Abstract
Influenza is a worldwide plague caused by the influenza virus (IAV) infection, which is initiated by specific recognition with sialic acids on host cell surface. Bovine lactoferrin (bLf) is a sialoglycoprotein belonging to the transferrin family, and it plays an important role in immune regulation. It also shows toxicity against cancer cells and pathogenic microorganisms including bacteria, fungi, and virus. The purpose of this study is to assess the roles of the sialylated glycans on bLf against IAV. To this end, bLf were first treated with sodium periodate to destroy its sialylated glycans. Then, the binding activity of native or desialylated bLf with various IAV was assessed by blotting assay. Finally, their ability to inhibit IAV attachment to host cells was analyzed in vitro. Our result showed that the sialylated glycans on bLf were almost completely destroyed by sodium periodate treatment. Furthermore, the binding activity of desialylated bLf to IAV and the ability to inhibit IAV mimics binding to MDCK cells were significantly reduced compared to that of native bLf. These results demonstrated that the sialylated glycans on bLf could serve as competitive substrates to block IAV attachment to host cells during the early stages of viral infection. Our findings make an important contribute for the fully understanding of the mechanism of bLf in the prevention of IAV infections and their possible applications in antiviral infection.
Keywords: Influenza virus, Bovine lactoferrin, Sialylated glycans, Antiviral infection
Introduction
Influenza is an important cause of morbidity and mortality both in humans and animals caused by IAV infection. The binding of viral coat glycoprotein hemagglutinin (HA) to sialylated glycan receptor on host epithelial cells is a critical step in the infection and transmission of these viruses [1, 2]. Currently, vaccination and neuraminidase inhibitors are available methods to prevent and treat influenza [3, 4]. However, Influenza vaccines must be updated periodically due to the continuous antigenic drift and sporadic antigenic shift of the viral surface glycoproteins [5]. Unfortunately, the existing vaccines are unable to keep up with the mutation rates of virus [6, 7]. In addition, it also takes several years to develop vaccine for newly emerged viruses. At the same time, the virus is developing resistance to the currently available drugs [8]. Therefore, there are no immediate response drugs to the newly emerging viral outbreaks. In this context, there is an urgently need for the development of novel preventive and therapeutic compounds to control the viral outbreaks.
Attachment of pathogens to the cellular receptor is a prerequisite for most viral infections. Therefore, the use of receptor analogs as inhibitors is a simple antiviral strategy. In the presence of an excessed analogs at viral infection sites, there will be competition between cellular receptors and analogs for binding with viruses and subsequently reduces the interaction between host cell and viruses, thereby reducing the infection [9]. It is well known that IAV uses sialylated glycans on cell surface as a receptor to entry into the host cells. Using this property, studies have demonstrated that the sialylated oligosaccharides could function as influenza hemagglutinin blockers against influenza virus both in vitro and in vivo [10, 11].
Bovine lactoferrin (bLf), a nonheme iron-binding glycoprotein with a molecular weight of 80 kDa, was discovered in 1937 and isolated in 1960 from bovine milk [12]. It is composed of 696 amino acids that folded into globular carboxyl (C) and amino (N) terminal lobes [13]. The two lobes are further divided into two domains (C1 and C2, N1 and N2) and each lobe binds one Fe(III) ion in a deep cleft between two domains. The iron-binding ability is associated with its inhibitory effects on microbial growth and regulation of the motility, aggregation and biofilm formation of pathogenic bacteria [14]. BLf is mainly secreted by epithelial cells and is present in most mucosal secretions, including tears, saliva, vaginal and seminal fluids, nasal and bronchial secretions, and bile and gastric juices [15]. However, its highest concentration was found in milk, especially in colostrum up to 0.8 g/L [16].
BLf has shown toxicity against various enveloped and naked viruses such as influenza virus [17], human cytomegalovirus [18], herpes simplex virus 1 and 2 [19, 20], human immunodeficiency virus [18, 21], human hepatitis virus B and C [22, 23], respiratory syncytial virus [24], and newly emerging SARS-CoV-2 [25, 26]. Previous studies have been demonstrated that bLf exerts anti-influenza effects from several aspects. For example, bLf can bind to the HA of influenza virus, therefore preventing the fusion of the viral envelope with the cell membrane [27, 28]. bLf also inhibits IAV induced programmed cell death by interfering with caspase 3 and blocks nuclear export of viral ribonucleoproteins, therefore preventing viral assembly [29]. Moreover, bLf is able to prevent IAV cytopathic effects in desialylated, deglycosylated, apo, and ion-saturated forms [30]. However, the previous studies mainly focused on the late stages of viral infection. As for the roles of the sialylated glycans on bLf in the early stages of viral infection are still not completely clear.
Glycosylation is one of the most common post-translational modification of proteins, and plays an important role in protein’s biological function. It has been reported that bLf possess five potential glycosylation sites (Asn233, Asn281, Asn368, Asn476, Asn545), which are mostly exposed on the outer surface of the molecule and may contribute to its antiviral activity [31]. It was reported that the glycosylation of bLf has no effects on the inhibition of influenza virus replication [30]. It is possible that sialylated glycans contribute the antiviral effects of bLf by affecting the early stages of viral infection, which however yet to be explored. In this context, the present study aimed to determine the role of sialylated glycans on bLf in the early phases of influenza viral infection, and help to understand the biological significance of protein sialylation in the prevention of IAV infections and their possible applications in the antiviral infection.
Materials and methods
IAV preparation and cell culture
IAV strains including two H5N1 subtypes (A/Chicken/Guangxi/4/2009 (H5N1 CK), A/Duck/Guangdong/17/2008 (H5N1 DK)), two H5N2 subtypes (A/Mallard/Jiangxi/16/2005 (H5N2 M), A/Ostrich/Denmark/96–72,420/1996 (H5N2 Os)), one H7N1 subtype (A/Fowl/Rostock/45/1934 (H7N1 KP)), and one H7N2 subtype (A/Chicken/Hebei/1/2002 (H7N2 CK)) were kindly gifted by Dr. Xiurong Wang, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Science, Harbin, China. These viruses were cultured in allantoic fluid of 10 days old embryonated hen eggs and then purified on a discontinuous sucrose-density gradient as methods described previously[32, 33]. The MDCK (Madin-Darby canine kidney) cells (Cat No. CBP60575, Cobioer Biosciences Co., LTD, Nanjing, China) were cultured in DMEM medium (HyClone, Waltham, MA, USA) containing 10% (v/v) fetal bovine serum (GIBCO, Grand Island, NY, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C under 5% CO2.
Destruction of the sialylated glycans on bLf
BLf (Sigma-Aldrich) was treated with 10 mM sodium periodate at 4 °C for 30 min to destroy their sialylated glycans [34], the rest of sodium periodate was removed by an Ultra Centrifugal filter (Ultracel-10 k, Millipore Corp., Billerica, MA). After that, 5 μg native or desialylated bLf was subjected to 10% SDS-PAGE and silver nitrate staining or transferred to PVDF membranes. Subsequently, these membranes were blocked with 1 × Carbo-Free Blocking Solution and then incubated with 1 μg mL−1 of Cy5-labelled SNA or MAL-II (vector labs, Burlingame, CA, USA), Finally, these membranes were scanned by Storm 480 at the wavelength of 635 nm and further analyzed by Image J software (ImageJ 1.46r, National Institutes of Health, USA) fluorescence spec-troscopy.
Structure analysis of native and desialylated bLf
The effect of NaIO4 treatment on the protein structure of bLf was analyzed by fluorescence spectroscopy. Native or desialylated bLf was dissolved in deionized water. The fluorescence spectra of protein solution were recorded in the emission wavelength range from 300 to 600 nm (5 nm slit), with an excitation wavelength at 290 nm (5 nm slit) and a photomultiplier tube voltage of 600 V. The data were further analyzed by FL solution software.
Quantification of the sialic acids on native or desialylated bLf
The sialic acids on native or desialylated bLf were quantified using our previously modified periodate-resorcinol method[35]. In brief, the concentration of native or desialylated bLf were first quantified using the BCA assay, and adjusted to 1 mg mL−1. The bovine fetuin (F3385, Sigma-Aldrich) was used as a standard sialoglycoprotein, which was prepared with an initial concentration of 4 mg mL−1 and then serially diluted to 2, 1, 0.5, 0.25, 0.125, 0.0625, 0 mg mL−1. The assay was performed as follows: 100 μL of each sample was added to 20 μL of 0.04 M sodium periodate solution. The solutions were thoroughly mixed and allowed to stand in a 37 °C water bath for 60 min. After the addition of 250 μL of resorcinol reagent (0.06 g resorcinol in a solution containing 6 mL of 28% HCl, 4 mL of water, and 25 μmol of CuSO4), the solutions were mixed, placed in an ice bath for 5 min, heated at 100 °C for 15 min, cooled to room temperature, and 250 μL of tertbutyl alcohol were added. The solutions were mixed vigorously and placed in a 37 °C water bath for 3 min to stabilize the color, cooled to room temperature, and the absorbances were determined at 630 nm.
Evaluation of the binding ability of native or desialylated bLf to IAV
The viral proteins were extracted by an ether/ethanol solution, the protein concentration was quantified by BCA assay. Then, 100 μg viral proteins were incubated with 5μL Cy5 dye (GE healthcare, Amersham Biosciences) under gentle shaking at room temperature for 3 h without light. The Cy5 labelled proteins were purified by a G-25 desalt column (GE healthcare) according to the manufacturer’s instructions. After that, 5 μg native or desialylated bLf was subjected to 10% SDS-PAGE and transferred to PVDF membranes. Subsequently, these membranes were blocked with 1 × Carbo-Free Blocking Solution and then parallelly incubated with Cy5-labelled viral proteins (20 μg/mL in 1 × Carbo-Free Blocking Solution), and Cy5-labeled H1N1 influenza A vaccine (Split Virion, inactivated) (Sinovac Biotech Ltd., Beijing). Finally, these membranes were scanned by Storm 480 and further analyzed by Image J software.
Assessment of the role of sialylated glycans on bLf to against IAV
To assess the ability of native or desialylated bLf to against IAV. Maackia amurensis lectin II (MAL-II) and Sambucus nigra lectin (SNA) were used as HA mimics for avian and human IAV, respectively [35, 36]. The inhibitory effect of native or desialylated bLf on IAV attachment to MDCK cells was evaluated by lectin histochemistry [35]. In brief, the MDCK cells were seed into confocal culture dishes (JingAn Biotechnology Co., Ltd Shanghai China) and cultured in medium until 70–80% confluence was reached. After washing with PBS and immobilized by 4% paraformaldehyde, the cells in the dishes were blocked with PBS containing 5% BSA at room temperature for 1 h. The mixtures comprised 25 μg mL−1 of Cy5 labelled lectins (MAL-II or SNA) and 20 μg mL−1 of native or desialylated bLf were added into a parallel set of dishes and incubated without light at 4 °C overnight. Next, the MDCK cells in the culture dishes were stained by DAPI (Thermo Fisher Scientific, Waltham, MA, USA) and then photographed using a laser scanning confocal microscope (FV 1000, Olympus, Tokyo, Japan) under the merged channels of Cy5 and DAPI. The fluorescent intensity was analyzed by ImageJ software.
Statistical analysis
Data were expressed as mean ± SD and analyzed with GraphPad Prism 8 software. Comparations between two groups were measured by t-test. One-way ANOVA and post-LSD were used to analyze data among three groups.
Results
Verification of the sialylated glycans on native or desialylated bLf
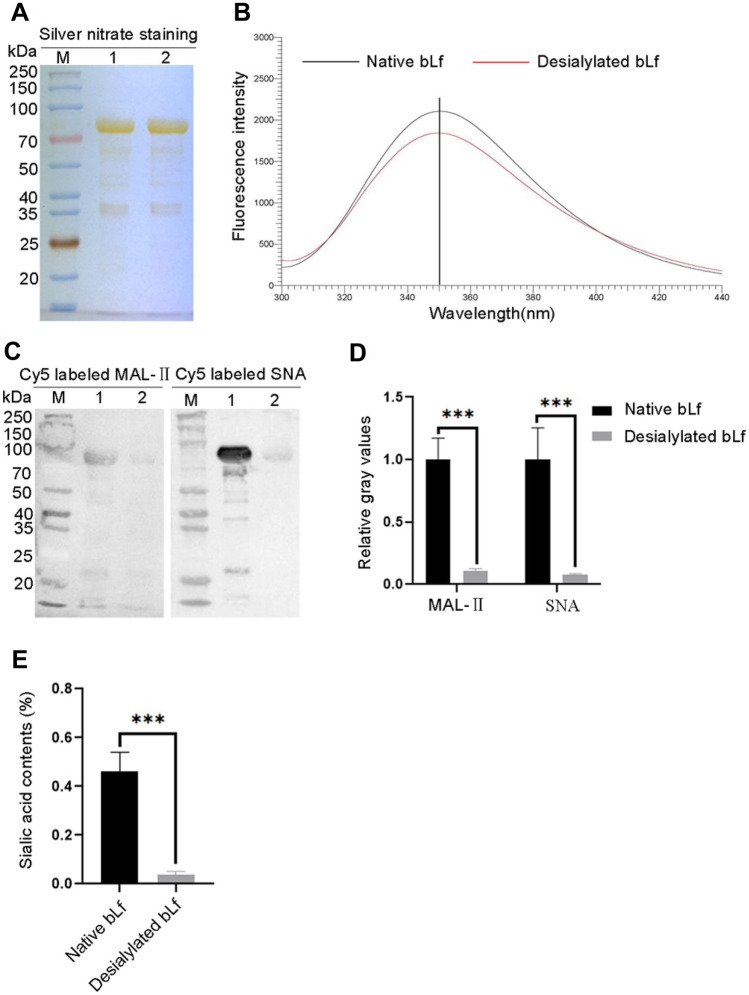
To confirm the destructive effect of sodium periodate treatment on the sialylated glycans of bLf, the binding ability of native or desialylated bLf with sialic acid-binding lectins (SNA and MAL-II) was evaluated by blotting assay. The results showed no obviously difference in molecular weight between native bLf and desialylated bLf (Fig. 1A). In addition, NaIO4 treatment did not affect the protein structure of bLf (Fig. 1B). However, the binding ability of desialylated bLf to SNA or MAL-II was significantly decreased compared to that of native bLf (Fig. 1C, 1D). As for the absolute content of the sialic acids on native or desialylated bLf, the sialic acids were quantified by periodate-resorcinol assay. The sialic acid content in native bLf was 0.462% of the protein compared 0.037% in the desialylated bLf, (Fig. 1E). These results indicated that the sialylated glycans on bLf were almost completely destroyed by sodium periodate treatment.
Fig. 1.
Verification of sialylated glycans on native or desialylated bLf. A Silver nitrate staining. B Fluorescence spectra. C Lectin blotting. Proteins were visualized by Cy5 labeled MAL-II (left) and Cy5 labeled SNA (right). D Gray value analysis. Relative gray values of lectin blotting were analysis from three replications by ImageJ software. E Quantification of sialic acid contents. Sialic acid contents of native or desialylated bLf were quantified and presented as a percentage of protein. M: protein molecular weight markers, lane 1: native bLf, lane 2: desialylated bLf. *P < 0.05, **P < 0.01, ***P < 0.001
Assessment of IAV bound to native or desialylated bLf
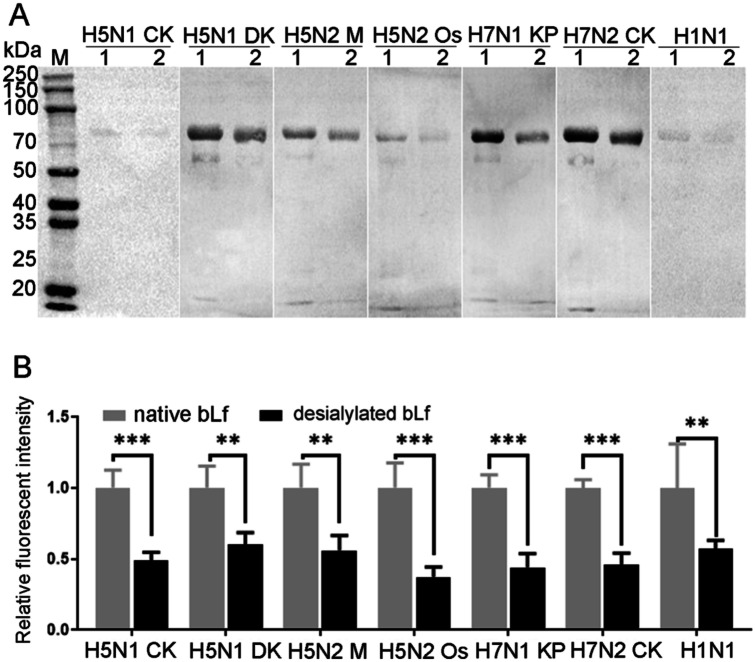
The binding activity of viral proteins extracted from avian IAV and human H1N1 vaccine to native or desialylated bLf were assessed by blotting analysis. The results showed that with the destruction of sialylated glycans on bLf, the binding ability of bLf to the viral proteins of six IAV strains (H5N1 CK, H5N1 DK, H5N2 M, H5N2 Os, H7N1 KP, H7N2 CK) as well as the human H1N1 vaccine decreased significantly (Fig. 2A). The fluorescence intensity of desialylated bLf bound to IAV or human H1N1 vaccine only retained 37 – 60% of that of native bLf (Fig. 2B). These results suggested that the sialylated glycans on bLf is vital for the binding ability of bLf with IAV.
Fig. 2.
Assessing the binding activity of native or desialylated bLf with IAV. A Viral proteins blotting. From left to right: Cy5-labeled viral proteins from H5N1 CK, H5N1 DK, H5N2 Os, H5N2 M, H7N1 KP, H7N2 CK, and H1N1 vaccine in turns. B The relative fluorescent intensity of native or desialylated bLf that bind with IAV. The fluorescent intensity in native bLf was deemed as 1 and compared to that of desialylated bLf. Data was measured from three replications by ImageJ software. M: protein molecular weight markers, lane 1: native bLf, lane 2: desialylated bLf. *P < 0.05, **P < 0.01, ***P < 0.001
Roles of the sialylated glycans on bLf against IAV
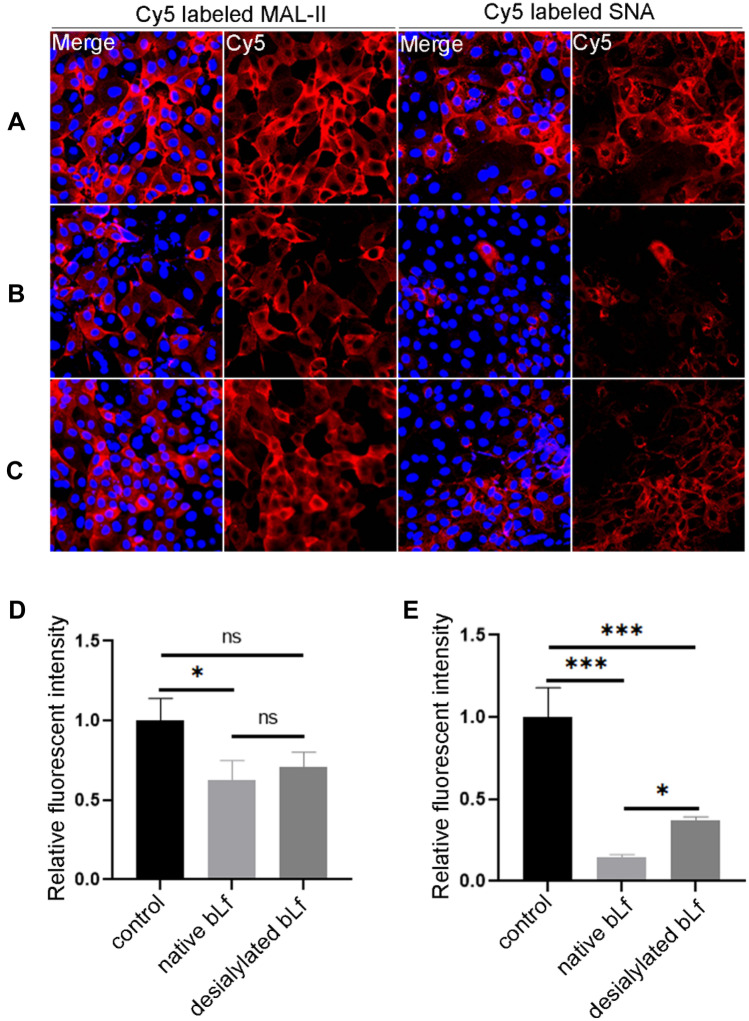
The ability of native or desialylated bLf to block the attachment of HA mimics to MDCK cells was assessed by lectin histochemistry. As shown in Fig. 3, both MAL-II and SNA could bind efficiently to the surface of MDCK cells (Fig. 3A). Notably, these binding can be inhibited when bLf was present in the system. Furthermore, the inhibitory effect of bLf was more pronounced on SNA than that of MAL-II (Fig. 3B). Interestingly, these inhibitions were reduced when the sialylated glycans of bLf were destroyed (Fig. 3C). The MAL-II and SNA bound to MDCK cells were reduced approximately 40% and 85% of the control when native bLf was present in the system. However, with the desialylation, the inhibition ability decreased to only 30% and 60% compared with that of control, respectively (Fig. 3D and 3E). These results indicated that the sialylated glycans on bLf could serves as competitive substrates to block IAV attachment to the host cells, therefore reducing the viral infection.
Fig. 3.
Evaluation of the roles of sialylated glycans on bLf against IAV. Histopathologic examination of Cy5 labeled MAL-II and SNA staining performed on MDCK cells. A Control: 25 μg mL−1 of Cy5 labeled MAL-II and SNA were incubated with immobilized MDCK cells. B 20 μg mL−1 native bLf, C 20 μg mL−1 desialylated bLf were added into the incubation solution. The images were acquired under the same conditions for the DAPI merge channel and the Cy5 channel. The relative fluorescent intensity of Cy5 labeled MAL-II D and SNA E bound to MDCK cells were analyzed by ImageJ software. The fluorescent intensity in control was deemed as 1 and compared to that of native or desialylated bLf. Data was measured from three replications. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Influenza is an acute respiratory disease in mammals and domestic poultry. About 20% of children and 5% of adults worldwide develop symptomatic influenza A or B each year [37]. The IAV infection is initiated by the binding of viral hemagglutinins to sialoglycoconjugates on the host cell surface [38]. Avian IAV preferentially recognizes α2,3-linked sialic acids, whereas human IAV preferentially recognizes α2,6-linked sialic acids [4]. Although vaccination and antiviral drugs are approved to the treatment and prevention of IAV infection, the emergence of resistant strains and the considerable side effects of drugs limit current therapeutic options [39]. Consequently, there is an urgently need to developed a novel preventive and therapeutic compound to control potential viral outbreaks. In addition to IAV, there are many human and animal pathogens including mumps, noro, rota, and DNA tumor viruses utilize sialic acid as a primary receptor to enter into the host cells[40]. In recent years, many studies have reported that sialoglycoconjugates as glycomimetics can be used to block infection by avian and human influenza viruses [10, 11, 41]. Here, we observed that bLf can bind with Cy5 labeled MAL-II and SNA (Fig. 1C), indicating that bLf possesses both α2,3-and α2,6-linked sialic acids. This result provides a reasonable probability of bLf acts as a receptor analogue for IAV and other sialic acid-binding viruses to block the attachment of virus to host cells.
BLf is a multifunctional glycoprotein and plays important roles in prevention of viral infection, such as influenza virus, canine herpesvirus, chikungunya and zika viruses, herpes simplex virus-1, and dengue virus [42–44]. As for the roles of the sialylated glycans on bLf in the early stages of viral infection are still not completely clear. Our results showed that bLf can directly bind with the viral proteins from avian IAV as well as human H1N1 vaccine (Fig. 2A). Moreover, bLf can block the attachment of IAV mimics to MDCK cells. And the inhibitory effect of bLf on SNA binding to MDCK cells was more pronounced than that of MAL-II (Fig. 3B). These may be due to the fact that bLf strongly binds to SNA than to MAL-II (Fig. 1C, 1D). As expected, the binding activity of bLf to viral proteins and inhibition of IAV mimics attachment to MDCK cells were reduced when the sialylated glycans on bLf were destroyed (Fig. 2 and Fig. 3). Although the inhibitory effect of native and desialylated bLf on MAL-II attachment to MDCK cells was not significantly different (Fig. 3C), the binding of Cy5 labelled MAL-II to MDCK cells tended to be enhanced after removal of sialic acids on bLf (Fig. 3D, 62% vs 71% of control). Together, our findings indicated that bLf can directly bind to the viral particles through its sialylated glycans at the early stages of viral infection, blocking the attachment of IAV to the host cells and thus reducing the viral infection.
In the present study, the native bLf was first treat with NaIO4 to destroy its sialylated glycans. Subsequently, the sialylated glycans were verified by lectin blotting and quantified by periodate-resorcinol method. Then, the binding ability of native or desialylated bLf to various IAV proteins were evaluated by blotting assay. Finally, the inhibitory effects of native or desialylated bLf on IAV attachment to MDCK cells were assessed by lectin histochemistry. The results showed that the binding ability of desialylated bLf to viral proteins as well as the ability to inhibit the attachment of IAV mimics to MDCK cells were diminished compared to that of native bLf. These results suggested that the sialylated glycans on bLf could serves as receptor analogs to block IAV attachment to host cells at early stage of viral infection.
Conclusions
This study provides evidences for the role of sialylated glycans on bLf prevent IAV infection. The sialylated glycans on bLf blocks IAV mimics attachment to MDCK cells at early stage of viral infection. The results provide new insights into the mechanism of bLf against IAV and possible roles of sialoglycoproteins in the anti-viral infection.
Author contributions
Zheng Li conceived and supervised the study. Xilong Wang, Jian Shu designed experiments. Lixin Yue, Jiajun Yang, and Zhuo Chen performed experiments. Xiurong Wang provided reagents. Xilong Wang, Lixin Yue, Liuyi Dang, Zheng Li analyzed data and wrote the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of Shaanxi Province (Grant No. 2021JQ-446).
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/10/2022
A Correction to this paper has been published: 10.1007/s10719-022-10071-x
References
- 1.Margine I, Krammer F. Animal models for influenza viruses: implications for universal vaccine development. Pathogens. 2014;3(4):845–874. doi: 10.3390/pathogens3040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo M. Influenza virus entry. Adv. Exp. Med. Biol. 2012;726:201–221. doi: 10.1007/978-1-4614-0980-9_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couch RB. A new antiviral agent for influenza—is there a clinical niche. N. Engl. J. Med. 1997;337(13):927–928. doi: 10.1056/NEJM199709253371310. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362(9397):1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster RG, Govorkova EA. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014;1323:115–139. doi: 10.1111/nyas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zappa, A., Amendola, A., Romano, L., Zanetti, A.: Emerging and re-emerging viruses in the era of globalisation. Blood transfusion = Trasfusione del sangue 7(3), 167–171 (2009). 10.2450/2009.0076-08 [DOI] [PMC free article] [PubMed]
- 7.Howard CR, Fletcher NF. Emerging virus diseases: can we ever expect the unexpected? Emerging microbes & infections. 2012;1(12):e46. doi: 10.1038/emi.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Vries E, Schutten M, Fraaij P, Boucher C, Osterhaus A. Influenza virus resistance to antiviral therapy. Adv. Pharmacol. 2013;67:217–246. doi: 10.1016/B978-0-12-405880-4.00006-8. [DOI] [PubMed] [Google Scholar]
- 9.Cozens D, Read RC. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert Rev. Anti Infect. Ther. 2012;10(12):1457–1468. doi: 10.1586/eri.12.145. [DOI] [PubMed] [Google Scholar]
- 10.Pandey RP, Kim DH, Woo J, Song J, Jang SH, Kim JB, Cheong KM, Oh JS, Sohng JK. Broad-spectrum neutralization of avian influenza viruses by sialylated human milk oligosaccharides: in vivo assessment of 3'-sialyllactose against H9N2 in chickens. Sci. Rep. 2018;8(1):2563–2575. doi: 10.1038/s41598-018-20955-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oka H, Onaga T, Koyama T, Guo CT, Suzuki Y, Esumi Y, Hatano K, Terunuma D, Matsuoka K. Sialyl alpha(2–>3) lactose clusters using carbosilane dendrimer core scaffolds as influenza hemagglutinin blockers. Bioorg. Med. Chem. Lett. 2008;18(15):4405–4408. doi: 10.1016/j.bmcl.2008.06.101. [DOI] [PubMed] [Google Scholar]
- 12.Groves ML. The Isolation of a Red Protein from Milk. J. Am. Chem. Soc. 1960;82(13):3345–3350. doi: 10.1021/ja01498a029. [DOI] [Google Scholar]
- 13.Wang B, Timilsena YP, Blanch E, Adhikari B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019;59(4):580–596. doi: 10.1080/10408398.2017.1381583. [DOI] [PubMed] [Google Scholar]
- 14.Berlutti F, Pantanella F, Natalizi T, Frioni A, Paesano R, Polimeni A, Valenti P. Antiviral properties of lactoferrin—a natural immunity molecule. Molecules. 2011;16(8):6992–7018. doi: 10.3390/molecules16086992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rascón-Cruz, Q., Espinoza-Sánchez, E.A., Siqueiros-Cendón, T.S., I.Nakamura-Bencomo, S., Arévalo-Gallegos, S., Iglesias-Figueroa, B.F.: Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules 26(1), 205–220 (2021). 10.3390/molecules26010205 [DOI] [PMC free article] [PubMed]
- 16.Kehoe SI, Jayarao BM, Heinrichs AJ. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J. Dairy Sci. 2007;90(9):4108–4116. doi: 10.3168/jds.2007-0040. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi, K., Wakabayashi, H., Shin, K., Takase, M.: Bovine lactoferrin: benefits and mechanism of action against infections. Biochemistry and cell biology = Biochimie et biologie cellulaire 84(3), 291–296 (2006). 10.1139/o06-054 [DOI] [PubMed]
- 18.Harmsen, M.C., Swart, P.J., Bethune, M.-P.d., Pauwels, R., Clercq, E.D., The, T.B., Meijer, D.K.F.: Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. The Journal of infectious diseases 172(2), 380–388 (1995). [DOI] [PubMed]
- 19.Fujihara.T, Hayashi.K: Lactoferrin inhibits herpes simplex virus type-1 (HSV-1) infection to mouse cornea. Archives of virology 140(8), 1469–1472 (1995). [DOI] [PubMed]
- 20.Marchetti M, Trybala E, Superti F, Johansson M, Bergstrom T. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology. 2004;318(1):405–413. doi: 10.1016/j.virol.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Puddu P, Borghi P, Gessani S, Valenti P, Belardelli F, Seganti L. Antiviral effect of bovine lactoferrin saturated with metal ions on early steps of human immunodeficiency virus type 1 infection. Int. J. Biochem. Cell Biol. 1998;30(9):1055–1062. doi: 10.1016/S1357-2725(98)00066-1. [DOI] [PubMed] [Google Scholar]
- 22.Hara K, Ikeda M, Saito S, Matsumoto S, Numata K, Kato N, Tanaka K, Sekihar H. Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol. Res. 2002;24(3):228–235. doi: 10.1016/S1386-6346(02)00088-8. [DOI] [PubMed] [Google Scholar]
- 23.Yi M, Kaneko S, Yu DY, Murakami S. Hepatitis C Virus Envelope Proteins Bind Lactoferrin. J. Virol. 1997;71(8):5597–6002. doi: 10.1128/jvi.71.8.5997-6002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sano H, Nagai K, Tsutsumi H, Kuroki Y. Lactoferrin and surfactant protein A exhibit distinct binding specificity to F protein and differently modulate respiratory syncytial virus infection. Eur. J. Immunol. 2003;33(10):2894–2902. doi: 10.1002/eji.200324218. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Meng X, Zhang F, Xiang Y, Wang J. The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerging microbes & infections. 2021;10(1):317–330. doi: 10.1080/22221751.2021.1888660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salaris C, Scarpa M, Elli M, Bertolini A, Guglielmetti S, Pregliasco F, Blandizzi C, Brun P, Castagliuolo I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients. 2021;13(2):328–339. doi: 10.3390/nu13020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammendolia MG, Agamennone M, Pietrantoni A, Lannutti F, Siciliano RA, De Giulio B, Amici C, Superti F. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus. Pathogens and Global Health. 2013;106(1):12–19. doi: 10.1179/2047773212y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Superti, F., Agamennone, M., Pietrantoni, A., Ammendolia, M.G.: Bovine Lactoferrin Prevents Influenza A Virus Infection by Interfering with the Fusogenic Function of Viral Hemagglutinin. Viruses 11(1) (2019). 10.3390/v11010051 [DOI] [PMC free article] [PubMed]
- 29.Pietrantoni A, Dofrelli E, Tinari A, Ammendolia MG, Puzelli S, Fabiani C, Donatelli I, Superti F. Bovine lactoferrin inhibits influenza A virus induced programmed cell death in vitro. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2010;23(3):465–475. doi: 10.1007/s10534-010-9323-3. [DOI] [PubMed] [Google Scholar]
- 30.Pietrantoni A, Ammendolia MG, Superti F. Bovine lactoferrin: involvement of metal saturation and carbohydrates in the inhibition of influenza virus infection. Biochem. Cell Biol. 2012;90(3):442–448. doi: 10.1139/o11-072. [DOI] [PubMed] [Google Scholar]
- 31.Karav S, German JB, Rouquie C, Le Parc A, Barile D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017;18(4):870–884. doi: 10.3390/ijms18040870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimer, C.B., Baker, R.S., Van Frank, R.M., Newlin, T.E., Cline, G.B., Anderson, N.G.: Purification of Large Quantities of Influenza Virus By Density Gradient Centrifugation. Journal of Virology 1(6), 1207–1216 (1967). [DOI] [PMC free article] [PubMed]
- 33.Steel J, Lowen AC, Pena L, Angel M, Solorzano A, Albrecht R, Perez DR, Garcia-Sastre A, Palese P. Live attenuated influenza viruses containing NS1 truncations as vaccine candidates against H5N1 highly pathogenic avian influenza. J Virol. 2009;83(4):1742–1753. doi: 10.1128/JVI.01920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim DS, Hosmillo M, Alfajaro MM, Kim JY, Park JG, Son KY, Ryu EH, Sorgeloos F, Kwon HJ, Park SJ, Lee WS, Cho D, Kwon J, Choi JS, Kang MI, Goodfellow I, Cho KO. Both alpha2,3- and alpha2,6-linked sialic acids on O-linked glycoproteins act as functional receptors for porcine Sapovirus. PLoS Pathog. 2014;10(6):e1004172. doi: 10.1371/journal.ppat.1004172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Ma T, Yu H, Chen Z, Zhu B, Chen W, Sun S, Li Z. Purification of sialoglycoproteins from bovine milk using serotonin-functionalized magnetic particles and their application against influenza A virus. Food Funct. 2020;11(8):6911–6920. doi: 10.1039/d0fo01447h. [DOI] [PubMed] [Google Scholar]
- 36.Yu H, Zhong Y, Zhang Z, Liu X, Zhang K, Zhang F, Zhang J, Shu J, Ding L, Chen W, Du H, Zhang C, Wang X, Li Z. Characterization of proteins with Siaalpha2-3/6Gal-linked glycans from bovine milk and role of their glycans against influenza A virus. Food Funct. 2018;9(10):5198–5208. doi: 10.1039/c8fo00950c. [DOI] [PubMed] [Google Scholar]
- 37.Turner, David, A., Wailoo, Allan, J., Nicholson, Karl, G., Cooper, Sutton, Alex, J.: Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technology Assessment 7(35), iii-iv, xi-xiii, 1–170 (2003). [DOI] [PubMed]
- 38.van Riel D, den Bakker MA, Leijten LM, Chutinimitkul S, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. Seasonal and pandemic human influenza viruses attach better to human upper respiratory tract epithelium than avian influenza viruses. Am. J. Pathol. 2010;176(4):1614–1618. doi: 10.2353/ajpath.2010.090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, Zhang Y, Shu Y, Gubareva LV, Cox NJ, Klimov AI. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007;196(2):249–257. doi: 10.1086/518936. [DOI] [PubMed] [Google Scholar]
- 40.Matrosovich M, Herrler G, Klenk HD. Sialic Acid Receptors of Viruses. In: Gerardy-Schahn R, Delannoy P, von Itzstein M, editors. SialoGlyco Chemistry and Biology II: Tools and Techniques to Identify and Capture Sialoglycans. Cham: Springer International Publishing; 2015. pp. 1–28. [Google Scholar]
- 41.Ogata M, Hidari KIPJ, Murata T, Shimada S, Kozaki W, Park EY, Suzuki T, Usui T. Chemoenzymatic Synthesis of Sialoglycopolypeptides As Glycomimetics to Block Infection by Avian and Human Influenza Viruses. Bioconjug. Chem. 2009;20(3):538–549. doi: 10.1021/bc800460p. [DOI] [PubMed] [Google Scholar]
- 42.Carvalho CAM, Casseb SMM, Goncalves RB, Silva EVP, Gomes AMO, Vasconcelos PFC. Bovine lactoferrin activity against Chikungunya and Zika viruses. J. Gen. Virol. 2017;98(7):1749–1754. doi: 10.1099/jgv.0.000849. [DOI] [PubMed] [Google Scholar]
- 43.Marr AK, Jenssen H, Moniri MR, Hancock RE, Pante N. Bovine lactoferrin and lactoferricin interfere with intracellular trafficking of Herpes simplex virus-1. Biochimie. 2009;91(1):160–164. doi: 10.1016/j.biochi.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Chen JM, Fan YC, Lin JW, Chen YY, Hsu WL, Chiou SS. Bovine Lactoferrin Inhibits Dengue Virus Infectivity by Interacting with Heparan Sulfate, Low-Density Lipoprotein Receptor, and DC-SIGN. Int. J. Mol. Sci. 2017;18(9):1957–1969. doi: 10.3390/ijms18091957. [DOI] [PMC free article] [PubMed] [Google Scholar]