Abstract
Background
Patients with epilepsy experience seizures, which have been reported to increase and worsen during the coronavirus disease (COVID-19) pandemic. However, the association between epilepsy and COVID-19 outcomes remains unclear. The aim of this study was to analyze whether patients with epilepsy have an increased risk of having poor COVID-19 outcomes.
Methods
We comprehensively evaluated potential articles extracted from the medRxiv, Europe PMC, and PubMed databases until June 30, 2021, using selected keywords. All published studies on epilepsy and COVID-19 were selected. We used the Review Manager 5.4 and Comprehensive Meta-Analysis 3 software for statistical analysis.
Results
Thirteen studies with 67,131 patients with COVID-19 were included in the analysis. Evaluation of the collated data revealed an association between epilepsy and increased severity of COVID-19 (OR, 1.69; 95%CI: 1.11–2.59; p = 0.010; I2 = 29%; random-effect modeling) and mortality from COVID-19 (OR, 1.71; 95%CI: 1.14–2.56; p = 0.010; I2 = 53%; random-effect modeling). The results also showed that the association between epilepsy and increased risk of developing severe COVID-19 is influenced by sex and neurodegenerative disease.
Conclusions
The findings of this study suggest that patients with epilepsy are at risk of having poor COVID-19 outcomes. Patients with epilepsy need special attention and should be prioritized for administration of the COVID-19 vaccine.
Registration details: PROSPERO (CRD42021264979).
Keywords: Coronavirus disease 2019, COVID-19, Epilepsy, Seizure, Neurology
1. Introduction
The outbreak of the coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the most recent catastrophic global pandemic. Since the start of the pandemic, over 186 million confirmed cases have been recorded, with more than 4 million deaths as of July 13, 2021 [1]. Although some patients with COVID-19 may develop mild, non-debilitating, self-limiting, upper-respiratory symptoms, a significant percentage of patients may also develop destructive and progressive symptoms that require hospitalization and intensive care treatment due to the threat of progression into acute respiratory distress syndrome, which may eventually advance to multi-organ failure [2], [3].
Recent studies have identified several comorbidities that can increase the probability of developing severe COVID-19. These comorbidities include chronic respiratory disease, diabetes, cardiovascular disease, obesity, and other immunocompromising conditions [4], [5], [6], [7], [8]. The findings of previous meta-analyses have also established that neurological comorbidities, such as stroke, dementia, and Parkinson’s Disease, are risk factors for poor COVID-19 outcomes [9], [10], [11], [12]. Epilepsy is another a neurological comorbidity that needs special attention. It has been reported that patients with epilepsy are included in the populations at risk during the ongoing COVID-19 pandemic. Several reports have shown that most patients with epilepsy experience worsened seizures during this pandemic, which may lead to higher morbidity and mortality rates [13], [14], [15]. However, studies on the association between epilepsy and COVID-19 outcomes are scarce; thus, comprehensive evidence regarding this topic remains unestablished. Therefore, the purpose of this systematic review and meta-analysis was to determine whether patients with epilepsy are at risk of having poor COVID-19 outcomes.
2. Materials and methods
2.1. Eligibility criteria
We conducted a systematic review and meta-analysis of observational studies. The study protocol was registered in PROSPERO (CRD42021264979). Append research in this systematic review and meta-analysis were chosen as most likely to attain the following criteria: studies that followed the PICO framework (P: Populations – hospitalized patients with COVID-19; I: Interventions – patients with a history of epilepsy or with active epilepsy as a comorbidity; C: Comparator/Control – patients without a history of epilepsy or without active epilepsy; O: Outcomes – severe COVID-19 or mortality), and cross-sectional, case-control, cohort, and case-series studies were included. Studies besides original articles (correspondence or review articles), randomized or non-randomized clinical trials, case reports, studies reported in a language other than in English, and research that focused on pregnant women or populations younger than 18 years old were excluded.
2.2. Search strategy and study selection
Systematic search of the medRxiv, Europe PMC, and PubMed databases was performed to identify relevant articles published in English language. The database search was conducted from December 2019 to June 30, 2021, using keywords, including “epilepsy” OR “epileptics” OR “epilepsia” OR “seizure disorders” OR “seizure syndrome” AND “SARS-CoV-2,” OR “coronavirus disease 2019” OR “COVID-19,” to identify potentially eligible studies for analysis. The details of the search strategy are outlined in Supplementary Table 1. The initial step was the identification of eligible articles through screening of titles and abstracts. The references in the eligible articles were additionally evaluated to identify other potentially eligible articles that may have been missed in the database search. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram shows the strategy employed in this study.
2.3. Data extraction and quality assessment
Two authors conducted the data extraction. An extraction form was developed to collate information about the studies, such as population characteristics; data on hypertension, diabetes, and stroke; number of patients with a history of epilepsy; details of the control groups; and COVID-19 outcomes.
We focused on the outcomes of severe COVID-19 and mortality. Severe COVID-19 outcomes were defined according to the Guidelines for the Diagnosis and Treatment of New Coronavirus Pneumonia (fifth edition) [16]. The guidelines stipulate that patients with severe COVID-19 outcomes are those who during disease progression (whether it was at the time of, during, or after admission) developed any of the following symptoms or features: (1) respiratory distress (defined as a respiratory rate ≥ 30 breaths per min); (2) resting oxygen saturation ≤ 93%; (3) ratio of partial pressure of arterial oxygen to fraction of inspired oxygen ≤ 300 mmHg; or (4) critical complications (respiratory failure, septic shock, or multiple organ dysfunction/failure) or admission to the intensive care unit. Mortality outcome was described as the number of patients with a history of positive SARS-CoV-2 infection who died during the follow-up period.
Two authors independently conducted a quality assessment of each study to be included in the analysis. The Newcastle–Ottawa Scale (NOS) was used to evaluate the qualities of the case-control and cohort studies. The assessment process included review of the comparability, selection, and outcome of each study. Thereafter, each study was assigned a total score ranging from zero to nine. A study is considered to be of good quality if it scores ≥ 7 [17]. Meanwhile, the qualities of the included case-series studies were assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Tools For Case-Series Studies [18].
2.4. Statistical analysis
The meta-analysis was performed using the Review Manager 5.4 (Cochrane Collaboration) and Comprehensive Meta-Analysis version 3 software. The Mantel Haenszel's formula with a random-effects model was used to calculate the odds ratios (OR) and 95% confidence intervals (95% CI) for severe COVID-19 and mortality outcomes. The I2 statistic was used to assess the heterogeneity of the studies. A value <25% is considered to indicate a low degree of heterogeneity, 26–50% indicates a moderate degree of heterogeneity, and >50% indicates a high degree of heterogeneity. Meta-regression with a random-effects model was performed using a restricted maximum likelihood for pre-specified variables, including age, sex, hypertension, diabetes, and stroke. Funnel plot analysis was utilized to assess the qualitative risk of publication bias, whereas Egger’s regression method was used to assess the quantitative risk of publication bias [19].
3. Results
3.1. Study selection and characteristics
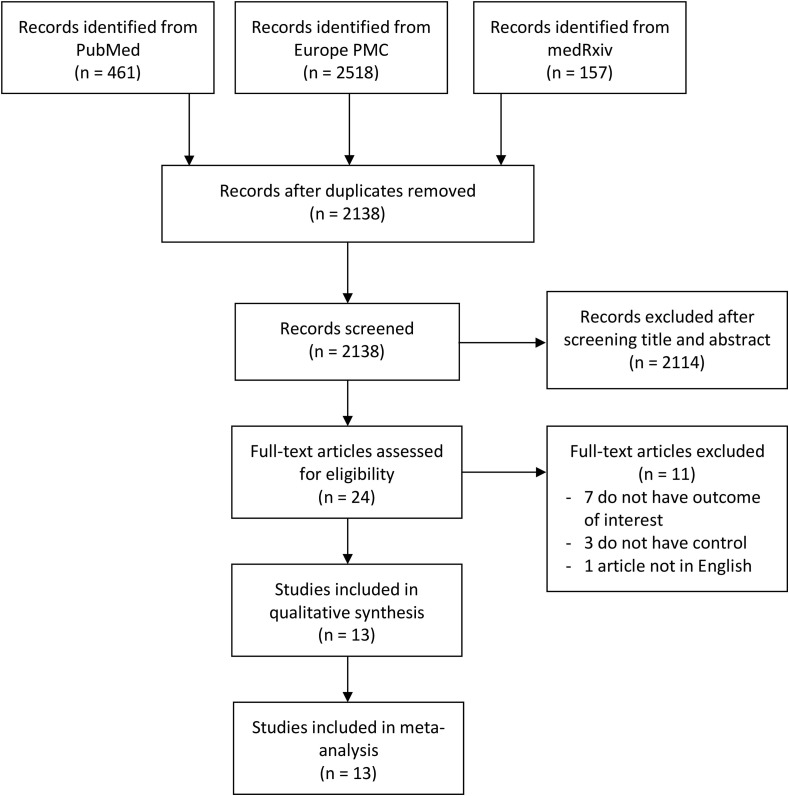
A total of 3,136 articles were identified after the initial database search. After duplicate articles were removed, 2,138 articles remained. An additional 2,114 articles were removed after the titles and abstracts were screened and inclusion and exclusion criteria were matched. The full texts of the remaining 24 articles were then assessed for eligibility. Eleven articles were excluded after the assessment because the outcomes outlined in seven of the articles did not meet the criteria of the present study, three articles had no information on a control group, and one article was not published in English. Thus, 13 studies [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], which included a total of 67,131 patients with COVID-19, were included in the analysis (Fig. 1 ). Of the 13 studies, eight were retrospective cohort studies, three were case-control studies, and two were case-series studies. The details of the included studies are outlined in Table 1 .
Fig. 1.
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis.
Table 1.
Characteristics of included studies.
| Study | Sample size | Design | Outcome | Age (years) | Male (%) | Hypertension (%) | Diabetes (%) | Stroke (%) | Neoplasm (%) | Neurodegenerative disease (%) | Patients with Epilepsy (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anand P et al. [20] 2020 | 7 | Case-series | Severitya | 75 ± 13 | 28.5% | N/A | N/A | 42.8% | N/A | 14.2% | 42.8% |
| Mortality | |||||||||||
| Asadi-Pooya AA et al. [21] 2021 | 37,968 | Case-control | Severitya | 53 ± 23 | 53.1% | N/A | N/A | 0.5% | N/A | 0.3% | 0.2% |
| Mortality | |||||||||||
| Cabezudo-Garcia P et al. [22] 2020 | 1537 | Retrospective cohort | Mortality | 67 ± 15 | 60.1% | 56.7% | 23.6% | N/A | N/A | N/A | 1.3% |
| Chou SHY et al. [23] 2021 | 3055 | Retrospective cohort | Mortality | 59.9 ± 0.9 | 57% | 58% | 35% | 3% | N/A | N/A | 1% |
| Clift AK et al. [24] 2020 | 10,776 | Retrospective cohort | Mortality | 69.6 ± 17.9 | 55.3% | N/A | 29.2% | 12.4% | 3.4% | 13.4% | 3.2% |
| Garcia-Azorin D et al. [25] 2021 | 233 | Retrospective cohort | Severityb | 51.1 ± 17.5 | 54.9% | 41.9% | 19.8% | 6.5% | 5.1% | 5.9% | 6% |
| Mortality | |||||||||||
| Ghaffari M et al. [26] 2021 | 361 | Retrospective cohort | Severityc | 61.9 ± 16.7 | 59.3% | 29.9% | 27.4% | 3.9% | 4.4% | 3.8% | 3.3% |
| Ji W et al. [27] 2020 | 7341 | Case-control | Severityc | 47 ± 19 | 40.5% | 22.2% | 14.2% | 6.6% | 4.6% | 11.4% | 1.8% |
| Poblador-Plou B et al. [28] 2020 | 4412 | Retrospective cohort | Mortality | 67.7 ± 20.7 | 41.2% | 34.4% | 11.9% | 6.7% | 6.8% | 15.6% | 1.5% |
| Romagnolo A et al. [29] 2021 | 344 | Case-series | Severityc | 61.5 ± 17.8 | 59.3% | 45.9% | 12.2% | 8.7% | 14.2% | 7.5% | 1.4% |
| Romero-Sanchez CM et al. [30] 2020 | 841 | Retrospective cohort | Severityc | 66.4 ± 14.9 | 56.2% | 55.2% | 25.1% | 6.3% | 8.6% | 8.4% | 2.5% |
| Tyson B et al. [31] 2021 | 150 | Case-control | Mortality | 77.6 ± 10.5 | 50% | N/A | 34% | 17.3% | 20% | 44.6% | 4.6% |
| Yin R et al. [32] 2020 | 106 | Retrospective cohort | Severityc | 72.7 ± 11.8 | 60.4% | 67.9% | 34.9% | 85.8% | 8.5% | 21.7% | 2.8% |
Admission into intensive care unit (ICU).
Respiratory distress (≥30 breaths per min) or admission into ICU.
Any of the following: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤ 93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (fiO2) ≤ 300 mmHg; (4) critical complications.
3.2. Assessment of the qualities of the studies
The NOS scale was used to evaluate the qualities of the cohort and case-control studies. The results indicated that all included studies are of good quality (Table 2 ). Meanwhile, the Joanna Briggs Institute Critical Appraisal checklist was used for the evaluation of case-series studies (Table 3 ). The results also showed that all included studies were fit to be included in the meta-analysis.
Table 2.
Newcastle-Ottawa quality assessment of observational studies.
| First author, year | Study design | Selectiona | Comparabilityb | Outcomec | Total score | Result |
|---|---|---|---|---|---|---|
| Asadi-Pooya AA et al. [21] 2021 | Case-control | *** | ** | ** | 7 | Good |
| Cabezudo-Garcia P et al. [22] 2020 | Cohort | *** | ** | ** | 7 | Good |
| Chou SHY et al. [23] 2021 | Cohort | **** | ** | *** | 9 | Good |
| Clift AK et al. [24] 2020 | Cohort | **** | ** | *** | 9 | Good |
| Garcia-Azorin D et al. [25] 2021 | Cohort | *** | ** | *** | 8 | Good |
| Ghaffari M et al. [26] 2021 | Cohort | *** | ** | ** | 7 | Good |
| Ji W et al. [27] 2020 | Case-control | *** | ** | *** | 8 | Good |
| Poblador-Plou B et al. [28] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Romero-Sanchez CM et al. [30] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Tyson B et al. [31] 2021 | Case-control | *** | ** | *** | 8 | Good |
| Yin R et al. [32] 2020 | Cohort | *** | ** | *** | 8 | Good |
(1) representativeness of the exposed cohort; (2) selection of the non-exposed cohort; (3) ascertainment of exposure; (4) demonstration that outcome of interest was not present at start of study.
(1) comparability of cohorts on the basis of design or analysis, (maximum two stars).
(1) assessment of outcome; (2) was follow-up long enough for outcomes to occur; (3) adequacy of follow-up of cohorts.
Table 3.
Joanna Briggs Institute Critical Appraisal tool for case-series study.
| Anand P et al. [20] 2020 | Romagnolo A et al. [29] 2021 | |
|---|---|---|
| 1. Were the criteria for inclusion in the sample clearly defined? | Yes | Yes |
| 2. Were the study subjects and the setting described in detail? | Yes | Yes |
| 3. Was the exposure measured in a valid and reliable way? | Yes | Yes |
| 4. Were objective, standard criteria used for measurement of the condition? | Yes | Yes |
| 5. Were confounding factors identified? | Yes | Yes |
| 6. Were strategies to deal with confounding factors stated? | No | Yes |
| 7. Were the outcomes measured in a valid and reliable way? | Yes | Yes |
| 8. Was appropriate statistical analysis used? | Yes | Yes |
| Quality | Include study | Include study |
3.3. Epilepsy and severe COVID-19
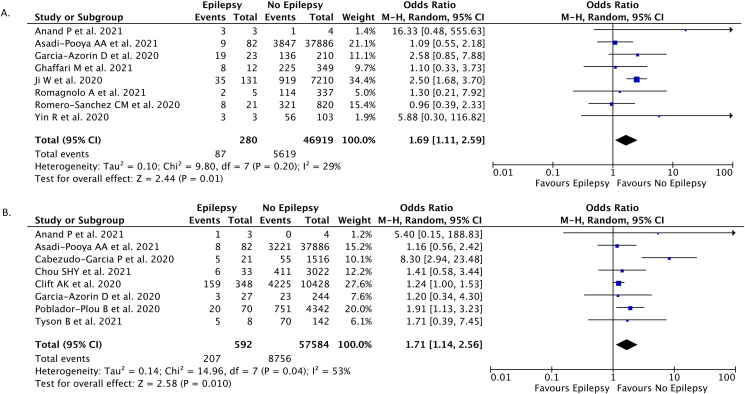
In eight studies (n = 47,199), severe COVID-19 was reported as the outcome of patients with epilepsy and COVID-19. Our pooled analysis revealed that epilepsy as a comorbidity was correlated with an enhanced risk of severe COVID-19 (OR, 1.69; 95%CI: 1.11–2.59; p = 0.010; I 2 = 29%; random-effect modeling) (Fig. 2 A).
Fig. 2.
Forest plot that demonstrates the association of epilepsy with severe Covid-19 (A) and mortality (B) outcomes.
3.4. Epilepsy and mortality of patients with COVID-19
Mortality outcomes were reported in eight studies (n = 58,176). The pooled estimate indicated that epilepsy was associated with increased mortality from COVID-19 (OR, 1.71; 95%CI: 1.14–2.56; p = 0.010; I 2 = 53%; random-effect modeling) (Fig. 2B).
3.5. Meta regression
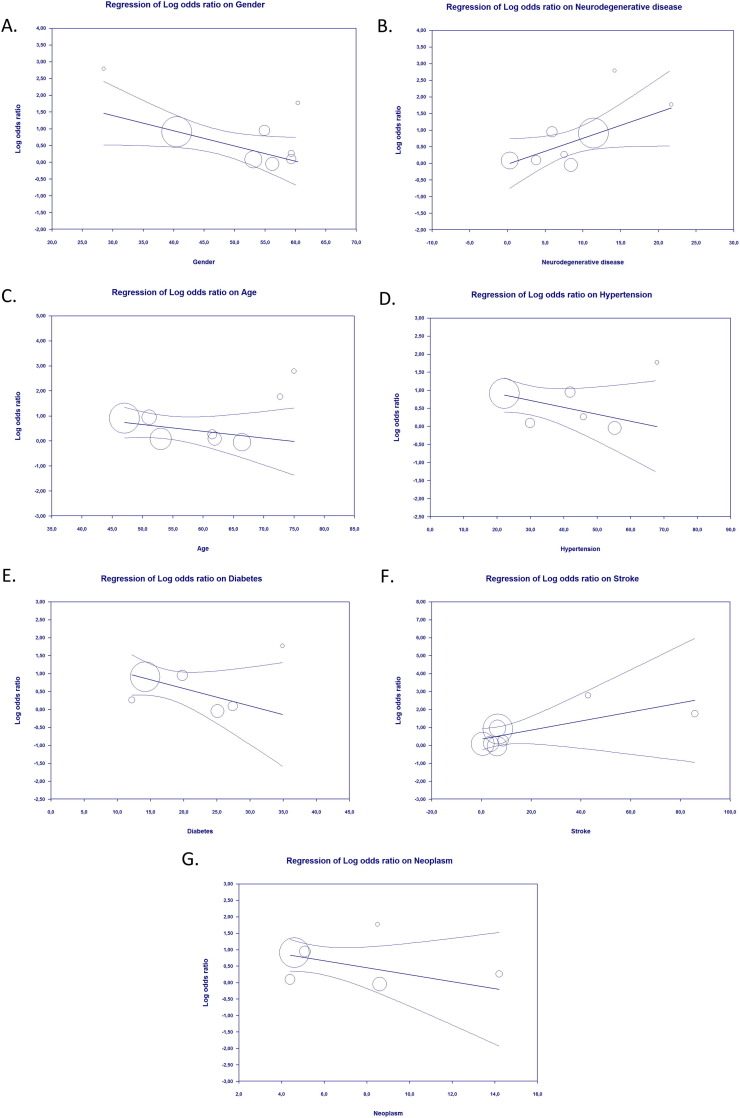
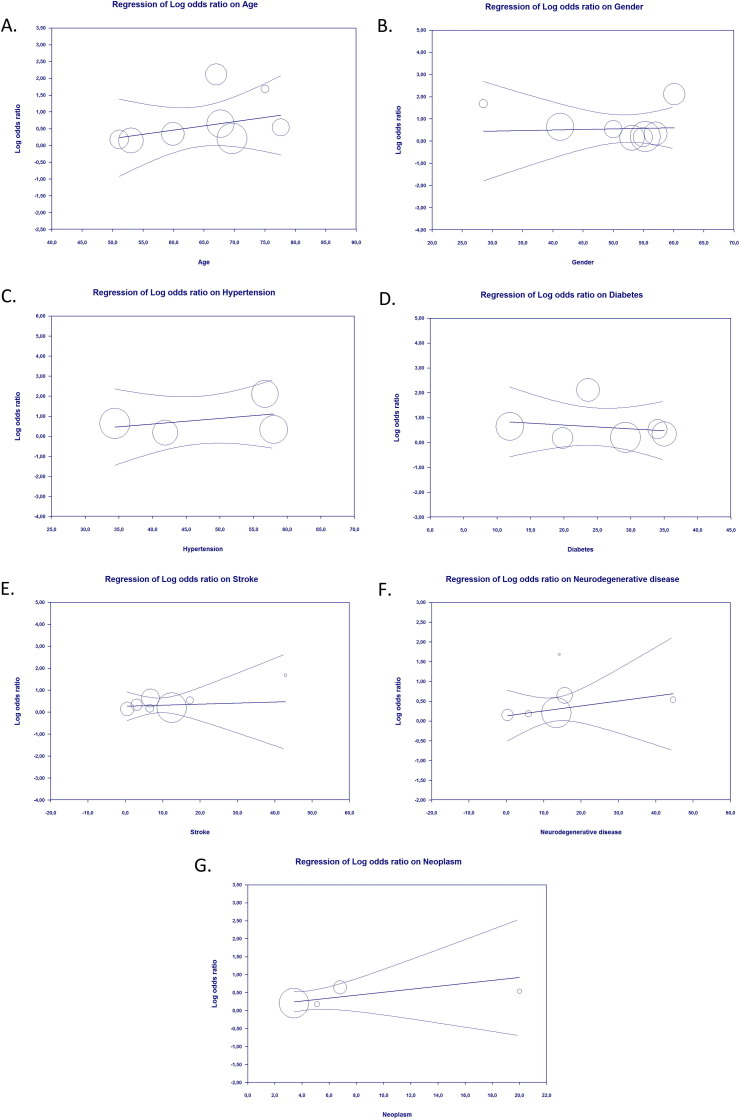
The results of meta-regression suggested that the association between epilepsy as a comorbidity and severe COVID-19 outcomes was affected by sex (p = 0.018) (Fig. 3 A) and neurodegenerative disease (p = 0.018) (Fig. 3B), but not by age (p = 0.266) (Fig. 3C), hypertension (p = 0.140) (Fig. 3D), diabetes (p = 0.128) (Fig. 3E), stroke (p = 0.154) (Fig. 3F) or neoplasm (p = 0.183) (Fig. 3G). The results also showed that the association between epilepsy as comorbidity and mortality from COVID-19 was not affected by age (p = 0.414) (Fig. 4 A), sex (p = 0.892) (Fig. 4B), hypertension (p = 0.554) (Fig. 4C), diabetes (p = 0.677) (Fig. 4D), stroke (p = 0.848) (Fig. 4E), neurodegenerative disease (p = 0.493) (Fig. 4F), or neoplasm (p = 0.326) (Fig. 4G).
Fig. 3.
Bubble-plot for Meta-regression. Meta-regression analysis showed that the association between epilepsy and severe Covid-19 was affected by sex (A) and neurodegenerative disease (B), but not by age (C), hypertension (D), diabetes (E), stroke (F), and neoplasm (G).
Fig. 4.
Bubble-plot for Meta-regression. Meta-regression analysis showed that the association between epilepsy and mortality from Covid-19 was not affected by age (A), sex (B), hypertension (C), diabetes (D), stroke (E), neurodegenerative disease (F), nor neoplasm (G).
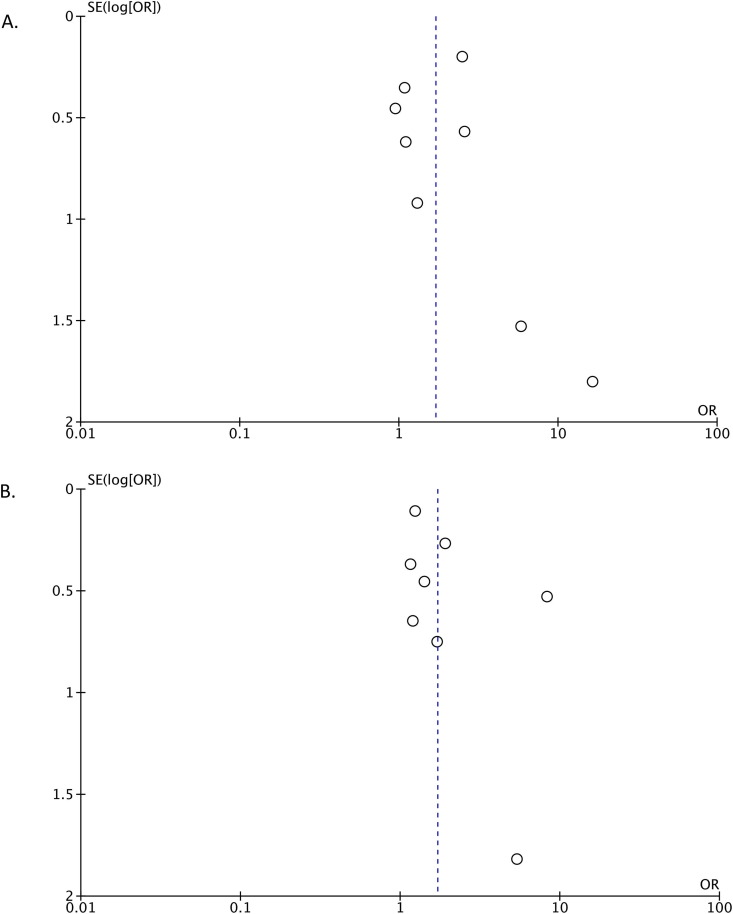
3.6. Publication bias
We used funnel plot analysis to evaluate severe COVID-19 (Fig. 5 A) and mortality outcomes (Fig. 5B). The results of the analysis showed a relatively symmetrical inverted plot, indicating no publication bias. The result of Egger’s regression test was not statistically significant for severe COVID-19 (p = 0.897) and mortality outcomes (p = 0.176), confirming the results of the funnel plot analysis, in which publication bias was not observed.
Fig. 5.
Funnel plot analysis for the association of epilepsy with severe Covid-19 (A) and mortality (B) outcomes.
4. Discussion
In this systematic review and meta-analysis, we investigated whether patients with epilepsy have an increased risk of having poor COVID-19 outcomes. After conducting pooled analyses, our results demonstrated that epilepsy as a comorbidity is associated with increased severity of COVID-19 and mortality from COVID-19. Sex was found to influence the association between epilepsy and severe COVID-19, whereas it had no influence mortality rate. In addition, age, hypertension, diabetes, and stroke were found to have no influence on association between epilepsy and both outcomes.
There are some plausible explanations for how epilepsy can affect the prognoses of patients with COVID-19. First, several experimental and clinical studies have shown that SARS-CoV-2 may have neuro-invasive and neurotropic properties, although the exact route for CNS entry is still unclear [33], [34]. The brain inflammation caused by SARS-CoV-2 may precipitate the development of status epilepticus (SE) in COVID-19 patients, especially in those who have epilepsy [35]. Moreover, systemic inflammatory response triggered by COVID-19 may give rise to the status epilepticus (SE) development because most cases of SE with SARS-CoV-2 infection described in the literature can be classified as cryptogenic New-Onset Refractory Status Epilepticus (NORSE), which are thought to be the clinical manifestation of a pro-inflammatory state in the CNS [35], [36]. The development of status epilepticus (SE) will surely worsen the outcomes in patients with COVID-19 and epilepsy. Second, brain inflammation is thought to be involved in the epileptogenesis process. Findings from immunohistochemical and biochemical studies have firmly established that certain inflammatory mediators rapidly increase within local brain areas affected by pro-epileptogenic brain injuries, including trauma, infection, and status epilepticus (febrile or non-febrile) [37], [38], [39]. An experimental study showed that the inflammatory response caused by these injuries can last from several days to weeks and is often followed by the development of epilepsy [40]. C-reactive protein, interleukin (IL)-1β (IL-1β), IL-6, and IL-8 are among the inflammatory markers that are increased in patients with epilepsy [41], [42]. According to the findings of several meta-analyses, patients with COVID-19 also have the increased levels of these inflammatory markers [43], [44]. Therefore, the pre-existing inflammatory state in patients with epilepsy (as evidenced by elevations of several inflammatory markers) will worsen if they are contracted with SARS-CoV-2 infection. Combination of these inflammatory conditions may lead to not only seizure exacerbation [35] but also the development of cytokine storm and poor COVID-19 outcomes [43], [44]. Third, some anti-epileptic drugs (AEDs) taken by patients with epilepsy may interact with drugs commonly used to treat COVID-19 (e.g., the combination of eslicarbazepine/lacosamide and atazanavir/lopinavir/ritonavir), which may cause potentially fatal arrhythmias. Other AEDs, such as carbamazepine, phenytoin, and phenobarbital, have also been found to interact with remdesivir, a well-known medication used to treat COVID-19, when they are taken together. This interaction leads to decreased remdesivir levels in the body. Thus, caution should be applied when AEDs and remdesivir are used together in treatment [45], [46]. However, we must bear in mind that not all AEDs have interaction with antiviral agents. There are still many AEDs which can be safely used together and do not interfere with antiviral agents. Fourth, the COVID-19 pandemic may cause psychological distress among patients with epilepsy, resulting in more frequent and worsened seizures [47], [48], [49]. An increase in the frequency and severity of seizures signifies that patients will have an increased risk of hypoxemia [50], [51], [52]. Hypoxemia can be fatal in cases of COVID-19 where respiratory functions are already compromised [53], [54]. Therefore, patients with epilepsy are at risk of developing severe hypoxemia, which may result in higher disease severity and mortality from COVID-19. Finally, in attempts of controlling COVID-19 pandemic, several countries implement national lockdown and heavy restrictions of healthcare services. This policy may delay the diagnosis and treatment for the patients, including those with epilepsy because they must limit their hospital visit [55]. Monitoring of the patients’ conditions and access to the AEDs may become impaired and these conditions will eventually lead to seizure exacerbations and worsening of epilepsy control during COVID-19 pandemic [55], [56].
This study has some limitations. Data regarding the duration of epilepsy, type of epilepsy, and AEDs used by patients were incomplete and not well-documented in the included studies, thereby making it unavailable for further analysis in the present study. Moreover, data regarding other potential confounders, such as motor disability, immunosuppressive conditions, and obesity prevalence were lacking in the included studies; therefore they cannot be further analyzed using meta-regression analysis. We also included some pre-print studies in our analysis. However, we ensured that meaningful research and several pre-print studies were included in the analysis to reduce publication bias risk. More studies with larger sample sizes that focus on the course of COVID-19 in patients with epilepsy are needed to confirm the results of the present study.
5. Conclusion
This systematic review and meta-analysis demonstrated that patients with epilepsy are at higher risk of having poorer COVID-19 outcomes than controls, specifically in terms of disease severity and mortality rate. The associations between epilepsy and severe COVID-19 are further affected by gender and neurodegenerative disease. Considering the findings of this study, we propose that patients with epilepsy need special attention and should be considered a population at risk during the COVID-19 pandemic. Patients with epilepsy should also be prioritized to receive COVID-19 vaccines, along with patients with other comorbidities that have already been established as risk factors for COVID-19.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Data analyzed in this study were a re-analysis of existing data, which are openly available at locations cited in the reference section.
Competing interest
The authors declare that they have no competing interests.
Funding
None.
Authors’ contributions
YMTS: conceptualization, methodology, formal analysis, data curation, writing‐original draft, visualization, writing‐review and editing. RJK: conceptualization, methodology, formal analysis, data curation, writing‐original draft, writing‐review and editing. VH: conceptualization, validation, supervision, writing‐review and editing. TIH: conceptualization, validation, supervision, writing‐review and editing. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yebeh.2021.108437.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization. Coronavirus disease (COVID-19): situation report. Accessed July 20, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---13-july-2021.
- 2.Hariyanto T.I., Rizki N.A., Kurniawan A. Anosmia/hyposmia is a good predictor of coronavirus disease 2019 (COVID-19) infection: a meta-analysis. Int Arch Otorhinolaryngol. 2020 doi: 10.1055/s-0040-1719120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwenandar F., Japar K.V., Damay V., Hariyanto T.I., Tanaka M., Lugito N.P.H., et al. Coronavirus disease 2019 and cardiovascular system: a narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. doi: 10.1016/j.ijcha.2020.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hariyanto T.I., Rosalind J., Christian K., Kurniawan A. Human immunodeficiency virus and mortality from coronavirus disease 2019: a systematic review and meta-analysis. South Afr J HIV Med. 2021;22(1):1220. doi: 10.4102/sajhivmed.v22i1.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold M.S., Sehayek D., Gabrielli S., Zhang X., McCusker C., Ben-Shoshan M. COVID-19 and comorbidities: a systematic review and meta-analysis. Postgrad Med. 2020;132(8):749–755. doi: 10.1080/00325481.2020.1786964. [DOI] [PubMed] [Google Scholar]
- 6.Hariyanto T.I., Kurniawan A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Sleep Med. 2021;82:47–53. doi: 10.1016/j.sleep.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020;19:100290. doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hariyanto T.I., Halim D.A., Rosalind J., Gunawan C., Kurniawan A. Ivermectin and outcomes from Covid-19 pneumonia: a systematic review and meta-analysis of randomized clinical trial studies. Rev Med Virol. 2021;6 doi: 10.1002/rmv.2265. [DOI] [Google Scholar]
- 9.Nannoni S., de Groot R., Bell S., Markus H.S. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16(2):137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariyanto T.I., Putri C., Arisa J., Situmeang R.F.V., Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2021;93:104299. doi: 10.1016/j.archger.2020.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariyanto T.I., Putri C., Situmeang R.F.V., Kurniawan A. Dementia is a predictor for mortality outcome from coronavirus disease 2019 (COVID-19) infection. Eur Arch Psychiatry Clin Neurosci. 2021;271(2):393–395. doi: 10.1007/s00406-020-01205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putri C., Hariyanto T.I., Hananto J.E., Christian K., Situmeang R.F.V., Kurniawan A. Parkinson's disease may worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia in hospitalized patients: a systematic review, meta-analysis, and meta-regression. Parkinsonism Relat Disord. 2021;S1353–8020(21):00152–158. doi: 10.1016/j.parkreldis.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonseca E., Quintana M., Lallana S., Luis Restrepo J., Abraira L., Santamarina E., et al. Epilepsy in time of COVID-19: A survey-based study. Acta Neurol Scand. 2020;142(6):545–554. doi: 10.1111/ane.v142.610.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assenza G., Lanzone J., Brigo F., Coppola A., Di Gennaro G., Di Lazzaro V., et al. Epilepsy care in the time of COVID-19 pandemic in Italy: risk factors for seizure worsening. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorpe J., Ashby S., Hallab A., Ding D., Andraus M., Dugan P., et al. Evaluating risk to people with epilepsy during the COVID-19 pandemic: preliminary findings from the COV-E study. Epilepsy Behav. 2021;115:107658. doi: 10.1016/j.yebeh.2020.107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Commission of the People’s Republic of China. Diagnosis and treatment of new coronavirus pneumonitis. (trial version 5). http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml.
- 17.Margulis A.V., Pladevall M., Riera-Guardia N., Varas-Lorenzo C., Hazell L., Berkman N.D., et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359–368. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munn Z., Barker T.H., Moola S., Tufanaru C., Stern C., McArthur A., et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;10:2127–2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anand P., Al-Faraj A., Sader E., Dashkoff J., Abdennadher M., Murugesan R., et al. Seizure as the presenting symptom of COVID-19: A retrospective case series. Epilepsy Behav. 2020;112:107335. doi: 10.1016/j.yebeh.2020.107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asadi‐Pooya A.A., Emami A., Akbari A., Javanmardi F. COVID-19 presentations and outcome in patients with epilepsy. Acta Neurol Scand. 2021;143(6):624–628. doi: 10.1111/ane.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabezudo-García P., Ciano-Petersen N.L., Mena-Vázquez N., Pons-Pons G., Castro-Sánchez M.V., Serrano-Castro P.J. Incidence and case fatality rate of COVID-19 in patients with active epilepsy. Neurology. 2020;95(10):e1417–e1425. doi: 10.1212/WNL.0000000000010033. [DOI] [PubMed] [Google Scholar]
- 23.Chou S.H.Y., Beghi E., Helbok R., Moro E., Sampson J., Altamirano V., et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-A report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open. 2021;4(5):e2112131. doi: 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clift AK, Coupland CAC, Keogh RH, Diaz-Ordaz K, Williamson E, Harrison EM, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. https://doi.org/10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed]
- 25.García-Azorín D., Abildúa M.J.A., Aguirre M.E.E., Fernández S.F., Moncó J.C.G., Guijarro-Castro C., et al. Neurological presentations of COVID-19: findings from the Spanish Society of Neurology neuroCOVID-19 registry. J Neurol Sci. 2021;423:117283. doi: 10.1016/j.jns.2020.117283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghaffari M., Ansari H., Beladimoghadam N., Aghamiri S.H., Haghighi M., Nabavi M., et al. Neurological features and outcome in COVID-19: dementia can predict severe disease. J Neurovirol. 2021;27(1):86–93. doi: 10.1007/s13365-020-00918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji W., Huh K., Kang M., Hong J., Bae G.H., Lee R., et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35(25):e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., Poncel-Falcó A., Bliek-Bueno K., Cano-Del Pozo M., et al. Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID study in Spain. Int J Environ Res Public Health. 2020;17(14):5171. doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romagnolo A., Balestrino R., Imbalzano G., Ciccone G., Riccardini F., Artusi C.A., et al. Neurological comorbidity and severity of COVID-19. J Neurol. 2021;268(3):762–769. doi: 10.1007/s00415-020-10123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Álvaro, Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyson B., Erdodi L., Shahein A., Kamrun S., Eckles M., Agarwal P. Predictors of survival in older adults hospitalized with COVID-19. Neurol Sci. 2021;42(10):3953–3958. doi: 10.1007/s10072-021-05435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin R., Yang Z.Q., Wei Y.X., Li Y.M., Chen H., Liu Z., et al. Clinical characteristics of 106 patients with neurological diseases and co-morbid coronavirus disease 2019: a retrospective study. medRxiv. 2020 doi: 10.1101/2020.04.29.20085415. [DOI] [Google Scholar]
- 33.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 34.DosSantos M.F., Devalle S., Aran V., Capra D., Roque N.R., Coelho-Aguiar J.M., et al. Neuromechanisms of SARS-CoV-2: a review. Front Neuroanat. 2020;14 doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dono F., Nucera B., Lanzone J., Evangelista G., Rinaldi F., Speranza R., et al. Status epilepticus and COVID-19: a systematic review. Epilepsy Behav. 2021;118:107887. doi: 10.1016/j.yebeh.2021.107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikbakht F., Mohammadkhanizadeh A., Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult Scler Relat Disord. 2020;46:102535. doi: 10.1016/j.msard.2020.102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Temkin N.R. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(Suppl 2):10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 38.Bartfai T., Sanchez-Alavez M., Andell-Jonsson S., Schultzberg M., Vezzani A., Danielsson E., et al. Interleukin-1 system in CNS stress: seizures, fever, and neurotrauma. Ann N Y Acad Sci. 2007;1113:173–177. doi: 10.1196/annals.1391.022. [DOI] [PubMed] [Google Scholar]
- 39.Vezzani A., Friedman A. Brain inflammation as a biomarker in epilepsy. Biomark Med. 2011 Oct;5(5):607–614. doi: 10.2217/bmm.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vezzani A., French J., Bartfai T., Baram T.Z. The role of inflammation in epilepsy. Nat Rev Neurol. 2011 Jan;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobylarek D., Iwanowski P., Lewandowska Z., Limphaibool N., Szafranek S., Labrzycka A., Kozubski W. Advances in the potential biomarkers of epilepsy. Front Neurol. 2019;10:685. doi: 10.3389/fneur.2019.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishikawa N., Kobayashi Y., Fujii Y., Kobayashi M. Increased interleukin-6 and high-sensitivity C-reactive protein levels in pediatric epilepsy patients with frequent, refractory generalized motor seizures. Seizure. 2015;25:136–140. doi: 10.1016/j.seizure.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Hariyanto T.I., Japar K.V., Kwenandar F., Damay V., Siregar J.I., Lugito N.P.H., et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am J Emerg Med. 2021;41:110–119. doi: 10.1016/j.ajem.2020.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asadi-Pooya A.A., Attar A., Moghadami M., Karimzadeh I. Management of COVID-19 in people with epilepsy: drug considerations. Neurol Sci. 2020;41(8):2005–2011. doi: 10.1007/s10072-020-04549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fırat O., Yalçın N., Demirkan K. COVID-19 & antiepileptic drugs: Should we pay attention? Seizure. 2020;80:240–241. doi: 10.1016/j.seizure.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S., Wu C., Jia Y., Li G., Zhu Z., Lu K., et al. COVID-19 outbreak: THE impact of stress on seizures in patients with epilepsy. Epilepsia. 2020;61(9):1884–1893. doi: 10.1111/epi.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao X., Zhou D., Li Z., Zeng G., Hao N., Li E., et al. Severe psychological distress among patients with epilepsy during the COVID-19 outbreak in southwest China. Epilepsia. 2020;61(6):1166–1173. doi: 10.1111/epi.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez-Larsen A., Gonzalez-Villar E., Díaz-Maroto I., Layos-Romero A., Martínez-Martín Álvaro, Alcahut-Rodriguez C., et al. Influence of the COVID-19 outbreak in people with epilepsy: analysis of a Spanish population (EPICOVID registry) Epilepsy Behav. 2020;112:107396. doi: 10.1016/j.yebeh.2020.107396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blum A.S., Ives J.R., Goldberger A.L., Al-Aweel I.C., Krishnamurthy K.B., Drislane F.W., et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia. 2000;41(5):536–541. doi: 10.1111/j.1528-1157.2000.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 51.Rheims S., Alvarez B.M., Alexandre V., Curot J., Maillard L., Bartolomei F., et al. Hypoxemia following generalized convulsive seizures: Risk factors and effect of oxygen therapy. Neurology. 2019;92(3):e183–e193. doi: 10.1212/WNL.0000000000006777. [DOI] [PubMed] [Google Scholar]
- 52.Bruno E., Maira G., Biondi A., Richardson M.P., RADAR-CNS Consortium Ictal hypoxemia: a systematic review and meta-analysis. Seizure. 2018;63:7–13. doi: 10.1016/j.seizure.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Kashani K.B. Hypoxia in COVID-19: sign of severity or cause for poor outcomes. Mayo Clin Proc. 2020;95(6):1094–1096. doi: 10.1016/j.mayocp.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jahani M., Dokaneheifard S., Mansouri K. Hypoxia: A key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J Inflamm (Lond) 2020;17:33. doi: 10.1186/s12950-020-00263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assenza G., Lanzone J., Ricci L., Boscarino M., Tombini M., Galimberti C.A., et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41(8):1999–2004. doi: 10.1007/s10072-020-04546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosengard J.L., Donato J., Ferastraoaru V., Zhao D., Molinero I., Boro A., et al. Seizure control, stress, and access to care during the COVID-19 pandemic in New York City: the patient perspective. Epilepsia. 2021;62(1):41–50. doi: 10.1111/epi.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data analyzed in this study were a re-analysis of existing data, which are openly available at locations cited in the reference section.