar]
- Frank RN, 1984. On the pathogenesis of diabetic retinopathy. Ophthalmology 91, 626–634. [DOI] [PubMed] [Google Scholar]
- Frank RN, Keirn RJ, Kennedy A, Frank KW, 1983. Galactose-induced retinal capillary basement membrane thickening: prevention by Sorbinil. Invest Ophthalmol Vis Sci 24, 1519–1524. [PubMed] [Google Scholar]
- Fruttiger M, 2002. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci 43, 522–527. [PubMed] [Google Scholar]
- Furcht LT, McCarthy JB, Palm SL, Basara ML, Enenstein J, 1984. Peptide fragments of laminin and fibronectin promote migration (haptotaxis and chemotaxis) of metastatic cells. Ciba Found Symp 108, 130–145. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez N, Jacobs-Cacha C, Mora-Gutierrez JM, Vergara A, Orbe J, Soler MJ, 2020. Matrix Metalloproteinases in Diabetic Kidney Disease. J Clin Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner TA, Anderson HR, Degenhardt T, Thorpe SR, Baynes JW, Archer DB, Stitt AW, 2003a. Prevention of retinal capillary basement membrane thickening in diabetic dogs by a non-steroidal anti-inflammatory drug. Diabetologia 46, 1269–1275. [DOI] [PubMed] [Google Scholar]
- Gardiner TA, Anderson HR, Stitt AW, 2003b. Inhibition of advanced glycation end-products protects against retinal capillary basement membrane expansion during long-term diabetes. J Pathol 201, 328–333. [DOI] [PubMed] [Google Scholar]
- Gay S, Martinez-Hernandez A, Rhodes RK, Miller EJ, 1981. The collagenous exocytoskeleton of smooth muscle cells. Coll Relat Res 1, 377–384. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C, 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs GE, Wilson RB, Gifford H, 1966. Glomerulosclerosis in the long-term alloxan diabetic monkey. Diabetes 15, 258–261. [DOI] [PubMed] [Google Scholar]
- Giebel SJ, Menicucci G, McGuire PG, Das A, 2005. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest 85, 597–607. [DOI] [PubMed] [Google Scholar]
- Grant MB, Caballero S, Tarnuzzer RW, Bass KE, Ljubimov AV, Spoerri PE, Galardy RE, 1998. Matrix metalloproteinase expression in human retinal microvascular cells. Diabetes 47, 1311–1317. [DOI] [PubMed] [Google Scholar]
- Grant MB, Ellis EA, Caballero S, Mames RN, 1996. Plasminogen activator inhibitor-1 overexpression in nonproliferative diabetic retinopathy. Exp Eye Res 63, 233–244. [DOI] [PubMed] [Google Scholar]
- Grant WP, Sullivan R, Sonenshine DE, Adam M, Slusser JH, Carson KA, Vinik AI, 1997. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg 36, 272–278; discussion 330. [DOI] [PubMed] [Google Scholar]
- Groggel GC, Stevenson J, Hovingh P, Linker A, Border WA, 1988. Changes in heparan sulfate correlate with increased glomerular permeability. Kidney Int 33, 517–523. [DOI] [PubMed] [Google Scholar]
- Gubbiotti MA, Neill T, Iozzo RV, 2017. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biol 57–58, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TS, Duraisamy S, Faulkner JL, Kasinath BS, 2004. Regulation of glomerular endothelial cell proteoglycans by glucose. J Korean Med Sci 19, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth DP, Katz ML, Sanders DA, Sanders DN, Wright EJ, Sturek M, 2002. Retinal capillary basement membrane thickening in a porcine model of diabetes mellitus. Comp Med 52, 523–529. [PubMed] [Google Scholar]
- Halfter W, Moes S, Asgeirsson DO, Halfter K, Oertle P, Melo Herraiz E, Plodinec M, Jenoe P, Henrich PB, 2017. Diabetes-related changes in the protein composition and the biomechanical properties of human retinal vascular basement membranes. PLoS One 12, e0189857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill KJ, Kligys K, Hopkinson SB, Jones JC, 2009. Laminin deposition in the extracellular matrix: a complex picture emerges. J Cell Sci 122, 4409–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj NS, Antonetti DA, 2004. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 36, 1206–1237. [DOI] [PubMed] [Google Scholar]
- Hay ED, 2013. Cell Biology of Extracellular Matrix : Second Edition. [Google Scholar]
- Hayden MR, Sowers JR, Tyagi SC, 2005. The central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloaded. Cardiovasc Diabetol 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez C, Bogdanov P, Corraliza L, Garcia-Ramirez M, Sola-Adell C, Arranz JA, Arroba AI, Valverde AM, Simo R, 2016. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 65, 172–187. [DOI] [PubMed] [Google Scholar]
- Hernandez C, Bogdanov P, Sola-Adell C, Sampedro J, Valeri M, Genis X, Simo-Servat O, Garcia-Ramirez M, Simo R, 2017. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 60, 2285–2298. [DOI] [PubMed] [Google Scholar]
- Hernandez C, Garcia-Ramirez M, Corraliza L, Fernandez-Carneado J, Farrera-Sinfreu J, Ponsati B, Gonzalez-Rodriguez A, Valverde AM, Simo R, 2013. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 62, 2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez C, Porta M, Bandello F, Grauslund J, Harding SP, Aldington SJ, Egan C, Frydkjaer-Olsen U, Garcia-Arumi J, Gibson J, Lang GE, Lattanzio R, Massin P, Midena E, Ponsati B, Ribeiro L, Scanlon P, Cunha-Vaz J, Simo R, 2020. The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. J Clin Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MS, Bose K, Mokkapati S, Nischt R, Smyth N, 2008. Nidogens-Extracellular matrix linker molecules. Microsc Res Tech 71, 387–395. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Yurchenco PD, 2013. Laminins in basement membrane assembly. Cell Adh Migr 7, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu TT, Vanhove M, Porcu M, Van Hove I, Van Bergen T, Jonckx B, Barbeaux P, Vermassen E, Feyen JHM, 2019. The potent small molecule integrin antagonist THR-687 is a promising next-generation therapy for retinal vascular disorders. Exp Eye Res 180, 43–52. [DOI] [PubMed] [Google Scholar]
- Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA, 2011. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci 52, 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudacko RM, Sciancalepore JP, Fyfe BS, 2009. Diabetic microangiopathy in the liver: an autopsy study of incidence and association with other diabetic complications. Am J Clin Pathol 132, 494–499. [DOI] [PubMed] [Google Scholar]
- Huet C, Monget P, Pisselet C, Hennequet C, Locatelli A, Monniaux D, 1998. Chronology of events accompanying follicular atresia in hypophysectomized ewes. Changes in levels of steroidogenic enzymes, connexin 43, insulin-like growth factor II/mannose 6 phosphate receptor, extracellular matrix components, and matrix metalloproteinases. Biology of reproduction 58, 175–185. [DOI] [PubMed] [Google Scholar]
- Hynes RO, 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Hynes RO, 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Schwarzbauer JE, Tamkun JW, 1984. Fibronectin: a versatile gene for a versatile protein. Ciba Found Symp 108, 75–92. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Olsen CE, Boitano S, 2006. Laminin-332 alters connexin profile, dye coupling and intercellular Ca2+ waves in ciliated tracheal epithelial cells. Respiratory research 7, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara J, Ishihara A, Fukunaga K, Sasaki K, White MJV, Briquez PS, Hubbell JA, 2018. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat Commun 9, 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacqueminet S, Ben Abdesselam O, Chapman MJ, Nicolay N, Foglietti MJ, Grimaldi A, Beaudeux JL, 2006. Elevated circulating levels of matrix metalloproteinase-9 in type 1 diabetic patients with and without retinopathy. Clin Chim Acta 367, 103–107. [DOI] [PubMed] [Google Scholar]
- Jayadev R, Chi Q, Keeley DP, Hastie EL, Kelley LC, Sherwood DR, 2019. alpha-Integrins dictate distinct modes of type IV collagen recruitment to basement membranes. J Cell Biol 218, 3098–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev R, Sherwood DR, 2017. Basement membranes. Curr Biol 27, R207–R211. [DOI] [PubMed] [Google Scholar]
- Jayashree K, Yasir M, Senthilkumar GP, Ramesh Babu K, Mehalingam V, Mohanraj PS, 2018. Circulating matrix modulators (MMP-9 and TIMP-1) and their association with severity of diabetic retinopathy. Diabetes Metab Syndr 12, 869–873. [DOI] [PubMed] [Google Scholar]
- Jerdan JA, Glaser BM, 1986. Retinal microvessel extracellular matrix: an immunofluorescent study. Invest Ophthalmol Vis Sci 27, 194–203. [PubMed] [Google Scholar]
- Kalb E, Engel J, 1991. Binding and calcium-induced aggregation of laminin onto lipid bilayers. J Biol Chem 266, 19047–19052. [PubMed] [Google Scholar]
- Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M, investigators F.s., 2005. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366, 1849–1861. [DOI] [PubMed] [Google Scholar]
- Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d’Emden MC, Crimet DC, O’Connell RL, Colman PG, investigators F.s., 2007. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370, 1687–1697. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG, 2008. Mammalian collagen IV. Microsc Res Tech 71, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen PD, Burbelo P, Sakurai Y, Yamada Y, 1988. Structure of the amino-terminal portion of the murine alpha 1(IV) collagen chain and the corresponding region of the gene. J Biol Chem 263, 8706–8709. [PubMed] [Google Scholar]
- Kilo C, Vogler N, Williamson JR, 1972. Muscle capillary basement membrane changes related to aging and to diabetes mellitus. Diabetes 21, 881–905. [DOI] [PubMed] [Google Scholar]
- Kim D, Lee D, Trackman PC, Roy S, 2019a. Effects of High Glucose-Induced Lysyl Oxidase Propeptide on Retinal Endothelial Cell Survival: Implications for Diabetic Retinopathy. Am J Pathol 189, 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Mecham RP, Nguyen NH, Roy S, 2019b. Decreased lysyl oxidase level protects against development of retinal vascular lesions in diabetic retinopathy. Exp Eye Res 184, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Mecham RP, Trackman PC, Roy S, 2017. Downregulation of lysyl oxidase protects retinal endothelial cells from high glucose–induced apoptosis. Investigative ophthalmology & visual science 58, 2725–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen I, Hughes JM, Vogels IM, Schalkwijk CG, Van Noorden CJ, Schlingemann RO, 2009. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res 89, 4–15. [DOI] [PubMed] [Google Scholar]
- Klaassen I, van Geest RJ, Kuiper EJ, van Noorden CJ, Schlingemann RO, 2015. The role of CTGF in diabetic retinopathy. Exp Eye Res 133, 37–48. [DOI] [PubMed] [Google Scholar]
- Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L, 1991. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol 115, 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RF, Feingold KR, Morgan C, Stern WH, Siperstein MD, 1987. Relationship of muscle capillary basement membrane thickness and diabetic retinopathy. Diabetes Care 10, 195–199. [DOI] [PubMed] [Google Scholar]
- Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V, 2009. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A 106, 18267–18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Ono M, Hara ES, Ueda J, Nguyen HTT, Nguyen HT, Yonezawa T, Maeba T, Kimura-Ono A, Takarada T, Momota R, Maekawa K, Kuboki T, Oohashi T, 2018. Type IV collagen alpha6 chain is a regulator of keratin 10 in keratinization of oral mucosal epithelium. Sci Rep 8, 2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotajima N, Kanda T, Yuuki N, Kimura T, Kishi S, Fukumura Y, Tamura I, Kobayashi I, 2001. Type IV collagen serum and vitreous fluid levels in patients with diabetic retinopathy. J Int Med Res 29, 292–296. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Mishra M, 2017. Regulation of Matrix Metalloproteinase in the Pathogenesis of Diabetic Retinopathy. Prog Mol Biol Transl Sci 148, 67–85. [DOI] [PubMed] [Google Scholar]
- Krag S, Danielsen CC, Carmeliet P, Nyengaard J, Wogensen L, 2005. Plasminogen activator inhibitor-1 gene deficiency attenuates TGF-beta1-induced kidney disease. Kidney Int 68, 2651–2666. [DOI] [PubMed] [Google Scholar]
- Lakshmanan HHS, Melrose AR, Sepp AI, Mitrugno A, Ngo ATP, Khader A, Thompson R, Sallee D, Pang J, Mangin PH, Jandrot-Perrus M, Aslan JE, McCarty OJT, 2020. The basement membrane protein nidogen-1 supports platelet adhesion and activation. Platelets, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Ma W, Rattigan EM, Aleshin A, Chen L, Johnson LL, D’Agati VD, Schmidt AM, Barile GR, 2010. Ultrastructural features of retinal capillary basement membrane thickening in diabetic swine. Ultrastruct Pathol 34, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Hsieh ST, Chiang HY, 2019. Fibronectin inhibitor pUR4 attenuates tumor necrosis factor alpha-induced endothelial hyperpermeability by modulating beta1 integrin activation. J Biomed Sci 26, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin EG, Santell L, 1987. Association of a plasminogen activator inhibitor (PAI-1) with the growth substratum and membrane of human endothelial cells. J Cell Biol 105, 2543–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AF, Roy S, 2009. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci 50, 1400–1407. [DOI] [PubMed] [Google Scholar]
- Li J, Wang P, Chen Z, Yu S, Xu H, 2018. Fenofibrate Ameliorates Oxidative Stress-Induced Retinal Microvascular Dysfunction in Diabetic Rats. Curr Eye Res 43, 1395–1403. [DOI] [PubMed] [Google Scholar]
- Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fassler R, 2005. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci 118, 2913–2921. [DOI] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD, 2002. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol 157, 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, Kenney MC, 1996. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem 44, 1469–1479. [DOI] [PubMed] [Google Scholar]
- Longhurst J, Capone RJ, Zelis R, 1975. Evaluation of skeletal muscle capillary basement membrane thickness in congestive heart failure. Chest 67, 195–198. [DOI] [PubMed] [Google Scholar]
- Lorenzi M, Podesta F, Mizutani M, Roy S, 1998. Cellular effects of elevated glucose concentrations and diabetic retinopathy. Front Diabetes 14, 105–112. [Google Scholar]
- Lu J, Doyle AD, Shinsato Y, Wang S, Bodendorfer MA, Zheng M, Yamada KM, 2020. Basement Membrane Regulates Fibronectin Organization Using Sliding Focal Adhesions Driven by a Contractile Winch. Dev Cell 52, 631–646 e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandarino LJ, Sundarraj N, Finlayson J, Hassell HR, 1993. Regulation of fibronectin and laminin synthesis by retinal capillary endothelial cells and pericytes in vitro. Exp Eye Res 57, 609–621. [DOI] [PubMed] [Google Scholar]
- Marchand M, Monnot C, Muller L, Germain S, 2019. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin Cell Dev Biol 89, 147–156. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Amenta PS, 1983. The basement membrane in pathology. Lab Invest 48, 656–677. [PubMed] [Google Scholar]
- Martinez-Hernandez A, Gay S, Miller EJ, 1982. Ultrastructural localization of type V collagen in rat kidney. J Cell Biol 92, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Marsh CA, Clark CC, Macarak EJ, Brownell AG, 1981a. Fibronectin: its relationship to basement membranes. II. Ultrastructural studies in rat kidney. Coll Relat Res 1, 405–418. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Marsh CA, Horn JF, Munoz E, 1981b. Glomerular basement membrane: lamina rara, lamina densa. Ren Physiol 4, 137–144. [DOI] [PubMed] [Google Scholar]
- Masmiquel L, Segura RM, Mateo C, Calatayud M, Marti R, Mesa J, Simo R, 2000. Serum laminin as a marker of diabetic retinopathy development: a 4-year follow-up study. Am J Ophthalmol 129, 347–352. [DOI] [PubMed] [Google Scholar]
- Masmiquel LL, Burgos R, Mateo C, Marti R, Segura RM, Simo R, 1999. Effect of panretinal photocoagulation on serum levels of laminin in patients with diabetes: a prospective study. Br J Ophthalmol 83, 1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PR, Timms PM, Chandran S, Gordon D, 2001. Peripheral blood level alterations of TIMP-1, MMP-2 and MMP-9 in patients with type 1 diabetes. Diabet Med 18, 777–780. [DOI] [PubMed] [Google Scholar]
- McCaleb ML, McKean ML, Hohman TC, Laver N, Robison WG Jr., 1991. Intervention with the aldose reductase inhibitor, tolrestat, in renal and retinal lesions of streptozotocin-diabetic rats. Diabetologia 34, 695–701. [DOI] [PubMed] [Google Scholar]
- McKee KK, Capizzi S, Yurchenco PD, 2009. Scaffold-forming and Adhesive Contributions of Synthetic Laminin-binding Proteins to Basement Membrane Assembly. J Biol Chem 284, 8984–8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee KK, Harrison D, Capizzi S, Yurchenco PD, 2007. Role of laminin terminal globular domains in basement membrane assembly. J Biol Chem 282, 21437–21447. [DOI] [PubMed] [Google Scholar]
- McLennan SV, Martell SY, Yue DK, 2000. High glucose concentration inhibits the expression of membrane type metalloproteinase by mesangial cells: possible role in mesangium accumulation. Diabetologia 43, 642–648. [DOI] [PubMed] [Google Scholar]
- Miller CG, Budoff G, Prenner JL, Schwarzbauer JE, 2017. Minireview: Fibronectin in retinal disease. Exp Biol Med (Maywood) 242, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad G, Siddiquei MM, 2012. Role of matrix metalloproteinase-2 and −9 in the development of diabetic retinopathy. J Ocul Biol Dis Infor 5, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Knack HP, Roy S, 2011. Effect of Inhibiting High Glucose-induced Upregulation of ECM on Connexin 43 (Cx43) Expression in Retinal Endothelial Cells. Investigative Ophthalmology & Visual Science; ARVO Annual Meeting Abstract 52, 3554. [Google Scholar]
- Mori R, Power KT, Wang CM, Martin P, Becker DL, 2006. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci 119, 5193–5203. [DOI] [PubMed] [Google Scholar]
- Morrissey MA, Sherwood DR, 2015. An active role for basement membrane assembly and modification in tissue sculpting. J Cell Sci 128, 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T, Tien T, Kim D, Sarthy VP, Roy S, 2014. High glucose alters Cx43 expression and gap junction intercellular communication in retinal Muller cells: promotes Muller cell and pericyte apoptosis. Invest Ophthalmol Vis Sci 55, 4327–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynderse LA, Hassell JR, Kleinman HK, Martin GR, Martinez-Hernandez A, 1983. Loss of heparan sulfate proteoglycan from glomerular basement membrane of nephrotic rats. Lab Invest 48, 292–302. [PubMed] [Google Scholar]
- Naduk-Kik J, Hrabec E, 2008. [The role of matrix metalloproteinases in the pathogenesis of diabetes mellitus and progression of diabetes retinopathy]. Postepy Hig Med Dosw (Online) 62, 442–450. [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G, 2006. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69, 562–573. [DOI] [PubMed] [Google Scholar]
- Nagata M, Katz ML, Robison WG Jr., 1986. Age-related thickening of retinal capillary basement membranes. Invest Ophthalmol Vis Sci 27, 437–440. [PubMed] [Google Scholar]
- Nakamura T, Fukui M, Ebihara I, Osada S, Tomino Y, Koide H, 1994. Abnormal gene expression of matrix metalloproteinases and their inhibitor in glomeruli from diabetic rats. Ren Physiol Biochem 17, 316–325. [DOI] [PubMed] [Google Scholar]
- Niland S, Eble JA, 2012. Integrin-mediated cell-matrix interaction in physiological and pathological blood vessel formation. J Oncol 2012, 125278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizono H, Murata Y, Tanaka M, Soji T, Herbert DC, 1993. Evidence that Muller cells can phagocytize egg-lecithin-coated silicone particles. Tissue Cell 25, 305–310. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Kador PF, 2011. Aldose reductase / polyol inhibitors for diabetic retinopathy. Curr Pharm Biotechnol 12, 373–385. [DOI] [PubMed] [Google Scholar]
- Oloris SC, Mesnil M, Reis VN, Sakai M, Matsuzaki P, Fonseca Ede S, da Silva TC, Avanzo JL, Sinhorini IL, Guerra JL, Costa-Pinto FA, Maiorka PC, Dagli ML, 2007. Hepatic granulomas induced by Schistosoma mansoni in mice deficient for connexin 43 present lower cell proliferation and higher collagen content. Life Sci 80, 1228–1235. [DOI] [PubMed] [Google Scholar]
- Ortolan EV, Spadella CT, Caramori C, Machado JL, Gregorio EA, Rabello K, 2008. Microscopic, morphometric and ultrastructural analysis of anastomotic healing in the intestine of normal and diabetic rats. Exp Clin Endocrinol Diabetes 116, 198–202. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Brown D, Roy S, 2005. SiRNA strategy against overexpression of extracellular matrix in diabetic retinopathy. Exp Eye Res 81, 32–37. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S, 2006. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes 55, 86–92. [PubMed] [Google Scholar]
- Pankov R, Yamada KM, 2002. Fibronectin at a glance. J Cell Sci 115, 3861–3863. [DOI] [PubMed] [Google Scholar]
- Parkin JD, San Antonio JD, Pedchenko V, Hudson B, Jensen ST, Savige J, 2011. Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum Mutat 32, 127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters SA, Engelen L, Buijs J, Chaturvedi N, Fuller JH, Schalkwijk CG, Stehouwer CD, Group EPCS, 2015. Plasma levels of matrix metalloproteinase-2, −3, −10, and tissue inhibitor of metalloproteinase-1 are associated with vascular complications in patients with type 1 diabetes: the EURODIAB Prospective Complications Study. Cardiovasc Diabetol 14, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro A, Roy S, 2007. High Glucose-induced upregulation of collagen type IV and laminin expression regulates connexin 43 expression in microvascular endothelial cells, 67th American Diabetes Association Conference. Chicago, IL, Abstract # 0838-P. [Google Scholar]
- Plantner JJ, Smine A, Quinn TA, 1998. Matrix metalloproteinases and metalloproteinase inhibitors in human interphotoreceptor matrix and vitreous. Curr Eye Res 17, 132–140. [DOI] [PubMed] [Google Scholar]
- Podesta F, Roth T, Ferrara F, Cagliero E, Lorenzi M, 1997. Cytoskeletal changes induced by excess extracellular matrix impair endothelial cell replication. Diabetologia 40, 879–886. [DOI] [PubMed] [Google Scholar]
- Randles MJ, Lausecker F, Humphries JD, Byron A, Clark SJ, Miner JH, Zent R, Humphries MJ, Lennon R, 2020. Basement membrane ligands initiate distinct signalling networks to direct cell shape. Matrix Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner M, Ferreira N, Dudele A, Jensen TS, Vaegter CB, Goncalves NP, 2018. Functional and Structural Changes of the Blood-Nerve-Barrier in Diabetic Neuropathy. Front Neurosci 12, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J, Bachinger HP, Engel J, Furthmayr H, Timpl R, 1980. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem 108, 239–250. [DOI] [PubMed] [Google Scholar]
- Robison WG Jr., Kador PF, Akagi Y, Kinoshita JH, Gonzalez R, Dvornik D, 1986. Prevention of basement membrane thickening in retinal capillaries by a novel inhibitor of aldose reductase, tolrestat. Diabetes 35, 295–299. [DOI] [PubMed] [Google Scholar]
- Robison WG Jr., Kador PF, Kinoshita JH, 1983. Retinal capillaries: basement membrane thickening by galactosemia prevented with aldose reductase inhibitor. Science 221, 1177–1179. [DOI] [PubMed] [Google Scholar]
- Robison WG Jr., Nagata M, Kinoshita JH, 1988. Aldose reductase and retinal capillary basement membrane thickening. Exp Eye Res 46, 343–348. [DOI] [PubMed] [Google Scholar]
- Rodman HM, Doershuk CF, Roland JM, 1986. The interaction of 2 diseases: diabetes mellitus and cystic fibrosis. Medicine (Baltimore) 65, 389–397. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Xin X, Jee K, Babapoor-Farrokhran S, Kashiwabuchi F, Ma T, Bhutto I, Hassan SJ, Daoud Y, Baranano D, Solomon S, Lutty G, Semenza GL, Montaner S, Sodhi A, 2013. VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes 62, 3863–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Cagliero E, Lorenzi M, 1996. Fibronectin overexpression in retinal microvessels of patients with diabetes. Invest Ophthalmol Vis Sci 37, 258–266. [PubMed] [Google Scholar]
- Roy S, Ha J, Trudeau K, Beglova E, 2010. Vascular basement membrane thickening in diabetic retinopathy. Curr Eye Res 35, 1045–1056. [DOI] [PubMed] [Google Scholar]
- Roy S, Jiang JX, Li AF, Kim D, 2017. Connexin channel and its role in diabetic retinopathy. Prog Retin Eye Res 61, 35–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Kim D, Hernandez C, Simo R, Roy S, 2015. Beneficial effects of fenofibric acid on overexpression of extracellular matrix components, COX-2, and impairment of endothelial permeability associated with diabetic retinopathy. Exp Eye Res 140, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Maiello M, Lorenzi M, 1994. Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest 93, 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Nasser S, Yee M, Graves DT, Roy S, 2011. A long-term siRNA strategy regulates fibronectin overexpression and improves vascular lesions in retinas of diabetic rats. Mol Vis 17, 3166–3174. [PMC free article] [PubMed] [Google Scholar]
- Roy S, Sato T, Paryani G, Kao R, 2003. Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes 52, 1229–1234. [DOI] [PubMed] [Google Scholar]
- Roy S, Zhang K, Roth T, Vinogradov S, Kao RS, Kabanov A, 1999. Reduction of fibronectin expression by intravitreal administration of antisense oligonucleotides. Nat Biotechnol 17, 476–479. [DOI] [PubMed] [Google Scholar]
- Rucker RB, Kosonen T, Clegg MS, Mitchell AE, Rucker BR, Uriu-Hare JY, Keen CL, 1998. Copper, lysyl oxidase, and extracellular matrix protein crosslinking. Am J Clin Nutr 67, 996S–1002S. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT, 2002. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 16, 2684–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, 1996. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12, 697–715. [DOI] [PubMed] [Google Scholar]
- Sato T, Haimovici R, Kao R, Li AF, Roy S, 2002. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes 51, 1565–1571. [DOI] [PubMed] [Google Scholar]
- Savige J, Liu J, DeBuc DC, Handa JT, Hageman GS, Wang YY, Parkin JD, Vote B, Fassett R, Sarks S, Colville D, 2010. Retinal basement membrane abnormalities and the retinopathy of Alport syndrome. Invest Ophthalmol Vis Sci 51, 1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder SM, Burridge K, 1999. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol 11, 274–286. [DOI] [PubMed] [Google Scholar]
- Schuppan D, Timpl R, Glanville RW, 1980. Discontinuities in the triple helical sequence Gly-X-Y of basement membrane (type IV) collagen. FEBS Lett 115, 297–300. [DOI] [PubMed] [Google Scholar]
- Schwarz U, Schuppan D, Oberbaumer I, Glanville RW, Deutzmann R, Timpl R, Kuhn K, 1986. Structure of mouse type IV collagen. Amino-acid sequence of the C-terminal 511-residue-long triple-helical segment of the alpha 2(IV) chain and its comparison with the alpha 1(IV) chain. Eur J Biochem 157, 49–56. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE, 1991. Alternative splicing of fibronectin: three variants, three functions. Bioessays 13, 527–533. [DOI] [PubMed] [Google Scholar]
- Seon YD, Lee TH, Lee MC, 1999. Changes of glomerular basement membrane components in Vacor-induced diabetic nephropathy. Korean J Intern Med 14, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SM, Talbott GA, Juliano RL, 1998. Integrin-mediated signaling events in human endothelial cells. Mol Biol Cell 9, 1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima AA, Chakrabarti S, Tze WJ, Tai J, 1988. Pancreatic islet allograft prevents basement membrane thickening in the diabetic rat retina. Diabetologia 31, 175–181. [DOI] [PubMed] [Google Scholar]
- Simo-Servat O, Simo R, Hernandez C, 2016. Circulating Biomarkers of Diabetic Retinopathy: An Overview Based on Physiopathology. J Diabetes Res 2016, 5263798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo R, Hernandez C, 2015. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res 48, 160–180. [DOI] [PubMed] [Google Scholar]
- Simo R, Roy S, Behar-Cohen F, Keech A, Mitchell P, Wong TY, 2013. Fenofibrate: a new treatment for diabetic retinopathy. Molecular mechanisms and future perspectives. Curr Med Chem 20, 3258–3266. [DOI] [PubMed] [Google Scholar]
- Simo R, Stitt AW, Gardner TW, 2018. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 61, 1902–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siperstein MD, Unger RH, Madison LL, 1968. Studies of muscle capillary basement membranes in normal subjects, diabetic, and prediabetic patients. J Clin Invest 47, 1973–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TL, Raynor E, Prazma J, Buenting JE, Pillsbury HC, 1995. Insul-independent diabetic microangiopathy in the inner ear. Laryngoscope 105, 236–240. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Meyer M, Frie C, Paulsson M, Edgar D, 1998. The targeted deletion of the LAMC1 gene. Ann N Y Acad Sci 857, 283–286. [DOI] [PubMed] [Google Scholar]
- Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F, 1999. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J 18, 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Kim D, Nguyen NH, Roy S, 2018. Inhibition of Diabetes-Induced Lysyl Oxidase Overexpression Prevents Retinal Vascular Lesions Associated With Diabetic Retinopathy. Invest Ophthalmol Vis Sci 59, 5965–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RH, Singh AK, Leehey DJ, 1999. Decreased glomerular proteinase activity in the streptozotocin diabetic rat. Am J Nephrol 19, 441–446. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simo R, Lois N, 2016. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51, 156–186. [DOI] [PubMed] [Google Scholar]
- Stout LC, Folse DS, Meier J, Crosby WM, Kling R, Williams GR, Price WE, Geyer JR, Padula R, Whorton E, et al. , 1986. Quantitative glomerular morphology of the normal and diabetic baboon kidney. Diabetologia 29, 734–740. [DOI] [PubMed] [Google Scholar]
- Subramanian ML, Stein TD, Siegel N, Ness S, Fiorello MG, Kim D, Roy S, 2019. Upregulation of Lysyl Oxidase Expression in Vitreous of Diabetic Subjects: Implications for Diabetic Retinopathy. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A, Aiello LP, King GL, 2011. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care 34, 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Kern TS, 2011. Inflammation in diabetic retinopathy. Prog Retin Eye Res 30, 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarallo S, Beltramo E, Berrone E, Dentelli P, Porta M, 2010. Effects of high glucose and thiamine on the balance between matrix metalloproteinases and their tissue inhibitors in vascular cells. Acta Diabetol 47, 105–111. [DOI] [PubMed] [Google Scholar]
- Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noel LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA, Renal Pathology S, 2010. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21, 556–563. [DOI] [PubMed] [Google Scholar]
- Thomson SE, McLennan SV, Kirwan PD, Heffernan SJ, Hennessy A, Yue DK, Twigg SM, 2008. Renal connective tissue growth factor correlates with glomerular basement membrane thickness and prospective albuminuria in a non-human primate model of diabetes: possible predictive marker for incipient diabetic nephropathy. J Diabetes Complications 22, 284–294. [DOI] [PubMed] [Google Scholar]
- Tien T, Barrette KF, Chronopoulos A, Roy S, 2013. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Invest Ophthalmol Vis Sci 54, 6518–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien T, Muto T, Barrette K, Challyandra L, Roy S, 2014. Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascular lesions in the diabetic retina. Mol Vis 20, 732–741. [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G, 1983. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem 137, 455–465. [DOI] [PubMed] [Google Scholar]
- Timpl R, Martin GR, Bruckner P, Wick G, Wiedemann H, 1978. Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem 84, 43–52. [DOI] [PubMed] [Google Scholar]
- Timpl R, Oberbaumer I, Furthmayr H, Kuehn K, 1982. Macromolecular organization of type IV collagen., In New Trends in Basement Membrane Research ed. Press Raven, York New. [Google Scholar]
- Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K, 1981. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem 120, 203–211. [DOI] [PubMed] [Google Scholar]
- To M, Goz A, Camenzind L, Oertle P, Candiello J, Sullivan M, Henrich PB, Loparic M, Safi F, Eller A, Halfter W, 2013. Diabetes-induced morphological, biomechanical, and compositional changes in ocular basement membranes. Exp Eye Res 116, 298–307. [DOI] [PubMed] [Google Scholar]
- Toussaint D, Dustin P, 1963. Electron microscopy of normal and diabetic retinal capillaries. Arch Ophthalmol 70, 96–108. [DOI] [PubMed] [Google Scholar]
- Trost A, Lange S, Schroedl F, Bruckner D, Motloch KA, Bogner B, Kaser-Eichberger A, Strohmaier C, Runge C, Aigner L, Rivera FJ, Reitsamer HA, 2016. Brain and Retinal Pericytes: Origin, Function and Role. Front Cell Neurosci 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau K, Roy S, Guo W, Hernandez C, Villarroel M, Simo R, Roy S, 2011. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci 52, 6348–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilibary EC, 2003. Microvascular basement membranes in diabetes mellitus. J Pathol 200, 537–546. [DOI] [PubMed] [Google Scholar]
- Vaca DJ, Thibau A, Schutz M, Kraiczy P, Happonen L, Malmstrom J, Kempf VAJ, 2020. Interaction with the host: the role of fibronectin and extracellular matrix proteins in the adhesion of Gram-negative bacteria. Med Microbiol Immunol 209, 277–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerven JM, Boot JP, Lemkes HH, van Best JA, 1994. Effects of aldose reductase inhibition with tolrestat on diabetic retinopathy in a six months double blind trial. Doc Ophthalmol 87, 355–365. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Derevjanik NL, Mahlow J, Berkowitz BA, Wilson CA, 1998. Electron microscopic evidence for the mechanism of blood-retinal barrier breakdown in diabetic rabbits: comparison with magnetic resonance imaging. Pathol Res Pract 194, 497–505. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Van Niel E, Swerdloff JL, Campochiaro PA, 1993. Electron microscopic immunocytochemical evidence for the mechanism of blood-retinal barrier breakdown in galactosemic rats and its association with aldose reductase expression and inhibition. Exp Eye Res 57, 723–735. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Folkman J, Sullivan R, Fridman R, Ishai-Michaeli R, Sasse J, Klagsbrun M, 1987. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A 84, 2292–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener HP, Wilder RM, 1921. The Retinitis of Diabetes Mellitus: Preliminary Report. JAMA Journal of the American Medical Association 76, 515–517. [Google Scholar]
- Waggett AD, Benjamin M, Ralphs JR, 2006. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. European journal of cell biology 85, 1145–1154. [DOI] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL, 2008. Type IV collagens regulate BMP signalling in Drosophila. Nature 455, 72–77. [DOI] [PubMed] [Google Scholar]
- Welling LW, Grantham JJ, 1972. Physical properties of isolated perfused renal tubules and tubular basement membranes. J Clin Invest 51, 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weynand B, Jonckheere A, Frans A, Rahier J, 1999. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration 66, 14–19. [DOI] [PubMed] [Google Scholar]
- White ES, Baralle FE, Muro AF, 2008. New insights into form and function of fibronectin splice variants. J Pathol 216, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JR, Kilo C, 1977. Current status of capillary basement-membrane disease in diabetes mellitus. Diabetes 26, 65–73. [DOI] [PubMed] [Google Scholar]
- Williamson JR, Kilo C, 1983. Capillary basement membranes in diabetes. Diabetes 32 Suppl 2, 96–100. [DOI] [PubMed] [Google Scholar]
- Williamson JR, Tilton RG, Chang K, Kilo C, 1988. Basement membrane abnormalities in diabetes mellitus: relationship to clinical microangiopathy. Diabetes Metab Rev 4, 339–370. [DOI] [PubMed] [Google Scholar]
- Williamson JR, Vogler NJ, Kilo C, 1969. Estimation of vascular basement membrane thickness. Theoretical and practical considerations. Diabetes 18, 567–578. [DOI] [PubMed] [Google Scholar]
- Witmer AN, van den Born J, Vrensen GF, Schlingemann RO, 2001. Vascular localization of heparan sulfate proteoglycans in retinas of patients with diabetes mellitus and in VEGF-induced retinopathy using domain-specific antibodies. Curr Eye Res 22, 190–197. [DOI] [PubMed] [Google Scholar]
- Wolfstetter G, Dahlitz I, Pfeifer K, Topfer U, Alt JA, Pfeifer DC, Lakes-Harlan R, Baumgartner S, Palmer RH, Holz A, 2019. Characterization of Drosophila Nidogen/entactin reveals roles in basement membrane stability, barrier function and nervous system patterning. Development 146. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Nakada S, Yoshihara T, Nara T, Furuya N, Miida T, Hattori N, Arikawa-Hirasawa E, 2018. Perlecan, a heparan sulfate proteoglycan, regulates systemic metabolism with dynamic changes in adipose tissue and skeletal muscle. Sci Rep 8, 7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Huang Y, Chen X, Liu J, Lu Y, Bu L, Xia L, Xiao W, Chen M, Nie Q, Liu Z, 2010. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-beta(2), elucidated by treatment with CTGFsiRNA. Acta Ophthalmol 88, 652–659. [DOI] [PubMed] [Google Scholar]
- Yang X, Scott HA, Monickaraj F, Xu J, Ardekani S, Nitta CF, Cabrera A, McGuire PG, Mohideen U, Das A, Ghosh K, 2016. Basement membrane stiffening promotes retinal endothelial activation associated with diabetes. FASEB J 30, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, 2015. Integrating Activities of Laminins that Drive Basement Membrane Assembly and Function. Curr Top Membr 76, 1–30. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL, 2004. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol 22, 521–538. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu G, Ling Q, Da C, 2011. Expression of aquaporin 4 and Kir4.1 in diabetic rat retina: treatment with minocycline. J Int Med Res 39, 464–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.